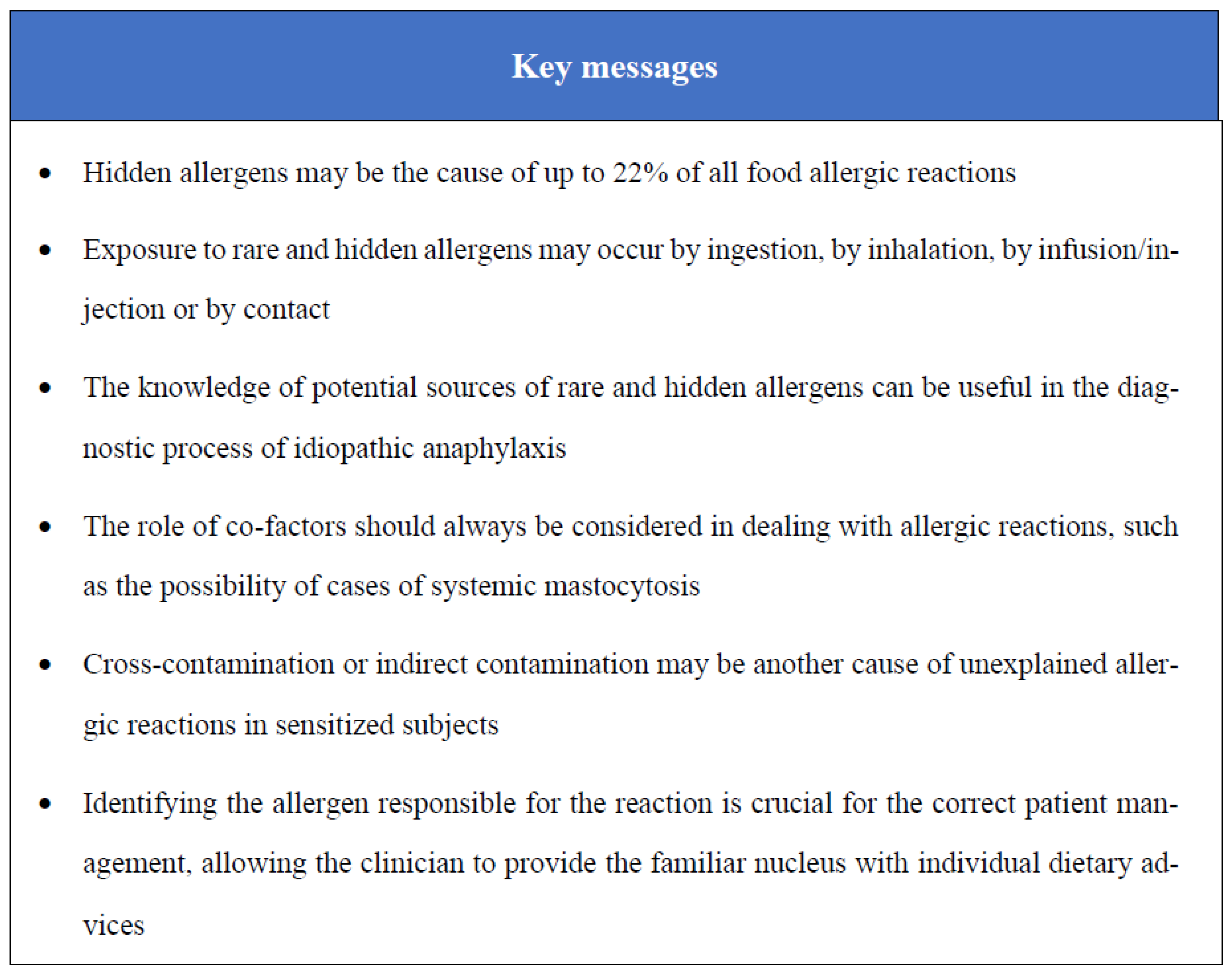
Hidden and Rare Food Allergens in Pediatric Age
Abstract
1. Introduction
2. Ingestion
2.1. Food Additives
2.2. Colorants
2.3. Preservatives
2.4. Thickening Agents
2.5. Flavorings
2.6. Cow’s Milk Proteins
2.7. Hidden Allergens in Other Food Products
2.8. Plant Food Allergens
2.9. Uncommon Food Allergens
2.9.1. Alpha-Gal Syndrome
2.9.2. Cat-Pork Syndrome
2.9.3. Bird-Egg Syndrome
2.9.4. Novel Allergic Cross-Recognitions
3. Inhalation
4. Infusion/Injection
5. Contact
| Allergen | Potential Source of Exposure | Reference |
|---|---|---|
| Ingestion | ||
| Food Additives | ||
| CARMINE (E120) | e.g., several commercially prepared foods including cheese, fruit, and vegetables preparations, processed fish and fishery products, jams, meat products, soups, sauces, desserts, and drinks | [13,14] |
| ANNATTO (E160B) | e.g., several commercially prepared foods including cheese, processed fish and fishery products, desserts, jams, meat products, soup, and sauces | [15,16] |
| TARTRAZINE (E102) | e.g., several commercially prepared foods including cheese, canned and bottled fruits and vegetables, soups, processed fish and fishery products, sauces, and non-alcoholic drinks | [17,18] |
| SULFITES (E220-228) | e.g., several foods including dried fruits, fruit juices, dried potatoes, desserts, processed fish and fishery products and alcoholic and non-alcoholic drinks | [21] |
| SODIUM BENZOATE (E211) | e.g., foods, pharmaceutical preparations, cosmetics | [22,23] |
| PECTIN (E440) | e.g., thickening agent in several food including candies | [24,25] |
| CARBOXYMETHYLCELLULOSE (E466) | e.g., pharmaceutical preparations, wound dressings, cosmetics, and foods such as chocolate products, ice creams, frozen cakes, instant pasta, condiments | [32] |
| Spices | ||
| PEPPER | e.g., seasoning in several foods | [35] |
| FENUGREEK | e.g., seasoning in several foods | [36] |
| MUSTARD | e.g., seasoning in several foods including baby foods and commercial foods for toddlers | [39,40] |
| LAMIACEE FAMILY | e.g., spice mixes and curry powder | [41] |
| Cow’s Milk Proteins | ||
| BETA-LACTOGLOBULIN | e.g., alternatives milk formulas | [42] |
| ALPHA-LACTALBUMIN | e.g., polio Sabin vaccine | [43] |
| COW’S MILK PROTEINS | e.g., probiotics | [44,45] |
| Other Food Products | ||
| STREPTOMYCIN | e.g., foods containing antibiotic residues | [46] |
| LATEX ALLERGENS | e.g., foods processed with latex gloves | [47] |
| DOMESTIC MITES | e.g., foods prepared by wheat flour | [48] |
| ANISAKIS SIMPLEX | e.g., commercial fishes and cephalopods | [49,50] |
| POTATO PEEL PROTEIN | e.g., candies | [51] |
| Meat Allergens | ||
| ALPHA-GAL SYNDROME | e.g., meat from non-primate mammals, intravenous drugs (such as cetuximab) | [59,60] |
| CAT PORK SYNDROME | e.g., red meat | [61,62,63,64] |
