Bile Acids and Short-Chain Fatty Acids Are Modulated after Onion and Apple Consumption in Obese Zucker Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Onion and Apple Powder Preparation
2.2. Animal Model and Experimental Design
2.3. Faeces Collection and Caecum Sampling
2.4. Caecum pH and Antioxidant Activity Determination
2.5. Microbial Analysis
2.6. Short-Chain Fatty Acid Analysis
2.6.1. Short-Chain Fatty Acid Extraction
2.6.2. Short-Chain Fatty Acid Identification and Quantification Using Gas Chromatography–Mass Spectrometry (GC–MS)
2.7. Bile Acid Analysis
2.7.1. Bile Acid Extraction
2.7.2. Bile Acid Identification Using High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (HPLC–QTOF MS)
2.8. Statistical Analysis
3. Results
3.1. Prebiotic Effect
3.2. Faecal Short-Chain Fatty Acid Composition in Response to Dietary Onion and Apple Intake
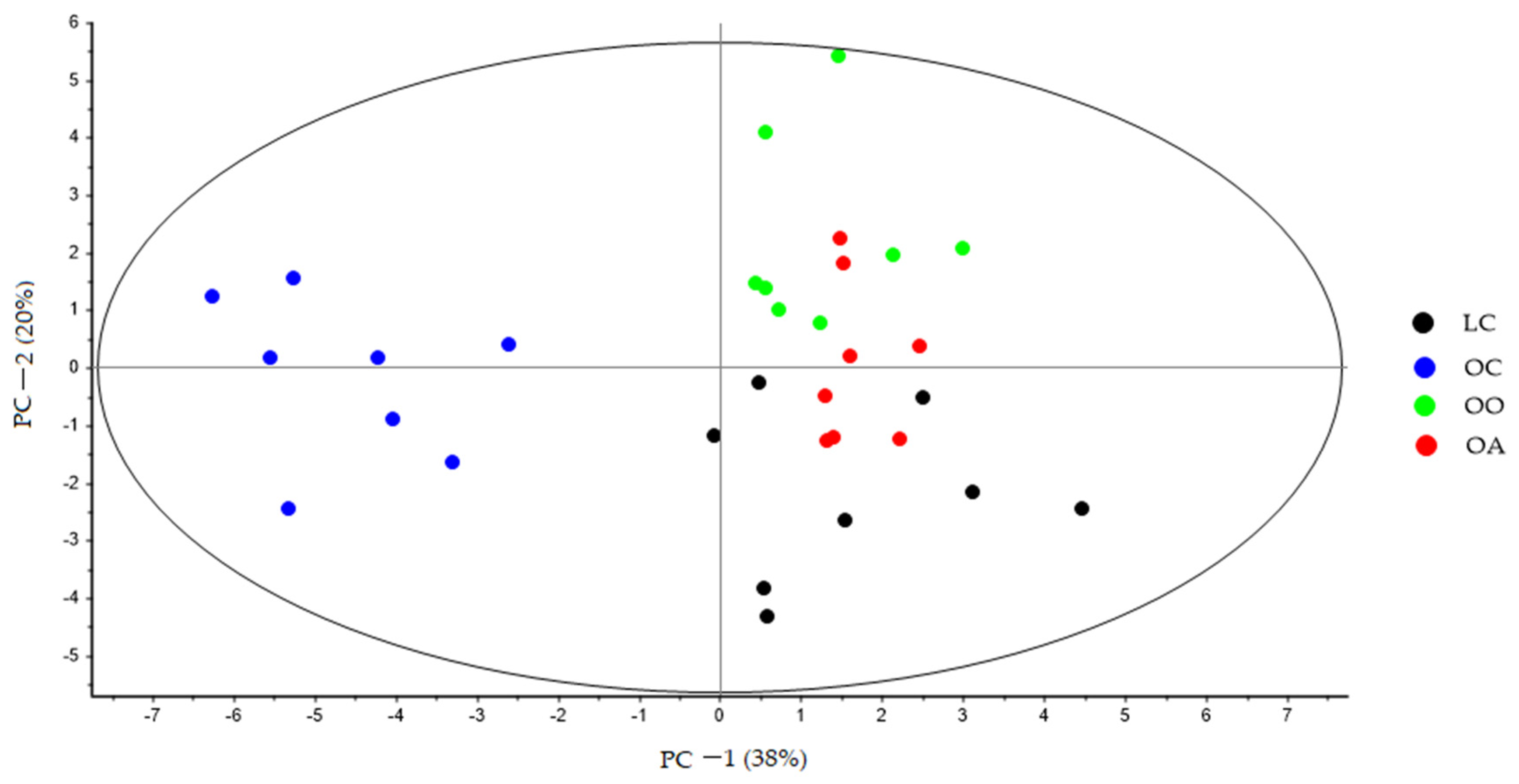
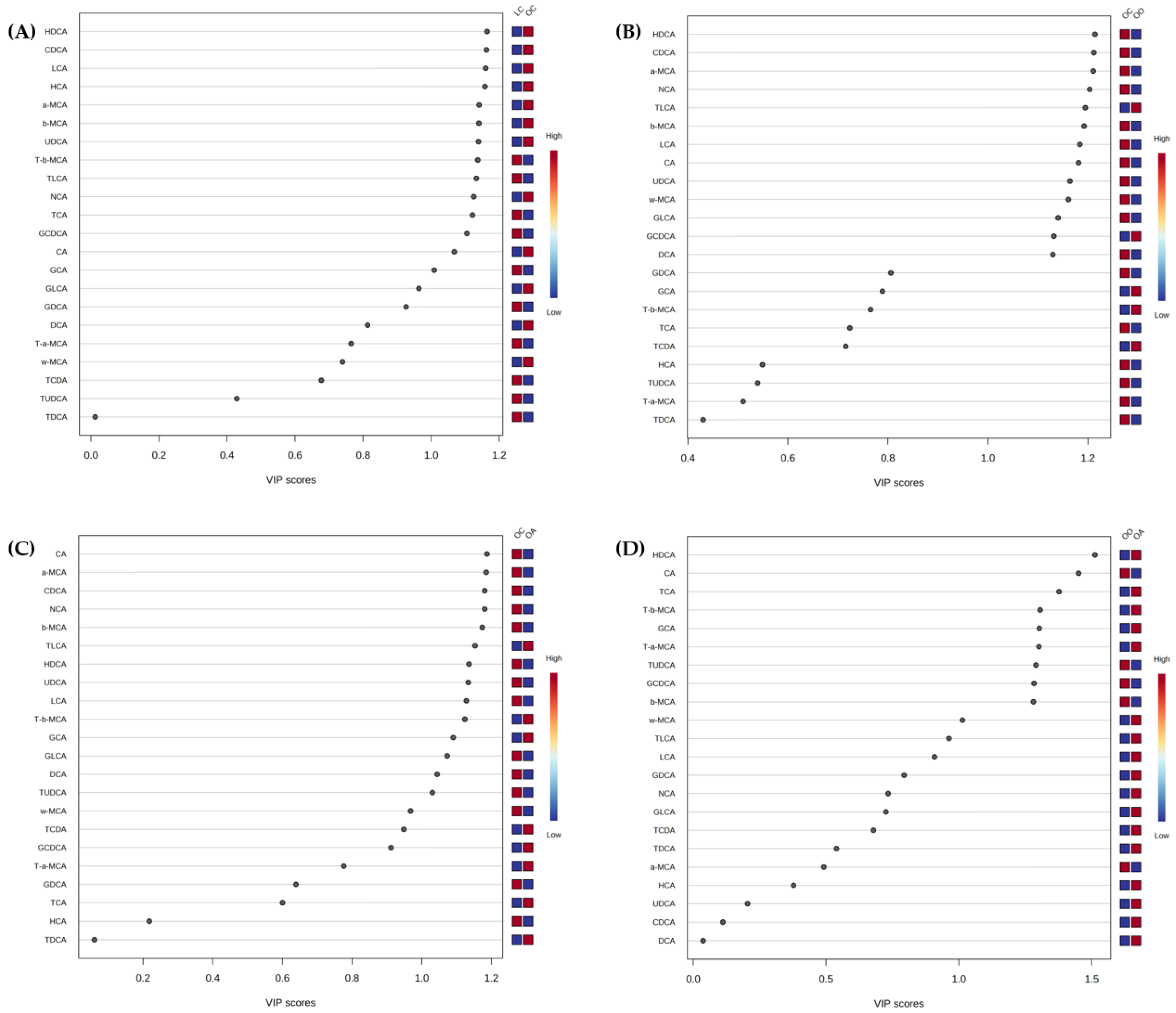
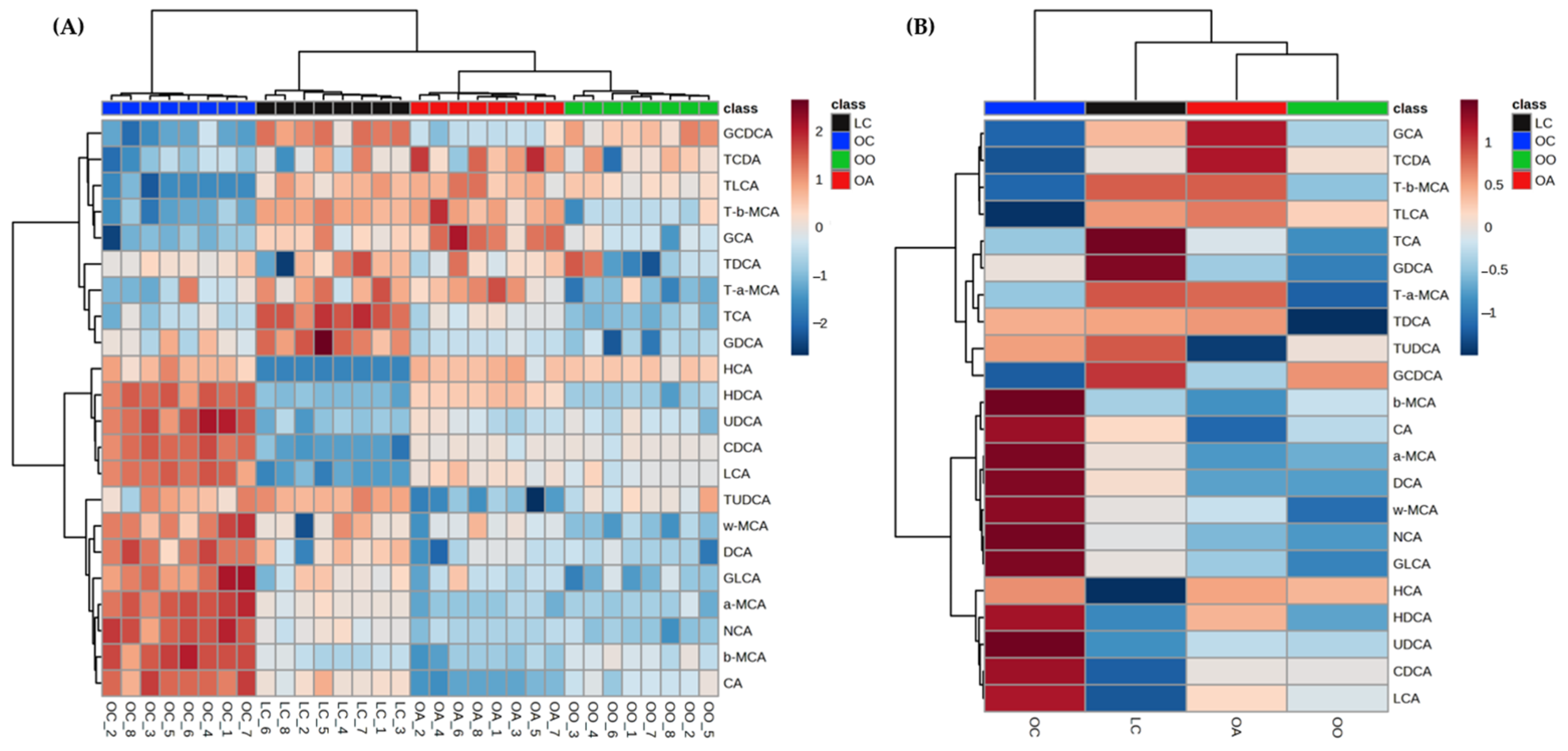
3.3. Bile Acid Content in Response to Dietary Onion and Apple Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, J.Y. Regulation of bile acid synthesis. Front. Biosci. 1998, 3, 176–193. [Google Scholar] [CrossRef]
- Meier, P.J.; Stieger, B. Bile salt transporters. Annu. Rev. Physiol. 2002, 64, 635–661. [Google Scholar] [CrossRef]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Li, J.; Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef]
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of te gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef]
- Li, R.; Andreu-Sánchez, S.; Kuipers, F.; Fu, J. Gut microbiome and bile acids in obesity-related diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101493. [Google Scholar] [CrossRef]
- Murphy, K.; O’Donovan, A.N.; Caplice, N.M.; Ross, R.P.; Stanton, C. Exploring the gut microbiota and cardiovascular disease. Metabolites 2021, 11, 493. [Google Scholar] [CrossRef]
- Islam, K.B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Yang, J.F.; Palmiotti, A.; Kuipers, F. Emerging roles of bile acids in control of intestinal functions. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 127–133. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of dietary interventions on chronic inflammatory diseases in relation to the microbiome: A systematic review. Nutrients 2021, 13, 3208. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Ghaffarzadegan, T.; Essen, S.; Verbrugghe, P.; Marungruang, N.; Hallenius, F.F.; Nyman, M.; Sandahl, M. Determination of free and conjugated bile acids in serum of Apoe(−/−) mice fed different lingonberry fractions by UHPLC-MS. Sci. Rep. 2019, 9, 3800. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Liao, W. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef]
