Food Network Analysis in Non-Obese Patients with or without Steatosis
Abstract
1. Introduction
2. Materials and Methods
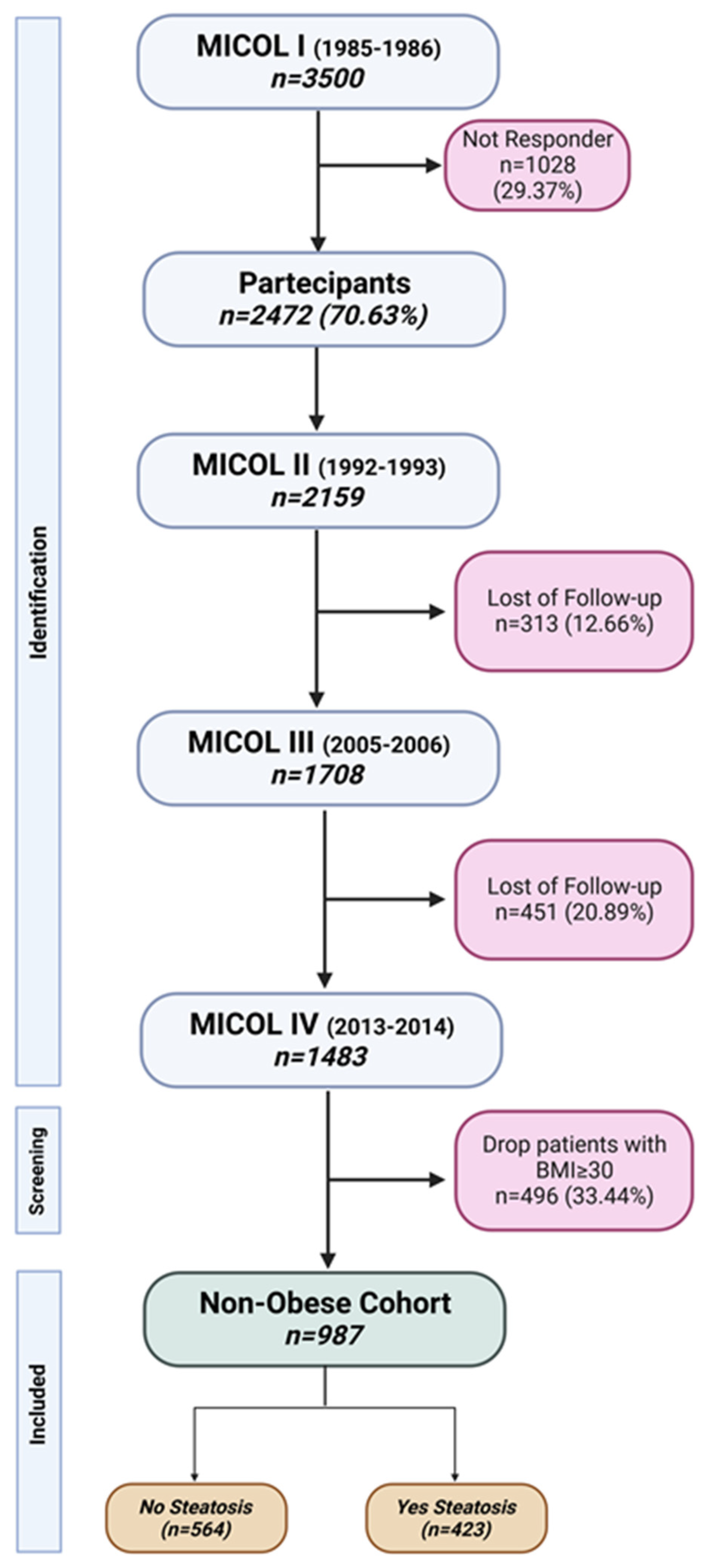
2.1. Study Population
2.2. Dietary Assessments
2.3. Statistical Analysis
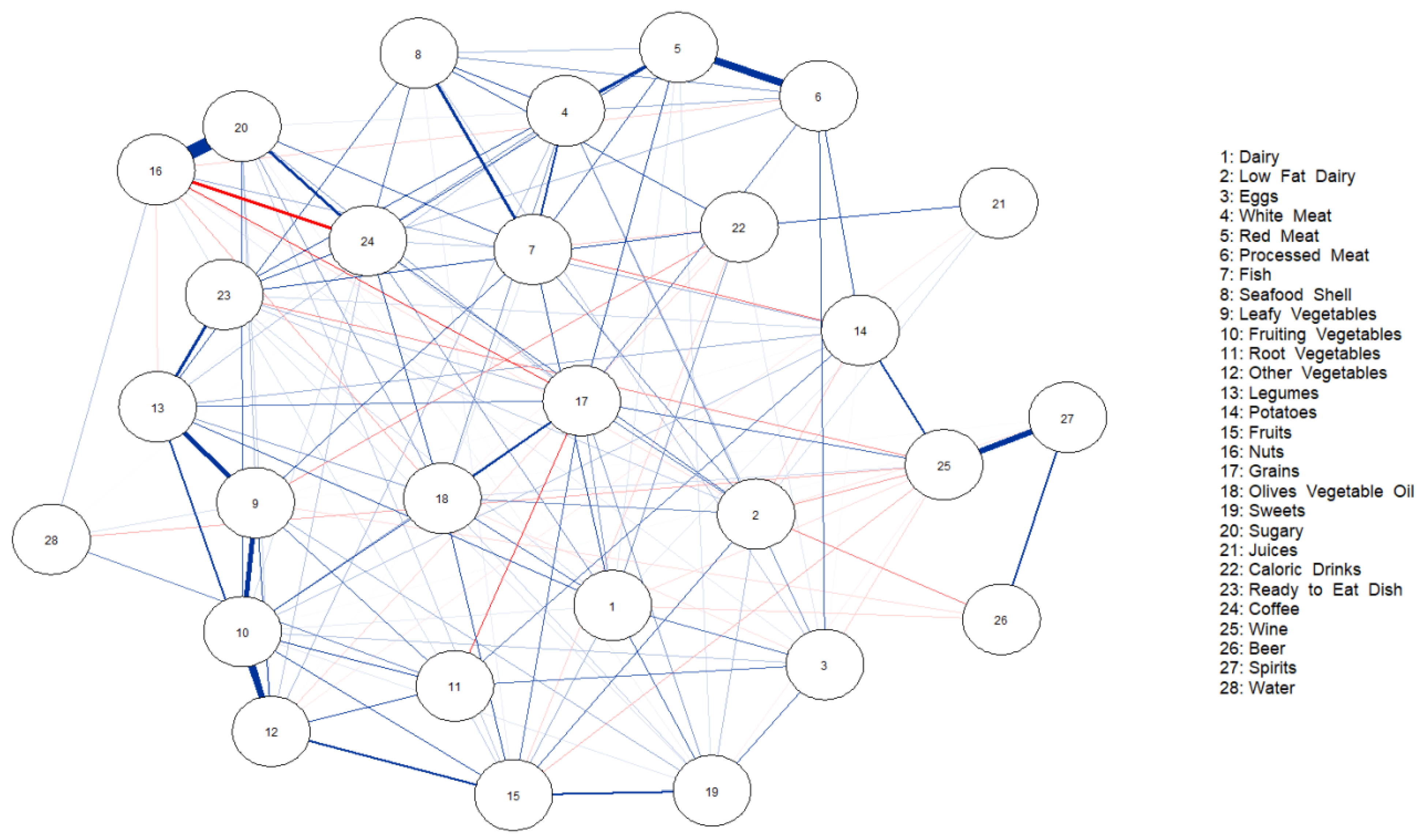
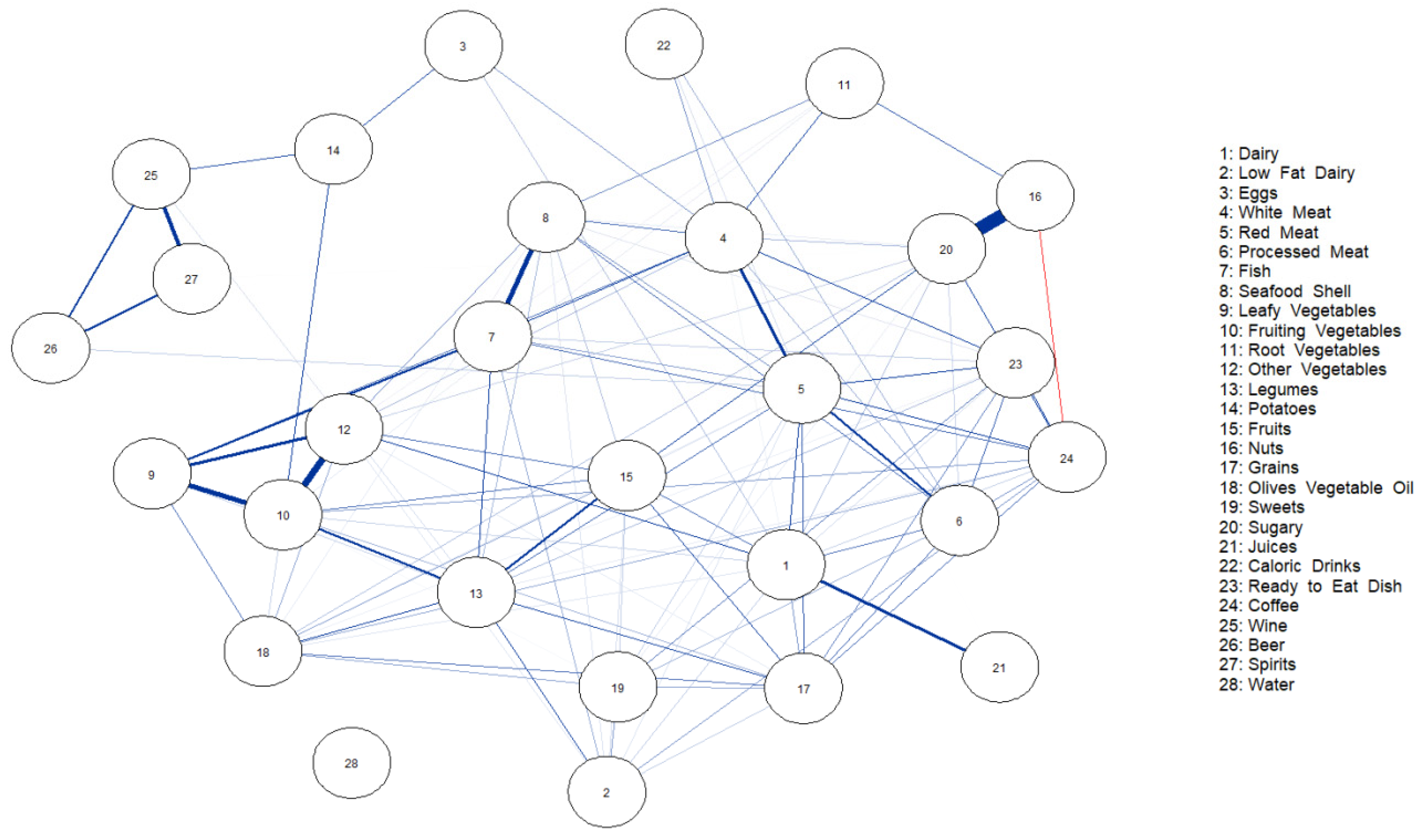
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younozzi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; Geroge, J.; Bugianesi, E. Global burden of NALFD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.M. Nonalcoholic fatty liver disease in lean subject: Is it all metabolic-associated fatty liver diasease? Hepatoma Res. 2020, 6, 84. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Steoanova, M.; Negro, F.; Hallaji, S.; Younossi, Y.; Lam, B.; Srishord, M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine 2012, 91, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, J.; Zhou, W.; Huang, J.; Wu, G.; Yang, F.; Yuan, S.; Fang, J.; Liu, J.; Jin, Y.; et al. Lean non-alcoholic fatty liver disease (lean-NAFLD) and the development of metabolic syndrome: A retrospective study. Sci. Rep. 2022, 12, 10977. [Google Scholar] [CrossRef] [PubMed]
- Hangström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagis, S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follo-up study. Hepatol. Commun. 2017, 2, 48–57. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, Y.K.; Cho, J.; Jung, H.; Yun, K.E.; Ahn, J.; Sohn, C.; Shin, H.; Ryu, S. Alcoholic and nonalcoholic fatty liver disease and liver-related mortality: A cohort study. Am. J. Gastroenterol. 2019, 114, 620–629. [Google Scholar] [CrossRef]
- Golabi, P.; Paik, J.; Fukui, N.; Locklear, C.T.; de Avilla, L.; Younossi, Z.M. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher rick for mortality. Clin. Diabetes. 2019, 37, 65–72. [Google Scholar] [CrossRef]
- Zou, B.; Yeo, Y.H.; Nguyen, V.H.; Cheung, R.; Ingelsson, E.; Nguyen, M.H. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J. Intern. Med. 2020, 288, 139–151. [Google Scholar] [CrossRef]
- Younes, R.; Govaere, O.; Petta, S.; Miele, L.; Tiniakos, D.; Burt, A.; David, E.; Vecchio, F.M.; Maggioni, M.; Cabibi, D.; et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: Time for reappraisal of BMI-driven approach? Gut 2022, 71, 382–390. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nurember, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Chan, W. Comaprison between obese and non-obese nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S58–S67. [Google Scholar] [CrossRef] [PubMed]