| BIRD-EGG SYNDROME | e.g., poultry meat | [65,66] |
| Uncommon Food Allergens | ||
| PARVALBUMINS | e.g., crocodile meat | [69,70] |
| Inhalation | ||
| LUPIN | e.g., fertilizer for plant | [73] |
| COW’S MILK PROTEIN | e.g., dry powder inhalers | [74] |
| WHEAT FLOUR | e.g., toy fingerprint kit | [75] |
| Infusion | ||
| COW’S MILK PROTEIN | e.g., methylprednisolone powder and solvent for solution for injection | [76] |
| SOY PROTEIN | e.g., benzylpenicillin | [77] |
| MANNITOL | e.g., paracetamol | [78] |
| PEANUT ALLERGENS | e.g., blood transfusion | [79] |
| Contact | ||
| WHEAT PROTEINS | e.g., facial cleanser, mouldable plastic materials | [80,81] |
| POLYVINILPIRROLIDONE (E1201) | e.g., corticosteroid eye drops | [83] |
6. The Role of Co-Factors
7. Mastocytosis
8. Cross-Contamination (Indirect Contamination)
9. Diagnosis
10. Management
11. Conclusions
Abbreviation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cafarotti, A.; Giovannini, M.; Begìn, P.; Brough, H.A.; Arasi, S. Management of IgE-mediated food allergy in the 21st century. Clin. Exp. allergy J. Br. Soc. Allergy Clin. Immunol. 2023, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Tropeano, A.; Giovannini, M. Allergen immunotherapy for food allergy: Evidence and outlook. Allergol. Sel. 2022, 6, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Giovannini, M.; Barni, S.; Jiménez-Saiz, R.; Munblit, D.; Biagioni, B.; Liccioli, G.; Sarti, L.; Liotti, L.; Ricci, S.; et al. Oral Immunotherapy for Food-Allergic Children: A Pro-Con Debate. Front. Immunol. 2021, 12, 636612. [Google Scholar] [CrossRef]
- Añíbarro, B.; Seoane, F.J.; Múgica, M.V. Involvement of hidden allergens in food allergic reactions. J. Investig. Allergol. Clin. Immunol. 2007, 17, 168–172. [Google Scholar]
- Taylor, S.L.; Baumert, J.L. Worldwide food allergy labeling and detection of allergens in processed foods. Chem. Immunol. Allergy 2015, 101, 227–234. [Google Scholar]
- Pekar, J.; Ret, D.; Untersmayr, E. Stability of allergens. Mol. Immunol. 2018, 100, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Do, A.B.; Khuda, S.E.; Sharma, G.M. Undeclared Food Allergens and Gluten in Commercial Food Products Analyzed by ELISA. J. AOAC Int. 2018, 101, 23–35. [Google Scholar] [CrossRef]
- Di Girolamo, F.; Muraca, M.; Mazzina, O.; Lante, I.; Dahdah, L. Proteomic applications in food allergy: Food allergenomics. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 259–266. [Google Scholar] [CrossRef]
- Cristina, L.; Elena, A.; Davide, C.; Marzia, G.; Lucia, D.; Cristiano, G.; Marco, A.; Carlo, R.; Laura, C.; Gabriella, G.M. Validation of a mass spectrometry-based method for milk traces detection in baked food. Food Chem. 2016, 199, 119–127. [Google Scholar] [CrossRef]
- WHO. Food Additives; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/food-additives (accessed on 25 January 2023).