- O’Connor, K.M.; Lucking, E.F.; Bastiaanssen, T.F.S.; Peterson, V.L.; Crispie, F.; Cotter, P.D.; Clarke, G.; Cryan, J.F.; O’Halloran, K.D. Prebiotic administration modulates gut microbiota and faecal short-chain fatty acid concentrations but does not prevent chronic intermittent hypoxia-induced apnoea and hypertension in adult rats. EBioMedicine 2020, 59, 102968. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Chan, J.Y.H.; Wu, K.L.H.; Yu, H.-R.; Lee, W.-C.; Hou, C.-Y.; Tain, Y.-L. Altered gut microbiota and its metabolites in hypertension of developmental origins: Exploring differences between fructose and antibiotics exposure. Int. J. Mol. Sci. 2021, 22, 2674. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-D.; Tung, T.-H.; Teng, C.-Y.; Chang, C.-H.; Chen, Y.-C.; Huang, H.-Y.; Lee, H.-C.; Huang, S.-Y. Fish oil ameliorates neuropsychiatric behaviors and gut dysbiosis by elevating selected microbiota–derived metabolites and tissue tight junctions in rats under chronic sleep deprivation. Food Funct. 2022, 13, 2662. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.Y.; Chen, L.; Wang, Y.; He, D.; Zhao, D.; Zhang, S.H.; Yu, R.; Huang, J.H. The potential mechanism of Liu–Wei–Di–Huang Pills in treatment of type 2 diabetic mellitus: From gut microbiota to short-chain fatty acids metabolism. Acta Diabetol. 2022, 59, 1295–1308. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, S.; Wu, J.; Luo, L.; Qiao, S.; Li, R.; Xu, W.; Wang, N.; Zhao, B.; Wang, X.; et al. A specific gut microbiota and metabolomic profiles shifts related to antidiabetic action: The similar and complementary antidiabetic properties of type 3 resistant starch from Canna edulis and metformin. Pharmacol. Res. 2020, 159, 104985. [Google Scholar] [CrossRef]
- Thipart, K.; Gruneck, L.; Phunikhom, K.; Sharpton, T.J.; Sattayasai, J.; Popluechai, S. Dark-purple rice extract modulates gut microbiota composition in acetic acid- and indomethacin-induced inflammatory bowel disease in rats. Int. Microbiol. 2023, 26, 423–434. [Google Scholar] [CrossRef]
- Lee, J.; Mitchell, A.E. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): Varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J. Agric. Food Chem. 2011, 59, 857–863. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol-microbiome interactions unifying health effects across geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef]
- Aisara, J.; Wongputtisin, P.; Deejing, S.; Maneewong, C.; Unban, K.; Khanongnuch, C.; Kanpiengjai, A. Potential of inulin-fructooligosaccharides extract produced from red onion. Plants 2021, 10, 2401. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The potential of pectins to modulate the human gut microbiota evaluated by in vitro fermentation: A systematic review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, L.; Meng, Q.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs. J. Anim. Sci. 2018, 96, 4743–4754. [Google Scholar] [CrossRef]