- Lim, U.; Monroe, K.R.; Buchthal, S.; Fan, B.; Cheng, I.; Kristal, B.S.; Lampe, J.W.; Hullar, M.A.; Franke, A.A.; Stram, D.O.; et al. Propensity for intra-abdominal and hepatic adiposity varies among ethnic groups. Gastroentrology 2019, 156, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-linke phospholipase domain containing 3 gene (PNOLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef]
- Trépo, E.M.; Romeo, S.; Zucman-Rossi, J.; Nahon, P. PNPLA3 gene in liver disease. J. Hepatol. 2016, 65, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Kong, A.S.; Zain, S.M.; Chan, W.; Tan, H.; Mohamed, Z.; Pung, Y.; Mohamed, R. Loss-of-function HSD17B13 variants, non-alòcoholic steatohepatitis and adverse liver outcomes: Results from a multi-ethnic Asian cohort. Clin. Mol. Hepatol. 2021, 27, 486–498. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Petta, S.; Lombardi, R.; Pisano, G.; Russello, M.; Consonni, D.; Di Marco, V.; Cammà, C.; Mensi, L.; Dongiovanni, P.; et al. Liver and cardiovascular damage in aptients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin. Gastroenterol. Hepatol. 2017, 15, 1604–1611. [Google Scholar] [CrossRef]
- Mouzaki, M.; Shah, A.; Arce-Clachar, A.C.; Hardy, J.; Bramlage, K.; Xanthakos, S.A. Extremely low levels of low-density lipoprotein potentially suggestive of familial hypobetalipoproteinemia: A separate phenotype of NAFLD? J. Clin. Lipidol. 2019, 13, 425–432. [Google Scholar] [CrossRef]
- Carter, A.; Brackeley, S.M.; Gao, J.; Mann, J.P. The global prevalence and genetic spectrum of lysosomal acid lipase deficiency: A rare condition that mimics NAFLD. J. Hepatol. 2019, 70, 142–150. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Systematic review with meta-analysis: Risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment. Pharmacol. Ther. 2017, 46, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. GI epidemiology: Nonalcoholic fatty liver diasease. Aliment. Pharmacol. Ther. 2007, 25, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Sahlabadi, A.; Teymoori, F.; Ahmadirad, H.; Mokhtari, E.; Azadi, M.; Seraj, S.S.; Hekmatdoost, A. Nutrients patterns and non-alcoholic fatty liver disease in Iranian Adul: A case-control study. Front. Nutr. 2022, 9, 977403. [Google Scholar] [CrossRef]
- Mokhtari, E.; Farhadnejad, H.; Salehi-Sahlabadi, A.; Najibi, N.; Azadi, M.; Teymoori, F.; Mirmiran, P. Spinach consumption and nonalcoholic fatty liver diasese among adults: A case-control study. BMC Gastroenterol. 2021, 21, 196. [Google Scholar] [CrossRef]
