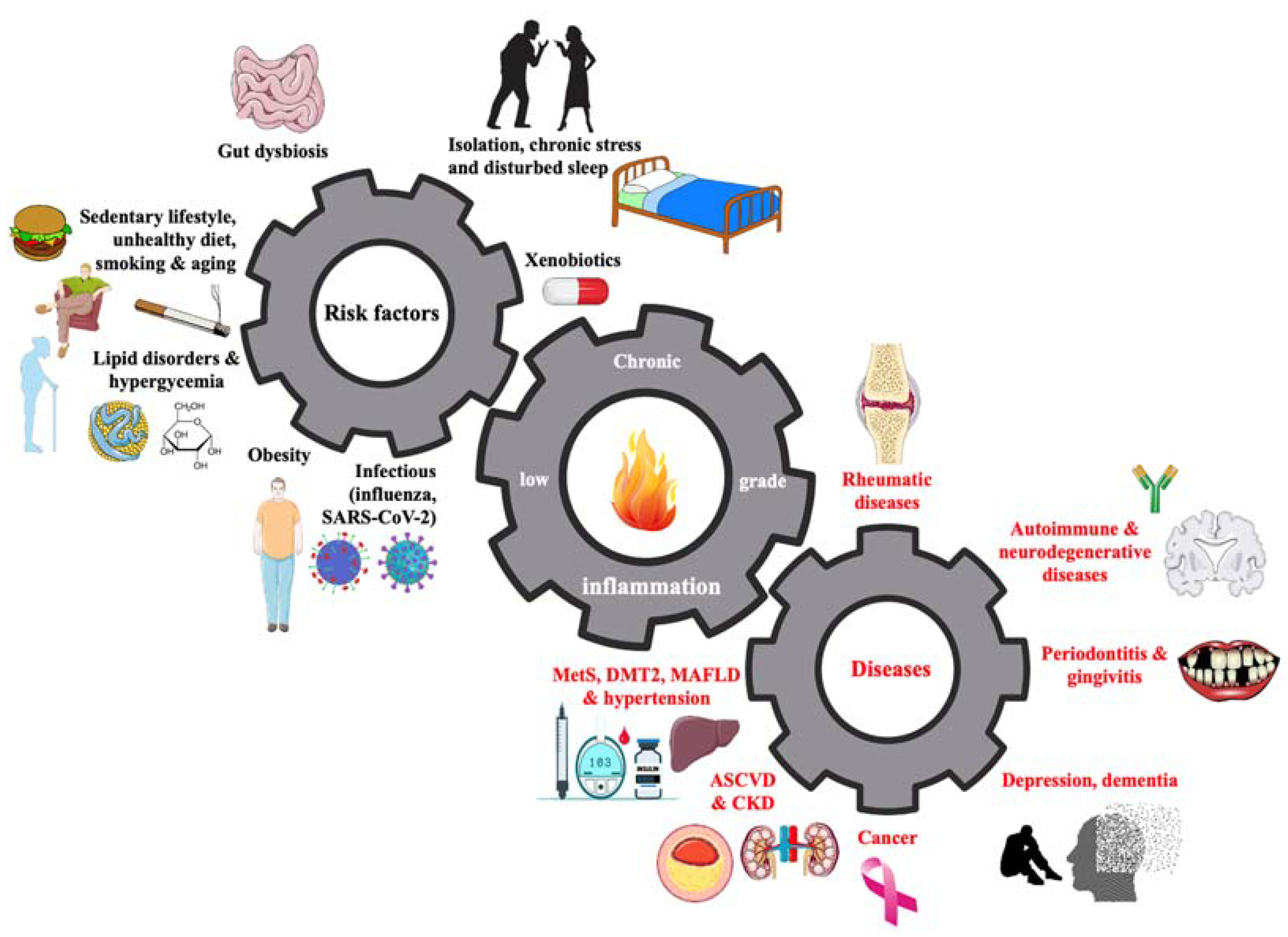
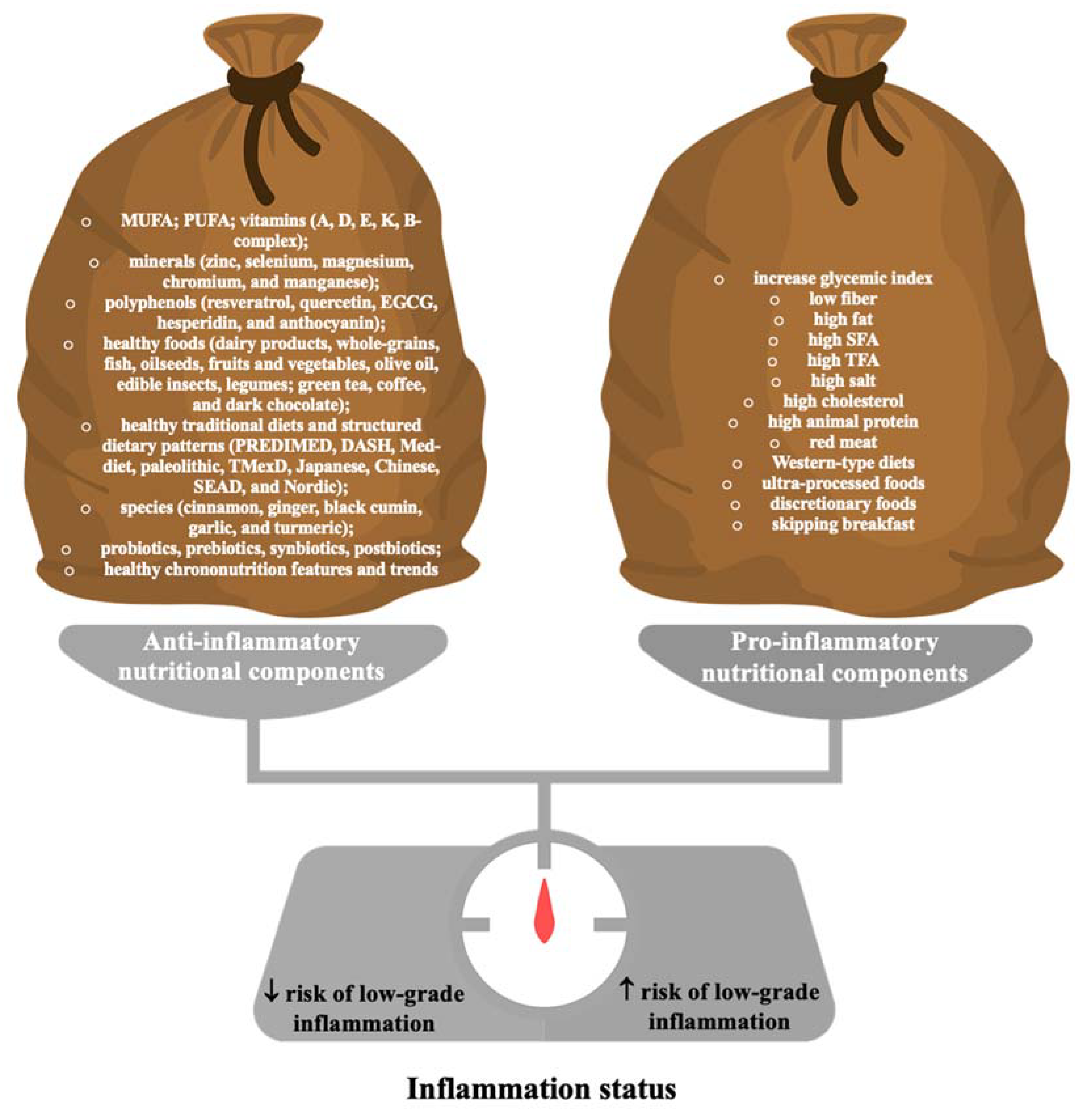
Nutrition, Nutraceuticals and Bioactive Compounds in the Prevention and Fight against Inflammation
Author Contributions
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Martinez-Urbistondo, D.; Vargas-Nuñez, J.A.; Martinez, J.A. The role of nutrition on meta-inflammation: Insights and potential targets in communicable and chronic disease management. Curr. Obes. Rep. 2022, 11, 305–335. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Chapter Two—Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Dzibla, T., Butterfield, A., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 35–58. [Google Scholar]
- Cantero, I.; Abete, I.; Bullón-Vela, V.; Crujeiras, A.B.; Casanueva, F.F.; Zulet, M.A.; Martinez, J.A. Fibroblast growth factor 21 levels and liver inflammatory biomarkers in obese subjects after weight loss. Arch. Med. Sci. 2022, 18, 36–44. [Google Scholar]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, aging, and cardiovascular disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Leontsinis, I.; Papademetriou, V.; Chrysohoou, C.; Kariori, M.; Dalakouras, I.; Tolis, P.; Fragoulis, C.; Kalos, T.; Tatakis, F.P.; Dimitriadis, K.; et al. Hypertensive urgencies during the first wave of the COVID-19 pandemic in a tertiary hospital setting: A U-shaped alarming curve. Arch. Med. Sci. 2022, 18, 982–990. [Google Scholar] [CrossRef]
- Lewek, J.; Jatczak-Pawlik, I.; Maciejewski, M.; Jankowski, P.; Banach, M. COVID-19 and cardiovascular complications—Preliminary results of the LATE-COVID study. Arch. Med. Sci. 2021, 17, 818–822. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar]
- Ni, P.; Yu, M.; Zhang, R.; Cheng, C.; He, M.; Wang, H.; Chen, S.; Duan, G. Dose-response association between C-reactive protein and risk of all-cause and cause-specific mortality: A systematic review and meta-analysis of cohort studies. Ann. Epidemiol. 2020, 51, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Bajraktari, G.; Penson, P.E.; Henein, M.Y.; Banach, M. Efficacy and safety of colchicine in patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2022, 88, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Penson, P.E.; Farnier, M.; Fras, Z.; Latkovskis, G.; Laufs, U.; Paneni, F.; Parini, P.; Pirro, M.; Reiner, Ž.; et al. Bempedoic acid in the management of lipid disorders and cardiovascular risk. 2023 position paper of the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2023, 77. [Google Scholar] [CrossRef]