- Balderas, C.; Angulo, J.; Sevilleja-Ortiz, A.; Peiró, C.; Vallejo, S.; Dongil, P.; de Ancos, B.; Sánchez-Moreno, C. Onion and apple functional ingredients intake improves antioxidant and inflammatory status and vascular injury in obese Zucker rats. Antioxidants 2022, 11, 1953. [Google Scholar] [CrossRef]
- González-Peña, D.; Giménez, L.; de Ancos, B.; Sánchez-Moreno, C. Role of dietary onion in modifying the faecal bile acid content in rats fed a high-cholesterol diet. Food Funct. 2017, 8, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jalao, I.; Balderas, C.; Calvo, M.V.; Fontecha, J.; Sánchez-Moreno, C.; de Ancos, B. Impact of high-pressure processed onion on colonic metabolism using a dynamic gastrointestinal digestion simulator. Metabolites 2021, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Colina-Coca, C.; González-Peña, D.; de Ancos, B.; Sánchez-Moreno, C. Dietary onion ameliorates antioxidant defence, inflammatory response, and cardiovascular risk biomarkers in hypercholesterolemic Wistar rats. J. Funct. Food 2017, 36, 300–309. [Google Scholar] [CrossRef]
- Roldán-Marín, E.; Krath, B.N.; Poulsen, M.; Binderup, M.L.; Nielsen, T.H.; Hansen, M.; Langkilde, S.; Cano, M.P.; Sánchez-Moreno, C.; Dragsted, L.O. Effects of an onion by-product on bioactivity and safety markers in healthy rats. Br. J. Nutr. 2009, 102, 1574–1582. [Google Scholar] [CrossRef]
- Roldán-Marín, E.; Jensen, R.I.; Krath, B.N.; Kristensen, M.; Poulsen, M.; Cano, M.P.; Sánchez-Moreno, C.; Dragsted, L.O. An onion byproduct affects plasma lipids in healthy rats. J. Agric. Food Chem. 2010, 58, 5308–5314. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Cho, K.D.; Han, C.K.; Lee, B.H. Loss of body weight and fat and improved lipid profiles in obese rats fed apple pomace or apple juice concentrate. J. Med. Food 2013, 16, 823–830. [Google Scholar] [CrossRef]
- Yoshinari, O.; Shiojima, Y.; Igarashi, K. Anti-obesity effects of onion extract in Zucker diabetic fatty rats. Nutrients 2012, 4, 1518–1526. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Denis, M.C.; Roy, D.; Yeganeh, P.R.; Desjardins, Y.; Varin, T.; Haddad, N.; Amre, D.; Sane, A.T.; Garofalo, C.; Furtos, A.; et al. Apple peel polyphenols: A key player in the prevention and treatment of experimental inflammatory bowel disease. Clin. Sci. 2016, 130, 2217–2237. [Google Scholar] [CrossRef]
- Tilg, H. Obesity, metabolic syndrome, and microbiota: Multiple interactions. J. Clin. Gastroenterol. 2010, 44 (Suppl. S1), S16–S18. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Waldram, A.; Holmes, E.; Wang, Y.; Rantalainen, M.; Wilson, I.D.; Tuohy, K.M.; McCartney, A.L.; Gibson, G.R.; Nicholson. J.K. Top-down systems biology modeling of host metabotype-microbiome associations in obese rodents. J. Proteome Res. 2009, 8, 2361–2375. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. Biofactors 2022, 48, 255–273. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Jauregui, O.; Amezqueta, S.; Dasilva, G.; Medina, I.; Nogues, M.R.; Romeu, M.; et al. Mechanistically different effects of fat and sugar on insulin resistance, hypertension, and gut microbiota in rats. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E552–E563. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef]