- Yasutake, K.; Kohjima, M.; Kotoh, K.; Nakashima, M.; Nakamuta, M.; Enjoji, M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1756–1767. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L.; Keshteli, A.H.; Feizi, A.; Feinle-Bisset, C.; Adibi, P. Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: Findings from the SEPAHAN study. Eur. J. Nutr. 2015, 55, 505–518. [Google Scholar] [CrossRef]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, M.S.; Patterson, B.W.; Klein, S. Dietary Fat and Carbohydrates Differentially Alter Insulin Sensitivity During Caloric Restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O′Sullivan, T.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef]
- Younes, R.; Bugianesi, E. NASH in lean individuals. Semin. Liver Dis. 2019, 39, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependet insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commum. 2005, 326, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.N.B.; Stefano, J.T.; Miele, L.; Ponziani, F.R.; Souza-Basqueira, M.; Okada, L.S.R.R.; de Barros Costa, F.G.; Toda, K.; Mazo, D.F.C.; Sabino, E.C.; et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 369–384. [Google Scholar] [CrossRef]
- Yamakado, M.; Tanaka, T.; Nagao, K.; Ishizaka, Y.; Mitushima, T.; Tani, M.; Toda, A.; Toda, E.; Okada, M.; Miyano, H.; et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin. Obes. 2012, 2, 29–40. [Google Scholar] [CrossRef]
- Chen, H.T.; Huang, H.L.; Li, Y.Q.; Xu, H.M.; Zhou, Y.J. Therapeutic advances in non-alcoholic fatty liver disease: A microbiota-centered view. World J. Gastroenterol. 2020, 26, 1901–1911. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Wong, V.W.; Chan, R.S.; Wong, G.L.; Cheung, B.H.; Chu, W.C.; Yeung, D.K.; Chim, A.M.; Lai, J.W.; Li, L.S.; Sea, M.M.; et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: A randomized controlled trial. J. Hepatol. 2013, 59, 536–542. [Google Scholar] [CrossRef]
- Misciagna, G.; Leoci, C.; Guerra, V.; Chiloiro, M.; Elba, S.; Petruzzi, J.; Mossa, A.; Noviello, M.R.; Coviello, A.; Minutolo, M.C.; et al. Epidemiology of cholelithiasis in southern Italy. Part II: Risk factors. Eur. J. Gastroenterol. Hepatol. 1996, 6, 585–593. [Google Scholar] [CrossRef]
- Attili, A.F.; Capocaccia, R.; Carulli, N.; Festi, D.; Rosa, E.; Barbara, L.; Capocaccia, L.; Menotti, A.; Okolicsanyi, L.; Ricci, G.; et al. Factors associated with gallstone diasease in the MICOL experience. Multicenter Italian Study on epidemiology of cholelithiasis. Hepatology 1997, 26, 809–818. [Google Scholar] [CrossRef]
