Association between the Preoperative Dietary Antioxidant Index and Postoperative Quality of Life in Patients with Esophageal Squamous Cell Carcinoma: A Prospective Study Based on the TTD Model
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Health-Related Quality
2.3. Questionnaire Assessment
2.4. Dietary Antioxidant Index (DAI)
2.5. Statistical Methods
3. Results
3.1. Association with Demographic and Clinical Characteristics
3.2. Baseline Quality-of-Life Scores
3.3. Effects of Preoperative DAI on Postoperative Quality of Life in Patients with ESCC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Short, M.W.; Burgers, K.G.; Fry, V.T. Esophageal Cancer. Am. Fam. Physician 2017, 95, 22–28. [Google Scholar] [PubMed]
- Xia, X.; Wu, M.; Gao, Q.; Sun, X.; Ge, X. Consolidation Chemotherapy Rather than Induction Chemotherapy Can Prolong the Survival Rate of Inoperable Esophageal Cancer Patients Who Received Concurrent Chemoradiotherapy. Curr. Oncol. 2022, 29, 6342–6349. [Google Scholar] [CrossRef]
- Tadrous, R.; O’Rourke, D.; Mockler, D.; Broderick, J. Health-related quality of life in narcolepsy: A systematic review and meta-analysis. J. Sleep Res. 2021, 30, e13383. [Google Scholar] [CrossRef]
- Sitlinger, A.; Zafar, S.Y. Health-Related Quality of Life: The Impact on Morbidity and Mortality. Surg. Oncol. Clin. N. Am. 2018, 27, 675–684. [Google Scholar] [CrossRef]
- Lagergren, P.; Johar, A.; Ness-Jensen, E.; Schandl, A. Prediction of severe reflux after oesophageal cancer surgery. Eur. J. Surg. Oncol. (EJSO) 2022, 48, 1011–1016. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; Brandt, P.A.v.D. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int. J. Cancer 2011, 129, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Q.; Lu, X.; Li, W. Post-Diagnosis use of Antioxidant Vitamin Supplements and Breast Cancer Prognosis: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2021, 21, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, Z.; Liu, X.; Agarwal, P.; Zhao, S.; Conroy, D.W.; Ji, G.; Yu, J.; Jaroniec, C.P.; Liu, Z.; et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562. [Google Scholar] [CrossRef]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Vitamin C intake and breast cancer mortality in a cohort of Swedish women. Br. J. Cancer 2013, 109, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Beetch, M.; Harandi-Zadeh, S.; Shen, K.; Lubecka, K.; Kitts, D.D.; O’Hagan, H.; Stefanska, B. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br. J. Pharmacol. 