Effect of a Probiotic and a Synbiotic on Body Fat Mass, Body Weight and Traits of Metabolic Syndrome in Individuals with Abdominal Overweight: A Human, Double-Blind, Randomised, Controlled Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
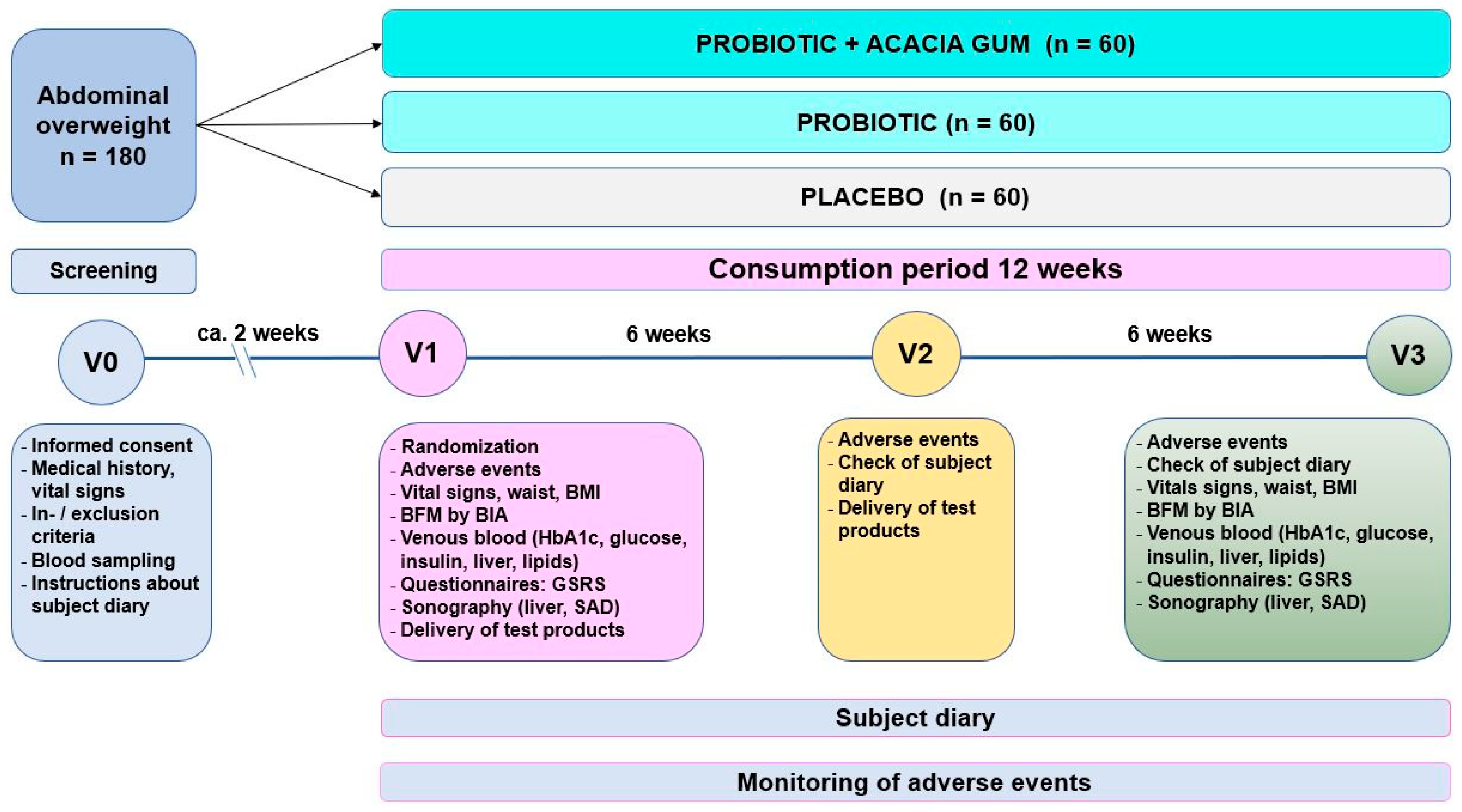
2.2. Study Design and Conduct
2.3. Subject Selection and Withdrawal
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Test Products
2.6.1. Placebo
2.6.2. Probiotic Test Product
2.6.3. Synbiotic Test Product
2.6.4. Mode of Consumption
2.7. Assessments
2.7.1. Bioelectrical Impedance Analysis (BIA)
2.7.2. Visceral Adiposity Index (VAI)
2.7.3. Anthropometry
2.7.4. Blood Pressure
2.7.5. Serum Parameters
2.7.6. Sonography
2.7.7. Medication
2.7.8. Gastrointestinal Symptoms
2.7.9. Compliance
2.7.10. Adverse Events (AEs)
- Not related: No investigational product was taken, or the AE could be ascribed with reasonable certainty to another cause.
- Unlikely: There were good reasons to think that there was no relationship.
- Possible: Equally valid arguments could be considered for or against an implication of the study product.
- Probable: The relationship was likely.
- Certain (definitely): There was strong relationship.
2.8. Statistics
2.8.1. Determination of Sample Size
2.8.2. Definition of Sets to Be Analysed
Full Analysis Set (FAS)
- Violation of an essential and, before randomization, objectively measurable inclusion criterion.
- Not taking a single dose of the test substance (without knowledge of the assigned test group).
- Lack of any dates for the assessment of effectiveness after randomization.
Intention-To-Treat (ITT) Set
Per-Protocol (PP) Set
2.8.3. Statistical Tests
2.8.4. Definition of Primary, Secondary and Exploratory Data
2.8.5. Data Screening and Transformation
2.8.6. Approach to Treatment of Missing Values
2.8.7. Database Generation and Management
- The database was compiled after the last test person had completed the study.
- Data from paper documentation (CRFs, questionnaires) were transferred to electronic files by two persons each (double data entry), and the files were compared for discrepancies.
- Discrepancies between entries were corrected.