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. Obesity in school-aged children and its correlation with gut E. coli and Bifidobacteria: A case-control study. BMC Pediatr. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastroint. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Fei, N.; Zhao, L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef]
- Larrosa, M.; Yanéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- O’Connor, K.; Morrissette, M.; Strandwitz, P.; Ghiglieri, M.; Caboni, M.; Liu, H.; Khoo, C.; D’Onofrio, A.; Lewis, K. Cranberry extracts promote growth of Bacteroidaceae and decrease abundance of Enterobacteriaceae in a human gut simulator model. PLoS ONE 2019, 14, e0224836. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and bioavailability of diet polyphenols and their modulation of gut microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Yang, H.-W.; Tain, Y.-L. Perinatal resveratrol therapy prevents hypertension programmed by maternal chronic kidney disease in adult male offspring: Implications of the gut microbiome and their metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Karaki, S.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Sembries, S.; Dongowski, G.; Mehrländer, K.; Will, F.; Dietrich, H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. J. Agric. Food Chem. 2006, 54, 10269–10280. [Google Scholar] [CrossRef]
- Aprikian, O.; Levrat-Verny, M.A.; Besson, C.; Busserolles, J.; Remesy, C.; Demigne, C. Apple favourably affects parameters of cholesterol metabolism and of anti-oxidative protection in cholesterol-fed rats. Food Chem. 2001, 75, 445–452. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Krath, B.N.; Markowski, J.; Poulsen, M.; Hansen, M.; Kołodziejczyk, K.; Kosmala, M.; Dragsted, L.O. Apple pomace improves gut health in Fisher rats independent of seed content. Food Funct. 2018, 9, 2931. [Google Scholar] [CrossRef]
- Martinez, O.D.M.; Gomes, M.J.C.; Grancieri, M.; de São José, V.P.B.; Toledo, R.C.L.; Queiroz, V.A.V.; da Silva, B.P.; Martino, H.S.D. Sorghum flour BRS 305 hybrid has the potential to modulate the intestinal microbiota of rats fed with a high-fat high-fructose diet. Eur. J. Nutr. 2023, 62, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Fukami, T.; Masuo, Y.; Brocker, C.N.; Xie, C.; Krausz, K.W.; Wolf, C.R.; Henderson, C.J.; Gonzalez, F.J. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res. 2016, 57, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile acid physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi. J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- De Boever, P.; Wouters, R.; Verschaeve, L.; Berckmans, P.; Schoeters, G.; Verstraete, W. Protective effect of the bile salt hydrolase-active Lactobacillus reuteri against bile salt cytotoxicity. Appl. Microbiol. Biotechnol. 2000, 53, 709–714. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Johnston, I.; Nolan, J.; Pattni, S.S.; Walters, J.R. New insights into bile acid malabsorption. Curr. Gastroenterol. Rep. 2011, 13, 418–425. [Google Scholar] [CrossRef]
