The Role of Proton-Coupled Amino Acid Transporter 2 (SLC36A2) in Cold-Induced Thermogenesis of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Usage and Ethics
2.2. Preadipocyte Isolation and Adipogenic Differentiation In Vitro
2.3. Body Composition Measurement
2.4. Indirect Calorimetry
2.5. Treadmill
2.6. Lipid Measurement in Serum
2.7. Lipid Measurement in Liver
2.8. RNA Extraction and Relative Gene Expression
2.9. Statistical Analysis
3. Results
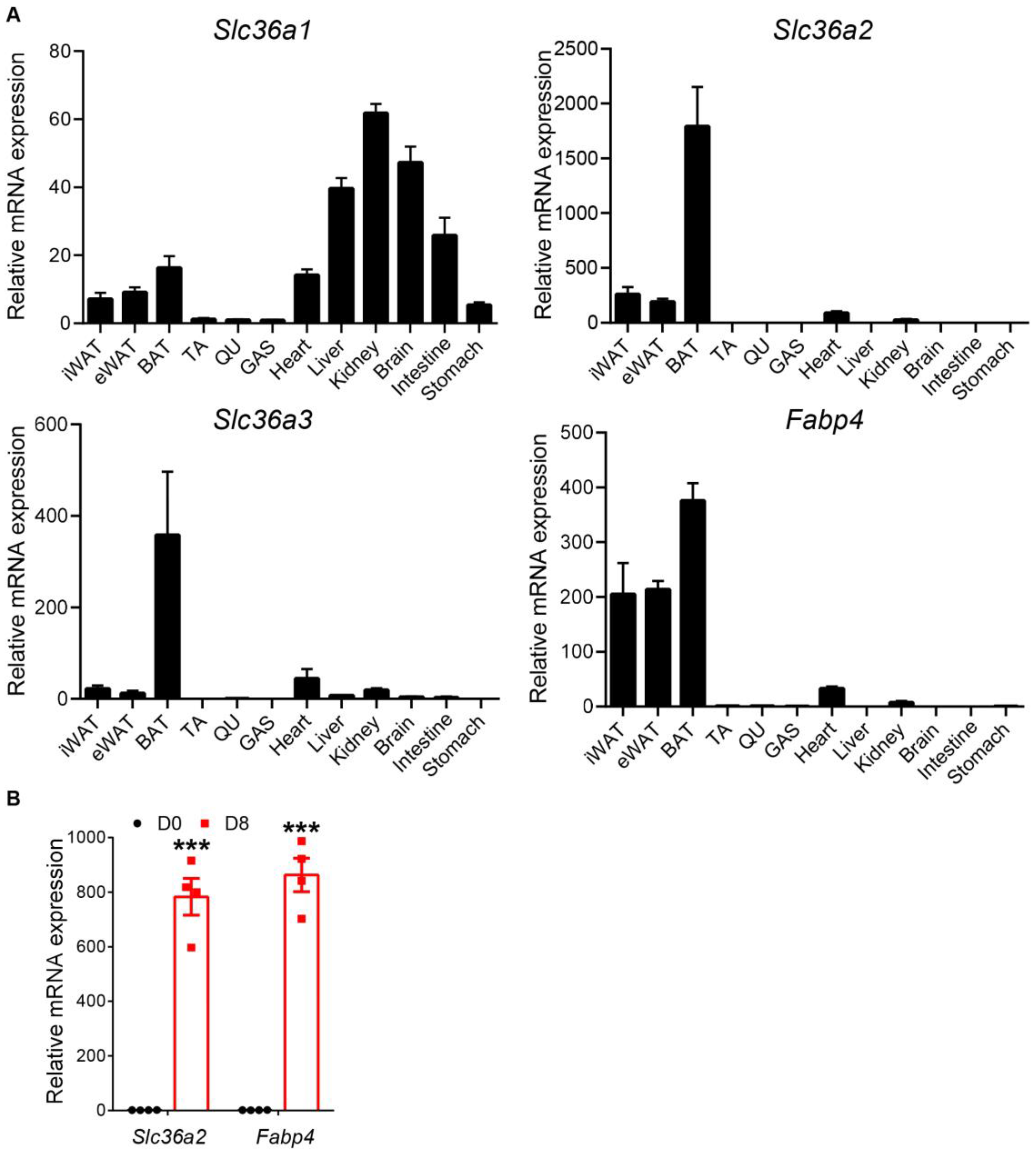
3.1. Slc36a2 and Slc36a3 Are Highly Expressed in Adipose Tissue and Upregulated during Adipocyte Differentiation
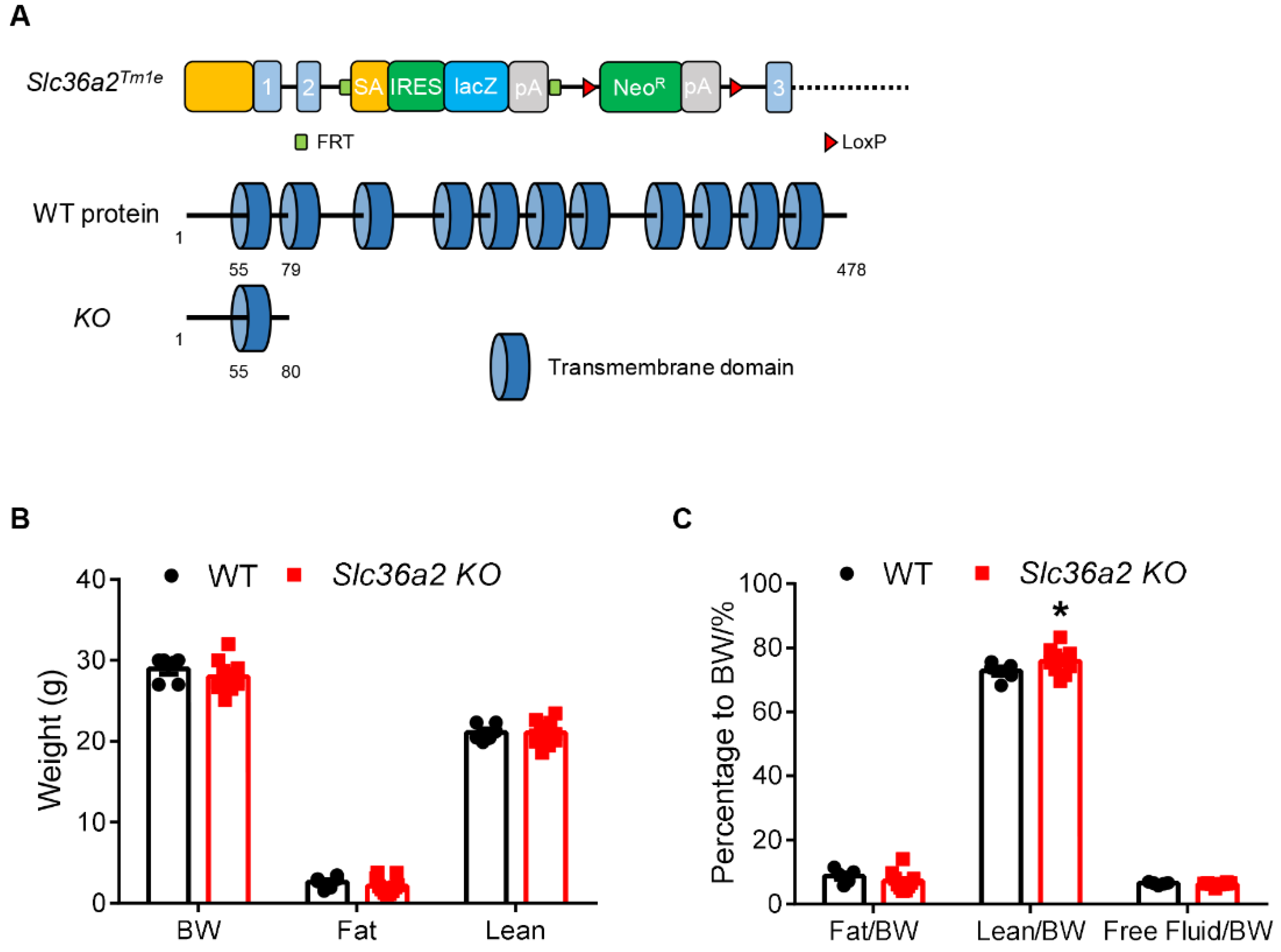
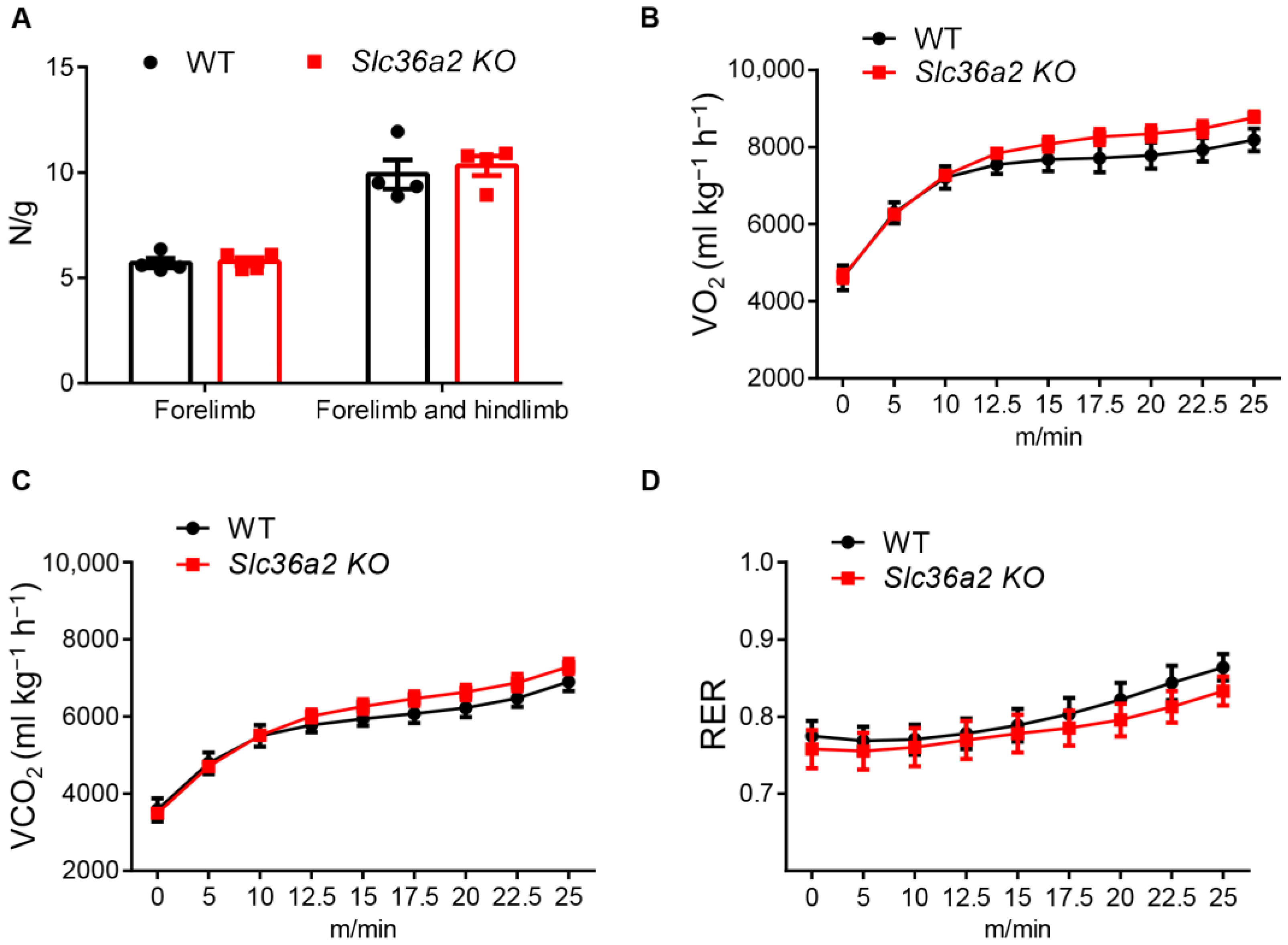
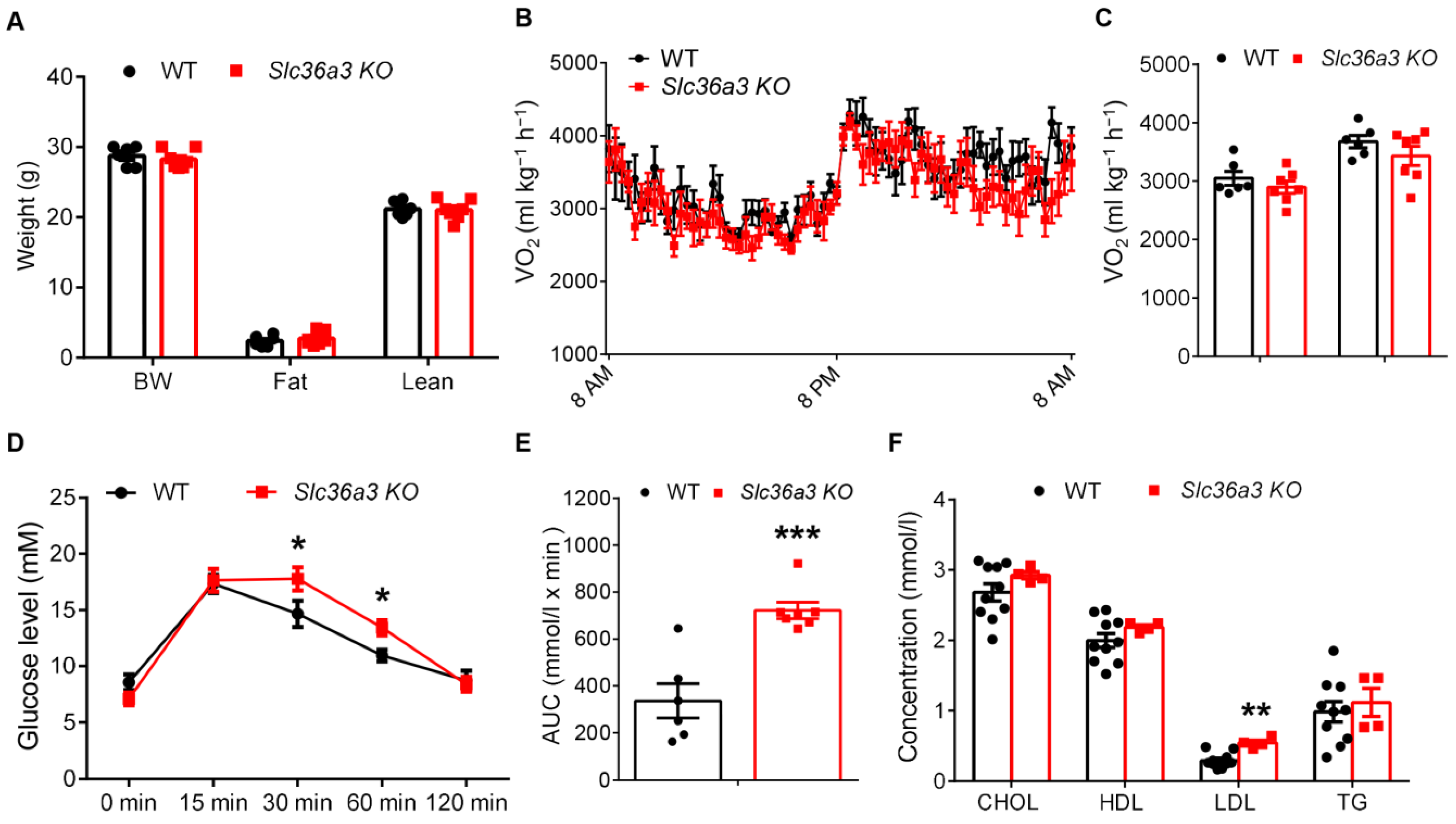
3.2. Global Knockout of Slc36a2 Has Minor Effect on Body Composition and Muscle Performance
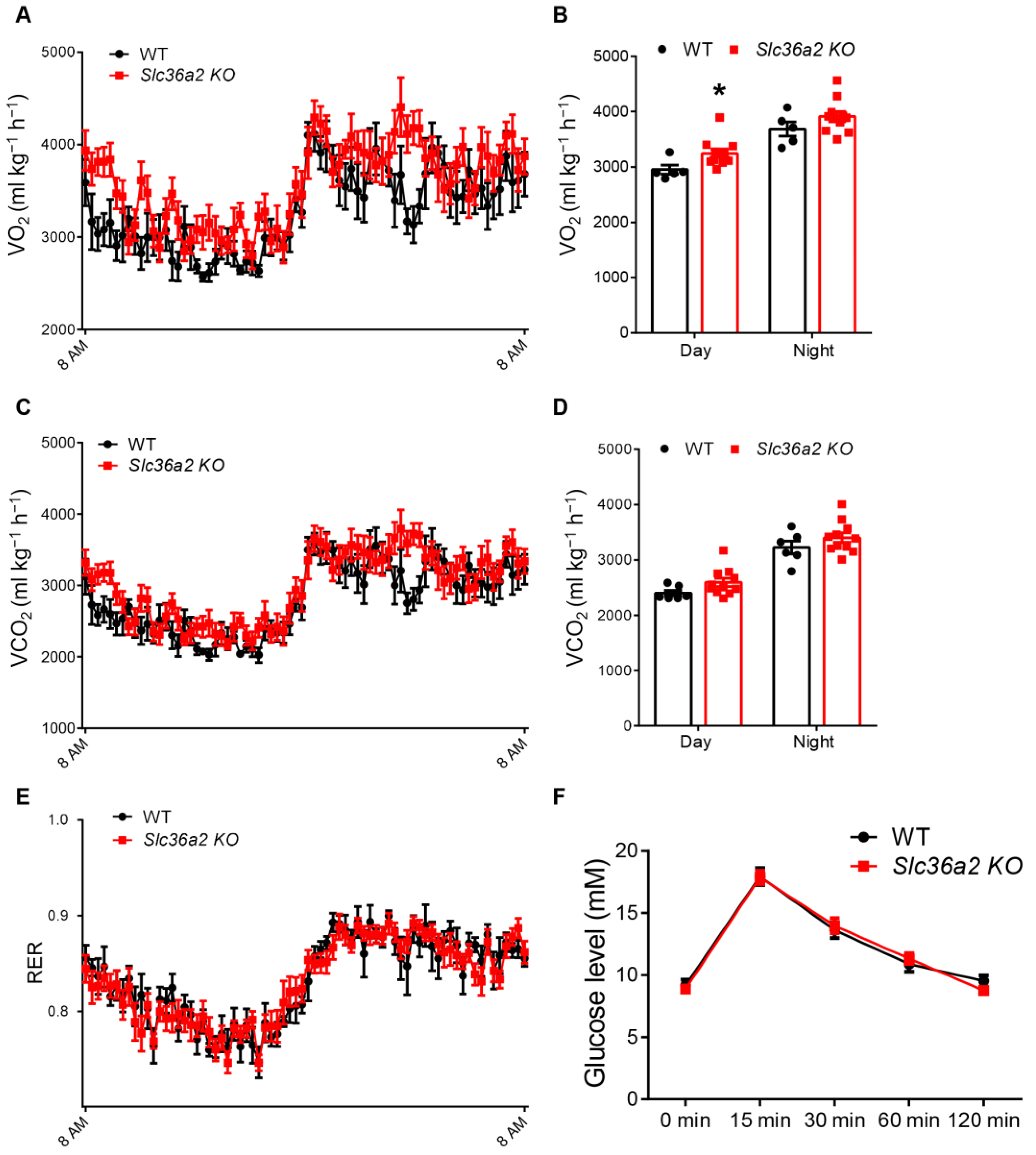
3.3. Slc36a2 Knockout Elevates the Oxygen Consumption at Daytime
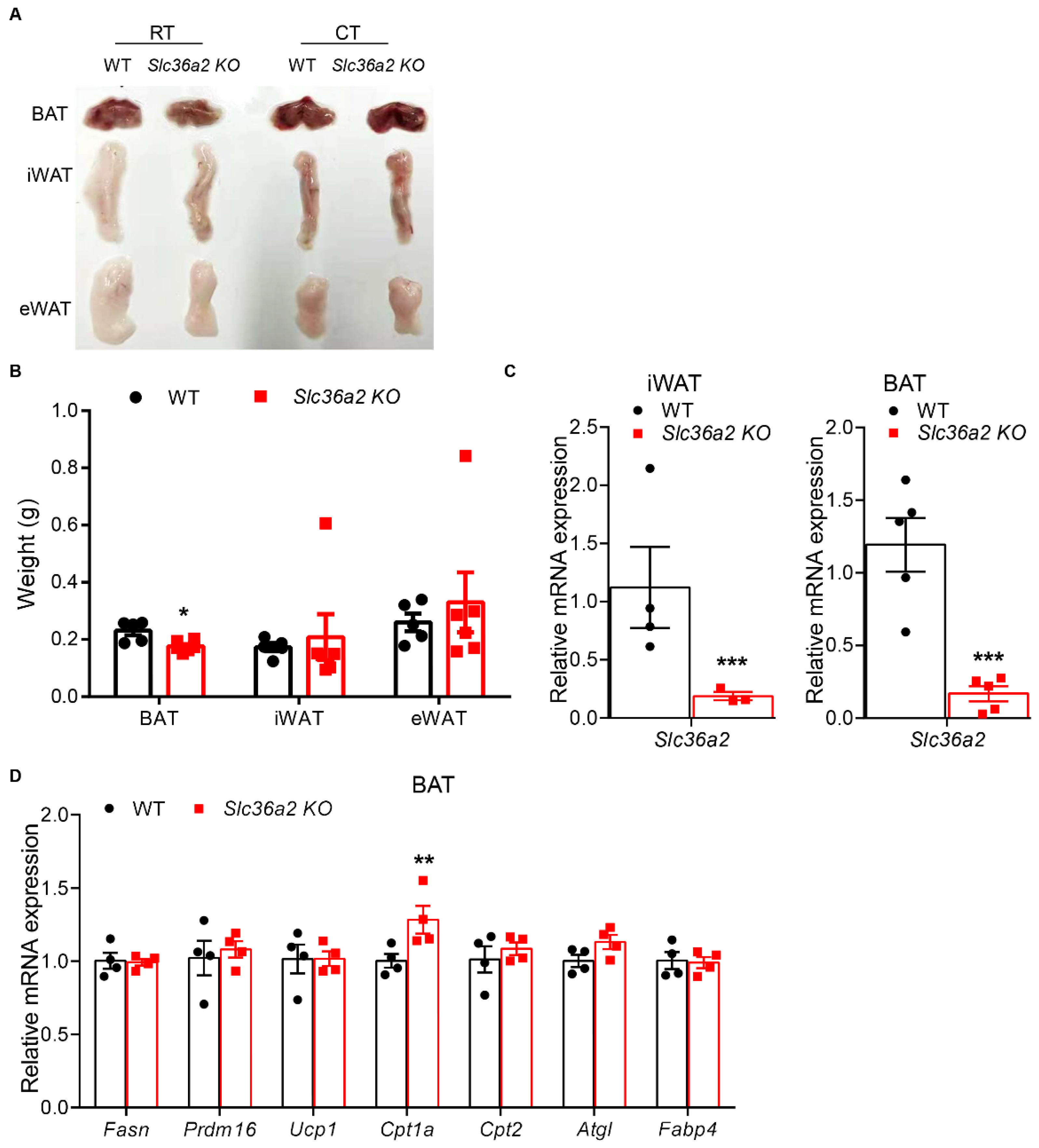
3.4. Slc36a2 Knockout Mice Had Smaller BAT and Increased Cpt1α Expression after Cold Treatment
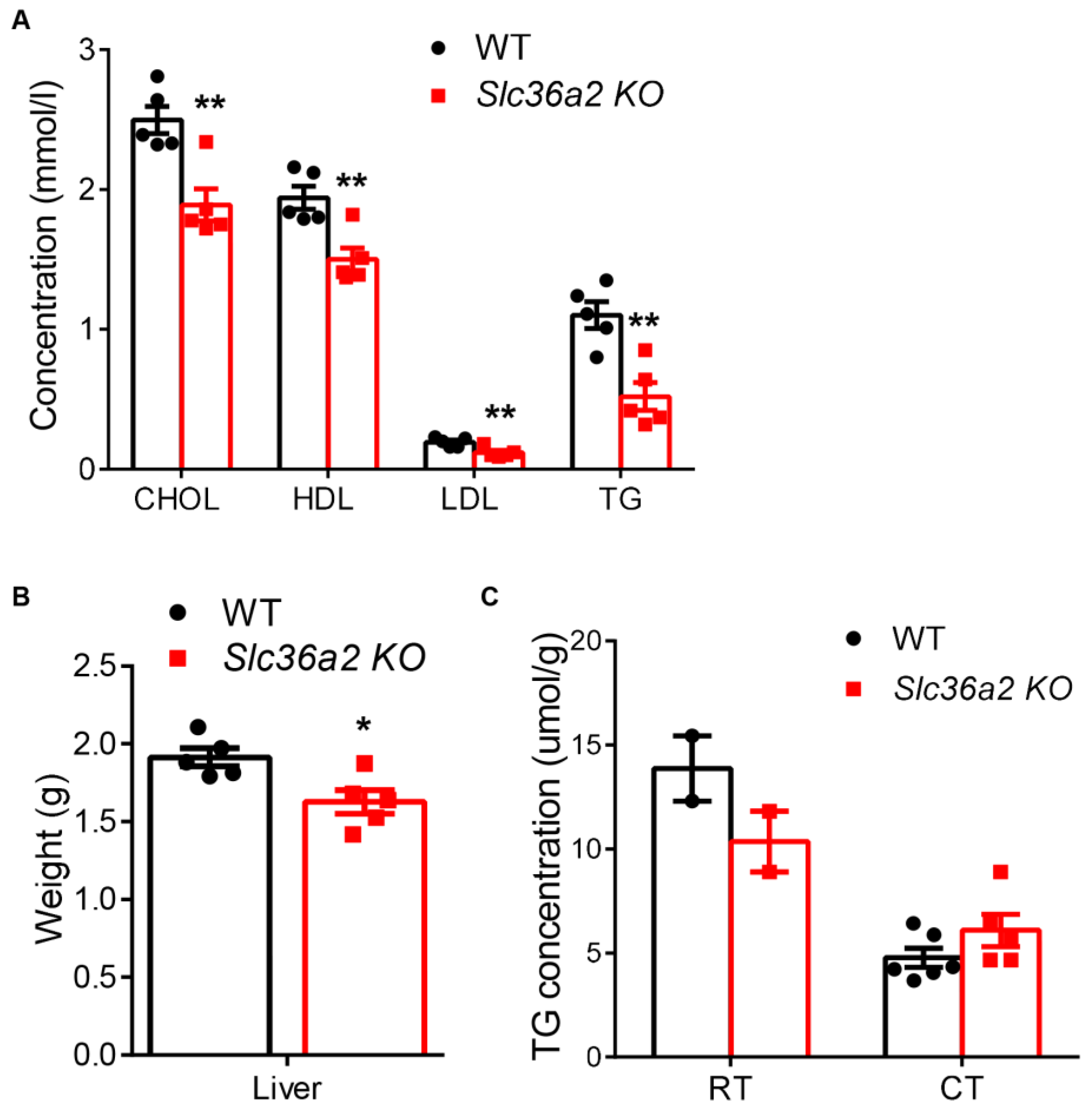
3.5. Loss of Slc36a2 Lowers Lipid Concentrations in Circulation and Liver after Cold Treatment
3.6. Knockout of Slc36a3 Impairs Glucose Tolerance and Increases Serum Lipid
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikeda, K.; Maretich, P.; Kajimura, S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Carpentier, A.C.; Richard, D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 2013, 24, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, A.; Grønning, L.M.; Ahrén, B.; Taskén, K.; Carlsson, P.; Enerbäck, S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of white fat: Novel insight into factors, mechanisms, and therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef]
- Roh, H.C.; Tsai, L.T.; Shao, M.; Tenen, D.; Shen, Y.; Kumari, M.; Lyubetskaya, A.; Jacobs, C.; Dawes, B.; Gupta, R.K.; et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab. 2018, 27, 1121–1137.e5. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Peijs, L.; Beaudry, J.L.; Jespersen, N.Z.; Nielsen, C.H.; Ma, T.; Brunner, A.D.; Larsen, T.J.; Bayarri-Olmos, R.; Prabhakar, B.S.; et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 2019, 30, 963–975.e7. [Google Scholar] [CrossRef]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and function of stress-induced IL-6 in murine models. Cell 2020, 182, 372–387.e14. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.A.; Liu, Y.; Jiang, L. Energy metabolism in brown adipose tissue. FEBS J. 2021, 288, 3647–3662. [Google Scholar] [CrossRef]
- Guo, F.; Cavener, D.R. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007, 5, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.-J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. Biochem. 2009, 139, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Onogi, Y.; Ussar, S. Regulatory networks determining substrate utilization in brown adipocytes. Trends Endocrinol. Metab. 