2020, 177, 1382–1408. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E.; Mayne, S.T.; Stolzenberg-Solomon, R.Z.; Li, Z.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Development of a Comprehensive Dietary Antioxidant Index and Application to Lung Cancer Risk in a Cohort of Male Smokers. Am. J. Epidemiology 2004, 160, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Nejati, M.; Vahid, F.; Almasi-Hashiani, A.; Saleh-Ghadimi, S.; Parsi, R.; Jafari-Vayghan, H.; Shivappa, N.; Hébert, J.R. The association between dietary inflammatory index, dietary antioxidant index, and mental health in adolescent girls: An analytical study. BMC Public Health 2022, 22, 1513. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: A prospective cohort study. Front. Cardiovasc. Med. 2022, 9, 2858. [Google Scholar] [CrossRef]
- Vahid, F.; Rahmani, D.; Davoodi, S.H. Validation of Dietary Antioxidant Index (DAI) and investigating the relationship between DAI and the odds of gastric cancer. Nutr. Metab. 2020, 17, 102. [Google Scholar] [CrossRef]
- Vahid, F.; Rahmani, W.; Khodabakhshi, A.; Davoodi, S.H. Associated between Dietary Antioxidant Index (DAI) and Odds of Breast Cancer and Correlation between DAI with Pathobiological Markers: Hospital-Based Incidence Case-Control Study. J. Am. Nutr. Assoc. 2022, 42, 386–392. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Z.; Zhou, J.; Huang, Y.; Chen, S.; Deng, Y.; Qiu, M.; Chen, Y.; Hu, Z. Effects of preoperative albumin-to-globulin ratio on overall survival and quality of life in esophageal cell squamous carcinoma patients: A prospective cohort study. BMC Cancer 2023, 23, 342. [Google Scholar] [CrossRef]
- Arraras, J.I.; Arias, F.; Tejedor, M.; Pruja, E.; Marcos, M.; Martinez, E.; Valerdi, J. The eortc QLQ-C30 (version 3.0) quality of life questionnaire: Validation study for Spain with head and neck cancer patients. Psycho-Oncology 2002, 11, 249–256. [Google Scholar] [CrossRef]
- Fujita, T.; Okada, N.; Sato, T.; Mayanagi, S.; Kanamori, J.; Daiko, H. Translation, validation of the EORTC esophageal cancer quality-of-life questionnaire for Japanese with esophageal squamous cell carcinoma: Analysis in thoraco-laparoscopic esophagectomy versus open esophagectomy. Jpn. J. Clin. Oncol. 2016, 46, 615–621. [Google Scholar] [CrossRef]