- Walters, J.R. Defining primary bile acid diarrhea: Making the diagnosis and recognizing the disorder. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 561–567. [Google Scholar] [CrossRef]
- Mullish, B.H.; Allegretti, J.R. The contribution of bile acid metabolism to the pathogenesis of Clostridioides difficile infection. Ther. Adv. Gastroenterol. 2021, 14, 17562848211017725. [Google Scholar] [CrossRef]
- Amaral, J.D.; Viana, R.J.; Ramalho, R.M.; Steer, C.J.; Rodrigues, C.M. Bile acids: Regulation of apoptosis by ursodeoxycholic acid. J. Lipid Res. 2009, 50, 1721–1734. [Google Scholar] [CrossRef]
- Wells, J.E.; Berr, F.; Thomas, L.A.; Dowling, R.H.; Hylemon, P.B. Isolation and characterization of cholic acid 7α-dehydroxylating fecal bacteria from cholesterol gallstone patients. J. Hepatol. 2000, 32, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, W.; Hu, D.; Xu, H.; Ji, G. Secondary bile acids and tumorigenesis in colorectal cancer. Front. Oncol. 2022, 12, 813745. [Google Scholar] [CrossRef]
- Thomas, C.E.; Luu, H.N.; Wang, R.; Xie, G.; Adams-Haduch, J.; Jin, A.; Koh, W.P.; Jia, W.; Behari, J.; Yuan, J.M. Association between pre-diagnostic serum bile acids and hepatocellular carcinoma: The Singapore Chinese health study. Cancers 2021, 13, 2648. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Zhao, A.; Lei, S.; Zhang, Y.; Xie, G.; Chen, T.; Qu, C.; Rajani, C.; Dong, B.; et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017, 15, 120. [Google Scholar] [CrossRef]
- Kumari, K.; Augusti, K.T. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J. Ethnopharmacol. 2007, 109, 367–371. [Google Scholar] [CrossRef]
- Hall, J.A.; Jewell, D.E.; Ephraim, E. Changes in the fecal metabolome are associated with feeding fiber not health status in cats with chronic kidney disease. Metabolites 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Hosoyamada, Y.; Yamada, M. Effects of dietary fish oil and apple polyphenol on the concentration serum lipids and excretion of fecal bile acids in rats. J. Nutr. Sci. Vitaminol. 2017, 63, 21–27. [Google Scholar] [CrossRef] [PubMed]


| LC | OC | OO | OA | |
|---|---|---|---|---|
| Caecum | ||||
| pH | 7.16 ± 0.39 a | 7.22 ± 0.4 a | 7.34 ± 0.42 a | 7.01 ± 0.29 a |
| Weight (g fw) | 0.55 ± 0.03 a | 0.55 ± 0.03 a | 0.54 ± 0.02 a | 0.54 ± 0.03 a |
| ABTS•+ (μmol TE/100 g fw) | 3.16 ± 0.06 b | 2.45 ± 0.15 a | 3.23 ± 0.13 b | 3.00 ± 0.17 b |
| FRAP (μmol TE/100 g fw) | 2.31 ± 0.06 b | 1.89 ± 0.05 a | 2.30 ± 0.05 b | 2.31 ± 0.06 b |
| Faeces | ||||