2022, 33, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016, 532, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Anderson, C.M. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011, 164, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-J.; Goberdhan, D.C. PATs and SNATs: Amino acid sensors in disguise. Front. Pharmacol. 2018, 9, 640. [Google Scholar] [CrossRef]
- Thwaites, D.T.; Anderson, C.M. Deciphering the mechanisms of intestinal imino (and amino) acid transport: The redemption of SLC36A1. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 179–197. [Google Scholar] [CrossRef]
- Larsen, M.; Larsen, B.B.; Frølund, B.; Nielsen, C.U. Transport of amino acids and GABA analogues via the human proton-coupled amino acid transporter, hPAT1: Characterization of conditions for affinity and transport experiments in Caco-2 cells. Eur. J. Pharm. Sci. 2008, 35, 86–95. [Google Scholar] [CrossRef]
- Larsen, M.; Holm, R.; Jensen, K.; Brodin, B.; Nielsen, C.U. Intestinal gaboxadol absorption via PAT1 (SLC36A1): Modified absorption in vivo following co-administration of L-tryptophan. Br. J. Pharmacol. 2009, 157, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Vanslambrouck, J.M.; Bröer, A.; Thavyogarajah, T.; Holst, J.; Bailey, C.G.; Bröer, S.; Rasko, J.E.J. Renal imino acid and glycine transport system ontogeny and involvement in developmental iminoglycinuria. Biochem. J. 2010, 428, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Al Harbi, M.; Al Mutairi, F. Heterozygous mutation in SLC36A2 gene causing hyperglycinuria and nephrolithiasis. J. Biochem. Clin. Genet. 