IgE-Mediated Shellfish Allergy in Children
Abstract
1. Introduction
2. Epidemiology
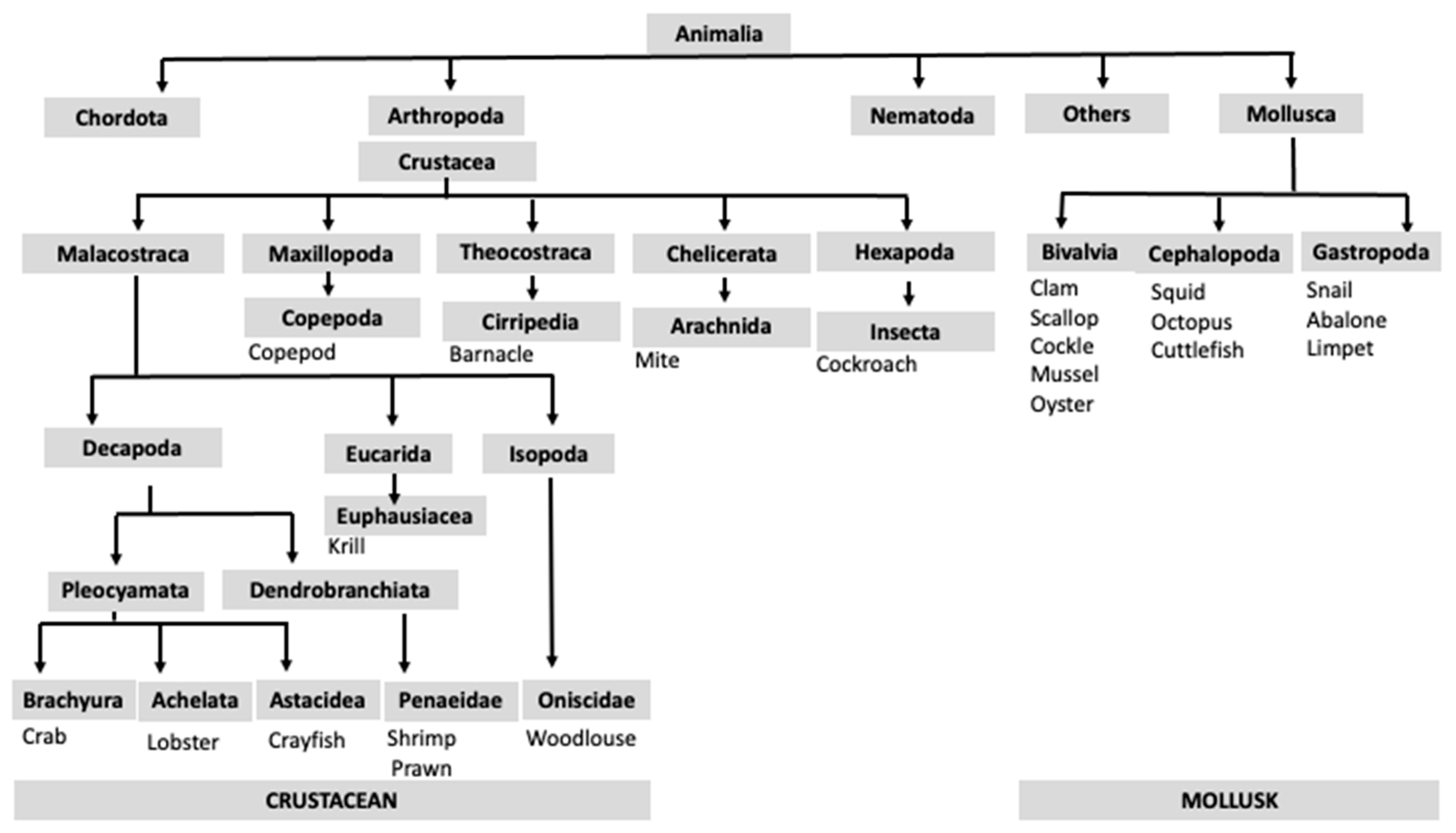
3. Classification of Shellfish Species
4. Adverse Reactions to Shellfish
4.1. Immunological Adverse Reactions
4.2. Non-Immunological Adverse Reactions
- -
- Diarrhetic shellfish poisoning; caused by okadaic acid and dinophysis toxins. The clinical manifestations include nausea, vomiting, abdominal pain, and diarrhea [32].
- -
- Paralytic shellfish poisoning; caused by saxitoxins which inhibit the generation of action potentials in the membranes of neurons and muscles. Clinical manifestations classically begin with a tingling sensation or numbness of the mouth, neck, fingers, and toes and progress to weakness, limb incoordination, and respiratory difficulty [33].
- -
- Neurotoxic shellfish poisoning; caused by brevetoxins that target voltage-gated sodium channels and trigger depolarization of neurons, muscular, and cardiac cells [3]. The signs and symptoms include both neurological (e.g., paralysis and coma) and gastrointestinal clinical manifestations (e.g., nausea, vomiting, and diarrhea) [34].
- -
- Ciguatera fish poisoning; caused by the consumption of fish that have accumulated ciguatoxins in their tissues. These toxins target voltage-gated sodium channels, and they can cause gastrointestinal signs and symptoms before or coinciding with neurological and cardiovascular clinical manifestations [35].
- -
- Amnesic shellfish poisoning; caused by domoic acid (produced by planktonic diatoms), which targets glutamate receptors in the central nervous system [36]. Usually, gastrointestinal signs and symptoms start first (e.g., nausea, vomiting, diarrhea, and abdominal cramps), and then patients develop neurological clinical manifestations such as confusion, short-term memory loss and coma [37].