- Maierean, S.; Webb, R.; Banach, M.; Mazidi, M. The role of inflammation and the possibilities of inflammation reduction to prevent cardiovascular events. Eur. Heart J. Open 2022, 2, oeac039. [Google Scholar] [CrossRef]
- Banach, M.; Burchardt, P.; Chlebus, K.; Dobrowolski, P.; Dudek, D.; Dyrbuś, K.; Gąsior, M.; Jankowski, P.; Jóźwiak, J.; Kłosiewicz-Latoszek, L.; et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch. Med. Sci. 2021, 17, 1447–1547. [Google Scholar] [CrossRef]
- Banach, M.; Surma, S. A look to the past—What has had the biggest impact on lipids in the last four decades? A personal perspective. Arch. Med. Sci. 2023, 19, 559–564. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Tian, W.; Wang, T.; Jia, J.; Lai, R.; Wang, T.; Zhang, Z.; Song, L.; Ju, J.; et al. The effect of various types and doses of statins on C-reactive protein levels in patients with dyslipidemia or coronary heart disease: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 2022, 9, 936817. [Google Scholar] [CrossRef]
- Kandelouei, T.; Abbasifard, M.; Imani, D.; Aslani, S.; Razi, B.; Fasihi, M.; Shafiekhani, S.; Mohammadi, K.; Jamialahmadi, T.; Reiner, Ž.; et al. effect of statins on serum level of hs-CRP and CRP in patients with cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Mediators Inflamm. 2022, 2022, 8732360. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. Pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 (PROVE IT-TIMI 22) investigators. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. How common is residual inflammatory risk? Circ. Res. 2017, 120, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.P.; Lins, L.C.; Fonseca, F.A.; Matos, L.N.; Aguirre, A.C.; Bianco, H.T.; Amaral, J.B.; França, C.N.; Santana, J.M.; Izar, M.C. Effects of ezetimibe on markers of synthesis and absorption of cholesterol in high-risk patients with elevated C-reactive protein. Life Sci. 2013, 92, 845–851. [Google Scholar] [CrossRef]
- Awad, K.; Mikhailidis, D.P.; Katsiki, N.; Muntner, P.; Banach, M. Effect of ezetimibe monotherapy on plasma lipoprotein(a) concentrations in patients with primary hypercholesterolemia: A systematic review and meta-analysis of randomized controlled trials. Drugs 2018, 78, 453–462. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Houri, J.; Notarbartolo, A.; Melani, L.; Lipka, L.J.; Suresh, R.; Sun, S.; LeBeaut, A.P.; Sager, P.T.; Veltri, E.P. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: A prospective, randomized, double-blind trial. Circulation 2003, 107, 2409–2415. [Google Scholar] [CrossRef]