2019, 2, 74–76. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Boll, M.; Weisenhorn, D.M.V.; Foltz, M.; Kottra, G.; Daniel, H. The proton/amino acid cotransporter PAT2 is expressed in neurons with a different subcellular localization than its paralog PAT1. J. Biol. Chem. 2004, 279, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Ussar, S.; Lee, K.Y.; Dankel, S.N.; Boucher, J.; Haering, M.F.; Kleinridders, A.; Thomou, T.; Xue, R.; Macotela, Y.; Cypess, A.M.; et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 2014, 6, 247ra103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Onogi, Y.; Krueger, M.; Oeckl, J.; Karlina, R.; Singh, I.; Hauck, S.M.; Feederle, R.; Li, Y.; Ussar, S. PAT2 regulates vATPase assembly and lysosomal acidification in brown adipocytes. Mol. Metab. 2022, 61, 101508. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.M.; Meredith, D. SLC36A4 (hPAT4) is a high affinity amino acid transporter when expressed in Xenopus laevis oocytes. J. Biol. Chem. 2011, 286, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Boll, M.; Foltz, M.; Rubio-Aliaga, I.; Daniel, H. A cluster of proton/amino acid transporter genes in the human and mouse genomes☆. Genomics 2003, 82, 47–56. [Google Scholar] [CrossRef]