- Laura, A.; Arianna, G.; Francesca, C.; Carlo, C.; Carla, M.; Giampaolo, R. Hypersensitivity reactions to food and drug additives: Problem or myth? Acta Biomed. 2019, 90, 80–90. [Google Scholar]
- Wüthrich, B.; Kägi, M.K.; Stücker, W. Anaphylactic reactions to ingested carmine (E120). Allergy Eur. J. Allergy Clin. Immunol. 1997, 52, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Machler, B.C.; Jacob, S.E. Carmine Red: A Potentially Overlooked Allergen in Children. Dermatitis 2018, 29, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Catli, G.; Bostanci, I.; Ozmen, S.; Dibek Misirlioglu, E.; Duman, H.; Ertan, U. Is Patch Testing with Food Additives Useful in Children with Atopic Eczema? Pediatr. Dermatol. 2015, 32, 684–689. [Google Scholar] [CrossRef]
- Myles, I.A.; Beakes, D. An Allergy to Goldfish? Highlighting Labeling Laws for Food Additives. World Allergy Organ. J. 2009, 2, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, N.B.; Tuano KT, S.; Davis, C.M.; Dillard, K.; Hanson, C. Annatto seed hypersensitivity in a pediatric patient. Ann. Allergy Asthma Immunol. 2016, 117, 331–333. [Google Scholar] [CrossRef]
- Wilson, N.; Scott, A. A double-blind assessment of additive intolerance in children using a 12 day challenge period at home. Clin. Exp. Allergy 1989, 19, 267–272. [Google Scholar] [CrossRef]
- Devlin, J.; David, T.J. Tartrazine in atopic eczema. Arch. Dis. Child. 1992, 67, 709–711. [Google Scholar] [CrossRef][Green Version]
- Towns, S.J.; Mellis, C.M. Role of acetyl salicylic acid and sodium metabisulfite in chronic childhood asthma. Pediatrics 1984, 73, 631–637. [Google Scholar] [CrossRef]
- Boner, A.; Guarise, A.; Vallone, G.; Fornari, A.; Piacentini, F.; Sette, L. Metabisulfite oral challenge: Incidence of adverse responses in chronic childhood asthma and its relationship with bronchial hyperreactivity. J. Allergy Clin. Immunol. 1990, 85, 479–483. [Google Scholar] [CrossRef]
- Vitaliti, G.; Guglielmo, F.; Giunta, L.; Pavone, P.; Falsaperla, R. Sodium metabisulphite allergy with multiple food and drug hypersensitivities in a five-year-old child: A case report and literature review. Allergol. Immunopathol. 2015, 43, 106–108. [Google Scholar] [CrossRef]
- Jacob, S.E.; Hill, H.; Lucero, H.; Nedorost, S. Benzoate allergy in children—From foods to personal hygiene products. Pediatr. Dermatol. 2016, 33, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Barni, S.; Pucci, N.; Rossi, M.E.; De Martino, M.; Novembre, E. Cutaneous Adverse Reactions to Amoxicillin-Clavulanic Acid Suspension in Children: The Role of Sodium Benzoate. Curr. Drug Saf. 2012, 7, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Ferdman, R.M.; Ong, P.Y.; Church, J.A. Pectin anaphylaxis and possible association with cashew allergy. Ann. Allergy Asthma Immunol. 2006, 97, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Saf, S.; Tsuang, A.; Nowak-Wegrzyn, A. Hidden allergens in food allergy. Ann. Allergy Asthma Immunol. 2018, 121, 285–292. [Google Scholar] [CrossRef]
- Kraut, A.; Peng, Z.; Becker, A.B.; Warren, C.P. Christmas candy maker’s asthma; IgG4-mediated pectin allergy. Chest 1992, 102, 1605–1607. [Google Scholar] [CrossRef]
- Räsänen, L.; Mäkinen-Kiljunen, S.; Harvima, R.J. Pectin and cashew nut allergy: Cross-reacting allergens? Allergy Eur. J. Allergy Clin. Immunol. 1998, 53, 626–628. [Google Scholar] [CrossRef]
- Hernández-García, E.; de las Heras, M.; Bartolomé, B.; Compés, E.; Sastre, J.; Cuesta, J. Anaphylaxis caused by the pectin component of barium sulphate suspension*1. J. Allergy Clin. Immunol. 2004, 113, S243. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Izakovic, J.; Weber, J.M.; Bircher, A.J. Anaphylaxis to the carbohydrate carboxymethylcellulose in parenteral corticosteroid preparations. Dermatology 2003, 207, 100–103. [Google Scholar] [CrossRef]