2.8.8. Interim Analysis
2.8.9. Software
3. Results
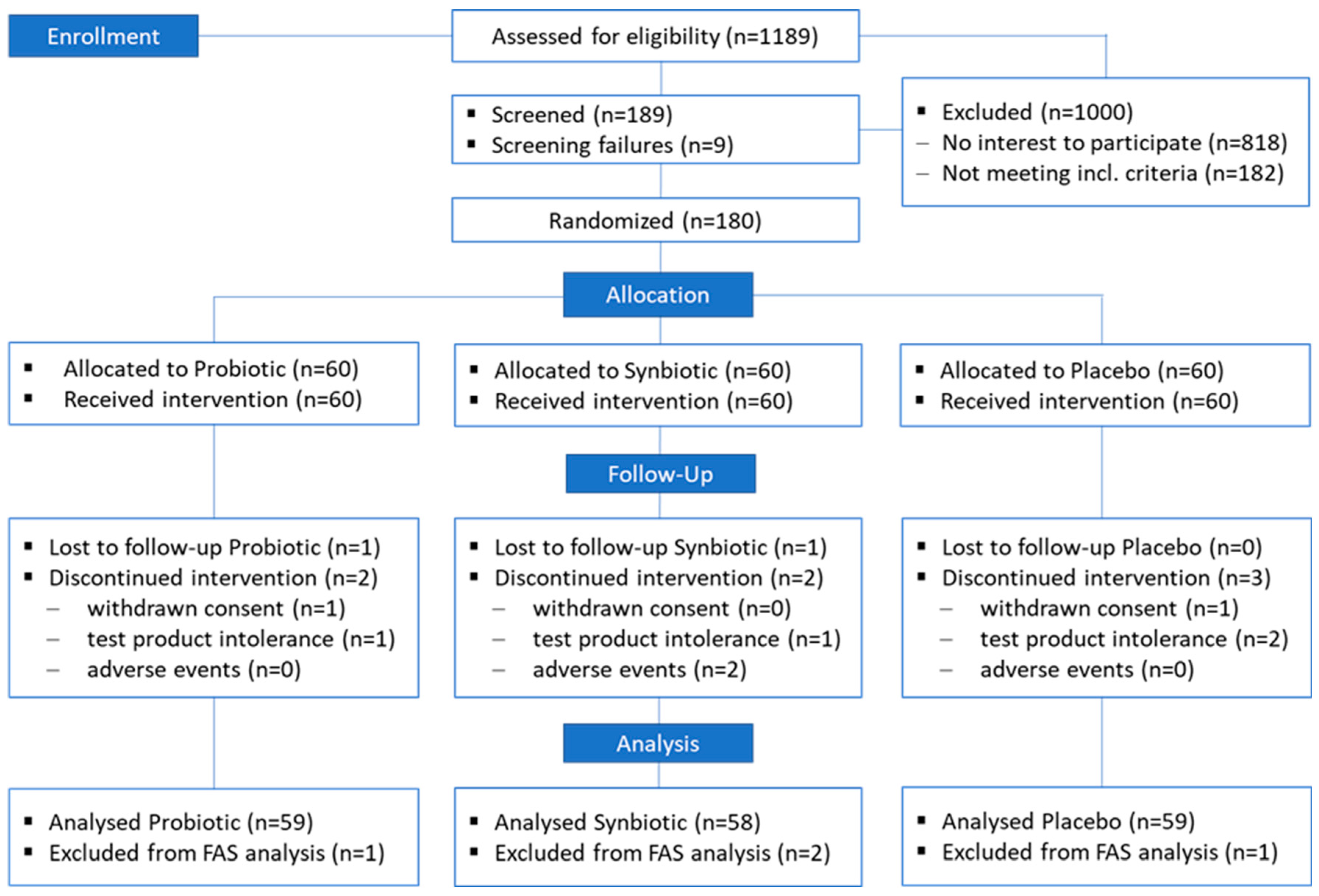
3.1. Key Data of Study Conduct
3.2. Study Populations
3.2.1. ITT Population
3.2.2. FAS Population
3.2.3. PP Population
3.3. Missing Values
3.4. Baseline Characteristics of the FAS Population
3.5. Alterations during Intervention
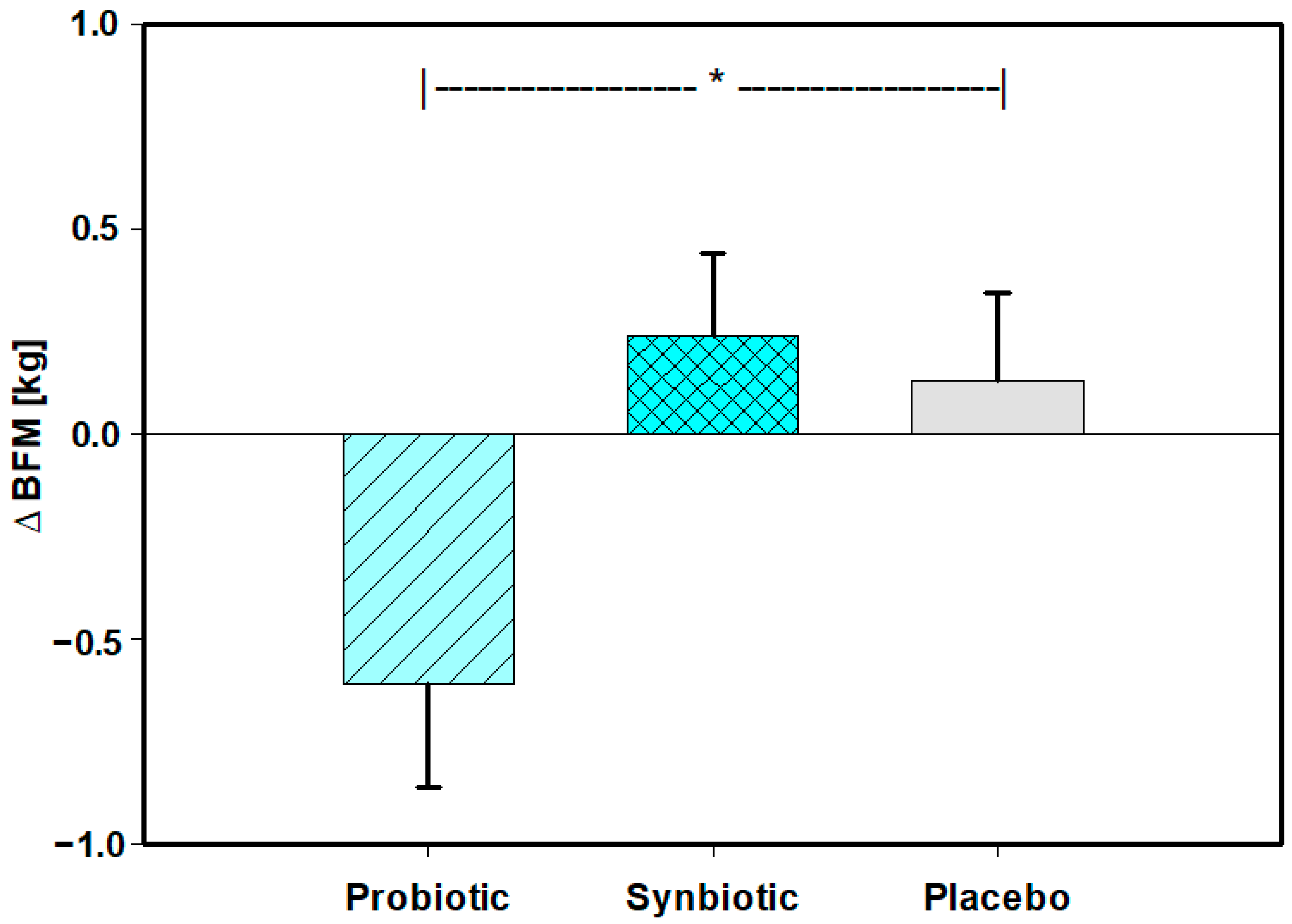
3.5.1. Body Composition as Assessed with Body Impedance Analysis (BIA) including Primary Parameter (Body Fat Mass (BFM))
3.5.2. Visceral Adiposity Index (VAI) According to Amato (Secondary Parameter)
3.5.3. Anthropometry and Blood Pressure
3.5.4. Laboratory Parameters
3.5.5. Sonography
3.5.6. Medication
3.5.7. Gastrointestinal Symptoms
3.5.8. Compliance
3.5.9. Adverse Events (AEs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Leverenz, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–590. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Solva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Li, L.; Yu, C.-H.; Shen, Z.; Chen, L.-H.; Li, Y.-M. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. 2013, 19, 6911–6918. [Google Scholar] [CrossRef]
- Sun, J.; Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015, 47, 430–440. [Google Scholar] [CrossRef]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on body weight and body-mass index: A systematic review and meta-analysis of randomized, controlled trials. Int. J. Food Sci. Nutr. 2016, 67, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2016, 52, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, F.; Yuan, Y.; Xu, Y. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med. Clínica 2017, 148, 362–370. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.-M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef]
- Xiao, M.-W.; Lin, S.-X.; Shen, Z.-H.; Luo, W.-W.; Wang, X.-Y. Systematic review with meta-analysis: The effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2019, 2019, 1484598. [Google Scholar] [CrossRef]
- Perna, S.; Ilyas, Z.; Giacosa, A.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Rigon, C.; Naso, M.; Riva, A.; Petrangolini, G.; et al. Is Probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients 2021, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Njeru, P.M.; Rösch, N.; Ghadimi, D.; Bockelmann, W.; de Vrese, M.; Schrezenmeir, J.; Heller, K.J. Identification and characterization of lactobacilli isolated from Kimere, a spontaneously fermented pearl millet dough from Mbeere, Kenya (East Africa). Benef. Microbes 2010, 1, 243–252. [Google Scholar] [CrossRef]
- Ghadimi, D.; Fölster-Holst, R.; de Vrese, M.; Winkler, P.; Heller, K.J.; Schrezenmeir, J. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology 2008, 213, 677–692. [Google Scholar] [CrossRef]
- Ghadimi, D.; Hassan, M.; Njeru, P.N.; de Vrese, M.; Geis, A.; Shalabi, A.I.; Abdel-Razek, S.T.; Abdel-Khair, A.A.; Heller, K.J.; Schrezenmeir, J. Suppression subtractive hybridization identifies bacterial genomic regions that are possibly involved in hBD-2 regulation by enterocytes. Mol. Nutr. Food Res. 2011, 55, 1533–1542. [Google Scholar] [CrossRef]
- Ghadimi, D.; Njeru, P.N.; Guigas, C.; Farghaly, M.H.; Fölster-Holst, R.; Geis, A.; de Vrese, M.; Schrezenmeir, J.; Heller, K.J. Molecular identification of potential Th1/Th2 responses-modulating bacterial genes using suppression subtractive DNA hybridization. Immunobiology 2014, 219, 208–217. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Bockelmann, W.; Meske, D.; de Vrese, M.; Walte, H.-G.; Schrezenmeir, J.; Heller, K.J. Ethanol Production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front. Microbiol. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Panwar, H.; Heller, K.J.; Schrezenmeir, J. Probiotics for dietary management of non-alcoholic fatty liver disease. Environ. Chem. Lett. 2019, 17, 1553–1563. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine ß-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Ghadimi, D.; Habermann, D.; de Vrese, M.; Bockelmann, W.; Kaatsch, H.J.; Heller, K.J.; Schrezenmeir, J. Effect of oral administration of Weissella confusa on fecal and plasma ethanol concentrations, lipids and glucose metabolism in Wistar rats fed high fructose and fat diet. Hepatic Med. Evid. Res. 2020, 12, 93–106. [Google Scholar] [CrossRef]
- Cherbut, C.; Michel, C.; Raison, V.; Kravtchenko, T.; Severine, M. Acacia gum is a bifidogenic dietary fibre with high digestive tolerance in healthy humans. Microb. Ecol. Health Dis. 2003, 15, 43–50. [Google Scholar] [CrossRef]
- Calame, W.; Weseler, A.R.; Viebke, C.; Flynn, C.; Siemensma, A.D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 2008, 100, 1269–1275. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. AJCN 2001, 73 (Suppl. S2), 361S–364S. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 11, 1147–1161. [Google Scholar] [CrossRef]
- Beserra, B.T.S.; Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.F.; Trindade, F.B.S.M. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 2015, 34, 845–858. [Google Scholar] [CrossRef]
- Gibb, R.D.; McRorie, J.W., Jr.; Russell, D.A.; Hasselblad, V.; D’Alessio, D.A. Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated 1 for type 2 diabetes mellitus. Am. J. Clin. Nutr. 2015, 102, 1604–1614. [Google Scholar] [CrossRef]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.-W. Effect of inulin-type fructans on blood lipid profile and glucose level: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 1514–1528. [Google Scholar] [CrossRef]
- Loman, B.R.; Hernàndesz-Saavedra, A.R.; Rector, S. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Babiker, R.; Merghani, T.H.; Elmunsharaf, K.; Badi, R.M.; Lang, F.; Saeed, A.M. Effects of gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: Two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 2012, 11, 111. Available online: http://www.nutritionj.com/content/11/1/111 (accessed on 17 April 2023). [CrossRef] [PubMed]
- Babiker, R.; Elmusharaf, K.; Keogh, M.B.; Banaga, A.S.I.; Saeed, A.M. Metabolic effects of Gum Arabic (Acacia Senegal) in patients with Type 2 Diabetes Mellitus (T2DM): Randomized, placebo controlled double blind trial. Funct. Foods Health Dis. 2017, 7, 219–231. [Google Scholar] [CrossRef]
- Babiker, R.; Elmusharaf, K.; Keogh, M.B.; Saeed, A.M. Effect of Gum Arabic (Acacia Senegal) supplementation on visceral adiposity index (VAI) and blood pressure in patients with type 2 diabetes mellitus as indicators of cardiovascular disease (CVD): A randomized and placebo-controlled clinical trial. Lipids Health Dis. 2018, 17, 56. [Google Scholar] [CrossRef]
- Nasir, O.; Artunc, F.; Wang, K.; Rexhepaj, R.; Föller, M.; Ebrahim, A.; Kempe, D.S.; Biswas, R.; Bhanduru, M.; Walter, M.; et al. Downregulation of Mouse Intestinal Na-coupled Glucose Transporter SGLT1 by Gum Arabic (Acacia Senegal), Cellular Physiology and Biochemistry. Cell. Physiol. Biochem. 2010, 25, 203–210. [Google Scholar] [CrossRef]
- Nasir, O. Effect of Gum Arabic (Acacia Senegal) on Glucose Metabolism and Body Weight Gain in Mice. J. Biol. Agric. Healthc. 2014, 4, 34–41. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA J. 2012, 10, 2604. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA J. 2011, 9, 2474. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206. [Google Scholar]
- IDF 223:2010, ISO 10932 I: 2010; Milk and Milk Products—Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB). ISO: Geneva, Switzerland, 2010.