5. Shellfish Allergens
5.1. Tropomyosin
5.2. Arginine Kinase
5.3. Myosin Light Chain
5.4. Sarcoplasmic Calcium-Binding Protein
5.5. Troponin C
5.6. Triosephosphate Isomerase
5.7. Other Allergens
5.8. Cross-Reactivity
5.8.1. Cross-Reactivity among Shellfish Species
5.8.2. Cross-Reactivity between Shellfish and Fish
5.8.3. Cross-Reactivity between Shellfish, HDM, and Cockroach
5.8.4. Cross-Reactivity between Shellfish and Anisakis simplex
5.8.5. Tropomyosin IgE Cross-Reactivity between Shellfish and Edible Insects
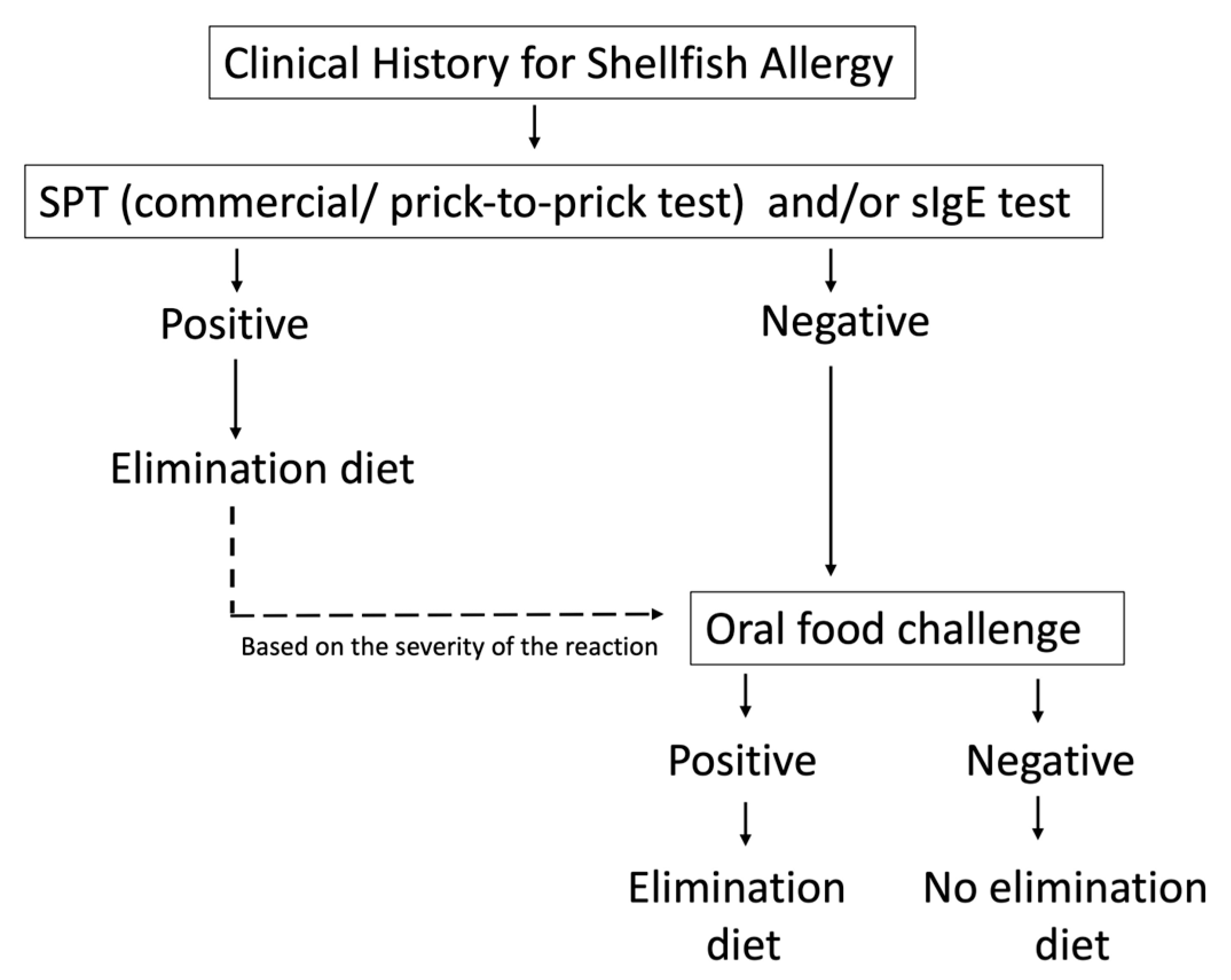
6. Diagnosis
6.1. Clinical History
6.2. Skin Prick Test
6.3. Specific IgE
6.4. OFC
6.5. Component-Resolved Diagnosis (CRD)
6.6. Basophil Activation Test (BAT) and IgE-Crosslinking-Induced Luciferase Expression (EXiLE)
7. Management
7.1. AIT
7.1.1. Shrimp Extract
7.1.2. Shrimp Allergen TPM Met e 1
7.1.3. Peptide-Based Immunotherapy
7.1.4. Hypoallergens
7.1.5. DNA Vaccine-Based Immunotherapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIT | allergen-specific immunotherapy |
| BAT | basophil activation test |
| CRD | component-resolved diagnosis |
| DBPCFC | double-blind placebo-controlled oral food challenge |
| HDM | house dust mite |
| EXiLE | IgE-crosslinking-induced luciferase expression |
| FBPA | fructose 1,6-bisphosphate aldolase |
| FDEIA | food-dependent exercise-induced anaphylaxis |
| FOXP3 | forkhead box P3 |
| FPIES | food protein-induced enterocolitis syndrome |
| IL | interleukin |
| MLC | myosin light chain |
| MrPTP | Macrobrachium rosenbergii prick-to-prick test |
| MrSPT | Macrobrachium rosenbergii skin prick test |
| NFAT | nuclear factor of activated T-cells |
| RBL | rat basophil leukemia |
| OFC | oral food challenge |
| OIT | oral immunotherapy |
| PmPTP | Penaeus monodon prick-to-prick test |
| PmSPT | Penaeus monodon skin prick test |
| PPV | positive predictive value |
| PTP | prick-to-prick test |
| sIgE | specific IgE |
| SCP | sarcoplasmic calcium-binding protein |
| SDS-PAGE | sodium dodecyl-sulfate polyacrylamide gel electrophoresis |
| SPT | skin prick test |
| TFGβ | transforming growth factor beta |
| TIM | triosephosphate isomerase |
| TpC | troponin C |
| TPM | tropomyosin |
References
- Adams, S.; Lopata, A.L.; Smuts, C.M.; Baatjies, R.; Jeebhay, M.F. Relationship between Serum Omega-3 Fatty Acid and Asthma Endpoints. Int. J. Environ. Res. Public Health 2018, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Smith, P.K.; Arshad, H. Dietary strategies for the prevention of asthma in children. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 123–131. [Google Scholar] [CrossRef]
- Xu, L.; Cai, J.; Gao, T.; Ma, A. Shellfish consumption and health: A comprehensive review of human studies and recommendations for enhanced public policy. Crit. Rev. Food Sci. Nutr. 2022, 62, 4656–4668. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of dietary fiber in promoting immune health-An EAACI position paper. Allergy 2022, 77, 3185–3198. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; The EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef]
- Rancé, F.; Grandmottet, X.; Grandjean, H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clin. Exp. Allergy 2005, 35, 167–172. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, A.S.Y.; Wong, G.W.K.; Leung, T.F. Seafood Allergy in Asia: Geographical Specificity and Beyond. Front. Allergy 2021, 2, 676903. [Google Scholar] [CrossRef]
- Li, J.; Ogorodova, L.M.; Mahesh, P.A.; Wang, M.H.; Fedorova, O.S.; Leung, T.F.; Fernandez-Rivas, M.; Mills, E.C.; Potts, J.; Kummeling, I.; et al. Comparative Study of Food Allergies in Children from China, India, and Russia: The EuroPrevall-INCO Surveys. J. Allergy Clin. Immunol. Pract. 2020, 8, 1349–1358.e16. [Google Scholar] [CrossRef]
- Le, T.T.K.; Nguyen, D.H.; Vu, A.T.L.; Ruethers, T.; Taki, A.C.; Lopata, A.L. A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatr. Allergy Immunol. 2019, 30, 348–355. [Google Scholar] [CrossRef]
- Ebisawa, M.; Ito, K.; Fujisawa, T.; Committee for Japanese Pediatric Guideline for Food Allergy TJSoPA; The Japanese Society of Pediatric Allergy and Clinical Immunology; The Japanese Society of Allergology. Japanese guidelines for food allergy 2020. Allergol. Int. 2020, 69, 370–386. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jeon, H.-Y.; Yang, H.-K.; Lee, K.-J.; Han, Y.; Kim, Y.H.; Kim, J.; Ahn, K. Prevalence of Immediate-Type Food Allergy in Korean Schoolchildren in 2015: A Nationwide, Population-based Study. Allergy Asthma Immunol. Res. 2017, 9, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Munoz-Furlong, A.; Sampson, H.A. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004, 114, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Warren, C.M.; Gupta, R.S.; Davis, C.M. Prevalence and Characteristics of Shellfish Allergy in the Pediatric Population of the United States. J. Allergy Clin. Immunol. Pract. 2020, 8, 1359–1370.e2. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.; Trendelenburg, V.; Bellach, J.; Yurek, S.; Reich, A.; Fiandor, A.; Rivero, D.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Frequency of food allergy in school-aged children in eight European countries—The EuroPrevall-iFAAM birth cohort. Allergy 2020, 75, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Hu, H.; Huang, Z.; Lin, Q.; Huang, H.; Liu, X.; Luo, W.; Sun, B. Shrimp and cockroach co-sensitization in Southern China: Association with moth sensitization. Allergy Asthma Proc. 2020, 41, e54–e60. [Google Scholar] [CrossRef] [PubMed]