- Laisuan, W.; Wongsa, C.; Dchapaphapeaktak, N.; Tongdee, M.; Chatmapanrangsee, J.; Rerkpattanapipat, T. Anaphylaxis following intralesional triamcinolone acetonide (Kenacort) injection. Asia Pac. Allergy 2012, 2, 76–85. [Google Scholar] [CrossRef]
- Dumond, P.; Franck, P.; Morisset, M.; Laudy, J.S.; Kanny, G.; A Moneret-Vautrin, D. Pre-lethal anaphylaxis to carboxymethylcellulose confirmed by identification of specific IgE—Review of the literature. Eur. Ann. Allergy Clin. Immunol. 2009, 41, 171–176. [Google Scholar]
- Ohnishi, A.; Hashimoto, K.; Ozono, E.; Sasaki, M.; Sakamoto, A.; Tashiro, K.; Moriuchi, H. Anaphylaxis to carboxymethylcellulose: Add food additives to the list of elicitors. Pediatrics 2019, 143, e20181180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Bahna, S.L. Spice allergy. Ann. Allergy Asthma. Immunol. 2011, 107, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Schöll, I.; Jensen-Jarolim, E. Allergenic potency of spices: Hot, medium hot, or very hot. Int. Arch. Allergy Immunol. 2004, 135, 247–261. [Google Scholar] [CrossRef]
- Gimenez, L.; Zacharisen, M. Severe pepper allergy in a young child. Wis. Med. J. 2011, 110, 138–139. [Google Scholar]
- Joseph, N.I.; Slavin, E.; Peppers, B.P.; Hostoffer, R.W. Fenugreek Anaphylaxis in a Pediatric Patient. Allergy Rhinol. 2018, 9, 215265671876413. [Google Scholar] [CrossRef]
- Fæste, C.K.; Christians, U.; Egaas, E.; Jonscher, K.R. Characterization of potential allergens in fenugreek (Trigonella foenum-graecum) using patient sera and MS-based proteomic analysis. J. Proteomics 2010, 73, 1321–1333. [Google Scholar] [CrossRef]
- Fæste, C.K.; Namork, E.; Lindvik, H. Allergenicity and antigenicity of fenugreek (Trigonella foenum-graecum) proteins in foods. J. Allergy Clin. Immunol. 2009, 123, 187–194. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, A.K.; Gupta, R.K.; Neelabh; Dwivedi, P.D. A Comprehensive Review on Mustard-Induced Allergy and Implications for Human Health. Clin. Rev. Allergy Immunol. 2019, 57, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Dalal, I.; Binson, I.; Reifen, R.; Amitai, Z.; Shohat, T.; Rahmani, S.; Levine, A.; Ballin, A.; Somekh, E. Food allergy is a matter of geography after all: Sesame as a major cause of severe IgE-mediated food allergic reactions among infants and young children in Israel. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 362–365. [Google Scholar] [CrossRef]
- Yazici, S.; Nacaroǧlu, H.T.; Erdem, S.B.; Karaman, S.; Can, D. Angioedema due to lamiaceae allergy. Iran. J. Allergy Asthma Immunol. 2018, 17, 97–99. [Google Scholar] [PubMed]
- Levin, M.E.; Motala, C.; Lopata, A.L. Anaphylaxis in a milk-allergic child after ingestion of soy formula cross-contaminated with cow’s milk protein. Pediatrics 2005, 116, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Parisi CA, S.; Smaldini, P.L.; Gervasoni, M.E.; Maspero, J.F.; Docena, G.H. Hypersensitivity reactions to the Sabin vaccine in children with cow’s milk allergy. Clin. Exp. Allergy 2013, 43, 249–254. [Google Scholar] [CrossRef]
- Martín-Muñoz, M.F.; Fortuni, M.; Caminoa, M.; Belver, T.; Quirce, S.; Caballero, T. Anaphylactic reaction to probiotics: Cow’s milk and hen’s egg allergens in probiotic compounds. Pediatr. Allergy Immunol. 2012, 23, 778–784. [Google Scholar] [CrossRef]
- Lee, T.-T.T.; Morisset, M.; Astier, C.; Moneret-Vautrin, D.-A.; Cordebar, V.; Beaudouin, E.; Codreanu, F.; Bihain, B.E.; Kanny, G. Contamination of probiotic preparations with milk allergens can cause anaphylaxis in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 119, 746–747. [Google Scholar] [PubMed]
