Cross-Sectional Association between Estimated Hardness of the Habitual Diet and Depressive Symptoms in Older Japanese Men
Abstract
1. Introduction
2. Materials and Methods
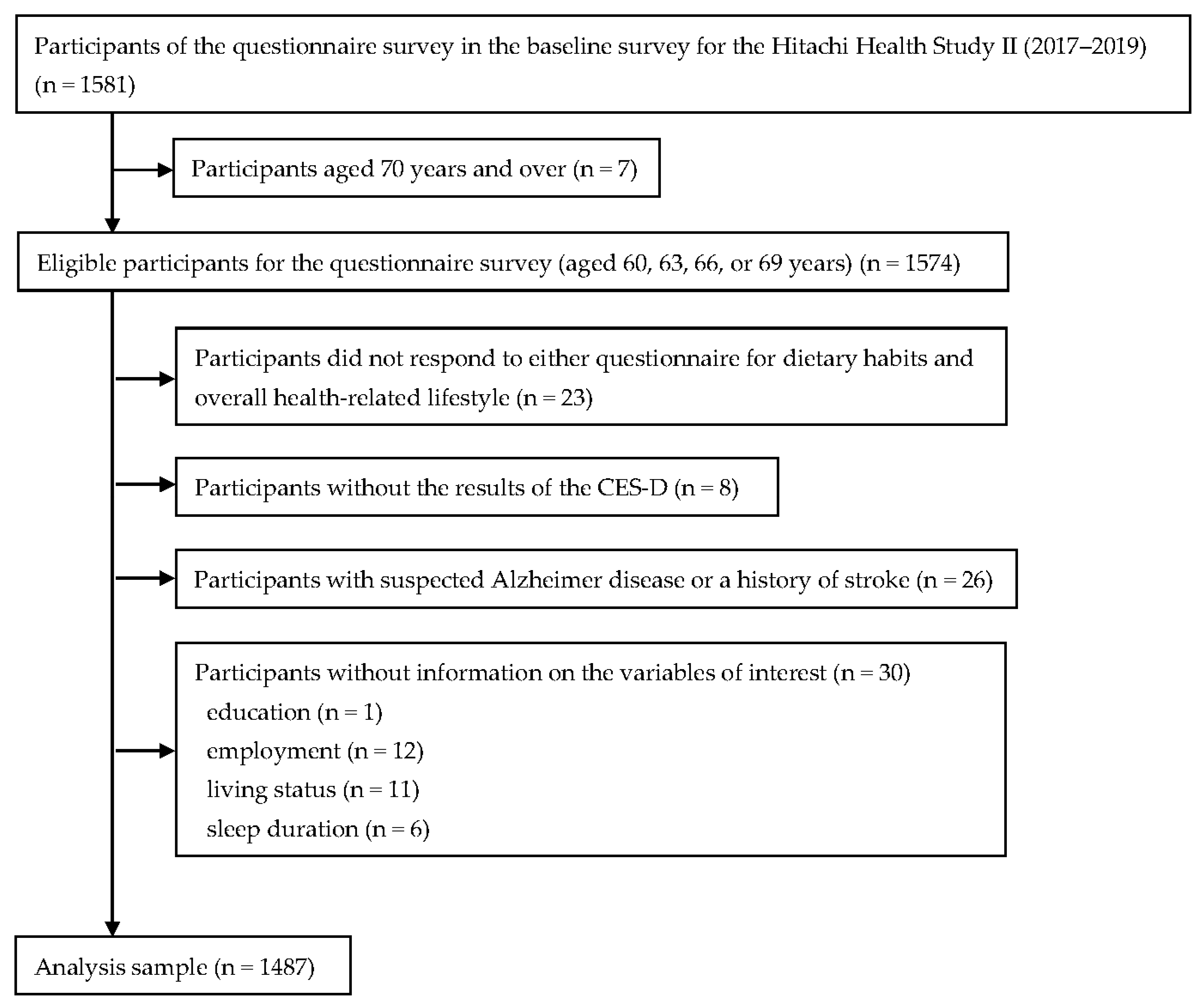
2.1. Study Design and Participants
2.2. Estimation of Dietary Hardness
2.3. Assessment of Depressive Symptoms
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Associations between Dietary Hardness and Selected Characteristics
3.2. Associations between Dietary Hardness and Dietary Intake
3.3. Associations between Dietary Hardness and the Prevalence of Depressive Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Population Division of the Department of Economic and Social Affairs of the United Nations. World Population Prospects. 2022. Available online: https://population.un.org/wpp/ (accessed on 21 May 2023).