- Ruethers, T.; Taki, A.C.; Johnston, E.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef]
- Shek, L.P.-C.; Cabrera-Morales, E.A.; Soh, S.E.; Gerez, I.; Ng, P.Z.; Yi, F.C.; Ma, S.; Lee, B.W. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J. Allergy Clin. Immunol. 2010, 126, 324–331.e7. [Google Scholar] [CrossRef]
- Moonesinghe, H.R.; Kilburn, S.; MacKenzie, H.; Turner, P.J.; Venter, C.; Lee, K.; Dean, T. Prevalence of fish and shellfish allergy: A systematic review. Ann. Allergy Asthma Immunol. 2016, 117, 264–272.e4. [Google Scholar] [CrossRef]
- Davis, C.M.; Gupta, R.S.; Aktas, O.N.; Diaz, V.; Kamath, S.D.; Lopata, A.L. Clinical Management of Seafood Allergy. J. Allergy Clin. Immunol. Pract. 2020, 8, 37–44. [Google Scholar] [CrossRef]
- Chokshi, N.Y.; Maskatia, Z.; Miller, S.; Guffey, D.; Minard, C.G.; Davis, C.M. Risk factors in pediatric shrimp allergy. Allergy Asthma Proc. 2015, 36, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Thalayasingam, M.; Gerez, I.F.A.; Yap, G.C.; Llanora, G.V.; Chia, I.P.; Chua, L.; Lee, C.J.A.O.; Ta, L.D.H.; Cheng, Y.K.; Thong, B.Y.H.; et al. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin. Exp. Allergy 2015, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Tuano, K.T.S.; Davis, C.M. Oral allergy syndrome in shrimp and house dust mite allergies. J. Allergy Clin. Immunol. Pract. 2018, 6, 2163–2164. [Google Scholar] [CrossRef]
- Van Ree, R.; Antonicelli, L.; Akkerdaas, J.H.; Garritani, M.S.; Aalberse, R.C.; Bonifazi, F. Possible induction of food allergy during mite immunotherapy. Allergy 1996, 51, 108–113. [Google Scholar] [PubMed]
- Maulitz, R.M.; Pratt, D.S.; Schocket, A.L. Exercise-induced anaphylactic reaction to shellfish. J. Allergy Clin. Immunol. 1979, 63, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.X.; Giovannini, M.; du Toit, G. Food-dependent exercise-induced anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 224–228. [Google Scholar] [CrossRef]
- Akimoto, S.; Yokooji, T.; Ogino, R.; Chinuki, Y.; Taogoshi, T.; Adachi, A.; Morita, E.; Matsuo, H. Identification of allergens for food-dependent exercise-induced anaphylaxis to shrimp. Sci. Rep. 2021, 11, 5400. [Google Scholar] [CrossRef]
- Rosa, S.; Prates, S.; Piedade, S.; Marta, C.S.; Pinto, J.R. Are there shrimp allergens exclusive from the cephalothorax? Allergy 2007, 62, 85–87. [Google Scholar] [CrossRef]
- Sopo, S.M.; Monaco, S.; Badina, L.; Barni, S.; Longo, G.; Novembre, E.; Viola, S.; Monti, G. Food protein-induced enterocolitis syndrome caused by fish and/or shellfish in Italy. Pediatr. Allergy Immunol. 2015, 26, 731–736. [Google Scholar] [CrossRef]
- Ayuso, R.; Sánchez-Garcia, S.; Lin, J.; Fu, Z.; Ibáñez, M.D.; Carrillo, T.; Blanco, C.; Goldis, M.; Bardina, L.; Sastre, J.; et al. Greater epitope recognition of shrimp allergens by children than by adults suggests that shrimp sensitization decreases with age. J. Allergy Clin. Immunol. 2010, 125, 1286–1293.e3. [Google Scholar] [CrossRef]
- La Bella, G.; Martella, V.; Basanisi, M.G.; Nobili, G.; Terio, V.; La Salandra, G. Food-Borne Viruses in Shellfish: Investigation on Norovirus and HAV Presence in Apulia (SE Italy). Food Environ. Virol. 2017, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Morabito, S.; Silvestro, S.; Faggio, C. How the marine biotoxins affect human health. Nat. Prod. Res. 2018, 32, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Vilarino, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.S.; Zatorre, R.J.; Carpenter, S.; Gendron, D.; Evans, A.C.; Gjedde, A.; Cashman, N.R. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N. Engl. J. Med. 1990, 322, 1781–1787. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Day, E.D., Jr.; Miller, J.S. The major heat stable allergen of shrimp. Ann. Allergy 1981, 47, 17–22. [Google Scholar]
- Asturias, J.A.; Gómez-Bayón, N.; Arilla, M.C.; Martínez, A.; Palacios, R.; Sánchez-Gascón, F.; Martinez, J. Molecular characterization of American cockroach tropomyosin (Periplaneta americana allergen 7), a cross-reactive allergen. J. Immunol. 1999, 162, 4342–4348. [Google Scholar] [CrossRef]
- Arrieta, I.; del Barrio, M.; Vidarte, L.; del Pozo, V.; Pastor, C.; Gonzalez-Cabrero, J.; Cárdaba, B.; Rojo, M.; Mínguez, A.; Cortegano, I.; et al. Molecular cloning and characterization of an IgE-reactive protein from Anisakis simplex: Ani s 1. Mol. Biochem. Parasitol. 2000, 107, 263–268. [Google Scholar] [CrossRef]
- Yadzir, Z.H.M.; Misnan, R.; Bakhtiar, F.; Abdullah, N.; Murad, S. Tropomyosin and Actin Identified as Major Allergens of the Carpet Clam (Paphia textile) and the Effect of Cooking on Their Allergenicity. Biomed Res. Int. 2015, 2015, 254152. [Google Scholar]