| pH—Week 0 | 7.00 ± 0.57 aA | 6.63 ± 0.39 aA | 6.73 ± 0.41 aA | 6.71 ± 0.28 aA |
| pH—Week 8 | 7.07 ± 0.39 abA | 6.73 ± 0.28 aA | 7.35 ± 0.43 bB | 7.07 ± 0.37 abB |
| Weight—Week 0 (g fw) | 3.19 ± 0.24 aA | 3.89 ± 0.30 bB | 4.45 ± 0.24 cB | 4.30 ± 0.51 bcB |
| Weight—Week 8 (g fw) | 3.02 ± 0.20 aA | 3.36 ± 0.34 aA | 3.79 ± 0.32 bA | 3.35 ± 0.33 aA |
| Total aerobic bacteria count (log CFU/g fw)—Week 0 | 8.76 ± 0.07 aB | 8.92 ± 0.10 bB | 8.97 ± 0.08 bB | 8.98 ± 0.10 bB |
| Total aerobic bacteria count (log CFU/g fw)—Week 8 | 6.91 ± 0.42 aA | 8.79 ± 0.02 cA | 8.12 ± 0.06 bA | 7.88 ± 0.28 bA |
| Enterobacter spp. (log CFU/g fw)—Week 0 | 8.16 ± 0.01 aB | 8.10 ± 0.14 aA | 8.12 ± 0.68 aA | 8.08 ± 0.78 aB |
| Enterobacter spp. (log CFU/g fw)—Week 8 | 7.48 ± 0.16 abA | 8.06 ± 0.47 cA | 7.84 ± 0.13 bcA | 7.20 ± 0.60 aA |
| β-glucuronidase-positive Escherichia coli (log CFU/g fw)—Week 0 | 8.08 ± 0.65 aB | 8.60 ± 0.41 bB | 8.77 ± 0.10 bB | 8.78 ± 0.18 bA |
| β-glucuronidase-positive Escherichia coli (log CFU/g fw)—Week 8 | 7.52 ± 0.20 aA | 7.99 ± 0.47 bA | 8.44 ± 0.18 cA | 8.61 ± 0.15 cA |
| Total coliform bacteria count (log CFU/g fw)—Week 0 | 8.08 ± 0.65 aB | 8.60 ± 0.41 bA | 8.77 ± 0.10 bB | 8.78 ± 0.18 bA |
| Total coliform bacteria count (log CFU/g fw)—Week 8 | 7.52 ± 0.20 aA | 7.99 ± 0.47 bA | 8.44 ± 0.18 cA | 8.61 ± 0.15 cA |
| Sulfite-reducing Clostridium spp. (log CFU/g fw)—Week 0 | 6.64 ± 0.14 bB | 4.57 ± 0.39 aA | 4.55 ± 0.54 aA | 4.65 ± 0.53 aA |
| Sulfite-reducing Clostridium spp. (log CFU/g fw)—Week 8 | 6.23 ± 0.02 aA | 6.61 ± 0.05 bB | 6.42 ± 0.09 abB | 6.57 ± 0.33 bB |
| Bifidobacterium spp. (log CFU/g fw)—Week 0 | 9.09 ± 0.03 bB | 8.49 ± 0.13 aB | 8.50 ± 0.40 aB | 8.51 ± 0.35 aB |
| Bifidobacterium spp. (log CFU/g fw)—Week 8 | 8.32 ± 0.11 cA | 7.88 ± 0.04 abA | 7.77 ± 0.14 aA | 7.99 ± 0.03 bA |
| Lactobacillus spp. (log CFU/g fw)—Week 0 | 9.07 ± 0.09 bB | 8.58 ± 0.13 aB | 8.53 ± 0.24 aB | 8.55 ± 0.06 aB |
| Lactobacillus spp. (log CFU/g fw)—Week 8 | 8.35 ± 0.10 dA | 7.67 ± 0.02 aA | 7.85 ± 0.08 bA | 8.11 ± 0.02 cA |
| LC | OC | OO | OA | |
|---|---|---|---|---|
| Acetic acid (µmol/g fw) | 46.32 ± 4.54 b | 35.58 ± 1.45 a | 55.01 ± 4.98 c | 45.42 ± 3.92 b |
| Propionic acid (µmol/g fw) | 5.47 ± 0.75 c | 2.61 ± 0.03 a | 4.40 ± 0.17 b | 4.42 ± 1.02 b |
| Isobutyric acid (µmol/g fw) | 0.46 ± 0.06 c | 0.32 ± 0.01 a | 0.39 ± 0.04 b | 0.35 ± 0.02 ab |
| Butyric acid (µmol/g fw) | 0.27 ± 0.02 c | 0.13 ± 0.02 b | 0.10 ± 0.02 a | 0.13 ± 0.01 b |
| Isovaleric acid (µmol/g fw) | 0.31 ± 0.05 c | 0.19 ± 0.05 b | 0.19 ± 0.04 b | 0.12 ± 0.02 a |
| Valeric acid (µmol/g fw) | 0.11 ± 0.01 a | 0.15 ± 0.02 b | 0.24 ± 0.01 c | 0.37 ± 0.02 d |
| Caproic acid (µmol/g fw) | 0.13 ± 0.01 b | 0.12 ± 0.004 ab | 0.12 ± 0.01 ab | 0.11 ± 0.01 a |
| Bile Acid (Abbreviation) | Formula | RT (min) | Monoisotopic Mass | (M-H) | Fragments |
|---|---|---|---|---|---|