- Dang, L.; Ananthasubramaniam, A.; Mezuk, B. Spotlight on the Challenges of Depression Following Retirement and Opportunities for Interventions. Clin. Interv. Aging 2022, 17, 1037–1056. [Google Scholar] [CrossRef]
- Cole, M.G.; Dendukuri, N. Risk Factors for Depression among Elderly Community Subjects: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2003, 160, 1147–1156. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease. 2019. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 21 May 2023).
- Paluska, S.A.; Schwenk, T.L. Physical Activity and Mental Health. Sport. Med. 2000, 29, 167–180. [Google Scholar] [CrossRef]
- Smith, A.P.; Chaplin, K.; Wadsworth, E. Chewing Gum, Occupational Stress, Work Performance and Wellbeing. An Intervention Study. Appetite 2012, 58, 1083–1108. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Woods, M. Effects of Chewing Gum on the Stress and Work of University Students. Appetite 2012, 58, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Otomaru, A.; Sakuma, Y.; Mochizuki, Y.; Ishida, S.; Kanoya, Y.; Sato, C. Effect of Regular Gum Chewing on Levels of Anxiety, Mood, and Fatigue in Healthy Young Adults. Clin. Pract. Epidemiol. Ment. Health 2011, 7, 133–139. [Google Scholar] [CrossRef]
- Yaman-Sözbir, Ş.; Ayaz-Alkaya, S.; Bayrak-Kahraman, B. Effect of Chewing Gum on Stress, Anxiety, Depression, Self-Focused Attention, and Academic Success: A Randomized Controlled Study. Stress Health 2019, 35, 441–446. [Google Scholar] [CrossRef]
- Smith, A. Effects of Chewing Gum on Stress and Health: A Replication and Investigation of Dose-Response. Stress Health 2013, 29, 172–174. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Yamasaki, M.; Hayabuchi, H.; Goda, T.; Oka, J.; Baba, K.; Ohki, K.; et al. Hardness (Difficulty of Chewing) of the Habitual Diet in Relation to Body Mass Index and Waist Circumference in Free-Living Japanese Women Aged 18–22 Y. Am. J. Clin. Nutr. 2007, 86, 206–213. [Google Scholar] [CrossRef]
- Okubo, H.; Murakami, K.; Inagaki, H.; Gondo, Y.; Ikebe, K.; Kamide, K.; Masui, Y.; Arai, Y.; Ishizaki, T.; Sasaki, S.; et al. Hardness of the Habitual Diet and Its Relationship with Cognitive Function among 70-Year-Old Japanese Elderly: Findings from the SONIC Study. J. Oral Rehabil. 2019, 46, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Watanabe, T.; Kohri, T.; Yamasaki, M.; Watanabe, R.; Baba, K.; Shibata, K.; et al. Association between Hardness (Difficulty of Chewing) of the Habitual Diet and Premenstrual Symptoms in Young Japanese Women. Environ. Health Insights 2009, 3, 53–61. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of Relative Validity of Food Group Intakes Estimated by Comprehensive and Brief-Type Self-Administered Diet History Questionnaires against 16 d Dietary Records in Japanese Adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard Tables of Food Composition in Japan, 2010; National Printing Bureau: Tokyo, Japan, 2010. (In Japanese) [Google Scholar]
- Yanagisawa, Y.; Tamura, A.; Akasaka, M.; Teramoto, Y. A Study of the Physical Properties of Food and Ingestion Functions. The 1st Report: On Objective Method of Measurement of Physical Properties of Foods, and Classification of Foods. Shoni Shikagaku Zasshi 1985, 23, 962–983. (In Japanese) [Google Scholar]
- Yanagisawa, Y.; Tamura, A.; Teramoto, Y.; Akasaka, M. A Classification of Foods by the Amount of Masticatory Action Involved. Shoni Shikagaku Zasshi. 1989, 27, 74–84. (In Japanese) [Google Scholar] [PubMed]