- Veronese, N.; Notarnicola, M.; Cisternino, A.M.; Inguaggiato, R.; Guerra, V.; Reddavide, R.; Donghia, R.; Rotolo, O.; Zinzi, I.; Leandro, G.; et al. Trends in adherence to the Mediterranean diet in South Italy: A cross sectional study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Osella, A.R.; Misciagna, G.; Leone, A.; Di Leo, A.; Fiore, G. Epidemiology of hepatitis C virus infection in ad area of Southern Italy. J. Hepatol. 1997, 27, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the reporting of observational studies in epidemiology-nutritional epidemiology (STROBE-nut): An extension of the STROBE statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Qayyum, A.; Chen, D.M.; Breiman, R.S.; Westphalen, A.C.; Yeh, B.M.; Jones, K.D.; Lu, Y.; Coakley, F.V.; Callen, P.W. Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: Which modality is best? Clin. Imaging 2009, 33, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Leoci, C.C.S.; Guerra, V.; Cisternino, A.M.; MIscigna, G. Reliability and validity of a self administrered semi-quantitative food frequency questionnaire. Giorn. Italy Nutr. Clin. Prev. 1993, 71, 1269–1324. [Google Scholar]
- Castelló, A.; PolláN, M.; Buijsse, B.; Ruiz, A.; Casa, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish meditettanean diet and other dietary patterns and breast cancer risks: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Suzuki, J.; Kameo, Y.; Hase, T. Dietary supplementation with hydroxypropyl-distarch phosphate from waxy maize starch increases resting energy expensditure by lowering the postprandial glucose-dependent insulinotropic polypeptide response in human subjects. Br. J. Nutr. 2011, 106, 96–104. [Google Scholar] [CrossRef]
- Stewart, M.L.; Zimmer, J.P. A High Fiber Cookie Made with Resistant Starch Type 4 Reduces Post-Prandial Glucose and Insulin Responses in Healthy Adults. Nutrients 2017, 9, 237. [Google Scholar] [CrossRef]
- Chazelas, E.; Deschasaux, M.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Alles, B.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Food additives: Distribution and co-occurrence in 126,000 food products of the French market. Sci. Rep. 2020, 10, 3980. [Google Scholar] [CrossRef]
- Boccaletti, S.; Latora, V.; Moreno, Y.; Chavez, M.; Hwang, D.-U. Complex networks: Structure and dynamics. Phys. Rep. 2006, 424, 175–308. [Google Scholar] [CrossRef]
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Net. 1978, 1, 215–239. [Google Scholar] [CrossRef]
- Chang, Y.; Jung, H.S.; Yun, K.E.; Cho, J.; Ahn, J.; Chung, E.C.; Shin, H.; Ryu, S. Metabolically healthy obesity is associated with an increased risk of diabetes independently of nonalcoholic fatty liver disease. Obesity 2016, 24, 1996–2003. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2005, 128, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Albhaisi, S.; Chowdhury, A.; Sanyal, A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019, 1, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human Fatty Liver Disease: Old Questions and New Insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.K.C.; Kabbany, M.N.; Lopez, R.; Rayas, M.S.; Lynch, J.L.; Alkhouri, N. Prevalence of Suspected Nonalcoholic Fatty Liver Disease in Lean Adolescents in the United States. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 75–79. [Google Scholar] [CrossRef]
- Maier, S.; Wieland, A.; Cree-Green, M.; Nadeau, K.; Sullivan, S.; Lanaspa, M.A.; Johnson, R.J.; Jensen, T. Lean NAFLD: An underrecognized and challenging disorder in medicine. Rev. Endocr. Metab. Disord. 2021, 22, 351–366. [Google Scholar] [CrossRef]
- Dressler, H.; Smith, C. Food choice, eating behavior, and food liking differs between lean/normal and overweight/obese, low-income women. Appetite 2013, 65, 145–152. [Google Scholar] [CrossRef]
- Galindo, M.M.; Schneider, N.Y.; Stähler, F.; Töle, J.; Meyerhof, W. Taste preferences. Prog. Mol. Biol. Transl. Sci. 2012, 108, 383–426. [Google Scholar]
- Montmayeur, J.P.; le Coutre, J. Fat detection: Taste, texture, and post ingestive effects. Front. Neurosc. 2010, 1, 265–291. [Google Scholar]
- Yau, Y.H.C.; Potenza, M.N. Stress and eating behaviors. Minerva. Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Jin, H.; Nicodemus-Johnson, J. Gender and age stratified analyses of nutrient and dietary pattern associations with circulating lipid levels identify novel ender and age-specific correlations. Nutrients 2018, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, L.; Duizer, L.; Caldwell, T. Body Fat, Sweetness Sensitivity, and Preference: Determining the Relationship. Can. J. Diet. Pr. Res. 2012, 73, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Toews, I.; Lohner, S.; Küllenberg de Gaudry, D.; Sommer, H.; Meerpohl, J.J. Association between intake of non-sugar sweeteners and health outcomes: Systematic review and meta-analyses of randomized and non-randomised analyses of randomized and non-randomised controlled triales and observational studies. BMJ 2019, 364, k4718. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The macronutrients, appetite, and energy intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.; Camacho, M.; Santos, O.; Pontes, C.; Torres, S.; Oliveira-Maia, A.J. Association between hedonic hunger and body-mass index versus obesity status. Sci. Rep. 2018, 8, 5857. [Google Scholar] [CrossRef]
- Chen, F.; Esmaili, S.; Rogers, G.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A distrinct entity shaped by differential metabolic adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef]