- Musoro, J.Z.; Bottomley, A.; Coens, C.; Eggermont, A.M.; King, M.T.; Cocks, K.; Sprangers, M.A.; Groenvold, M.; Velikova, G.; Flechtner, H.-H.; et al. Interpreting European Organisation for Research and Treatment for Cancer Quality of life Questionnaire core 30 scores as minimally importantly different for patients with malignant melanoma. Eur. J. Cancer 2018, 104, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Próchnicki, M.; Rudzki, S.; Laskowska, B.; Brudniak, J. Quality of Life of Cancer Patients Treated with Chemotherapy. Int. J. Environ. Res. Public Health 2020, 17, 6938. [Google Scholar] [CrossRef] [PubMed]
- Gadisa, D.; Gebremariam, E.T.; Ali, G.Y. Reliability and validity of Amharic version of EORTC QLQ-C30 and QLQ-BR23 modules for assessing health-related quality of life among breast cancer patients in Ethiopia. Health Qual. Life Outcomes 2019, 17, 182. [Google Scholar] [CrossRef]
- Mounir, M.; Ibijbijen, A.; Farih, K.; Rabetafika, H.N.; Razafindralambo, H.L. Synbiotics and Their Antioxidant Properties, Mechanisms, and Benefits on Human and Animal Health: A Narrative Review. Biomolecules 2022, 12, 1443. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Lei, Y.-Y.; Ho, S.C.; Kwok, C.; Cheng, A.; Cheung, K.L.; Lee, R.; Mo, F.K.F.; Yeo, W. Association of high adherence to vegetables and fruits dietary pattern with quality of life among Chinese women with early-stage breast cancer. Qual. Life Res. 2022, 31, 1371–1384. [Google Scholar] [CrossRef]
- Kapela, I.; Bąk, E.; Krzemińska, S.A.; Foltyn, A. Evaluation of the level of acceptance of the disease and of satisfaction with life in patients with colorectal cancer treated with chemotherapy. Nurs. Public Health 2017, 7, 53–61. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- van Deudekom, F.J.; Klop, H.G.; Hartgrink, H.H.; Boonstra, J.J.; Lips, I.M.; Slingerland, M.; Mooijaart, S.P. Functional and cognitive impairment, social functioning, frailty and adverse health outcomes in older patients with esophageal cancer, a systematic review. J. Geriatr. Oncol. 2018, 9, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. Int. Rev. J. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Karami, R.; Valizadeh, R.; Rokhgireh, S.; Kabir, A.; Pakaneh, M.; Kassir, R.; Pazouki, A. Five-year outcomes of one anastomosis gastric bypass as conversional surgery following sleeve gastrectomy for weight loss failure. Sci. Rep. 2022, 12, 10304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hou, Z.-K.; Huang, Z.-B.; Chen, X.-L.; Liu, F.-B. Dietary and Lifestyle Factors Related to Gastroesophageal Reflux Disease: A Systematic Review. Ther. Clin. Risk Manag. 