- Song, A.; Dai, W.; Jang, M.J.; Medrano, L.; Li, Z.; Zhao, H.; Shao, M.; Tan, J.; Li, A.; Ning, T.; et al. Low-and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Investig. 2020, 130, 247–257. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Brer, S.; Bailey, C.G.; Kowalczuk, S.; Ng, C.; Rasko, J.E.J. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J. Clin. Investig. 2009, 118, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.; Anderson, C.M.; Gatfield, K.M.; Jevons, M.P.; Ganapathy, V.; Thwaites, D.T. Amino acid derivatives are substrates or non-transported inhibitors of the amino acid transporter PAT2 (slc36a2). Biochim. Biophys. Acta-Biomembr. 2011, 1808, 260–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagano, T.; Nakashima, A.; Onishi, K.; Kawai, K.; Awai, Y.; Kinugasa, M.; Iwasaki, T.; Kikkawa, U.; Kamada, S. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species authors. Developmental 2017, 144, 1413–1420. [Google Scholar]
- Goncalves, R.L.; Rothschild, D.E.; Quinlan, C.L.; Scott, G.K.; Benz, C.C.; Brand, M.D. Sources of superoxide/H2O2 during mitochondrial proline oxidation. Redox Biol. 2014, 2, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Lettieri Barbato, D.; Aquilano, K.; Baldelli, S.; Cannata, S.M.; Bernardini, S.; Rotilio, G.; Ciriolo, M.R. Proline oxidase-adipose triglyceride lipase pathway restrains adipose cell death and tissue inflammation. Cell Death Differ. 2014, 21, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Barbato, D.L.; Aquilano, K. Feast and Famine: Adipose Tissue Adaptations for Healthy Aging. Ageing Res. Rev. 2016, 28, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczyńska, M.G.; Paranjpe, I.; Wainwright, G.L.; Bétourné, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 2017, 25, 811–822. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Kirichok, Y. Mitochondrial H+ Leak and Thermogenesis. Annu. Rev. Physiol. 2022, 84, 381–407. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Kirichok, Y. UCP1: A transporter for H+ and fatty acid anions. Biochimie 2017, 134, 28–34. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Gatfield, K.M.; Winpenny, J.P.; Ganapathy, V.; Thwaites, D.T. Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2). Br. J. Pharmacol. 2010, 144, 28–41. [Google Scholar] [CrossRef]
- Zebisch, K.; Brandsch, M. Transport of l-proline by the proton-coupled amino acid transporter PAT2 in differentiated 3T3-L1 cells. Amino Acids 2013, 44, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F.; Nakamura, K.; Madden, C.J. Central control of thermogenesis in mammals. Exp. Physiol. 2008, 93, 773–797. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central control of brown adipose tissue thermogenesis. Front. Endocrinol. 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Choii, G.; Um, J.W. The balancing act of GABAergic synapse organizers. Trends Mol. Med. 2015, 21, 256–268. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kawabata, T.; Okazaki, K.; Suzuki, T.; Imai, D.; Hamamoto, T.; Matsumura, S.; Miyagawa, T. Oral administration of γ-aminobutyric acid affects heat production in a hot environment in resting humans. J. Physiol. Anthropol. 