- Sahebkar, A.; Di Giosia, P.; Stamerra, C.A.; Grassi, D.; Pedone, C.; Ferretti, G.; Bacchetti, T.; Ferri, C.; Giorgini, P. Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: A meta-analysis of 16 randomized controlled treatment arms. Br. J. Clin. Pharmacol. 2016, 81, 1175–1190. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol. 2020, 5, 1124–1135. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Hernandez, A.V.; Banach, M. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003121. [Google Scholar] [CrossRef]
- Penson, P.E.; Long, D.L.; Howard, G.; Toth, P.P.; Muntner, P.; Howard, V.J.; Safford, M.M.; Jones, S.R.; Martin, S.S.; Mazidi, M.; et al. Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C-reactive protein, and health outcomes in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study. Eur. Heart J. 2018, 39, 3641–3653. [Google Scholar] [CrossRef]
- Berkley, A.; Ferro, A. Changes in C-reactive protein in response to anti-inflammatory therapy as a predictor of cardiovascular outcomes: A systematic review and meta-analysis. JRSM Cardiovasc. Dis. 2020, 9, 2048004020929235. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic inflammation in the context of everyday life: Dietary changes as mitigating factors. Int. J. Environ. Res. Public. Health 2020, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Penson, P.E.; Ferri, N.; Sirtori, C.R.; Pirro, M.; Mancini, G.B.J.; Sattar, N.; Toth, P.P.; Sahebkar, A.; Lavie, C.J.; et al. Impact of nutraceuticals on markers of systemic inflammation: Potential relevance to cardiovascular diseases—A position paper from the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2021, 67, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, H.; Bazgir, B.; Shamsoddini, A.; Saeidi, A.; Tayebi, S.M.; Escobar, K.A.; Laher, I.; VanDusseldorp, T.A.; Weiss, K.; Knechtle, B.; et al. Oregano (Origanum vulgare) consumption reduces oxidative stress and markers of muscle damage after combat readiness tests in soldiers. Nutrients 2022, 15, 137. [Google Scholar] [CrossRef]