2021, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, J.; Li, S.; Li, B.; Jia, M.; Pang, B.; Cui, H. Resveratrol alleviates salivary gland dysfunction induced by ovariectomy in rats. Biochem. Biophys. Res. Commun. 2022, 630, 112–117. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- O’Callaghan, N.; Douglas, P.; Keaver, L. A qualitative study into cancer survivors’ relationship with nutrition post-cancer treatment. J. Hum. Nutr. Diet. 2023, 36, 406–414. [Google Scholar] [CrossRef]
- Mohseni, S.; Tabatabaei-Malazy, O.; Ejtahed, H.-S.; Qorbani, M.; Azadbakht, L.; Khashayar, P.; Larijani, B. Effect of vitamins C and E on cancer survival; a systematic review. DARU J. Pharm. Sci. 2022, 30, 427–441. [Google Scholar] [CrossRef]
- Dacrema, M.; Ali, A.; Ullah, H.; Khan, A.; Di Minno, A.; Xiao, J.; Martins, A.M.C.; Daglia, M. Spice-Derived Bioactive Compounds Confer Colorectal Cancer Prevention via Modulation of Gut Microbiota. Cancers 2022, 14, 5682. [Google Scholar] [CrossRef]
| Variables | DAI < 0.729 [n (%)] | DAI ≥ 0.729 [n (%)] | χ2 | p Value |
|---|---|---|---|---|
| Sex | 3.940 | 0.047 | ||
| Female | 79 (25.7%) | 10 (14.5%) | ||
| Male | 228 (74.3%) | 59 (85.5%) | ||
| Age (years) | 0.901 | 0.343 | ||
| <60 | 132 (43.0%) | 34 (49.3%) | ||
| ≥60 | 175 (57.0%) | 35 (50.7%) | ||
| Education level | 3.405 | 0.065 | ||
| Primary and below | 201 (65.5%) | 37 (53.6%) | ||
| Junior high school and above | 106 (34.5%) | 32 (46.4%) | ||
| Family income per month | 3.507 | 0.061 | ||
| <2000 | 112 (36.5%) | 17 (24.6%) | ||
| ≥2000 | 195 (63.5%) | 52 (75.4%) | ||
| Smoker | 2.326 | 0.127 | ||
| No | 100 (32.6%) | 16 (23.2%) | ||
| Yes | 207 (67.4%) | 53 (76.8%) | ||
| Drinker | 0.912 | 0.340 | ||
| No | 153 (49.8%) | 30 (43.5%) | ||
| Yes | 154 (50.2%) | 39 (56.5%) | ||
| Radiochemotherapy | 2.359 | 0.125 | ||
| No | 178 (58.0%) | 33 (47.8%) | ||
| Yes | 129 (42.0%) | 52.2%) | ||
| TNM stage | 0.651 | 0.420 | ||
| I–II | 141 (45.9%) | 28 (40.6%) | ||
| III–IV | 166 (54.1%) | 41 (59.4%) |
| Domain/Scale | Baseline HRQOL Scores [M (IQR)], n = 376 | |||
|---|---|---|---|---|
| DAI < 0.729 | DAI ≥ 0.729 | Z | p | |
| QLQ-C30 | ||||
| Global health status/QOL | 75.00 (66.67, 83.33) | 83.33 (66.67, 83.33) | −0.301 | 0.763 |
| Functional scales | ||||
| Physical functioning | 100.00 (93.33, 100.00) | 100.00 (93.33, 100.00) | −0.702 | 0.483 |
| Role functioning | 100.00 (100.00, 100.00) | 100.00 (100.00, 100.00) | −0.603 | 0.547 |
| Emotional functioning | 100.00 (83.33, 100.00) | 91.67 (75.00, 100.00) | −2.157 | 0.031 |
| Cognitive functioning | 100.00 (83.33, 100.00) | 100.00 (83.33, 100.00) | −0.889 | 0.374 |