- James, J.K.; Pike, D.H.; Khan, I.J.; Nanda, V. Structural and Dynamic Properties of Allergen and Non-Allergen Forms of Tropomyosin. Structure 2018, 26, 997–1006.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Huang, Y.Y.; Cai, Q.F.; Weng, W.Y.; Su, W.J.; Cao, M.J. Comparative study of in vitro digestibility of major allergen, tropomyosin and other proteins between Grass prawn (Penaeus monodon) and Pacific white shrimp (Litopenaeus vannamei). J. Sci. Food Agric. 2011, 91, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.D.; Rahman, A.M.; Voskamp, A.; Komoda, T.; Rolland, J.M.; O’Hehir, R.E.; Lopata, A.L. Effect of heat processing on antibody reactivity to allergen variants and fragments of black tiger prawn: A comprehensive allergenomic approach. Mol. Nutr. Food Res. 2014, 58, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Carnés, J.; Ferrer, A.; Huertas, A.J.; Andreu, C.; Larramendi, C.H.; Fernández-Caldas, E. The use of raw or boiled crustacean extracts for the diagnosis of seafood allergic individuals. Ann. Allergy Asthma Immunol. 2007, 98, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.D.; Rahman, A.M.A.; Komoda, T.; Lopata, A.L. Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies. Food Chem. 2013, 141, 4031–4039. [Google Scholar] [CrossRef] [PubMed]
- Gámez, C.; Zafra, M.P.; Sanz, V.; Mazzeo, C.; Ibáñez, M.D.; Sastre, J.; del Pozo, V. Simulated gastrointestinal digestion reduces the allergic reactivity of shrimp extract proteins and tropomyosin. Food Chem. 2015, 173, 475–481. [Google Scholar] [CrossRef]
- Khulal, U.; Stojadinovic, M.; Prodic, I.; Rajkovic, A.; Velickovic, T.C. Comparative digestion of thermally treated vertebrates and invertebrates allergen pairs in real food matrix. Food Chem. 2023, 405 Pt B, 134981. [Google Scholar] [CrossRef]
- Wild, L.G.; Lehrer, S.B. Fish and shellfish allergy. Curr. Allergy Asthma Rep. 2005, 5, 74–79. [Google Scholar] [CrossRef]
- Thalayasingam, M.; Lee, B.W. Fish and shellfish allergy. Chem. Immunol. Allergy 2015, 101, 152–161. [Google Scholar]
- Nugraha, R.; Ruethers, T.; Taki, A.C.; Johnston, E.B.; Karnaneedi, S.; Kamath, S.D.; Lopata, A.L. Recombinant Tropomyosin from the Pacific Oyster (Crassostrea gigas) for Better Diagnosis. Foods 2022, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Mistrello, G.; Amato, S.; Ariano, R.; Colombo, G.; Conte, M.E.; Crivellaro, M.; De Carli, M.; Della Torre, F.; Emiliani, F.; et al. Shrimp allergy in Italian adults: A multicenter study showing a high prevalence of sensitivity to novel high molecular weight allergens. Int. Arch. Allergy Immunol. 2012, 157, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Reese, G.; Ayuso, R.; Lehrer, S.B. Tropomyosin: An invertebrate pan-allergen. Int. Arch. Allergy Immunol. 1999, 119, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, R.; Kamath, S.D.; Johnston, E.; Karnaneedi, S.; Ruethers, T.; Lopata, A.L. Conservation Analysis of B-Cell Allergen Epitopes to Predict Clinical Cross-Reactivity between Shellfish and Inhalant Invertebrate Allergens. Front. Immunol. 2019, 10, 2676. [Google Scholar] [CrossRef]
- Yu, C.J.; Lin, Y.F.; Chiang, B.L.; Chow, L.P. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J. Immunol. 2003, 170, 445–453. [Google Scholar] [CrossRef]
- Misnan, R.; Murad, S.; Yadzir, Z.H.; Abdullah, N. Identification of the major allergens of Charybdis feriatus (red crab) and its cross-reactivity with Portunus pelagicus (blue crab). Asian Pac. J. Allergy Immunol. 2012, 30, 285–293. [Google Scholar]
- Shen, H.W.; Cao, M.J.; Cai, Q.F.; Ruan, M.M.; Mao, H.Y.; Su, W.J.; Liu, G.M. Purification, cloning, and immunological characterization of arginine kinase, a novel allergen of Octopus fangsiao. J. Agric. Food Chem. 2012, 60, 2190–2199. [Google Scholar] [CrossRef]
- Sookrung, N.; Chaicumpa, W.; Tungtrongchitr, A.; Vichyanond, P.; Bunnag, C.; Ramasoota, P.; Tongtawe, P.; Sakolvaree, Y.; Tapchaisri, P. Periplaneta americana arginine kinase as a major cockroach allergen among Thai patients with major cockroach allergies. Environ. Health Perspect. 2006, 114, 875–880. [Google Scholar] [CrossRef]
- Xing, P.; Yu, H.; Li, M.; Xiao, X.; Jiang, C.; Mo, L.; Zhang, M.; Yang, P.; Liu, Z. Characterization of arginine kinase, a novel allergen of dermatophagoides farinae (Der f 20). Am. J. Transl. Res. 2015, 7, 2815–2823. [Google Scholar]
- Rahman, A.M.A.; Kamath, S.D.; Gagne, S.; Lopata, A.L.; Helleur, R. Comprehensive proteomics approach in characterizing and quantifying allergenic proteins from northern shrimp: Toward better occupational asthma prevention. J. Proteome Res. 2013, 12, 647–656. [Google Scholar] [CrossRef]
- Jeebhay, M.F.; Lopata, A.L. Occupational allergies in seafood-processing workers. Adv. Food Nutr. Res. 2012, 66, 47–73. [Google Scholar] [PubMed]
- Giuffrida, M.G.; Villalta, D.; Mistrello, G.; Amato, S.; Asero, R. Shrimp allergy beyond Tropomyosin in Italy: Clinical relevance of Arginine Kinase, Sarcoplasmic calcium binding protein and Hemocyanin. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 172–177. [Google Scholar] [PubMed]
- Ayuso, R.; Grishina, G.; Bardina, L.; Carrillo, T.; Blanco, C.; Ibáñez, M.D.; Sampson, H.A.; Beyer, K. Myosin light chain is a novel shrimp allergen, Lit v 3. J. Allergy Clin. Immunol. 2008, 122, 795–802. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, K.S.; Oh, C.-W.; Mykles, D.L.; Lee, S.G.; Kim, H.J.; Kim, H.-W. Twelve actin-encoding cDNAs from the American lobster, Homarus americanus: Cloning and tissue expression of eight skeletal muscle, one heart, and three cytoplasmic isoforms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 178–184. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, H.L.; Maleki, S.J.; Cao, M.J.; Zhang, L.J.; Su, W.J.; Liu, G.M. Purification, Characterization, and Analysis of the Allergenic Properties of Myosin Light Chain in Procambarus clarkii. J. Agric. Food Chem. 2015, 63, 6271–6282. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, K.; Wangorsch, A.; Garoffo, L.P.; Reuter, A.; Conti, A.; Taylor, S.L.; Lidholm, J.; DeWitt, A.M.; Enrique, E.; Vieths, S.; et al. Generation of a comprehensive panel of crustacean allergens from the North Sea Shrimp Crangon crangon. Mol. Immunol. 2011, 48, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Grishina, G.; Yang, A.C.; Sanchez-Garcia, S.; Lin, J.; Towle, D.; Ibañez, M.D.; Sastre, J.; Sampson, H.A.; Ayuso, R. Molecular Diagnosis of Shrimp Allergy: Efficiency of Several Allergens to Predict Clinical Reactivity. J. Allergy Clin. Immunol. Pract. 2015, 3, 521–529.e10. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Sato, Y.; Hamamoto, S.; Mita, H.; Shimakura, K. Sarcoplasmic calcium-binding protein: Identification as a new allergen of the black tiger shrimp Penaeus monodon. Int. Arch. Allergy Immunol. 2008, 146, 91–98. [Google Scholar] [CrossRef]
- Ayuso, R.; Grishina, G.; Ibáñez, M.D.; Blanco, C.; Carrillo, T.; Bencharitiwong, R.; Sánchez, S.; Nowak-Wegrzyn, A.; Sampson, H.A. Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. J. Allergy Clin. Immunol. 2009, 124, 114–120. [Google Scholar] [CrossRef]
- Mita, H.; Koketsu, A.; Ishizaki, S.; Shiomi, K. Molecular cloning and functional expression of allergenic sarcoplasmic calcium-binding proteins from Penaeus shrimps. J. Sci. Food Agric. 2013, 93, 1737–1742. [Google Scholar] [CrossRef]
- Lopata, A.L.; Kleine-Tebbe, J.; Kamath, S.D. Allergens and molecular diagnostics of shellfish allergy: Part 22 of the Series Molecular Allergology. Allergo J. Int. 2016, 25, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gelis, S.; Rueda, M.; Valero, A.; Fernandez, E.A.; Moran, M.; Fernandez-Caldas, E. Shellfish Allergy: Unmet Needs in Diagnosis and Treatment. J. Investig. Allergol. Clin. Immunol. 2020, 30, 409–420. [Google Scholar] [CrossRef]
- Guilloux, L.; Vuitton, D.-A.; Delbourg, M.; Lagier, A.; Adessi, B.; Marchand, C.R.; Ville, G. Cross-reactivity between terrestrial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). II. In vitro study. Allergy 1998, 53, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, M.R.; Kamath, S.D.; Bang, B.E.; Nugraha, R.; Nie, S.; Williamson, N.A.; Lopata, A.L.; Aasmoe, L. Occupational Allergic Sensitization Among Workers Processing King Crab (Paralithodes camtschaticus) and Edible Crab (Cancer pagurus) in Norway and Identification of Novel Putative Allergenic Proteins. Front. Allergy 2021, 2, 718824. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Chu, K.H.; Leung, P.S.C.; Leung, A.S.Y.; Wong, G.W.K.; Leung, T.F. Overcoming Shellfish Allergy: How Far Have We Come? Int. J. Mol. Sci. 2020, 21, 2234. [Google Scholar] [CrossRef] [PubMed]
- Daul, C.B.; Slattery, M.; Reese, G.; Lehrer, S.B. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int. Arch. Allergy Immunol. 1994, 105, 49–55. [Google Scholar] [CrossRef]
- Lopata, A.L.; Zinn, C.; Potter, P.C. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J. Allergy Clin. Immunol. 1997, 100, 642–648. [Google Scholar] [CrossRef]
- Leung, P.S.; Chen, Y.C.; Gershwin, M.E.; Wong, S.H.; Kwan, H.S.; Chu, K.H. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J. Allergy Clin. Immunol. 1998, 102, 847–852. [Google Scholar] [CrossRef]
- Mykles, D.L.; Cotton, J.L.; Taniguchi, H.; Sano, K.; Maeda, Y. Cloning of tropomyosins from lobster (Homarus americanus) striated muscles: Fast and slow isoforms may be generated from the same transcript. J. Muscle Res. Cell Motil. 1998, 19, 105–115. [Google Scholar] [CrossRef]
- Azofra, J.; Echechipía, S.; Irazábal, B.; Muñoz, D.; Bernedo, N.; García, B.; Gastaminza, G.; Goikoetxea, M.; Joral, A.; Lasa, E.; et al. Heterogeneity in allergy to mollusks: A clinical-immunological study in a population from the North of Spain. J. Investig. Allergol. Clin. Immunol. 2017, 27, 252–260. [Google Scholar] [CrossRef]
- Rahman, A.M.A.; Kamath, S.D.; Lopata, A.L.; Robinson, J.J.; Helleur, R.J. Biomolecular characterization of allergenic proteins in snow crab (Chionoecetes opilio) and de novo sequencing of the second allergen arginine kinase using tandem mass spectrometry. J. Proteom. 2011, 74, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Mykles, D.L. Heterogeneity of myofibrillar proteins in lobster fast and slow muscles: Variants of troponin, paramyosin, and myosin light chains comprise four distinct protein assemblages. J. Exp. Zool. 1985, 234, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shimizu, K.; Kobayashi, Y.; Ishizaki, S.; Shiomi, K. Paramyosin from the Disc Abalone Haliotis Discus Discus. J. Food Biochem. 2014, 38, 444–451. [Google Scholar] [CrossRef]
- Múnera, M.; Martínez, D.; Wortmann, J.; Zakzuk, J.; Keller, W.; Caraballo, L.; Puerta, L. Structural and allergenic properties of the fatty acid binding protein from shrimp Litopenaeus vannamei. Allergy 2022, 77, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Piboonpocanun, S.; Jirapongsananuruk, O.; Tipayanon, T.; Boonchoo, S.; Goodman, R.E. Identification of hemocyanin as a novel non-cross-reactive allergen from the giant freshwater shrimp Macrobrachium rosenbergii. Mol. Nutr. Food Res. 2011, 55, 1492–1498. [Google Scholar] [CrossRef]