- Graham, F.; Paradis, L.; Bégin, P.; Paradis, J.; Babin, Y.; Roches, A.D. Risk of allergic reaction and sensitization to antibiotics in foods. Ann. Allergy Asthma Immunol. 2014, 113, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, R.; Novembre, E.; Lombardi, E.; Pucci, N.; Marcucci, F.; Vierucci, A. Anaphylaxis to latex after ingestion of a cream-filled doughnut contaminated with latex. J. Allergy Clin. Immunol. 2002, 110, 534–535. [Google Scholar] [CrossRef]
- Bódi, B.; Cojanu, C.; Gorbatovschi, V.; Ureche, C. Pancake syndrome—Oral mite anaphylaxis. Alergologia 2018, 4, 183. [Google Scholar] [CrossRef]
- Falcão, H.; Lunet, N.; Neves, E.; Iglésias, I.; Barros, H. Anisakis simplex as a risk factor for relapsing acute urticaria: A case-control study. J. Epidemiol. Community Health 2008, 62, 634–637. [Google Scholar] [CrossRef]
- Pontone, M.; Giovannini, M.; Barni, S.; Mori, F.; Venturini, E.; Galli, L.; Valleriani, C.; Vecillas, L.D.L.; Sackesen, C.; Lopata, A.L.; et al. IgE-mediated Anisakis allergy in children. Allergol. Immunopathol. 2023, 51, 98–109. [Google Scholar] [CrossRef]
- Martín-Muñoz, M.F.; Diaz-Perales, A.; Cannabal, J.; Quirce, S. Anaphylaxis to hidden potato allergens in a peach and egg allergic boy. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 45–48. [Google Scholar]
- Betancor, D.; Gomez-Lopez, A.; Villalobos-Vilda, C.; Nuñez-Borque, E.; Fernández-Bravo, S.; Gozalo, M.D.L.H.; Pastor-Vargas, C.; Esteban, V.; Cuesta-Herranz, J. Ltp allergy follow-up study: Development of allergy to new plant foods 10 years later. Nutrients 2021, 13, 2165. [Google Scholar] [CrossRef]
- Asero, R.; Piantanida, M.; Pinter, E.; Pravettoni, V. The clinical relevance of lipid transfer protein. Clin. Exp. Allergy 2018, 48, 6–12. [Google Scholar] [CrossRef]
- Skypala, I.J.; Bartra, J.; Ebo, D.G.; Faber, M.A.; Fernández-Rivas, M.; Gomez, F.; Luengo, O.; Till, S.J.; Asero, R.; Barber, D.; et al. The diagnosis and management of allergic reactions in patients sensitized to non-specific lipid transfer proteins. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 2433–2446. [Google Scholar] [CrossRef]
- Vereda, A.; van Hage, M.; Ahlstedt, S.; Ibañez, M.D.; Cuesta-Herranz, J.; van Odijk, J.; Wickman, M.; Sampson, H.A. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J. Allergy Clin. Immunol. 2011, 127, 603–607. [Google Scholar] [CrossRef]
- Asero, R.; Mistrello, G.; Roncarolo, D.; Amato, S.; Caldironi, G.; Barocci, F.; Van Ree, R. Immunological cross-reactivity between lipid transfer proteins from botanically unrelated plant-derived foods: A clinical study. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, B.; Tomei, L.; Valleriani, C.; Liccioli, G.; Barni, S.; Sarti, L.; Citera, F.; Giovannini, M.; Mori, F. Allergy to Gibberellin-Regulated Proteins (Peamaclein) in Children. Int. Arch. Allergy Immunol. 2021, 182, 1194–1199. [Google Scholar] [CrossRef]
- Inomata, N. Gibberellin-regulated protein allergy: Clinical features and cross-reactivity. Allergol. Int. 2020, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Caglayan-Sozmen, S.; Santoro, A.; Cipriani, F.; Mastrorilli, C.; Ricci, G.; Caffarelli, C. Hazardous Medications in Children with Egg, Red Meat, Gelatin, Fish, and Cow’s Milk Allergy. Medicina 2019, 55, 501. [Google Scholar] [CrossRef]
- Saretta, F.; Giovannini, M.; Mori, F.; Arasi, S.; Liotti, L.; Pecoraro, L.; Barni, S.; Castagnoli, R.; Mastrorilli, C.; Novembre, E. Alpha-Gal Syndrome in Children: Peculiarities of a “Tick-Borne” Allergic Disease. Front. Pediatr. 2021, 9, 801753. [Google Scholar] [CrossRef]