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. for the ALKAMESY STUDY GROUP. A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- WHO. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008; ISBN 978-92-4-150149-1. [Google Scholar]
- Armellini, F.; Zamboni, M.; Rigo, L.; Bergamo-Andreis, I.A.; Robbi, R.; De Marchi, M.; Bosello, O. Sonography detection of small intra-abdominal fat variations. Int. J. Obes. 1991, 15, 847–852. [Google Scholar]
- Saverymuttu, S.H.; Joseph, A.E.A.; Maxwell, J.D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Brit. Med. J. 1986, 292, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Alnumeiri, M.S.; Ayad, C.E.; Ahmed, B.H.; Abdalla, E.A. Evaluation of ascites and its etiology using ultrasonography. J. Res. Dev. 2015, 3, 1. [Google Scholar]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Dimenäs, E.; Glise, H.; Hallerbäck, B.; Hernqvist, H.; Svedlund, J.; Wiklund, I. Well-Being and Gastrointestinal Symptoms among Patients Referred to Endoscopy Owing to Suspected Duodenal Ulcer. Scand. J. Gastroenterol. 1995, 30, 1046–1052. [Google Scholar] [CrossRef]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual. Life Res. 1998, 7, 75–83. [Google Scholar] [CrossRef]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. Consens. Statement Int. Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar]
- Lorenzo, C.; Williams, K.; Hunt, K.J.; Haffner, S.M. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007, 30, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Masarone, V.; Aglitti, A.; Persico, M. The diagnostic conundrum in non-alcoholic fatty liver disease. Explor Med. 2020, 1, 259–286. [Google Scholar] [CrossRef]
- Li, C.; Sheng, J.; Zhang, H.; Qiu, C.; Huang, X.; Yang, L.; Yu, X. The development of a clinical score for the prediction of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease using routine parameters. Turk. J. Gastroenterol. 2015, 26, 408–416. [Google Scholar] [CrossRef]
- Pizarroso, N.A.; Fuciños, P.; Gonçalves, C.; Pastrana, L.; Amado, I.R. A review on the role of food-derived bioactive molecules and the microbiota-gut-brain axis in satiety regulation. Nutrients 2021, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. A newly identified protein from Akkermansia muciniphila stimulates GLP-1 secretion. Cell Metab. 2021, 33, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Déchelotte, P.; Breton, J.; Trotin-Picolo, C.; Grube, B.; Erlenbeck, C.; Bothe, G.; Fetissov, S.O.; Lambert, G. The probiotic strain H. alvei HA4597® improves weight loss in overweight subjects under moderate hypocaloric diet: A proof-of-concept, multicenter randomized, double-blind placebo-controlled study. Nutrients 2021, 13, 1902. [Google Scholar] [CrossRef]
| Ingredients Per Sachet | Placebo | Probiotic | Synbiotic | ||
|---|---|---|---|---|---|
| CFU a.p. | CFU a.e.s. | CFU a.p. | CFU a.e.s. | ||
| L. fermentum K7-Lb1 | 5 × 109 | 1 × 109 | 5 × 109 | 1 × 109 | |
| L. fermentum K8-Lb1 | 5 × 109 | 1 × 109 | 5 × 109 CFU | 1 × 109 CFU | |
| L. fermentum K11-Lb3 | 5 × 109 | 1 × 109 | 5 × 109 | 1 × 109 | |
| mg | mg | mg | |||
| Microcrystalline cellulose | 5555.6 | 5555.6 | |||
| Acacia gum | 5555.6 | ||||
| Sucralose | 11.3 | 11.3 | 11.3 | ||
| Cream flavour | 97.4 | 97.4 | 97.4 | ||
| Maltodextrin | 185.7 | 185.7 | 185.7 | ||
| TOTAL | 6000.0 | 6000.0 | 6000.0 | ||
| Time | Parameter | Mean | SD | Median | 25% | 75% |
|---|---|---|---|---|---|---|
| Point | ||||||
| V0 | Age (years) | 60.06 | 12.33 | 62.00 | 53.00 | 69.00 |
| Height (cm) | 170.1 | 9.1 | 170.0 | 163.3 | 176.0 | |
| V1 | Weight (kg) | 93.83 | 17.82 | 92.30 | 80.46 | 102.20 |
| BMI (kg/m2) | 32.33 | 5.45 | 30.84 | 28.42 | 34.87 | |
| Waist (cm) | 109.45 | 12.36 | 107.5 | 101.05 | 115.30 | |
| WHtR | 0.644 | 0.073 | 0.627 | 0.590 | 0.680 | |
| Bp syst (mmHg) | 131.2 | 15.99 | 130.0 | 120.0 | 140.0 | |
| Bp diast (mmHg) | 85.00 | 9.12 | 85.00 | 80.00 | 90.00 | |