| Social functioning | 100 (66.67, 100.00) | 91.67 (66.67, 100.00) | −0.493 | 0.622 |
| Symptom scales | ||||
| Fatigue | 00.00 (00.00, 22.22) | 11.11 (00.00, 22.22) | −0.210 | 0.833 |
| Nausea/vomiting | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −1.107 | 0.268 |
| Pain | 00.00 (00.00, 16.67) | 00.00 (00.00, 00.00) | −0.122 | 0.903 |
| Dyspnea | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −0.691 | 0.490 |
| Insomnia | 00.00 (00.00, 00.00) | 00.00 (00.00, 33.33) | −1.253 | 0.210 |
| Appetite loss | 00.00 (00.00, 00.00) | 00.00 (00.00, 33.33) | −2.339 | 0.019 |
| Constipation | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −0.050 | 0.960 |
| Diarrhea | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −1.267 | 0.205 |
| QLQ-QES18 | ||||
| General symptom scales | ||||
| Dysphagia | 88.89 (66.67, 100.00) | 88.89 (77.78, 100.00) | −0.316 | 0.752 |
| Eating problems | 00.00 (00.00, 16.67) | 8.33 (0.00, 16.67) | −1.379 | 0.168 |
| Reflux | 00.00 (00.00, 00.00) | 00.00 (00.00, 8.33) | −0.494 | 0.621 |
| Odynophagia | 11.11 (00.00, 22.22) | 11.11 (00.00, 22.22) | −0.133 | 0.894 |
| General symptom items | ||||
| Trouble swallowing saliva | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −0.744 | 0.457 |
| Choking when swallowing | 00.00 (00.00, 33.33) | 33.33 (00.00, 33.33) | −2.372 | 0.018 |
| Dry mouth | 00.00 (00.00, 00.00) | 00.00 (00.00, 25.00) | −0.603 | 0.546 |
| Trouble with taste | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −1.006 | 0.286 |
| Coughing | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −0.497 | 0.619 |
| Speech problems | 00.00 (00.00, 00.00) | 00.00 (00.00, 00.00) | −1.653 | 0.098 |
| Domain/Scale | Time to Deterioration [M (95% CI)], n = 376 | |||
|---|---|---|---|---|
| DAI < 0.729 | DAI ≥ 0.729 | χ2 | p | |
| QLQ-C30 | ||||
| Global health status/QOL | 11.072 (9.466–12.678) | 13.569 (11.481–15.656) | 4.094 | 0.043 |
| Functional scales | ||||
| Physical functioning | 11.828 (10.832–12.823) | 12.255 (9.369–15.141) | 2.198 | 0.138 |
| Role functioning | 14.127 (12.419–15.835) | 17.216 (11.712–22.720) | 1.951 | 0.163 |
| Emotional functioning | 18.497 (15.124–21.869) | 25.823 (19.396–32.251) | 3.582 | 0.058 |
| Cognitive functioning | 23.031 (18.392–27.669) | 40.378 (19.070–61.685) | 4.667 | 0.031 |
| Social functioning | 17.446 (14.956–19.935) | 21.848 (11.865–31.831) | 1.381 | 0.240 |
| Symptom scales | ||||
| Fatigue | 13.109 (11.367–14.851) | 14.719 (11.027–18.411) | 2.298 | 0.130 |