2012, 31, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikegami, R.; Shimizu, I.; Sato, T.; Yoshida, Y.; Hayashi, Y.; Suda, M.; Katsuumi, G.; Li, J.; Wakasugi, T.; Minokoshi, Y.; et al. Gamma-aminobutyric acid signaling in brown adipose tissue promotes systemic metabolic derangement in obesity. Cell Rep. 2018, 24, 2827–2837.e5. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, J.R.; Pennington, J. Organization and expression of the SLC36 cluster of amino acid transporter genes. Mamm. Genome 2004, 15, 114–125. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Z.; Zhang, C.; Liu, X.; Zhang, J.; Shu, H.; Ma, Y.; Liu, Z.; Feng, Y.; Chen, X.; et al. Hepatic Acat2 overexpression promotes systemic cholesterol metabolism and adipose lipid metabolism in mice. Diabetologia 2022, 66, 390–405. [Google Scholar] [CrossRef]
- Rigby, M.J.; Orefice, N.S.; Lawton, A.J.; Ma, M.; Shapiro, S.L.; Yi, S.Y.; Dieterich, I.A.; Frelka, A.; Miles, H.N.; Pearce, R.A.; et al. Increased expression of SLC25A1/CIC causes an autistic-like phenotype with altered neuron morphology. Brain 2022, 145, 500–516. [Google Scholar] [CrossRef]
| Primer | Sequence (5′–3′) |
|---|---|
| Real-time PCR | |
| qSlc36a1 | F: ATCAGGAACCTGCGTGTGTT |
| R: GTCTTCCATGGAGCCACCAA | |
| qSlc36a2 | F: GACCAAGAGTGCCAGGAGTC |
| R: CCGGTTATGCCCTTGGTCTT | |
| qSlc36a3 | F: AATGTGCCGCTGCTTAGAGA |
| R: TTGAGGAGGCTGTAGACCGA | |
| qSlc36a4 | F: TGGGATACGGTCCCTCTTGG |
| R: GGGCTAGTGTACTGCTGCTC | |
| qUcp1 | F: AGGCTTCCAGTACCATTAGGT |
| R: CTGAGTGAGGCAAAGCTGATTT | |