| Abs. BFM (kg) | 41.25 | 7.663 | 42.62 | 35.47 | 47.07 | |
| Rel. BFM (%) | 38.94 | 11.72 | 35.62 | 31.76 | 46.04 | |
| FFM (kg) | 54.89 | 11.36 | 52.55 | 45.31 | 64.20 | |
| VAT(BIA) (L) | 4.208 | 2.13 | 3.595 | 2.82 | 5.072 | |
| Glucose (mg/dL) | 110.3 | 17.58 | 107.0 | 101.0 | 117.0 | |
| HbA1c (%) | 5.54 | 0.44 | 5.50 | 5.30 | 5.78 | |
| HOMA-IR | 4.282 | 3.586 | 3.079 | 2.275 | 4.865 | |
| QUICKI | 0.321 | 0.0276 | 0.323 | 0.303 | 0.337 | |
| Cholesterol (mg/dL) | 227.8 | 44.23 | 225.5 | 195.3 | 257.5 | |
| HDL-C (mg/dL) | 62.43 | 14.70 | 61.5 | 51.00 | 70.75 | |
| LDL-C (mg/dL) | 146.0 | 33.38 | 141.5 | 124.0 | 164.75 | |
| Triglycerides (mg/dL) | 129.1 | 56.86 | 118.0 | 87.0 | 162.0 | |
| CRP | 0.395 | 0.742 | 0.23 | 0.13 | 0.46 | |
| AST (U/L) | 26.48 | 9.255 | 24.0 | 21.0 | 29.0 | |
| ALT (U/L) | 26.94 | 15.40 | 23.5 | 17.0 | 31.0 | |
| GGT (U/L) | 31.11 | 23.39 | 24.0 | 17.0 | 34.0 | |
| LSG | 1.148 | 0.637 | 1.00 | 0.67 | 1.67 | |
| SAD | 52.21 | 23.12 | 50.55 | 33.4 | 69.93 |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | Param. | Group | Size | Mean | SD | Median | 25% | 75% | p | Post Hoc Test If Sign (vs. Control) |
| V0 | Age (years) | Pro | 60 | 61.50 | 11.30 | 63.00 | 56.30 | 69.80 | 0.016 | |
| Syn | 60 | 56.90 | 14.60 | 59.00 | 48.00 | 68.00 | ||||
| Pla | 60 | 61.80 | 10.30 | 63.50 | 54.30 | 70.00 | ||||
| Height (cm) | Pro | 60 | 171.0 | 9.1 | 171.0 | 165.0 | 176.0 | 0.190 | ||
| Syn | 60 | 168.0 | 8.3 | 169.0 | 162.0 | 174.0 | Normality | |||
| Pla | 60 | 171.0 | 9.6 | 172.0 | 163.0 | 178.0 | (p ANOVA) | |||
| V1 | Weight (kg) | Pro | 59 | 95.1 | 19.1 | 91.3 | 82.7 | 102.2 | 0.282 | |
| Syn | 58 | 90.9 | 17.4 | 88.3 | 78.6 | 101.3 | ||||
| Pla | 59 | 95.5 | 16.9 | 93.5 | 83.1 | 106.2 | ||||
| BMI (kg/m2) | Pro | 59 | 32.37 | 5.18 | 31.07 | 28.90 | 34.80 | 0.612 | ||
| Syn | 58 | 31.98 | 5.73 | 30.54 | 28.25 | 34.56 | ||||
| Pla | 59 | 32.64 | 5.50 | 31.37 | 28.34 | 36.55 | ||||
| Waist (cm) | Pro | 59 | 110.0 | 12.2 | 107.2 | 102.3 | 114.4 | 0.291 | ||
| Syn | 58 | 107.6 | 12.8 | 105.8 | 97.5 | 115.1 | ||||
| Pla | 59 | 110.7 | 12.1 | 108.3 | 101.5 | 119.0 | ||||
| WHtR | Pro | 59 | 0.64 | 0.07 | 0.63 | 0.60 | 0.68 | 0.547 | ||
| Syn | 58 | 0.64 | 0.08 | 0.62 | 0.59 | 0.67 | ||||
| Pla | 59 | 0.65 | 0.08 | 0.64 | 0.60 | 0.71 | ||||
| BP syst (mmHg) | Pro | 59 | 133.5 | 16.56 | 130.0 | 120.0 | 140.0 | 0.259 | ||
| Syn | 58 | 128.6 | 17.09 | 130.0 | 118.8 | 140.0 | Normality | |||
| Pla | 59 | 131.4 | 14.08 | 130.0 | 120.0 | 140.0 | (p ANOVA) | |||
| BP diast (mmHg) | Pro | 59 | 86.4 | 9.51 | 90.0 | 80.0 | 90.0 | 0.426 | ||
| Syn | 58 | 83.9 | 8.43 | 85.0 | 80.0 | 90.0 | ||||
| Pla | 59 | 84.7 | 9.35 | 85.0 | 80.0 | 90.0 | ||||
| BFM (kg) | Pro | 59 | 38.60 | 11.24 | 35.37 | 31.75 | 45.19 | 0.610 | ||
| Syn | 58 | 38.44 | 12.34 | 34.67 | 31.64 | 47.00 | ||||
| Pla | 59 | 39.78 | 11.70 | 38.27 | 31.38 | 47.19 | ||||
| FFM (kg) | Pro | 59 | 56.47 | 12.77 | 54.85 | 45.26 | 66.24 | 0.190 | ||
| Syn | 58 | 52.47 | 9.63 | 49.78 | 44.77 | 59.76 | ||||
| Pla | 59 | 55.68 | 11.23 | 53.63 | 46.29 | 64.85 | ||||
| VATBIA (L) | Pro | 59 | 4.45 | 2.27 | 3.72 | 3.00 | 5.30 | 0.123 | ||
| Syn | 58 | 3.74 | 1.77 | 3.36 | 2.45 | 4.95 | ||||
| Pla | 59 | 4.43 | 2.27 | 3.74 | 2.82 | 5.10 | ||||
| SAD (mm) | Pro | 59 | 53.69 | 24.64 | 48.20 | 33.40 | 73.80 | 0.417 | ||
| Syn | 58 | 49.01 | 22.68 | 43.60 | 29.80 | 66.18 | ||||
| Pla | 59 | 53.87 | 22.03 | 54.90 | 37.60 | 71.70 | ||||
| LSG | Pro | 59 | 1.31 | 0.70 | 1.33 | 0.67 | 1.78 | 0.120 | ||
| Syn | 58 | 1.08 | 0.61 | 0.95 | 0.56 | 1.56 | ||||
| Pla | 59 | 1.05 | 0.57 | 0.89 | 0.67 | 1.33 | ||||
| (b) | ||||||||||
| Time Point | Param. | Group | Size | Mean | SD | Median | 25% | 75% | p | Post Hoc Test If Sign (vs. Control) |
| V1 | Glucose (mg/dL) | Pro | 59 | 112.1 | 24.4 | 106.0 | 101.0 | 122.0 | 0.877 | |
| Syn | 58 | 108.6 | 13.1 | 107.0 | 100.5 | 116.3 | ||||
| Pla | 59 | 110.1 | 12.7 | 108.0 | 100.0 | 117.0 | ||||