- Wilson, J.M.; Platts-Mills, T.A.E. Red meat allergy in children and adults. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Matsubara, K.; Chinuki, Y.; Hori, M.; Masaki, T. Early childhood-onset pork-cat syndrome due to sensitization by both cats and dogs—a case report. Arerugi 2019, 68, 1141–1147. [Google Scholar]
- Sagawa, N.; Inomata, N.; Suzuki, K.; Sano, S.; Watanabe, Y.; Aihara, M. Pork-cat syndrome caused by ingestion of beef intestines in an 8-year-old child. Allergol. Int. 2021, 70, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuki, R.; Chinuki, Y.; Fukushiro, S.; Morita, E. A case of pork-cat syndrome that developed as food-dependent exercise-induced anaphylaxis. Acta Derm. Venereol. 2020, 100, adv00233. [Google Scholar] [CrossRef] [PubMed]
- Mandallaz, M.M.; De Weck, A.L.; Dahinden, C.A. Bird-egg syndrome. Cross-reactivity between bird antigens and egg-yolk livetins in IgE-mediated hypersensitivity. Int. Arch. Allergy Appl. Immunol. 1988, 87, 143–150. [Google Scholar] [CrossRef]
- Szepfalusi, Z.; Ebner, C.; Pandjaitan, R.; Orlicek, F.; Scheiner, O.; Boltznitulescu, G.; Kraft, D.; Ebner, H. Egg yolk α-livetin (chicken serum albumin) is a cross-reactive allergen in the bird-egg syndrome. J. Allergy Clin. Immunol. 1994, 93, 932–942. [Google Scholar] [CrossRef]
- Hemmer, W.; Klug, C.; Swoboda, I. Update on the bird-egg syndrome and genuine poultry meat allergy. Allergo J. Int. 2016, 25, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Quirce, S.; Maranon, F.; Umpierrez, A.; Heras, M.D.L.; Fernandez-Caldas, E.; Sastre, J. Chicken serum albumin (Gal d 5*) is a partially heat-labile inhalant and food allergen implicated in the bird-egg syndrome. Allergy Eur. J. Allergy Clin. Immunol. 2001, 56, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, N.; Nopp, A.; Hamsten, C.; Vetander, M.; Melén, E.; Nilsson, C.; Ollert, M.; Flohr, C.; Kuehn, A.; van Hage, M. Anaphylactic reactions to novel foods: Case report of a child with severe crocodile meat allergy. Pediatrics 2017, 139, e20161404. [Google Scholar] [CrossRef]
- Haroun-Díaz, E.; Blanca-López, N.; de la Torre, M.V.; Ruano, F.J.; Álvarez, M.L.S.; Horrillo, M.L.; Bartolomé, B.; Blanca, M.; Díez, G.C. Severe anaphylaxis due to crocodile-meat allergy exhibiting wide cross-reactivity with fish allergens. J. Allergy Clin. Immunol. Pract. 2018, 6, 669–670.e1. [Google Scholar] [CrossRef]
- Hilger, C.; Thill, L.; Grigioni, F.; Lehners, C.; Falagiani, P.; Ferrara, A.; Romano, C.; Stevens, W.; Hentges, F. IgE-antibodies of fish allergic patients cross-react with frog parvalbumin. Allergy Eur. J. Allergy Clin. Immunol. 2004, 59, 653–660. [Google Scholar] [CrossRef]
- Christopoulou, G.; Tziotou, M.; Psomiadou, M.; Tzeli, K.; Karantoumanis, D.; Kostoudi, S.; Zisaki, V.; Georgiou, K.; Roumpedaki, E.; Douladiris, N.; et al. Allergy in products of non enzymatic browning reactions—A case report. Allergy 2015, 70, 612–613. [Google Scholar]
- Novembre, E.; Moriondo, M.; Bernardini, R.; Azzari, C.; Rossi, M.E.; Vierucci, A. Lupin allergy in a child. J. Allergy Clin. Immunol. 1999, 103, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.; Motheral, L. Hypersensitivity Reaction After Inhalation of a Lactose-Containing Dry Powder Inhaler. J. Pediatr. Pharmacol. Ther. 2014, 19, 206–211. [Google Scholar] [CrossRef]
- Taylor, S.L.; Baumert, J.L.; Boudreau-Romano, S.M. Allergic reaction from fingerprint kit attributable to unlabeled gluten, probable wheat flour. J. Allergy Clin. Immunol. Pract. 2017, 5, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Savvatianos, S.; Giavi, S.; Stefanaki, E.; Siragakis, G.; Manousakis, E.; Papadopoulos, N.G. Cow’s milk allergy as a cause of anaphylaxis to systemic corticosteroids. Allergy 2011, 66, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Mori, F.; Pantano, S.; Novembre, E. Adverse reaction to benzathine benzylpenicillin due to soy allergy: A case report. J. Med. Case Rep. 2015, 9, 10–12. [Google Scholar] [CrossRef]