| qFasn | F: GGAGGTGGTGATAGCCGGTAT |
| R: TGGGTAATCCATAGAGCCCAG | |
| qPrdm16 | F: CCACCAGCGAGGACTTCAC |
| R: CCACCAGCGAGGACTTCAC | |
| qFabp4 | F: AAGGTGAAGAGCATCATAACCCT |
| R: TCACGCCTTTCATAACACATTCC | |
| qAtgl | F: CTGAGAATCACCATTCCCACATC |
| R: CACAGCATGTAAGGGGGAGA | |
| qCpt2 | F: CAGCACAGCATCGTACCCA |
| R: TCCCAATGCCGTTCTCAAAAT | |
| qCpt1α | F: CTCCGCCTGAGCCATGAAG |
| R: CACCAGTGATGATGCCATTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.; Zhang, J.; Cheng, D.; Zhao, X.; Ma, Y.; Zhang, C.; Zhang, Y.; Jia, Z.; Liu, Z. The Role of Proton-Coupled Amino Acid Transporter 2 (SLC36A2) in Cold-Induced Thermogenesis of Mice. Nutrients 2023, 15, 3552. https://doi.org/10.3390/nu15163552
Shu H, Zhang J, Cheng D, Zhao X, Ma Y, Zhang C, Zhang Y, Jia Z, Liu Z. The Role of Proton-Coupled Amino Acid Transporter 2 (SLC36A2) in Cold-Induced Thermogenesis of Mice. Nutrients. 2023; 15(16):3552. https://doi.org/10.3390/nu15163552
Chicago/Turabian StyleShu, Hui, Jie Zhang, Dawei Cheng, Xiaorui Zhao, Yue Ma, Chi Zhang, Yong Zhang, Zhihao Jia, and Zhiwei Liu. 2023. "The Role of Proton-Coupled Amino Acid Transporter 2 (SLC36A2) in Cold-Induced Thermogenesis of Mice" Nutrients 15, no. 16: 3552. https://doi.org/10.3390/nu15163552
APA StyleShu, H., Zhang, J., Cheng, D., Zhao, X., Ma, Y., Zhang, C., Zhang, Y., Jia, Z., & Liu, Z. (2023). The Role of Proton-Coupled Amino Acid Transporter 2 (SLC36A2) in Cold-Induced Thermogenesis of Mice. Nutrients, 15(16), 3552. https://doi.org/10.3390/nu15163552