| HbA1c (%) | Pro | 59 | 5.58 | 0.40 | 5.60 | 5.30 | 5.80 | 0.353 | ||
| Syn | 58 | 5.47 | 0.32 | 5.50 | 5.28 | 5.60 | ||||
| Pla | 59 | 5.55 | 0.57 | 5.50 | 5.20 | 5.80 | ||||
| HOMA-IR | Pro | 59 | 5.11 | 4.43 | 3.30 | 2.31 | 7.16 | 0.196 | ||
| Syn | 58 | 4.03 | 3.76 | 2.83 | 2.19 | 4.45 | ||||
| Pla | 59 | 3.71 | 2.05 | 3.09 | 2.29 | 4.53 | ||||
| QUICKI | Pro | 59 | 0.316 | 0.030 | 0.320 | 0.289 | 0.337 | 0.164 | Normality | |
| Syn | 58 | 0.325 | 0.030 | 0.327 | 0.307 | 0.339 | (p—ANOVA) | |||
| Pla | 59 | 0.321 | 0.021 | 0.323 | 0.306 | 0.337 | ||||
| Cholesterol (mg/dL) | Pro | 59 | 228.17 | 46.66 | 219.00 | 199.00 | 251.00 | 0.472 | ||
| Syn | 58 | 232.72 | 44.82 | 228.00 | 198.25 | 265.00 | Normality | |||
| Pla | 59 | 222.68 | 41.25 | 226.00 | 192.00 | 254.00 | (p-ANOVA) | |||
| HDL-C (mg/dL) | Pro | 59 | 59.10 | 14.70 | 57.00 | 48.00 | 68.00 | 0.006 | Pro vs. Pla (p = 0.903) | |
| Syn | 58 | 66.86 | 13.64 | 65.00 | 58.00 | 76.25 | Syn vs. Pla (p = 0.041) | |||
| Pla | 59 | 61.41 | 14.86 | 60.00 | 52.00 | 70.00 | (Dunn’s method) | |||
| LDL-C (mg/dL) | Pro | 59 | 148.66 | 34.65 | 142.00 | 128.00 | 166.00 | 0.530 | ||
| Syn | 58 | 147.29 | 35.28 | 143.00 | 120.00 | 166.75 | ||||
| Pla | 59 | 142.07 | 30.23 | 140.00 | 120.00 | 163.00 | ||||
| Triglycerides (mg/dL) | Pro | 59 | 128.10 | 56.49 | 113.00 | 87.00 | 153.00 | 0.973 | ||
| Syn | 58 | 128.16 | 51.08 | 120.00 | 90.00 | 167.25 | ||||
| Pla | 59 | 130.98 | 63.19 | 119.00 | 87.00 | 164.00 | ||||
| CRP (mg/L) | Pro | 59 | 0.52 | 1.21 | 0.23 | 0.16 | 0.47 | 0.136 | ||
| Syn | 58 | 0.36 | 0.30 | 0.26 | 0.13 | 0.58 | ||||
| Pla | 59 | 0.31 | 0.28 | 0.21 | 0.12 | 0.40 | ||||
| AST (U/L) | Pro | 59 | 28.41 | 9.95 | 25.00 | 22.00 | 33.00 | 0.138 | ||
| Syn | 58 | 25.83 | 9.82 | 24.00 | 19.00 | 28.25 | ||||
| Pla | 59 | 25.20 | 7.66 | 24.00 | 20.00 | 28.00 | ||||
| ALT (U/L) | Pro | 59 | 30.27 | 15.26 | 26.00 | 21.00 | 34.00 | 0.011 | Pro vs. Pla (p = 0.025) | |
| Syn | 58 | 25.86 | 17.45 | 19.50 | 15.00 | 29.50 | Syn vs. Pla (p = 1.000) | |||
| Pla | 59 | 24.66 | 12.89 | 23.00 | 16.00 | 28.00 | (Dunn’s method) | |||
| GGT (U/L) | Pro | 59 | 32.48 | 20.96 | 27.00 | 20.00 | 37.00 | 0.142 | ||
| Syn | 58 | 27.16 | 16.64 | 21.00 | 16.00 | 31.25 | ||||
| Pla | 59 | 33.64 | 30.24 | 24.00 | 17.00 | 35.00 | ||||
| Time Period | Parameter | Group | Size | Mean | SD | Median | 25% | 75% | p | Post Hoc Test If Sign (vs. Control) |
|---|---|---|---|---|---|---|---|---|---|---|
| ∆(V3-V1) | BFM (kg) | Pro | 59 | −0.61 | 1.94 | −0.46 | −1.72 | 0.81 | 0.015 | Pro vs. Pla (p = 0.039) |
| Syn | 58 | 0.24 | 1.52 | 0.30 | −0.79 | 0.95 | Syn vs. Pla (p = 0.730) | |||
| Pla | 59 | 0.13 | 1.64 | 0.24 | −1.30 | 1.02 | Normality (Holm–Sidak) | |||
| BFM (%) | Pro | 59 | −0.43 | 1.41 | −0.33 | −1.31 | 0.57 | 0.045 | Pro vs. Pla (p = 0.546) | |
| Syn | 58 | 0.15 | 1.11 | 0.23 | −0.57 | 1.01 | Syn vs. Pla (p = 0.326) | |||
| Pla | 59 | −0.05 | 1.11 | −0.10 | −1.03 | 0.70 | (Dunn’s method) | |||
| FFM (kg) | Pro | 59 | −0.08 | 1.06 | −0.03 | −0.64 | 0.56 | 0.254 | ||
| Syn | 58 | 0.01 | 1.19 | −0.14 | −0.91 | 0.74 | Normality | |||
| Pla | 59 | 0.25 | 1.12 | 0.32 | −0.47 | 0.99 | ||||
| VATBIA (L) | Pro | 59 | −0.20 | 0.44 | −0.19 | −0.44 | 0.04 | 0.021 | Pro vs. Pla (p = 0.148) | |
| Syn | 58 | −0.02 | 0.39 | −0.03 | −0.15 | 0.15 | Syn vs. Pla (p = 0.675) | |||
| Pla | 59 | −0.04 | 0.36 | −0.10 | −0.32 | 0.13 | (Dunn’s method) |
| Time Period | Param. | Group | Size | Mean | SD | Median | 25% | 75% | p | Post Hoc Test If Sign (vs. Control) |
|---|---|---|---|---|---|---|---|---|---|---|
| ∆(V3-V1) | Weight (kg) | Pro | 59 | −0.69 | 2.17 | −0.60 | −1.90 | 0.75 | 0.013 | Pro vs. Pla (p = 0.012) |
| Syn | 58 | 0.25 | 2.02 | 0.28 | −0.84 | 1.49 | Syn vs. Pla (p = 1.000) | |||
| Pla | 59 | 0.37 | 1.87 | 0.10 | −0.60 | 1.80 | (Dunn’s method) | |||
| BMI (kg/m2) | Pro | 59 | −0.24 | 0.74 | −0.20 | −0.65 | 0.27 | 0.013 | Pro vs. Pla (p = 0.011) | |