- Zhu, L.; She, T.; Zhang, Y.; Li, S.; Xu, Z.; Yan, J.; Li, H. Identification and characterization of ovary development-related protein EJO1 (Eri s 2) from the ovary of Eriocheir sinensis as a new food allergen. Mol. Nutr. Food Res. 2016, 60, 2275–2287. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takagi, T.; Konishi, K.; Cox, J.A. Amino acid sequence of crayfish troponin I. J. Biol. Chem. 1989, 264, 1551–1557. [Google Scholar] [CrossRef]
- Leung, N.Y.H.; Wai, C.Y.Y.; Shu, S.; Wang, J.; Kenny, T.P.; Chu, K.H.; Leung, P.S.C. Current immunological and molecular biological perspectives on seafood allergy: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 46, 180–197. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef]
- Kamath, S.D.; Liu, T.; Giacomin, P.; Loukas, A.; Navarro, S.; Lopata, A.L. Mollusk allergy: Not simply cross-reactivity with crustacean allergens. Allergy 2022, 77, 3127–3130. [Google Scholar] [CrossRef]
- Vidal, C.; Bartolome, B.; Rodriguez, V.; Armisen, M.; Linneberg, A.; Gonzalez-Quintela, A. Sensitization pattern of crustacean-allergic individuals can indicate allergy to molluscs. Allergy 2015, 70, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical Relevance of Cross-Reactivity in Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Ng, I.; Kemp, A.; Campbell, D. Seafood allergy in children: A descriptive study. Ann. Allergy Asthma Immunol. 2011, 106, 494–501. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Karnaneedi, S.; Nie, S.; Kalic, T.; Dai, D.; Daduang, S.; Leeming, M.; Williamson, N.A.; Breiteneder, H.; et al. Expanding the allergen repertoire of salmon and catfish. Allergy 2021, 76, 1443–1453. [Google Scholar] [CrossRef]
- Xu, L.L.; Chen, J.; Sun, L.R.; Gao, X.; Lin, H.; Ahmed, I.; Pramod, S.; Li, Z.X. Analysis of the allergenicity and B cell epitopes in tropomyosin of shrimp (Litopenaeus vannamei) and correlation to cross-reactivity based on epitopes with fish (Larimichthys crocea) and clam (Ruditapes philippinarum). Food Chem. 2020, 323, 126763. [Google Scholar] [CrossRef]
- Xu, L.L.; Lin, H.; Li, Z.X.; Ahmed, I.; Pramod, S.; Lin, H.; Lv, L.T.; Tian, S.L.; Yu, Z.W. Influence of nonthermal extraction technique and allergenicity characteristics of tropomyosin from fish (Larimichthys crocea) in comparison with shrimp (Litopenaeus vannamei) and clam (Ruditapes philippinarum). Food Chem. 2020, 309, 125575. [Google Scholar] [CrossRef]
- Ayuso, R.; Lehrer, S.B.; Reese, G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin). Int. Arch. Allergy Immunol. 2002, 127, 27–37. [Google Scholar] [CrossRef]
- Santos, A.B.R.; Chapman, M.D.; Aalberse, R.C.; Vailes, L.D.; Ferriani, V.P.; Oliver, C.; Rizzo, M.; Naspitz, C.K.; Arruda, L. Cockroach allergens and asthma in Brazil: Identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J. Allergy Clin. Immunol. 1999, 104 Pt 1, 329–337. [Google Scholar] [CrossRef]
- Zheng, L.N.; Lin, H.; Pawar, R.; Li, Z.X.; Li, M.H. Mapping IgE binding epitopes of major shrimp (Penaeus monodon) allergen with immunoinformatics tools. Food Chem. Toxicol. 2011, 49, 2954–2960. [Google Scholar] [CrossRef]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int. Arch. Allergy Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Pontone, M.; Giovannini, M.; Barni, S.; Mori, F.; Venturini, E.; Galli, L.; Valleriani, C.; Vecillas, L.D.L.; Sackesen, C.; Lopata, A.L.; et al. IgE-mediated Anisakis allergy in children. Allergol. Immunopathol. 2023, 51, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.C.; Knulst, A.C.; Jager, C.F.D.H.; van Bilsen, J.H.; Raymakers, F.M.; Kruizinga, A.G.; Gaspari, M.; Gabriele, C.; Bruijnzeel-Koomen, C.A.; Houben, G.F.; et al. Primary respiratory and food allergy to mealworm. J. Allergy Clin. Immunol. 2017, 140, 600–603.e7. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Breiteneder, H.; Mills, E.N. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J. Allergy Clin. Immunol. 2007, 120, 1399–1405. [Google Scholar] [CrossRef]
- Aalberse, R.C. Structural biology of allergens. J. Allergy Clin. Immunol. 2000, 106, 228–238. [Google Scholar] [CrossRef]
- Palmer, L.K.; Marsh, J.T.; Lu, M.; Goodman, R.E.; Zeece, M.G.; Johnson, P.E. Shellfish Tropomyosin IgE Cross-Reactivity Differs Among Edible Insect Species. Mol. Nutr. Food Res. 2020, 64, e1900923. [Google Scholar] [CrossRef]
- Tong, W.S.; Yuen, A.W.; Wai, C.Y.; Leung, N.Y.; Chu, K.H.; Leung, P.S. Diagnosis of fish and shellfish allergies. J. Asthma Allergy 2018, 11, 247–260. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, P.S.C. Emerging approaches in the diagnosis and therapy in shellfish allergy. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 202–212. [Google Scholar] [CrossRef]
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina 2020, 56, 111. [Google Scholar] [CrossRef]
- Lewis, T.; Zotterman, Y. Vascular reactions of the skin to injury: Part VIII. The resistance of the human skin to constant currents, in relation to injury and vascular response. J. Physiol. 1927, 62, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Scala, E.; Villalta, D.; Pravettoni, V.; Arena, A.; Billeri, L.; Colombo, G.; Cortellini, G.; Cucinelli, F.; De Cristofaro, M.L.; et al. Shrimp Allergy: Analysis of Commercially Available Extracts for In Vivo Diagnosis. J. Investig. Allergol. Clin. Immunol. 2017, 27, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jirapongsananuruk, O.; Sripramong, C.; Pacharn, P.; Udompunturak, S.; Chinratanapisit, S.; Piboonpocanun, S.; Visitsunthorn, N.; Vichyanond, P. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin. Exp. Allergy 2008, 38, 1038–1047. [Google Scholar] [CrossRef]