- Ganpule, A.A.; Tanaka, S.; Ishikawa-Takata, K.; Tabata, I. Interindividual Variability in Sleeping Metabolic Rate in Japanese Subjects. Eur. J. Clin. Nutr. 2007, 61, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.E.; Black, A.E. Markers of the Validity of Reported Energy Intake. J. Nutr. 2003, 133, 895S–920S. [Google Scholar] [CrossRef]
- Willett, W.C. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Kohout, F.J.; Berkman, L.F.; Evans, D.A.; Cornoni-Huntley, J. Two Shorter Forms of the CES-D (Center for Epidemiological Studies Depression) Depression Symptoms Index. J. Aging Health 1993, 5, 179–193. [Google Scholar] [CrossRef]
- Shima, S.; Shikano, T.; Kitamura, T.; Asai, M. New Self-Rating Scale for Depression. Clin. Psychiatry 1985, 27, 717–723. (In Japanese) [Google Scholar]
- Torres, E. Psychometric Properties of the Center for Epidemiologic Studies Depression Scale in African American and Black Caribbean US Adults. Issues Ment. Health Nurs. 2012, 33, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Jinbo, D.; Nakamura, Y.; Taniguchi, M.; Urakami, K. Development and Evaluation of a Computerised Test Battery for Alzheimer’s Disease Screening in Community-Based Settings. Am. J. Alzheimers. Dis. Other Demen. 2009, 24, 129–135. [Google Scholar] [CrossRef]
- Katoh, S.; Shimogaki, H.; Onodera, A.; Ueda, H.; Oikawa, K.; Ikeda, K.; Kosaka, A.; Imai, Y.; Hasegawa, K. Development of the Revised Version of Hasegawa’s Dementia Scale. Jpn. J. Geriatr. Psychiatry Geriatr. Psychiatry 1991, 2, 1339–1347. [Google Scholar]
- Ito, Y.; Urakami, K. Evaluation of Dementia-Prevention Classes for Community-Dwelling Older Adults with Mild Cognitive Impairment. Psychogeriatrics 2012, 12, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Jimbo, D.; Taniguchi, M.; Urakami, K. Touch Panel-Type Dementia Assessment Scale: A New Computer-Based Rating Scale for Alzheimer’s Disease. Psychogeriatrics 2011, 11, 28–33. [Google Scholar] [CrossRef]
- Rosen, W.G.; Mohs, R.C.; Davis, K.L. A New Rating Scale for Alzheimer’s Disease. Am. J. Psychiatry 1984, 141, 1356–1364. [Google Scholar]
- Kashino, I.; Kochi, T.; Imamura, F.; Eguchi, M.; Kuwahara, K.; Nanri, A.; Kurotani, K.; Akter, S.; Hu, H.; Miki, T.; et al. Prospective Association of Soft Drink Consumption with Depressive Symptoms. Nutrition 2021, 81, 110860. [Google Scholar] [CrossRef]
- Shimmura, N.; Nanri, A.; Kashino, I.; Kochi, T.; Eguchi, M.; Kabe, I.; Mizoue, T. Prospective Association of Confectionery Intake with Depressive Symptoms among Japanese Workers: The Furukawa Nutrition and Health Study. Br. J. Nutr. 2022, 128, 139–144. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S. Dietary Intake and Depressive Symptoms: A Systematic Review of Observational Studies. Mol. Nutr. Food Res. 2010, 54, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Hayabuchi, H.; Ohta, M.; Sato, M.; Mishima, N.; Mizoue, T. Serum Folate and Depressive Symptoms Among Japanese Men and Women: A Cross-Sectional and Prospective Study. Psychiatry Res. 2012, 200, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Kochi, T.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Kurotani, K.; Ito, R.; Akter, S.; Kashino, I.; Pham, N.M.; et al. Dietary Intake of Minerals in Relation to Depressive Symptoms in Japanese Employees: The Furukawa Nutrition and Health Study. Nutrition 2015, 31, 686–690. [Google Scholar] [CrossRef]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Butterworth, P. Does Reverse Causality Explain the Relationship between Diet and Depression? J. Affect. Disord. 2015, 175, 248–250. [Google Scholar] [CrossRef]