| Syn | 58 | 0.08 | 0.70 | 0.10 | −0.32 | 0.51 | Syn vs. Pla (p = 1.000) | |||
| Pla | 59 | 0.13 | 0.63 | 0.04 | −0.22 | 0.55 | (Dunn’s method) | |||
| Waist (cm) | Pro | 59 | −1.57 | 2.70 | −1.80 | −3.00 | 0.00 | 0.016 | Pro vs. Pla (p = 0.033) | |
| Syn | 58 | −0.53 | 2.57 | −0.45 | −1.83 | 1.00 | Syn vs. Pla (p = 1.000) | |||
| Pla | 59 | −0.40 | 2.55 | −0.50 | −2.30 | 0.70 | (Dunn’s method) | |||
| WHtR | Pro | 59 | −0.009 | 0.016 | −0.011 | −0.018 | 0.000 | 0.018 | Pro vs. Pla (p = 0.033) | |
| Syn | 58 | −0.003 | 0.015 | −0.003 | −0.011 | 0.006 | Syn vs. Pla (p = 1.000) | |||
| Pla | 59 | −0.002 | 0.015 | −0.003 | −0.013 | 0.004 | (Dunn’s method) | |||
| BP syst (mmHg) | Pro | 58 | −1.93 | 14.64 | 0.00 | −10.00 | 5.00 | 0.414 | ||
| Syn | 57 | −0.18 | 10.80 | 0.00 | −5.00 | 5.00 | ||||
| Pla | 59 | −2.88 | 11.97 | 0.00 | −10.00 | 5.00 | ||||
| BP diast (mmHg) | Pro | 58 | −3.22 | 8.05 | −5.00 | −6.75 | 0.00 | 0.049 | Pro vs. Pla (p = 0.243) | |
| Syn | 57 | −0.83 | 6.52 | 0.00 | −5.00 | 5.00 | Syn vs. Pla (p = 0.845) | |||
| Pla | 59 | −1.51 | 7.64 | 0.00 | −5.00 | 0.00 | (Dunn’s method) |
| Time Period | Param. | Group | Size | Mean | SD | Median | 25% | 75% | p |
|---|---|---|---|---|---|---|---|---|---|
| ∆(V3-V1) | Glucose (mg/dL) | Pro | 57 | −1.77 | 10.69 | −2.00 | −6.50 | 3.00 | 0.388 |
| Syn | 57 | −1.75 | 8.08 | −2.00 | −6.00 | 3.00 | |||
| Pla | 58 | −0.03 | 8.72 | −1.00 | −5.00 | 3.00 | |||
| HbA1c (%) | Pro | 56 | −0.04 | 0.22 | 0.00 | −0.10 | 0.10 | 0.266 | |
| Syn | 57 | 0.02 | 0.18 | 0.00 | −0.10 | 0.20 | |||
| Pla | 58 | 0.01 | 0.24 | 0.00 | −0.10 | 0.13 | |||
| Insulin (mU/L) | Pro | 57 | −2.45 | 7.80 | −1.60 | −4.05 | 2.00 | 0.164 | |
| Syn | 57 | −0.30 | 6.41 | 0.00 | −2.40 | 2.40 | |||
| Pla | 58 | −0.23 | 3.78 | −0.70 | −2.35 | 1.88 | |||
| HOMA-IR | Pro | 57 | −0.77 | 2.88 | −0.42 | −1.15 | 0.54 | 0.164 | |
| Syn | 57 | −0.16 | 2.06 | −0.11 | −0.58 | 0.59 | |||
| Pla | 58 | −0.05 | 1.15 | −0.11 | −0.68 | 0.67 | |||
| QUICKI | Pro | 57 | 0.004 | 0.022 | 0.008 | −0.003 | 0.014 | 0.283 | |
| Syn | 57 | 0.000 | 0.018 | 0.002 | −0.010 | 0.013 | |||
| Pla | 58 | 0.003 | 0.015 | 0.002 | −0.007 | 0.013 | |||
| Cholesterol (mg/dL) | Pro | 57 | −3.11 | 24.97 | −6.00 | −16.00 | 14.50 | 0.653 | |
| Syn | 57 | −6.58 | 24.53 | −4.00 | −19.00 | 6.00 | |||
| Pla | 58 | −7.16 | 21.40 | −7.00 | −18.25 | 3.50 | |||
| HDL Chol. (mg/dL) | Pro | 57 | 0.28 | 8.53 | 1.00 | −2.50 | 4.50 | 0.468 | |
| Syn | 57 | −0.77 | 8.21 | 0.00 | −5.00 | 4.00 | |||
| Pla | 58 | 0.72 | 6.51 | 1.00 | −3.00 | 4.25 | |||
| LDL Chol. (mg/dL) | Pro | 57 | 2.02 | 20.10 | 2.00 | −8.00 | 16.00 | 0.416 | |
| Syn | 57 | −0.65 | 19.63 | 0.00 | −9.50 | 10.50 | |||
| Pla | 58 | −2.03 | 17.19 | −2.00 | −12.25 | 8.00 | |||
| Triglycerides (mg/dL) | Pro | 57 | −8.16 | 47.12 | −1.00 | −27.50 | 17.00 | 0.353 | |
| Syn | 57 | −15.33 | 45.66 | −10.00 | −30.50 | 6.00 | |||
| Pla | 58 | −14.29 | 36.22 | −15.00 | −34.00 | 2.25 | |||
| CRP (mg/L) | Pro | 57 | −0.18 | 1.24 | 0.00 | −0.11 | 0.04 | 0.626 | |
| Syn | 57 | −0.01 | 0.28 | 0.00 | −0.09 | 0.06 | |||
| Pla | 58 | 0.02 | 0.22 | 0.00 | −0.06 | 0.05 | |||
| AST (U/L) | Pro | 57 | −0.12 | 13.00 | 0.00 | −4.00 | 2.50 | 0.137 | |
| Syn | 57 | −0.60 | 6.63 | −1.00 | −4.00 | 2.00 | |||
| Pla | 58 | 0.74 | 6.03 | 0.00 | −2.00 | 3.00 | |||
| ALT (U/L) | Pro | 57 | −0.54 | 10.51 | 0.00 | −5.50 | 2.00 | 0.260 | |
| Syn | 57 | 1.21 | 12.39 | 1.00 | −4.00 | 3.00 | |||
| Pla | 58 | 1.72 | 9.75 | 1.00 | −2.00 | 4.00 | |||
| GGT (U/L) | Pro | 57 | 0.97 | 17.88 | −1.00 | −3.50 | 2.00 | 0.812 | |
| Syn | 57 | −0.49 | 5.90 | 0.00 | −2.00 | 1.50 | |||
| Pla | 58 | 0.85 | 12.67 | −1.00 | −2.25 | 2.00 |
| Time Period | Param. | Group | Size | Mean | SD | Median | 25% | 75% | p | Post Hoc Test If Sign (vs. Control) |
|---|---|---|---|---|---|---|---|---|---|---|
| ∆(V3-V1) | VATsono SAD (mm) | Pro | 57 | −6.28 | 9.34 | −5.30 | −10.85 | −1.20 | <0.001 | Pro vs. Pla (p < 0.001) |