| Tauro-α-muricholic acid (T-α-MCA) | C26H45NO7S | 2.88 | 515.2917 | 514.284398 | 514.2897; 496.3058; 479.3058; 358.7687; 80.9621; 65.6705 |
| Tauroursodeoxycholic acid (TUDCA) | C26H45NO6S | 3.27 | 499.2968 | 498.289483 | 498.2880; 479.9107; 465.9335; 393.2521; 159.0796; 96.9606 |
| Tauro-β-muricholic acid (T-β-MCA) | C26H45NO7S | 3.29 | 515.2917 | 514.284398 | 514.2897; 496.3058; 479.3058; 358.7687; 80.9621; 65.6705 |
| ω-muricholic acid (ω-MCA) | C24H40O5 | 3.35 | 408.2876 | 407.280298 | 407.2799; 391.2347; 373.1968; 345.2663; 179.0411; 59.011 |
| Taurocholic acid (TCA) | C26H45NO7S | 3.37 | 515.2917 | 514.284398 | 514.2897; 496.9256; 479.3105; 357.2196; 80.9621 |
| Glycocholic acid (GCA) | C26H43NO6 | 3.40 | 465.3090 | 464.301762 | 464.3091; 447.1784; 405.2479; 379.2552; 357.1239 |
| α-Muricholic acid (α-MCA) | C24H40O5 | 4.00 | 408.2876 | 407.280298 | 407.2791; 389.2682; 373.2247; 345.2770; 60.0348 |
| β-muricholic acid (β-MCA) | C24H40O5 | 4.17 | 408.2876 | 407.280298 | 407.2805; 389.2735 |
| Taurochenodeoxycholic acid (TCDA) | C26H45NO6S | 4.35 | 499.2968 | 498.289483 | 498.2881; 480.2670; 466.2540; 388.2578; 374.1424; 80.9626 |
| Hyocholic acid (HCA) | C24H40O5 | 4.70 | 408.2876 | 407.280298 | 407.2805; 389.2735 |
| Ursodeoxycholic acid (UDCA) | C24H40O4 | 4.85 | 392.2927 | 391.285384 | 391.2526; 373.9815; 357.7511; 329.7645; 221.0703; 59.0103 |
| Glycodeoxycholic acid (GDCA) | C26H43NO5 | 4.93 | 449.3141 | 448.306847 | 448.3165; 433.2967; 407.2899; 389.2625; 329.0834; 74.0232 |
| Glycochenodeoxycholic acid (GCDCA) | C26H43NO5 | 5.16 | 449.3141 | 448.306847 | 448.3165; 433.2967; 407.2899; 389.2625; 329.0834; 74.0232 |
| Taurodeoxycholic acid (TDCA) | C26H45NO6S | 5.33 | 499.2968 | 498.289483 | 498.2881; 480.2670; 466.2540; 388.2578; 374.1424; 80.9626 |
| Cholic acid (CA) | C24H40O5 | 5.53 | 408.2876 | 407.280298 | 407.2808; 391.2378; 371.2674; 345.2730; 289.7773; 131.6716 |
| Hyodeoxycholic acid (HDCA) | C24H40O4 | 5.65 | 392.2927 | 391.285384 | 391.2839; 373.2686; 345.2772; 327.2668; 284.2067; 59.0152 |
| Nutriacholic acid (NCA) | C24H38O4 | 6.65 | 390.2770 | 389.269734 | |
| Taurolithocholic acid (TLCA) | C26H45NO5S | 6.75 | 483.3018 | 482.294569 | 482.2910; 464.4037; 389.2734; 349.1847; 79.9556 |
| Deoxycholic acid (DCA) | C24H40O4 | 7.66 | 392.2927 | 391.285384 | 391.2905; 355.2557; 345.2856; 327.2615; 140.6033; 57.0309 |
| Chenodeoxycholic acid (CDCA) | C24H40O4 | 9.22 | 392.2927 | 391.285384 | 391.2872; 373.9914; 345.2780; 329.2756; 140.6033; 59.0132 |
| Lithocholic acid (LCA) | C24H40O3 | 16.33 | 376.2977 | 375.290469 | 375.2906; 357.2796; 329.3094; 191.4657; 50.0429 |
| Glycolithocholic acid (GLCA) | C26H43NO4 | 22.69 | 433.3192 | 432.311933 | 433.3379; 405.2467; 387.2367; 373.2224; 359.1364; 59.0162 |
| Week 0 | Week 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Abbreviation | Formula | RT (min) | Monoisotopic Mass | % Change Lean vs. Obese | % Change LC vs. OC | % Change OO vs. OC | % Change OA vs. OC | % Change OO vs. OA |