- Cruz, A.C.D.; Bugianesi, E.; Geroge, J.; Day, C.P.; Liaquat, H.; Charatcharoenwitthaya, P.; Mills, P.R.; Dam-Larsen, S.; Bjornsoon, E.S.; Haflidadottir, S.; et al. 379 Charactestics and long-term progrosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, S909. [Google Scholar] [CrossRef]
- de Villiers, M.R.; de Villiers, P.J.T.; Kent, A.P. The Delphi technique in health sciences education research. Med. Teach. 2005, 27, 639–643. [Google Scholar] [CrossRef]
| Parameters * | Total Cohort (n = 987) | Steatosis | p ^ | |
|---|---|---|---|---|
| No (n = 564) | Yes (n = 423) | |||
| Gender (M) (%) | 579 (58.66) | 290 (51.42) | 289 (68.32) | <0.001 ѱ |
| Age (years) | 63.56 ± 11.57 | 63.72 ± 12.22 | 63.34 ± 10.66 | 0.68 |
| Degree of Education (%) | 0.11 ѱ | |||
| No | 262 (28.20) | 160 (30.02) | 102 (25.76) | |
| Elementary School | 295 (31.75) | 151 (28.33) | 144 (36.36) | |
| Secondary School | 245 (26.37) | 147 (27.58) | 98 (24.75) | |
| High School | 83 (8.93) | 51 (9.57) | 32 (8.08) | |
| Short Degree | 44 (4.74) | 24 (4.50) | 20 (5.05) | |
| Smoker (Yes) (%) | 139 (14.96) | 81 (15.53) | 58 (14.61) | 0.79 ѱ |
| BMI (Kg/m2) | 25.66 ± 2.69 | 24.87 ± 2.65 | 26.72 ± 2.37 | <0.0001 |
| DBP (mmHg) | 77.81 ± 7.75 | 76.87 ± 7.62 | 79.06 ± 7.76 | <0.0001 |
| SBP (mmHg) | 125.30 ± 15.28 | 124.02 ± 15.76 | 127.01 ± 14.44 | 0.001 |
| Diabetes (Yes) (%) | 66 (8.71) | 34 (7.71) | 32 (10.09) | 0.25 ѱ |
| Hypertension (Yes) (%) | 345 (45.39) | 194 (43.79) | 151 (47.63) | 0.29 ѱ |
| MetS (Yes) (%) | 279 (28.27) | 116 (20.57) | 163 (38.53) | <0.001 ѱ |
| Blood Parameters | ||||
| Glucose (mg/dL) | 97.84 ± 20.86 | 94.69 ± 16.43 | 102.04 ± 25.01 | <0.0001 |
| Cholesterol (mg/mL) | 193.26 ± 37.67 | 192.18 ± 37.78 | 194.71 ± 37.52 | 0.35 |
| HDL (mg/dL) | 50.95 ± 13.24 | 53.39 ± 13.34 | 47.71 ± 12.40 | <0.0001 |
| LDL (mg/dL) | 122.95 ± 33.05 | 122.17 ± 32.62 | 123.92 ± 33.61 | 0.31 |
| Triglycerides (mg/dL) | 98.94 ± 57.79 | 86.25 ± 47.72 | 115.81 ± 65.28 | <0.0001 |
| Insulin (U/L) | 8.10 ± 27.48 | 7.71 ± 35.99 | 8.63 ± 5.98 | <0.0001 |
| HOMA-IR | 2.12 ± 8.84 | 2.01 ± 11.56 | 2.26 ± 2.11 | <0.0001 |
| RBCs (M/mcL) | 4.87 ± 0.52 | 4.81 ± 0.53 | 4.95 ± 0.49 | <0.0001 |
| Hemoglobin (g/dL) | 14.06 ± 1.49 | 13.85 ± 1.51 | 14.35 ± 1.42 | <0.0001 |
| HCT (%) | 42.41 ± 3.38 | 41.96 ± 3.40 | 42.94 ± 3.29 | 0.0003 |
| MCV (fL) | 85.56 ± 6.81 | 85.75 ± 7.29 | 85.34 ± 6.19 | 0.04 |
| MCH (pg) | 28.92 ± 2.57 | 28.81 ± 2.72 | 29.05 ± 2.37 | 0.25 |
| MCHC (g/dL) | 33.79 ± 1.15 | 33.58 ± 1.13 | 34.03 ± 1.12 | <0.0001 |
| RDW-CV (%) | 13.63 ± 1.16 | 13.67 ± 1.24 | 13.60 ± 1.06 | 0.78 |
| Platelets (K/mcL) | 228.90 ± 59.83 | 230.44 ± 62.35 | 226.85 ± 56.32 | 0.39 |
| WBCs (K/mcL) | 5.98 ± 2.11 | 5.83 ± 2.19 | 6.17 ± 1.98 | 0.0001 |