- Piboonpocanun, S.; Boonchoo, S.; Pariyaprasert, W.; Visitsunthorn, N.; Jirapongsananuruk, O. Determination of storage conditions for shrimp extracts: Analysis of specific IgE-allergen profiles. Asian Pac. J. Allergy Immunol. 2010, 28, 47–52. [Google Scholar] [PubMed]
- Pariyaprasert, W.; Piboonpocanun, S.; Jirapongsananuruk, O.; Visitsunthorn, N. Stability and potency of raw and boiled shrimp extracts for skin prick test. Asian Pac. J. Allergy Immunol. 2015, 33, 136–142. [Google Scholar] [CrossRef]
- Gelis, S.; Rueda, M.; Pascal, M.; Fernández-Caldas, E.; Fernández, E.; Araujo-Sánchez, G.; Bartra, J.; Valero, A. Usefulness of the Nasal Allergen Provocation Test in the Diagnosis of Shellfish Allergy. J. Investig. Allergol. Clin. Immunol. 2022, 32, 460–470. [Google Scholar] [CrossRef]
- Scala, E.; Abeni, D.; Aruanno, A.; Boni, E.; Brusca, I.; Cappiello, F.; Caprini, E.; Buzzulini, F.; Deleonardi, G.; Demonte, A.; et al. Mollusk allergy in shrimp-allergic patients: Still a complex diagnosis. An Italian real-life cross-sectional multicenter study. World Allergy Organ. J. 2022, 15, 100685. [Google Scholar] [CrossRef]
- Bindslev-Jensen, C.; Ballmer-Weber, B.K.; Bengtsson, U.; Blanco, C.; Ebner, C.; Hourihane, J.; Knulst, A.C.; Moneret-Vautrin, D.A.; Nekam, K.; Niggemann, B.; et al. Standardization of food challenges in patients with immediate reactions to foods—Position paper from the European Academy of Allergology and Clinical Immunology. Allergy 2004, 59, 690–697. [Google Scholar] [CrossRef]
- Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Beyer, K.; Defernez, M.; Sperrin, M.; Mackie, A.R.; Salt, L.J.; Hourihane, J.O.B.; Asero, R.; Belohlavkova, S.; et al. How much is too much? Threshold dose distributions for 5 food allergens. J. Allergy Clin Immunol. 2015, 135, 964–971. [Google Scholar] [CrossRef]
- Fernández-Rivas, M.; Barreales, L.; Mackie, A.R.; Fritsche, P.; Vázquez-Cortés, S.; Jedrzejczak-Czechowicz, M.; Kowalski, M.L.; Clausen, M.; Gislason, D.; Sinaniotis, A.; et al. The EuroPrevall outpatient clinic study on food allergy: Background and methodology. Allergy 2015, 70, 576–584. [Google Scholar] [CrossRef]
- Yang, A.C.; Arruda, L.K.; Santos, A.B.R.; Barbosa, M.C.; Chapman, M.; Galvão, C.E.; Kalil, J.; Morato-Castro, F.F. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J. Allergy Clin. Immunol. 2010, 125, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Gamez, C.; Sánchez-García, S.; Ibáñez, M.D.; López, R.; Aguado, E.; López, E.; Sastre, B.; Sastre, J.; Del Pozo, V. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy 2011, 66, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Ukleja-Sokolowska, N.; Lis, K.; Zbikowska-Gotz, M.; Adamczak, R.; Kuzminski, A.; Bartuzi, Z. Clinical utility of immunological methods based on the singleplex and multiplex ImmunoCap systems for diagnosis of shrimp allergy. J. Int. Med. Res. 2021, 49, 3000605211006597. [Google Scholar] [CrossRef]
- Nakamura, R.; Uchida, Y.; Higuchi, M.; Tsuge, I.; Urisu, A.; Teshima, R. A convenient and sensitive allergy test: IgE crosslinking-induced luciferase expression in cultured mast cells. Allergy 2010, 65, 1266–1273. [Google Scholar] [CrossRef]
- Jarupalee, T.; Chatchatee, P.; Komolpis, K.; Suratannon, N.; Roytrakul, S.; Yingchutrakul, Y.; Yimchuen, W.; Butta, P.; Jacquet, A.; Palaga, T.; et al. Detecting Allergens From Black Tiger Shrimp Penaeus monodon That Can Bind and Cross-link IgE by ELISA, Western Blot, and a Humanized Rat Basophilic Leukemia Reporter Cell Line RS-ATL8. Allergy Asthma Immunol. Res. 2018, 10, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.; Leung, N.Y.; Leung, A.S.; Shum, Y.; Leung, P.S.; Chu, K.H.; Kwan, Y.W.; Lee, Q.U.; Wong, J.S.; Lam, I.C.; et al. Cell-Based Functional IgE Assays Are Superior to Conventional Allergy Tests for Shrimp Allergy Diagnosis. J. Allergy Clin. Immunol. Pract. 2021, 9, 236–244.e9. [Google Scholar] [CrossRef]
- Pecoraro, L.; Dalle Carbonare, L.; Castagnoli, R.; Marseglia, G.L.; Piacentini, G.; Pietrobelli, A. IgE-mediated fish allergy in children: Is omega-3 supplementation useful? Int. J. Food Sci. Nutr. 2022, 73, 154–157. [Google Scholar] [CrossRef]
- Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Alternative sources of omega-3 fats: Can we find a sustainable substitute for fish? Nutrients 2013, 5, 1301–1315. [Google Scholar] [CrossRef]
- Dunlop, J.H.; Keet, C.A. Goals and motivations of families pursuing oral immunotherapy for food allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 662–663.e18. [Google Scholar] [CrossRef]
- Refaat, M.; Attia, M.; Saber, H. Desensitization Efficacy by Sublingual Immunotherapy of Shrimps Extract in Asthmatic, Rhinitis and Urticaria Allergic Patients. Food Nutr. Sci. 2014, 5, 1704–1710. [Google Scholar] [CrossRef]
- Nguyen, D.I.; Sindher, S.B.; Chinthrajah, R.S.; Nadeau, K.; Davis, C.M. Shrimp-allergic patients in a multi-food oral immunotherapy trial. Pediatr. Allergy Immunol. 2022, 33, e13679. [Google Scholar] [CrossRef] [PubMed]
- Passanisi, S.; Caminiti, L.; Zirilli, G.; Lombardo, F.; Crisafulli, G.; Aversa, T.; Pajno, G.B. Biologics in food allergy: Up-to-date. Expert Opin. Biol. Ther. 2021, 21, 1227–1235. [Google Scholar] [CrossRef]