| Syn | 58 | −4.99 | 11.84 | −4.20 | −10.55 | 0.55 | Syn vs. Pla (p = 0.002) | |||
| Pla | 59 | 2.06 | 11.65 | 3.40 | −6.10 | 9.90 | (Dunn’s method) | |||
| LSG | Pro | 57 | −0.25 | 0.27 | −0.22 | −0.44 | −0.11 | <0.001 | Pro vs. Pla (p < 0.001) | |
| Syn | 58 | −0.17 | 0.24 | −0.11 | −0.23 | 0.00 | Syn vs. Pla (p < 0.001) | |||
| Pla | 59 | 0.05 | 0.29 | 0.00 | −0.11 | 0.22 | (Dunn’s method) |
| Time Point | Parameter | Group | N | Mean | SD | Median | 25% | 75% | p | Post Hoc Test If Sign (vs. Control) |
|---|---|---|---|---|---|---|---|---|---|---|
| ∆(V3-V1) | Total Score | Pro | 58 | −0.05 | 0.48 | −0.03 | −0.33 | 0.15 | 0.356 | |
| Syn | 58 | −0.16 | 0.59 | −0.07 | −0.27 | 0.07 | ||||
| Pla | 58 | −0.01 | 0.43 | 0.00 | −0.15 | 0.15 | ||||
| Pain Score | Pro | 58 | 0.04 | 0.77 | 0.00 | 0.00 | 0.00 | 0.119 | ||
| Syn | 58 | −0.16 | 0.62 | 0.00 | −0.33 | 0.00 | ||||
| Pla | 58 | 0.05 | 0.46 | 0.00 | 0.00 | 0.00 | ||||
| Reflux Score | Pro | 58 | −0.10 | 0.84 | 0.00 | 0.00 | 0.00 | 0.101 | ||
| Syn | 58 | −0.24 | 0.53 | 0.00 | −0.50 | 0.00 | ||||
| Pla | 58 | −0.05 | 0.79 | 0.00 | −0.50 | 0.00 | ||||
| Indigestion Score | Pro | 58 | −0.15 | 0.88 | 0.00 | −0.56 | 0.25 | 0.544 | ||
| Syn | 58 | −0.02 | 0.96 | 0.00 | −0.25 | 0.50 | ||||
| Pla | 58 | −0.04 | 0.72 | 0.00 | −0.25 | 0.25 | ||||
| Constipation Score | Pro | 58 | −0.10 | 0.89 | 0.00 | −0.33 | 0.00 | 0.012 | Syn vs. Pla (p = 0.014) (Dunn’s method) | |
| Syn | 58 | −0.36 | 0.93 | 0.00 | −0.67 | 0.00 | ||||
| Pla | 58 | 0.11 | 0.69 | 0.00 | 0.00 | 0.42 | ||||
| Diarrhoea Score | Pro | 58 | 0.06 | 0.77 | 0.00 | −0.33 | 0.33 | 0.395 | ||
| Syn | 58 | −0.10 | 0.87 | 0.00 | −0.33 | 0.08 | ||||
| Pla | 58 | −0.12 | 0.76 | 0.00 | −0.33 | 0.33 |
| Occurrence | Probiotic | Synbiotic | Placebo | p |
|---|---|---|---|---|
| UTI | 0 | 0 | 1 | 0.369 * |
| GITI | 13 | 8 | 12 | 0.484 |
| RTI | 4 | 6 | 8 | 0.478 |
| Other infections | 4 | 5 | 6 | 0.804 |
| Laboratory | 2 | 0 | 1 | 0.367 * |
| Pain | 4 | 12 | 8 | 0.090 |
| Allergy | 1 | 1 | 0 | 0.600 * |
| Other AEs | 23 | 15 | 16 | 0.235 |
| Total number of individuals with AEs | 34 | 32 | 33 | 0.963 |
| Incidence | Probiotic | Synbiotic | Placebo | p |
| UTI | 0 | 0 | 3 | 0.371 |
| GITI | 15 | 11 | 16 | 0.512 |
| RTI | 5 | 7 | 9 | 0.492 |
| Other infections | 4 | 5 | 6 | 0.805 |
| Laboratory | 2 | 0 | 1 | 0.369 |
| Pain | 4 | 14 | 11 | 0.090 |
| Allergy | 1 | 1 | 0 | 0.602 |
| Other AEs | 33 | 19 | 22 | 0.180 |
| Total number of AEs | 64 | 57 | 68 | 0.762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laue, C.; Papazova, E.; Pannenbeckers, A.; Schrezenmeir, J. Effect of a Probiotic and a Synbiotic on Body Fat Mass, Body Weight and Traits of Metabolic Syndrome in Individuals with Abdominal Overweight: A Human, Double-Blind, Randomised, Controlled Clinical Study. Nutrients 2023, 15, 3039. https://doi.org/10.3390/nu15133039
Laue C, Papazova E, Pannenbeckers A, Schrezenmeir J. Effect of a Probiotic and a Synbiotic on Body Fat Mass, Body Weight and Traits of Metabolic Syndrome in Individuals with Abdominal Overweight: A Human, Double-Blind, Randomised, Controlled Clinical Study. Nutrients. 2023; 15(13):3039. https://doi.org/10.3390/nu15133039
Chicago/Turabian StyleLaue, Christiane, Ekaterina Papazova, Angelika Pannenbeckers, and Jürgen Schrezenmeir. 2023. "Effect of a Probiotic and a Synbiotic on Body Fat Mass, Body Weight and Traits of Metabolic Syndrome in Individuals with Abdominal Overweight: A Human, Double-Blind, Randomised, Controlled Clinical Study" Nutrients 15, no. 13: 3039. https://doi.org/10.3390/nu15133039
APA StyleLaue, C., Papazova, E., Pannenbeckers, A., & Schrezenmeir, J. (2023). Effect of a Probiotic and a Synbiotic on Body Fat Mass, Body Weight and Traits of Metabolic Syndrome in Individuals with Abdominal Overweight: A Human, Double-Blind, Randomised, Controlled Clinical Study. Nutrients, 15(13), 3039. https://doi.org/10.3390/nu15133039