| Nausea/vomiting | 19.088 (15.517–22.659) | 20.534 (11.432–29.636) | 0.958 | 0.328 |
| Pain | 20.140 (15.433–24.846) | 26.349 (24.340–28.358) | 1.509 | 0.219 |
| Dyspnea | 19.844 (16.274–23.414) | 24.312 (13.268–35.356) | 1.188 | 0.276 |
| Insomnia | 21.158 (17.521–24.796) | 20.797 (10.874–30.720) | 0.110 | 0.740 |
| Appetite loss | 20.698 (16.461–24.935) | 17.183 (5.474–28.891) | 0.336 | 0.562 |
| Constipation | 32.657 (28.272–37.042) | 26.612 (13.509–39.714) | 0.049 | 0.825 |
| Diarrhea | 21.717 (18.311–25.122) | 20.172 (10.784–29.560) | 0.020 | 0.887 |
| QLQ-QES18 | ||||
| General symptom scales | ||||
| Dysphagia | 12.123 (10.936–13.311) | 13.372 (10.808–15.935) | 0.751 | 0.386 |
| Eating problems | 14.883 (13.354–16.412) | 17.183 (10.807–23.559) | 2.861 | 0.091 |
| Reflux | 14.127 (12.147–16.107) | 14.653 (12.474–16.832) | 0.343 | 0.558 |
| Odynophagia | 20.994 (16.282–25.705) | 26.612 (16.847–36.377) | 1.357 | 0.244 |
| General symptom items | ||||
| Trouble swallowing saliva | 31.014 (24.899–37.130) | 37.651 (24.651–50.651) | 1.133 | 0.287 |
| Choking when swallowing | 20.534 (15.617–25.450) | 27.992 (22.081–33.903) | 2.917 | 0.088 |
| Dry mouth | 25.856 (20.067–31.645) | 45.667 (15.969–75.365) | 5.479 | 0.019 |
| Trouble with taste | 32.526 (27.953–37.458) | 40.378 (3.537–77.219) | 0.638 | 0.425 |
| Coughing | 23.589 (19.014–28.165) | 29.142 (12.518–45.765) | 2.254 | 0.133 |
| Speech problems | 30.324 (24.508–36.141) | 45.667 (32.126–59.209) | 4.685 | 0.030 |
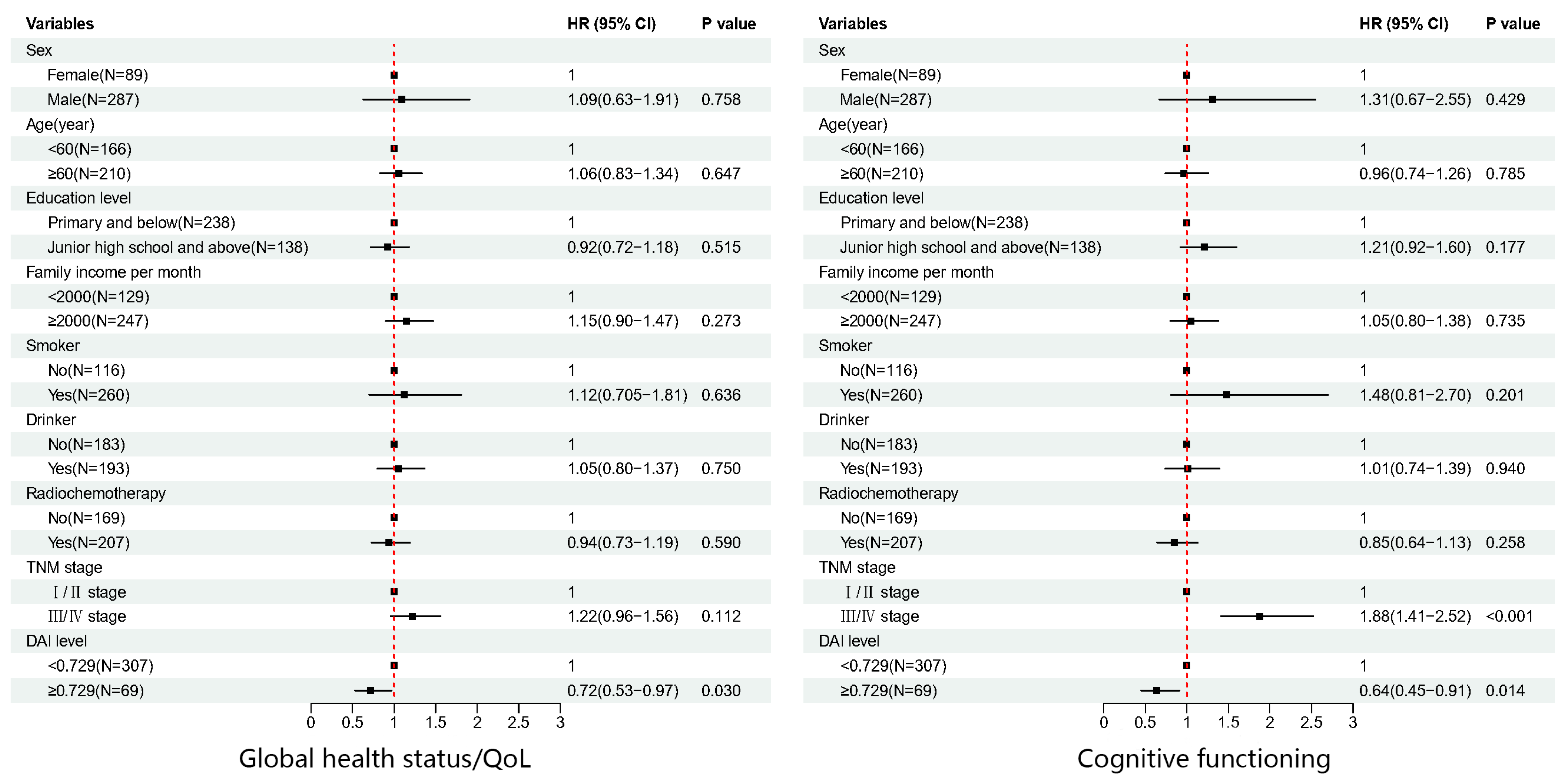
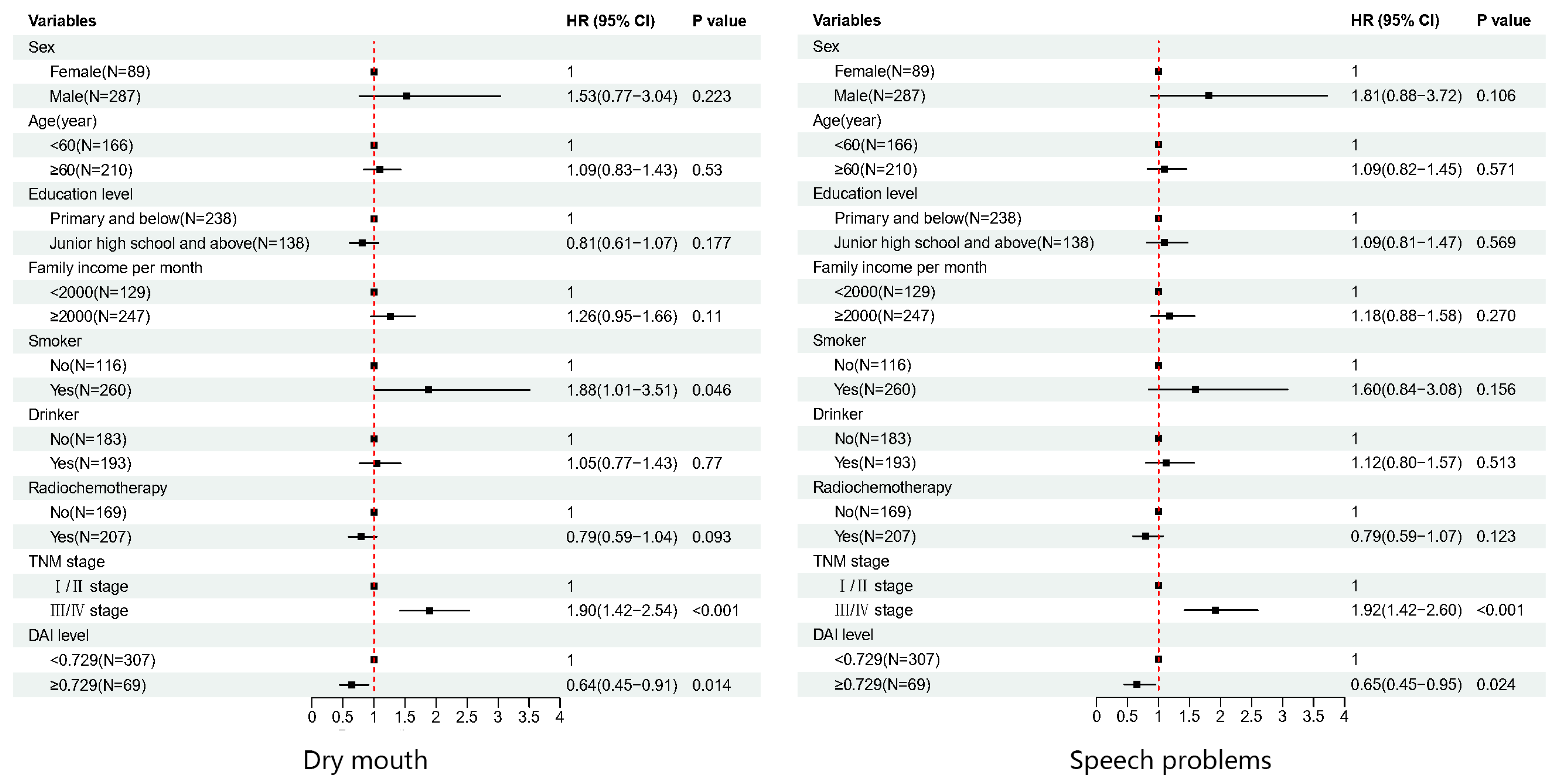
| Domain/Scale | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) * | p Value | |
| QLQ-C30 | ||||
| Global health status/QOL | 0.739 (0.550–0.992) | 0.044 | 0.718 (0.532–0.969) | 0.030 |
| Physical functioning | 0.807 (0.607–1.073) | 0.139 | 0.771 (0.737–2.110) | 0.077 |
| Role functioning | 0.807 (0.598–1.091) | 0.164 | 0.809 (0.595–1.100) | 0.176 |
| Emotional functioning | 0.727 (0.522–1.013) | 0.060 | 0.717 (0.514–1.001) | 0.051 |
| Cognitive functioning | 0.682 (0.480–0.967) | 0.032 | 0.641 (0.450–0.913) | 0.014 |
| Social functioning | 0.827 (0.601–1.136) | 0.241 | 0.846 (0.612–1.169) | 0.310 |
| Fatigue | 0.790 (0.582–1.073) | 0.131 | 0.779 (0.572–1.061) | 0.113 |
| Nausea/vomiting | 0.856 (0.628–1.169) | 0.329 | 0.877 (0.639–1.204) | 0.417 |
| Pain | 0.819 (0.594–1.127) | 0.220 | 0.750 (0.541–1.040) | 0.085 |
| Dyspnea | 0.834 (0.601–1.157) | 0.277 | 0.836 (0.599–1.167) | 0.292 |