- Kamiya, K.; Fumoto, M.; Kikuchi, H.; Sekiyama, T.; Mohri-Ikuzawaa, Y.; Umino, M.; Arita, H. Prolonged Gum Chewing Evokes Activation of the Ventral Part of Prefrontal Cortex and Suppression of Nociceptive Responses: Involvement of the Serotonergic System. J. Med. Dent. Sci. 2010, 57, 35–43. [Google Scholar]
- Scholey, A.; Haskell, C.; Robertson, B.; Kennedy, D.; Milne, A.; Wetherell, M. Chewing Gum Alleviates Negative Mood and Reduces Cortisol during Acute Laboratory Psychological Stress. Physiol. Behav. 2009, 97, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Zhou, Q.; Niwa, M.; Kubo, K. ya Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1687. [Google Scholar] [CrossRef] [PubMed]
- Fukushima-Nakayama, Y.; Ono, T.; Ono, T.; Hayashi, M.; Inoue, M.; Wake, H.; Nakashima, T. Reduced Mastication Impairs Memory Function. J. Dent. Res. 2017, 96, 1058–1066. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hirayama, A. Effects of Soft-Diet Feeding on Synaptic Density in the Hippocampus and Parietal Cortex of Senescence-Accelerated Mice. Brain Res. 2001, 902, 255–263. [Google Scholar] [CrossRef]
- Vicario-Abejón, C.; Owens, D.; McKay, R.; Segal, M. Role of Neurotrophins in Central Synapse Formation and Stabilization. Nat. Rev. Neurosci. 2002, 3, 965–974. [Google Scholar] [CrossRef]
| T1 (n = 495) | T2 (n = 496) | T3 (n = 496) | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Dietary hardness (mV·s/4184 kJ), mean, SD * | 192 | 13 | 219 | 6 | 253 | 22 |
| Age (years), mean, SD | 63.2 | 3.5 | 63.5 | 3.6 | 63.8 | 3.5 |
| BMI (kg/m2), mean, SD | 24.4 | 3.3 | 24.2 | 3.2 | 23.9 | 2.8 |
| Education (years) | ||||||
| <10 | 17 | (3.4) | 24 | (4.8) | 25 | (5.0) |
| 10 to 12 | 275 | (55.6) | 237 | (47.8) | 255 | (51.4) |
| ≥13 | 203 | (41.0) | 235 | (47.4) | 216 | (43.5) |
| Current employment | 357 | (72.1) | 355 | (71.6) | 349 | (70.4) |
| Living alone | 37 | (7.5) | 36 | (7.3) | 30 | (6.0) |
| Smoking status | ||||||
| Never | 151 | (30.5) | 147 | (29.6) | 136 | (27.4) |
| Former | 239 | (48.3) | 244 | (49.2) | 242 | (48.8) |
| Current | 105 | (21.2) | 105 | (21.2) | 118 | (23.8) |
| Alcohol consumption (g of ethanol/day) | ||||||
| None | 146 | (29.5) | 124 | (25.0) | 99 | (20.0) |
| >0 to <46 | 316 | (63.8) | 327 | (65.9) | 335 | (67.5) |
| ≥46 | 33 | (6.7) | 45 | (9.1) | 62 | (12.5) |
| Sleep duration (h/d) | ||||||
| <6 | 51 | (10.3) | 44 | (8.9) | 37 | (7.5) |
| 6 to <7 | 145 | (29.3) | 152 | (30.6) | 125 | (25.2) |
| ≥7 | 299 | (60.4) | 300 | (60.5) | 334 | (67.3) |
| Habitual exercise | 216 | (43.6) | 223 | (45.0) | 287 | (57.9) |
| History of chronic diseases † | 62 | (12.5) | 49 | (9.9) | 44 | (8.9) |
| Diabetes ‡ | 104 | (21.0) | 94 | (19.0) | 128 | (25.8) |
| Cognitive dysfunction § | 41 | (8.3) | 32 | (6.5) | 38 | (7.7) |
| T1 (n = 495) | T2 (n = 496) | T3 (n = 496) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | P for Trend * | |
| Dietary hardness (mV·s/4184 kJ) | |||||||
| Median | 195 | 219 | 246 | ||||
| Energy intake (kJ/day) | 8222 | 104 | 8231 | 104 | 8174 | 104 | 0.73 |
| Nutrients † | |||||||
| n-3 PUFA (g/4184 kJ) | 1.3 | 0.0 | 1.4 | 0.0 | 1.5 | 0.0 | <0.001 |