| Neutrophils (%) | 57.05 ± 8.65 | 57.35 ± 8.72 | 56.69 ± 8.56 | 0.23 |
| Lymphocytes (%) | 32.20 ± 8.27 | 31.99 ± 8.39 | 32.45 ± 8.13 | 0.37 |
| Eosinophils (%) | 2.88 ± 1.82 | 2.88 ± 1.83 | 2.88 ± 1.80 | 0.96 |
| Monocytes (%) | 7.34 ± 1.84 | 7.21 ± 1.73 | 7.59 ± 1.95 | 0.07 |
| Basophils (%) | 0.52 ± 0.30 | 0.56 ± 0.34 | 0.48 ± 0.24 | 0.05 |
| Neutrophils (103/µL) | 3.44 ± 1.46 | 3.34 ± 1.17 | 3.57 ± 1.75 | 0.04 |
| Lymphocytes (103/µL) | 1.95 ± 1.71 | 1.90 ± 2.08 | 2.01 ± 1.12 | 0.0004 |
| Monocytes (103/µL) | 0.43 ± 0.17 | 0.41 ± 0.16 | 0.46 ± 0.17 | 0.0001 |
| Eosinophils (103/µL) | 0.17 ± 0.11 | 0.16 ± 0.11 | 0.17 ± 0.11 | 0.15 |
| Basophils (103/µL) | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.87 |
| HbA1c (mmol/mol) | 36.56 ± 7.43 | 35.10 ± 6.33 | 38.30 ± 8.23 | <0.0001 |
| Fractional total bilirubinemia (mg/dL) | 0.72 ± 0.37 | 0.71 ± 0.38 | 0.74 ± 0.37 | 0.32 |
| Direct fractional bilirubinemia (mg/dL) | 0.16 ± 0.05 | 0.16 ± 0.05 | 0.16 ± 0.05 | 0.24 |
| Indirect fractional bilirubinemia (mg/dL) | 0.50 ± 0.28 | 0.46 ± 0.24 | 0.55 ± 0.33 | 0.33 |
| GOT (U/L) | 22.90 ± 20.08 | 23.14 ± 25.50 | 22.58 ± 8.70 | 0.01 |
| SGPT (U/L) | 22.68 ± 18.08 | 21.61 ± 21.25 | 24.10 ± 12.56 | <0.0001 |
| GGT (U/I) | 19.71 ± 15.83 | 17.89 ± 13.20 | 22.14 ± 18.49 | <0.0001 |
| Albumin (%) | 4.15 ± 0.26 | 4.13 ± 0.26 | 4.17 ± 0.25 | 0.10 |
| Iron (mg/dL) | 90.02 ± 30.94 | 88.76 ± 30.97 | 91.52 ± 30.90 | 0.35 |
| Urea (mg/dL) | 40.02 ± 10.80 | 40.18 ± 11.87 | 39.80 ± 9.19 | 0.71 |
| Creatinine (mg/dL) | 0.82 ± 0.34 | 0.81 ± 0.42 | 0.83 ± 0.18 | 0.001 |
| eGFR (mL/min) | 84.97 ± 9.98 | 85.47 ± 10.41 | 84.39 ± 9.44 | 0.14 |
| AAT (mg/dL) | 184.50 ± 40.52 | 183.68 ± 39.58 | 185.48 ± 41.67 | 0.78 |
| Folate (ng/mL) | 8.46 ± 4.95 | 8.64 ± 4.97 | 8.23 ± 4.91 | 0.08 |
| Vitamin B12 (pg/mL) | 368.02 ± 513.64 | 385.44 ± 575.76 | 344.86 ± 416.57 | 0.64 |
| TSH (mUI/mL) | 959.08 ± 1395.93 | 961.07 ± 1501.29 | 956.44 ± 1245.06 | 0.89 |
| FT3 (pg/mL) | 3.32 ± 0.47 | 3.29 ± 0.45 | 3.36 ± 0.48 | 0.01 |
| FT4 (ng/mL) | 0.87 ± 0.32 | 0.86 ± 0.15 | 0.87 ± 0.45 | 0.52 |
| CRP (mg/L) | 0.23 ± 0.49 | 0.17 ± 0.26 | 0.30 ± 0.65 | <0.0001 |
| Food Groups * | Total Cohort | Steatosis | p ^ | |
|---|---|---|---|---|
| No | Yes | |||
| Dairy | 76.30 ± 30 | 70.63 ± 92.14 | 83.87 ± 114.95 | 0.06 |
| Low-Fat Dairy | 66.17 ± 98.33 | 65.28 ± 98.41 | 67.36 ± 98.32 | 0.39 |
| Eggs | 9.19 ± 8.12 | 9.39 ± 8.50 | 8.93 ± 7.59 | 0.37 |
| White Meat | 20.97 ± 26.32 | 20.56 ± 25.27 | 21.51 ± 27.68 | 0.79 |
| Red Meat | 24.06 ± 28.28 | 23.32 ± 31.80 | 25.05 ± 22.76 | 0.01 |
| Processed Meat | 4.64 ± 8.76 | 4.48 ± 9.96 | 4.84 ± 6.84 | 0.03 |