| Insomnia | 0.949 (0.698–1.291) | 0.741 | 0.925 (0.676–1.265) | 0.625 |
| Appetite loss | 0.912 (0.669–1.245) | 0.563 | 0.897 (0.655–1.229) | 0.499 |
| Constipation | 1.040 (0.735–1.470) | 0.825 | 1.000 (0.705–1.418) | 1.000 |
| Diarrhea | 1.023 (0.745–1.406) | 0.887 | 1.014 (0.734–1.401) | 0.934 |
| QLQ-QES18 | ||||
| Dysphagia | 0.877 (0.652–1.181) | 0.387 | 0.882 (0.652–1.194) | 0.418 |
| Eating problems | 0.765 (0.560–1.045) | 0.092 | 0.757 (0.551–1.039) | 0.085 |
| Reflux | 0.919 (0.692–1.220) | 0.559 | 0.895 (0.671–1.192) | 0.448 |
| Odynophagia | 0.822 (0.591–1.144) | 0.245 | 0.790 (0.563–1.108) | 0.171 |
| Trouble swallowing saliva | 0.826 (0.581–1.175) | 0.288 | 0.776 (0.544–1.108) | 0.162 |
| Choking when swallowing | 0.744 (0.529–1.046) | 0.089 | 0.768 (0.544–1.084) | 0.133 |
| Dry mouth | 0.658 (0.462–0.937) | 0.020 | 0.637 (0.445–0.911) | 0.014 |
| Trouble with taste | 0.862 (0.598–1.242) | 0.425 | 0.878 (0.606–1.272) | 0.491 |
| Coughing | 0.766 (0.541–1.086) | 0.135 | 0.751 (0.527–1.069) | 0.112 |
| Speech problems | 0.668 (0.462–0.965) | 0.032 | 0.651 (0.449–0.945) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, J.; Huang, Y.; Lin, Z.; Zhang, S.; Qiu, M.; Xiang, Z.; Hu, Z. Association between the Preoperative Dietary Antioxidant Index and Postoperative Quality of Life in Patients with Esophageal Squamous Cell Carcinoma: A Prospective Study Based on the TTD Model. Nutrients 2023, 15, 2828. https://doi.org/10.3390/nu15132828
Zhang J, Zhou J, Huang Y, Lin Z, Zhang S, Qiu M, Xiang Z, Hu Z. Association between the Preoperative Dietary Antioxidant Index and Postoperative Quality of Life in Patients with Esophageal Squamous Cell Carcinoma: A Prospective Study Based on the TTD Model. Nutrients. 2023; 15(13):2828. https://doi.org/10.3390/nu15132828
Chicago/Turabian StyleZhang, Juwei, Jinsong Zhou, Yue Huang, Zheng Lin, Suhong Zhang, Minglian Qiu, Zhisheng Xiang, and Zhijian Hu. 2023. "Association between the Preoperative Dietary Antioxidant Index and Postoperative Quality of Life in Patients with Esophageal Squamous Cell Carcinoma: A Prospective Study Based on the TTD Model" Nutrients 15, no. 13: 2828. https://doi.org/10.3390/nu15132828
APA StyleZhang, J., Zhou, J., Huang, Y., Lin, Z., Zhang, S., Qiu, M., Xiang, Z., & Hu, Z. (2023). Association between the Preoperative Dietary Antioxidant Index and Postoperative Quality of Life in Patients with Esophageal Squamous Cell Carcinoma: A Prospective Study Based on the TTD Model. Nutrients, 15(13), 2828. https://doi.org/10.3390/nu15132828