- Leung, N.Y.H.; Wai, C.Y.Y.; Shu, S.A.; Chang, C.C.; Chu, K.H.; Leung, P.S.C. Low-Dose Allergen-Specific Immunotherapy Induces Tolerance in a Murine Model of Shrimp Allergy. Int. Arch. Allergy Immunol. 2017, 174, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Myrset, H.R.; Faeste, C.K.; Kristiansen, P.E.; Dooper, M.M. Mapping of the immunodominant regions of shrimp tropomyosin Pan b 1 by human IgE-binding and IgE receptor crosslinking studies. Int. Arch. Allergy Immunol. 2013, 162, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.; Leung, N.Y.; Leung, P.S.; Chu, K.H. T cell epitope immunotherapy ameliorates allergic responses in a murine model of shrimp allergy. Clin. Exp. Allergy 2016, 46, 491–503. [Google Scholar] [CrossRef]
- Reese, G.; Viebranz, J.; Leong-Kee, S.M.; Plante, M.; Lauer, I.; Randow, S.; Moncin, M.S.-M.; Ayuso, R.; Lehrer, S.B.; Vieths, S. Reduced allergenic potency of VR9-1, a mutant of the major shrimp allergen Pen a 1 (tropomyosin). J. Immunol. 2005, 175, 8354–8364. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Ho, M.H.K.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.C.; Chu, K.H. Immunization with Hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, P.S.C.; Chu, K.H. Modulating Shrimp Tropomyosin-Mediated Allergy: Hypoallergen DNA Vaccines Induce Regulatory T Cells to Reduce Hypersensitivity in Mouse Model. Int. J. Mol. Sci. 2019, 20, 4656. [Google Scholar] [CrossRef]
| Biochemical Name | Molecular Weight | Heat Stability | Route of Exposure | Physiological Function | Sources (Examples) | Allergen | IgE Sensitization (%) | References |
|---|---|---|---|---|---|---|---|---|
| Tropomyosin | 33–38 kDA | Stable | Ingestion Inhalation | Binds to actin and regulates the interaction of troponin and myosin | Shrimp Lobster Crab Octopus SnailWhelk Abalone Clam Mussels | Pen a 1 Lit v 1 Pen m 1 Hal m 1 Cra c 1 Mel l 1 Pan b 1 Pen i 1 Met e 1 Por p 1 Hom a 1 Scy o 1 Scy p 1 Scy s 1 Cha f 1 | 72–98 | [76,77,78,79,80] |
| Arginine kinase | 38–41 kDA | Labile | Ingestion Inhalation | Catalyzes the reversible transfer of phosphoryl group from ATP to arginine | Shrimp Crab Octopus | Pen a 2 Pen m 2 Cra c 1 Lit v 2 Scy o 2 Scy p 2 Scy s 2 Cha f 2 Met e 2 Por p 2 | 10–51 | [55,81] |
| Myosin light chain | 17–20 kDA | Stable | Ingestion | Regulates smooth muscle contraction | Shrimp Lobster | Pen m 3 Lit v 3 Cra c 3 Hom a 3 | 19–55 | [63,82] |
| Sarcoplasmic calcium-binding protein | 20–25 kDA | Stable | Ingestion | Acts as a calcium buffer regulating calcium-based signalling | Shrimp | Pen m 4 Lit v 4 Cra c 4 Mel l 4 Pon l 4 Scy p 4 Cha f 4 Met e 4 | 29–50 | [68,69] |
| Troponin C | 20–21 kDA | Unknown | Ingestion | Regulates interaction of actin and myosin during muscle contraction | Shrimp Lobster | Lit v 6 Cra c 6 Hom a 6 Pen m 6 Scy o 6 Pan b 6 | 12–29 | [66,83] |
| Triosephosphate isomerase | 25 kDA | Labile | Ingestion Inhalation | Catalyses conversion of dihydroxyacetone phosphate to glyceraldehyde 3-phosphate in glycolysis | Shrimp | Pen m 8 Cra c 8 Arc s 8 Pro c 8 Scy p 8 | 15–23 | [66] |
| Paramyosin | 99 kDA | Unknown | Ingestion | Functions as a cytoplasmic protein that plays an essential role in the processes of myoblast fusion | Octopus Abalone Turban Shell Mussels | Myt g PM Oct v PM | * NR | [83] |
| Fatty acid-binding protein | 15 kDA | Stable | Ingestion | Coordinates lipid trafficking and signalling in cells | Lit v 13 | 10.3 | [84] | |
| Hemocyanin | 72–75 kDA | Stable | Ingestion | Binding, transportation, and storage of dioxygen within the blood of many invertebrates | Shrimp | Lit v 1 Hemocyanin Pan b Hemocyanin Mac r Hemocyanin | 29–47 | [85] |
| Myosin heavy chain | 225 kDA | Unknown | Ingestion | Muscle contraction | Shrimp Snail | Pan b Myosin | * NR | [60] |
| α-actine | 31–42 kDA | Unknown | Ingestion | Muscle contraction | Shrimp | * NR | [60,81] | |
| Smooth endoplasmic reticulum Ca+2 ATP ase | 113 kDA | Unknown | Ingestion | Enzyme | Crab | Chi o SERCA | * NR | [81] |
| Glyceraldehyde-3-phosphate dehydrogenase | 37 kDA | Unknown | Ingestion | Enzyme for anaerobic glycolysis | Shrimp | * NR | [60] | |
| Ovary development-related protein | 28 kDA | Unknown | Ingestion | Ovary development | Crab | Eri s 2 | * NR | [86] |
| Troponin I | 30 kDA | Unknown | Ingestion | Calcium-binding protein | Crayfish | Pon I 7 | * NR | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, M.; Beken, B.; Buyuktiryaki, B.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Pontone, M.; Bartha, I.; Mori, F.; et al. IgE-Mediated Shellfish Allergy in Children. Nutrients 2023, 15, 2714. https://doi.org/10.3390/nu15122714
Giovannini M, Beken B, Buyuktiryaki B, Barni S, Liccioli G, Sarti L, Lodi L, Pontone M, Bartha I, Mori F, et al. IgE-Mediated Shellfish Allergy in Children. Nutrients. 2023; 15(12):2714. https://doi.org/10.3390/nu15122714
Chicago/Turabian StyleGiovannini, Mattia, Burcin Beken, Betul Buyuktiryaki, Simona Barni, Giulia Liccioli, Lucrezia Sarti, Lorenzo Lodi, Matteo Pontone, Irene Bartha, Francesca Mori, and et al. 2023. "IgE-Mediated Shellfish Allergy in Children" Nutrients 15, no. 12: 2714. https://doi.org/10.3390/nu15122714
APA StyleGiovannini, M., Beken, B., Buyuktiryaki, B., Barni, S., Liccioli, G., Sarti, L., Lodi, L., Pontone, M., Bartha, I., Mori, F., Sackesen, C., du Toit, G., Lopata, A. L., & Muraro, A. (2023). IgE-Mediated Shellfish Allergy in Children. Nutrients, 15(12), 2714. https://doi.org/10.3390/nu15122714