| Vitamin B6 (mg/4184 kJ) | 0.56 | 0.01 | 0.64 | 0.01 | 0.76 | 0.01 | <0.001 |
| Vitamin B12 (μg/4184 kJ) | 4.2 | 0.1 | 5.0 | 0.1 | 6.1 | 0.1 | <0.001 |
| Folate (μg/4184 kJ) | 145 | 2 | 167 | 2 | 214 | 2 | <0.001 |
| Magnesium (mg/4184 kJ) | 123 | 1 | 134 | 1 | 154 | 1 | <0.001 |
| Zinc (mg/4184 kJ) | 4.1 | 0.0 | 4.3 | 0.0 | 4.6 | 0.0 | <0.001 |
| Food groups (g/4184 kJ) † | |||||||
| Rice | 162.3 | 2.9 | 181.5 | 2.9 | 166.5 | 2.9 | 0.44 |
| Bread | 23.7 | 0.7 | 17.3 | 0.7 | 11.9 | 0.7 | <0.001 |
| Noodles | 41.6 | 1.3 | 45.6 | 1.3 | 44.0 | 1.3 | 0.22 |
| Potatoes | 14.5 | 0.7 | 17.9 | 0.7 | 19.2 | 0.7 | <0.001 |
| Sugar and sweeteners | 2.9 | 0.1 | 2.5 | 0.1 | 2.6 | 0.1 | 0.37 |
| Pulses | 31.8 | 1.0 | 35.1 | 1.0 | 41.4 | 1.0 | <0001 |
| Vegetables | 83.2 | 2.4 | 110.1 | 2.3 | 170.0 | 2.4 | <0.001 |
| Fruits | 31.4 | 1.4 | 36.5 | 1.4 | 43.5 | 1.4 | <0.001 |
| Fish and shellfish | 32.8 | 1.0 | 40.8 | 1.0 | 52.1 | 1.0 | <0.001 |
| Meats | 32.9 | 0.9 | 34.6 | 0.9 | 37.2 | 0.9 | <0.001 |
| Eggs | 20.8 | 0.6 | 20.1 | 0.6 | 22.3 | 0.6 | 0.09 |
| Dairy products | 66.6 | 2.6 | 60.0 | 2.6 | 70.6 | 2.6 | 0.22 |
| Fat and oil | 9.4 | 0.2 | 9.1 | 0.2 | 9.0 | 0.2 | 0.15 |
| Confectioneries | 42.5 | 0.9 | 26.2 | 0.9 | 17.0 | 0.9 | <0.001 |
| Fruit and vegetable juices | 24.9 | 2.1 | 23.9 | 2.1 | 27.2 | 2.1 | 0.41 |
| Alcoholic beverages | 85.8 | 5.4 | 101.3 | 5.4 | 117.5 | 5.4 | <0.001 |
| Unsweetened tea and coffee | 286.9 | 8.3 | 298.5 | 8.3 | 302.7 | 8.3 | 0.18 |
| Sugar-sweetened beverages | 34.6 | 2.2 | 23.3 | 2.2 | 21.0 | 2.2 | <0.001 |
| Seasonings | 10.8 | 0.2 | 11.7 | 0.2 | 11.8 | 0.2 | <0.001 |
| T1 (n = 495) | T2 (n = 496) | T3 (n = 496) | P for Trend * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dietary hardness (mV·s/4184 kJ), median | 195 | 219 | 246 | ||||||
| Depressive symptoms, n (%) † | 76 | (15.4) | 70 | (14.1) | 43 | (8.7) | |||
| Model 1 ‡ | 1 | (reference) | 0.93 | (0.65 | 1.32) | 0.55 | (0.37 | 0.81) | 0.003 |
| Model 2 § | 1 | (reference) | 0.92 | (0.64 | 1.33) | 0.58 | (0.38 | 0.87) | 0.01 |
| Model 3 ** | 1 | (reference) | 0.93 | (0.63 | 1.36) | 0.58 | (0.35 | 0.97) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, A.; Fukunaga, A.; Murakami, K.; Inoue, Y.; Nakagawa, T.; Yamamoto, S.; Konishi, M.; Mizoue, T. Cross-Sectional Association between Estimated Hardness of the Habitual Diet and Depressive Symptoms in Older Japanese Men. Nutrients 2023, 15, 3034. https://doi.org/10.3390/nu15133034
Fujiwara A, Fukunaga A, Murakami K, Inoue Y, Nakagawa T, Yamamoto S, Konishi M, Mizoue T. Cross-Sectional Association between Estimated Hardness of the Habitual Diet and Depressive Symptoms in Older Japanese Men. Nutrients. 2023; 15(13):3034. https://doi.org/10.3390/nu15133034
Chicago/Turabian StyleFujiwara, Aya, Ami Fukunaga, Kentaro Murakami, Yosuke Inoue, Tohru Nakagawa, Shuichiro Yamamoto, Maki Konishi, and Tetsuya Mizoue. 2023. "Cross-Sectional Association between Estimated Hardness of the Habitual Diet and Depressive Symptoms in Older Japanese Men" Nutrients 15, no. 13: 3034. https://doi.org/10.3390/nu15133034
APA StyleFujiwara, A., Fukunaga, A., Murakami, K., Inoue, Y., Nakagawa, T., Yamamoto, S., Konishi, M., & Mizoue, T. (2023). Cross-Sectional Association between Estimated Hardness of the Habitual Diet and Depressive Symptoms in Older Japanese Men. Nutrients, 15(13), 3034. https://doi.org/10.3390/nu15133034