- Jain, S.S.; Green, S.; Rose, M. Anaphylaxis following intravenous paracetamol: The problem is the solution. Anaesth. Intensive Care 2015, 43, 779–781. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Baumert, J.L.; Brons, P.P.; Joosten, I.; Koppelman, S.J.; van Pampus, E.C. Anaphylaxis from passive transfer of peanut allergen in a blood product. New Engl. J. Med. 2011, 364, 1981–1982. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Taniguchi, M.; Nakamura, H.; Akiyama, K. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1405–1411. [Google Scholar] [CrossRef]
- Ponda, P.; Sicherer, S. An Allergic Reaction to Play-Doh in a Child With Wheat Hypersensitivity. J. Allergy Clin. Immunol. 2005, 115, S32. [Google Scholar] [CrossRef]
- Liccioli, G.; Mori, F.; Barni, S.; Pucci, N.; Novembre, E. Anaphylaxis to polyvinylpyrrolidone in eye drops administered to an adolescent. J. Investig. Allergol. Clin. Immunol. 2018, 28, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sakurai, Y.; Kawahara, S.; Takeda, T.; Ishikawa, T.; Murakami, T.; Yoshioka, A. Anaphylaxis to polyvinylpyrrolidone in povidone-iodine for impetigo contagiosum in a boy with atopic dermatitis. Int. Arch. Allergy Immunol. 2008, 146, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Mori, F.; Giovannini, M.; de Luca, M.; Novembre, E. In situ simulation in the management of anaphylaxis in a pediatric emergency department. Intern. Emerg. Med. 2019, 14, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wölbing, F.; Fischer, J.; Köberle, M.; Kaesler, S.; Biedermann, T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 1085–1092. [Google Scholar] [CrossRef]
- Foong, R.X.; Giovannini, M.; du Toit, G. Food-dependent exercise-induced anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, J.; Araujo, G.; Cardona, V.; García-Moral, A.; Casas-Saucedo, R.; Guilarte, M.; Torres, M.J.; Doña, I.; Picado, C.; Pascal, M.; et al. Food-dependent NSAID-induced hypersensitivity (FDNIH) reactions: Unraveling the clinical features and risk factors. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 1480–1492. [Google Scholar] [CrossRef]
- Bilò, M.B.; Martini, M.; Tontini, C.; Mohamed, O.E.; Krishna, M.T. Idiopathic anaphylaxis. Clin. Exp. Allergy 2019, 49, 942–952. [Google Scholar] [CrossRef]
- Carter, M.C.; Desai, A.; Komarow, H.D.; Bai, Y.; Clayton, S.T.; Clark, A.S.; Ruiz-Esteves, K.N.; Long, L.M.; Cantave, D.; Wilson, T.M.; et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J. Allergy Clin. Immunol. 2018, 141, 180–188.e3. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Sander, B.; Dahlén, B.; Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 2014, 44, 1179–1187. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; González-De-Olano, D.; Sánchez-Muñoz, L.; Matito, A.; Jara-Acevedo, M.; Teodosio, C.; García-Montero, A.; Morgado, J.; Orfao, A.; Escribano, L. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int. Arch. Allergy Immunol. 2012, 157, 275–280. [Google Scholar] [CrossRef]
- Boyano-Martínez, T.; García-Ara, C.; Pedrosa, M.; Díaz-Pena, J.M.; Quirce, S. Accidental allergic reactions in children allergic to cow’s milk proteins. J. Allergy Clin. Immunol. 2009, 123, 883–888. [Google Scholar] [CrossRef]
- Furlong, T.J.; Desimone, J.; Sicherer, S.H. Peanut and tree nut allergic reactions in restaurants and other food establishments. J. Allergy Clin. Immunol. 2001, 108, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Sicherer, S.H. Living with Food Allergy: Allergen Avoidance. Pediatr. Clin. North Am. 2011, 58, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Baumert, J.L. Cross-contamination of foods and implications for food allergic patients. Curr. Allergy Asthma Rep. 2010, 10, 265–270. [Google Scholar] [CrossRef]