| Primary | CA | C24H40O5 | 5.53 | 408.2876 | ↑ | ↓ | ↓ | ↓ | ↑ |
| Primary | CDCA | C24H40O4 | 9.22 | 392.2927 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Primary (mouse) | α-MCA | C24H40O5 | 4.00 | 408.2876 | ↓ | ↓ | ↓ | ↓ | ↑ |
| Primary (mouse) | β-MCA | C24H40O5 | 4.17 | 408.2876 | ↓ | ↓ | ↓ | ↓ | ↑ |
| Secondary (mouse) | ω-MCA | C24H40O5 | 3.35 | 408.2876 | ↑ | ↓ | ↓ | ↓ | ↓ |
| Secondary | HCA | C24H40O5 | 4.70 | 408.2876 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Secondary | UDCA | C24H40O4 | 4.85 | 392.2927 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Secondary | HDCA | C24H40O4 | 5.65 | 392.2927 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Secondary | NCA | C24H38O4 | 6.65 | 390.2770 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Secondary | DCA | C24H40O4 | 7.66 | 392.2927 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Secondary | LCA | C24H40O3 | 16.33 | 376.2977 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Glycoconjugated | GCA | C26H43NO6 | 3.40 | 465.3090 | ↑ | ↑ | ↑ | ↑ | ↓ |
| Glycoconjugated | GDCA | C26H43NO5 | 4.93 | 449.3141 | ↑ | ↑ | ↓ | ↓ | ↓ |
| Glycoconjugated | GCDCA | C26H43NO5 | 5.16 | 449.3141 | ↑ | ↑ | ↑ | ↑ | ↑ |
| Glycoconjugated | GLCA | C26H43NO4 | 22.69 | 433.3192 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Tauroconjugated | TUDCA | C26H45NO6S | 3.27 | 499.2968 | ↑ | ↑ | ↓ | ↓ | ↑ |
| Tauroconjugated | TCA | C26H45NO7S | 3.37 | 515.2917 | ↑ | ↑ | ↓ | ↑ | ↓ |
| Tauroconjugated | TCDA | C26H45NO6S | 4.35 | 499.2968 | ↑ | ↑ | ↑ | ↑ | ↓ |
| Tauroconjugated | TDCA | C26H45NO6S | 5.33 | 499.2968 | ↑ | ↑ | ↓ | ↑ | ↓ |
| Tauroconjugated | TLCA | C26H45NO5S | 6.75 | 483.3018 | ↑ | ↑ | ↑ | ↑ | ↓ |
| Tauroconjugated (mouse) | T-α-MCA | C26H45NO7S | 2.88 | 515.2917 | ↑ | ↑ | ↓ | ↑ | ↓ |
| Tauroconjugated (mouse) | T-β-MCA | C26H45NO7S | 3.29 | 515.2917 | ↓ | ↑ | ↑ | ↑ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balderas, C.; de Ancos, B.; Sánchez-Moreno, C. Bile Acids and Short-Chain Fatty Acids Are Modulated after Onion and Apple Consumption in Obese Zucker Rats. Nutrients 2023, 15, 3035. https://doi.org/10.3390/nu15133035
Balderas C, de Ancos B, Sánchez-Moreno C. Bile Acids and Short-Chain Fatty Acids Are Modulated after Onion and Apple Consumption in Obese Zucker Rats. Nutrients. 2023; 15(13):3035. https://doi.org/10.3390/nu15133035
Chicago/Turabian StyleBalderas, Claudia, Begoña de Ancos, and Concepción Sánchez-Moreno. 2023. "Bile Acids and Short-Chain Fatty Acids Are Modulated after Onion and Apple Consumption in Obese Zucker Rats" Nutrients 15, no. 13: 3035. https://doi.org/10.3390/nu15133035
APA StyleBalderas, C., de Ancos, B., & Sánchez-Moreno, C. (2023). Bile Acids and Short-Chain Fatty Acids Are Modulated after Onion and Apple Consumption in Obese Zucker Rats. Nutrients, 15(13), 3035. https://doi.org/10.3390/nu15133035