| Fish | 19.07 ± 21.56 | 18.89 ± 22.41 | 19.31 ± 20.41 | 0.22 |
| Seafood/Shellfish | 4.10 ± 6.25 | 3.98 ± 6.49 | 4.25 ± 5.94 | 0.08 |
| Leafy Vegetables | 44.36 ± 58.07 | 45.37 ± 62.00 | 43.01 ± 52.40 | 0.82 |
| Fruiting Vegetables | 70.43 ± 81.68 | 69.30 ± 83.64 | 71.93 ± 79.08 | 0.33 |
| Root Vegetables | 14.38 ± 25.75 | 15.37 ± 25.36 | 13.07 ± 26.23 | 0.01 |
| Other Vegetables | 63.19 ± 82.08 | 65.58 ± 87.62 | 60.02 ± 74.06 | 0.80 |
| Legumes | 26.08 ± 29.67 | 26.03 ± 31.32 | 26.15 ± 27.35 | 0.81 |
| Potatoes | 12.99 ± 16.71 | 13.04 ± 15.97 | 12.94 ± 17.65 | 0.59 |
| Fruits | 360.35 ± 443.83 | 353.10 ± 445.82 | 370.01 ± 441.49 | 0.32 |
| Nuts | 3.66 ± 6.41 | 3.93 ± 6.67 | 3.31 ± 6.04 | 0.04 |
| Grains | 118.61 ± 122.41 | 113.64 ± 120.94 | 125.23 ± 124.17 | 0.09 |
| Olives and Vegetable Oil | 32.74 ± 32.04 | 31.40 ± 28.02 | 34.53 ± 36.68 | 0.18 |
| Sweets | 19.91 ± 39.50 | 19.79 ± 41.09 | 20.07 ± 37.33 | 0.49 |
| Sugars | 13.81 ± 19.97 | 14.16 ± 20.28 | 13.35 ± 19.56 | 0.71 |
| Juices | 8.89 ± 21.56 | 8.58 ± 19.40 | 9.32 ± 24.15 | 0.35 |
| High-Calorie Drinks | 9.86 ± 30.12 | 8.84 ± 25.51 | 11.22 ± 35.32 | 0.16 |
| Ready-to-Eat Dishes | 36.22 ± 45.55 | 35.10 ± 50.46 | 37.72 ± 38.04 | 0.01 |
| Coffee | 45.79 ± 41.65 | 43.98 ± 40.15 | 48.22 ± 43.50 | 0.15 |
| Wine | 107.32 ± 139.01 | 95.58 ± 122.60 | 122.97 ± 157.06 | 0.03 |
| Beer | 31.95 ± 84.09 | 22.10 ± 57.91 | 45.09 ± 108.38 | 0.03 |
| Spirits | 1.75 ± 4.93 | 1.48 ± 4.39 | 2.10 ± 5.55 | 0.22 |
| Water | 658.39 ± 269.12 | 667.34 ± 278.95 | 646.45 ± 255.27 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donghia, R.; Pesole, P.L.; Coletta, S.; Bonfiglio, C.; De Pergola, G.; De Nucci, S.; Rinaldi, R.; Giannelli, G. Food Network Analysis in Non-Obese Patients with or without Steatosis. Nutrients 2023, 15, 2713. https://doi.org/10.3390/nu15122713
Donghia R, Pesole PL, Coletta S, Bonfiglio C, De Pergola G, De Nucci S, Rinaldi R, Giannelli G. Food Network Analysis in Non-Obese Patients with or without Steatosis. Nutrients. 2023; 15(12):2713. https://doi.org/10.3390/nu15122713
Chicago/Turabian StyleDonghia, Rossella, Pasqua Letizia Pesole, Sergio Coletta, Caterina Bonfiglio, Giovanni De Pergola, Sara De Nucci, Roberta Rinaldi, and Gianluigi Giannelli. 2023. "Food Network Analysis in Non-Obese Patients with or without Steatosis" Nutrients 15, no. 12: 2713. https://doi.org/10.3390/nu15122713
APA StyleDonghia, R., Pesole, P. L., Coletta, S., Bonfiglio, C., De Pergola, G., De Nucci, S., Rinaldi, R., & Giannelli, G. (2023). Food Network Analysis in Non-Obese Patients with or without Steatosis. Nutrients, 15(12), 2713. https://doi.org/10.3390/nu15122713