- Cummings, A.J.; Knibb, R.C.; King, R.M.; Lucas, J.S. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: A review. Allergy Eur. J. Allergy Clin. Immunol. 2010, 65, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Pistiner, M.; LeBovidge, J.; Bantock, L.; James, L.; Harada, L. Living Confidently with Food Allergy Handbook: A Guide for Parents and Families; Anaphylaxis: Toronto, ON, Canada, 2013. [Google Scholar]
- Centers for Disease Control and Prevention. Voluntary Guidelines for Managing Food Allergies in Schools and Early Care and Education Programs; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Anaphylaxis Campaign. Model Policy for Allergy Management at School. Available online: https://www.anaphylaxis.org.uk/wp-content/uploads/2021/10/Model-Policy-for-allergy-management-at-school.pdf (accessed on 25 January 2023).
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-mediated food allergy in children: Epidemiology, pathogenesis, diagnosis, prevention, and management. Medicina 2020, 56, 111. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. EAACI Allergen Immunotherapy User’s Guide. Pediatr. allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S25), 1–101. [Google Scholar]
- Dantzer, J.A.; Wood, R.A. Next-Generation Approaches for the Treatment of Food Allergy. Curr. Allergy Asthma Rep. 2019, 19, 5. [Google Scholar] [CrossRef]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Passanisi, S.; Caminiti, L.; Zirilli, G.; Lombardo, F.; Crisafulli, G.; Aversa, T.; Pajno, G.B. Expert Opinion on Biological Therapy Biologics in food allergy: Up-to-date Biologics in food allergy: Up-to-date. Expert Opin. Biol. Ther. 2021, 21, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Francuzik, W.; Dölle-Bierke, S.; Alexiou, A. Use of biologics in food allergy management. Allergol. Sel. 2021, 5, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.; Du Toit, G.; Giovannini, M.; Turcanu, V.; Lack, G. Pediatric Allergic Diseases, Food Allergy, and Oral Tolerance. Annu. Rev. Cell Dev. Biol. 2020, 36, 511–528. [Google Scholar] [CrossRef]
- Feng, C.; Kim, J.H. Beyond Avoidance: The Psychosocial Impact of Food Allergies. Clin. Rev. Allergy Immunol. 2019, 57, 74–82. [Google Scholar] [CrossRef]
- Meyer, R.; De Koker, C.; Dziubak, R.; Venter, C.; Dominguez-Ortega, G.; Cutts, R.; Yerlett, N.; Skrapak, A.-K.; Fox, A.; Shah, N. Malnutrition in children with food allergies in the UK. J. Hum. Nutr. Diet. 2014, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomei, L.; Muraro, A.; Giovannini, M.; Barni, S.; Liccioli, G.; Paladini, E.; Sarti, L.; Pessina, B.; Skypala, I.; Novembre, E.; et al. Hidden and Rare Food Allergens in Pediatric Age. Nutrients 2023, 15, 1386. https://doi.org/10.3390/nu15061386
Tomei L, Muraro A, Giovannini M, Barni S, Liccioli G, Paladini E, Sarti L, Pessina B, Skypala I, Novembre E, et al. Hidden and Rare Food Allergens in Pediatric Age. Nutrients. 2023; 15(6):1386. https://doi.org/10.3390/nu15061386
Chicago/Turabian StyleTomei, Leonardo, Antonella Muraro, Mattia Giovannini, Simona Barni, Giulia Liccioli, Erika Paladini, Lucrezia Sarti, Benedetta Pessina, Isabel Skypala, Elio Novembre, and et al. 2023. "Hidden and Rare Food Allergens in Pediatric Age" Nutrients 15, no. 6: 1386. https://doi.org/10.3390/nu15061386
APA StyleTomei, L., Muraro, A., Giovannini, M., Barni, S., Liccioli, G., Paladini, E., Sarti, L., Pessina, B., Skypala, I., Novembre, E., & Mori, F. (2023). Hidden and Rare Food Allergens in Pediatric Age. Nutrients, 15(6), 1386. https://doi.org/10.3390/nu15061386