- Gunes-Bayir, A.; Guler, E.M.; Bilgin, M.G.; Ergun, I.S.; Kocyigit, A.; Dadak, A. Anti-inflammatory and antioxidant effects of carvacrol on N-Methyl-N’-Nitro-N-Nitrosoguanidine (MNNG) induced gastric carcinogenesis in Wistar rats. Nutrients 2022, 14, 2848. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, O.; Delli, E.; Vasila, M.E.; Loukas, T.; Magkoutis, A.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. The acute effect of a novel miso-type sauce, enhanced with a carotenoid-rich extract from fruit by-products, on postprandial biomarkers of oxidative stress and inflammation. Nutrients 2022, 14, 1316. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Atkin, S.L.; Jamialahmadi, T.; Banach, M.; Sahebkar, A. Effect of curcumin on attenuation of liver cirrhosis via genes/proteins and pathways: A system pharmacology study. Nutrients 2022, 14, 4344. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Butler, A.E.; Majeed, M.; Banach, M.; Sahebkar, A. Investigation of the effect of curcumin on protein targets in NAFLD using bioinformatic analysis. Nutrients 2022, 14, 1331. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Atkin, S.L.; Nikiforov, N.G.; Sahebkar, A. Therapeutic role of curcumin in diabetes: An analysis based on bioinformatic findings. Nutrients 2022, 14, 3244. [Google Scholar] [CrossRef]
- Koperska, A.; Wesołek, A.; Moszak, M.; Szulińska, M. Berberine in non-alcoholic fatty liver disease-a review. Nutrients 2022, 14, 3459. [Google Scholar] [CrossRef]
- Butler, A.E.; Obaid, J.; Wasif, P.; Varghese, J.V.; Abdulrahman, R.; Alromaihi, D.; Atkin, S.L.; Alamuddin, N. Effect of date fruit consumption on the glycemic control of patients with type 2 diabetes: A randomized clinical trial. Nutrients 2022, 14, 3491. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Inflammatory markers in non-obese women with polycystic ovary syndrome are not elevated and show no correlation with vitamin D metabolites. Nutrients 2022, 14, 3540. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Woo, J.K.; Heo, W.; Huang, W.Y.; Kim, Y.; Chung, S.; Lee, G.H.; Park, J.W.; Han, B.K.; Shin, E.C.; et al. Citrus junos Tanaka peel extract and its bioactive naringin reduce fine dust-induced respiratory injury markers in BALB/c male mice. Nutrients 2022, 14, 1101. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting cardiovascular diseases by flavonols: An update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Yan, W. Treatment effects of natural products on inflammatory bowel disease in vivo and their mechanisms: Based on animal experiments. Nutrients 2023, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Sahebkar, A.; Urbański, J.; Penson, P.E.; Banach, M. Curcumin—The nutraceutical with pleiotropic effects? Which cardiometabolic subjects might benefit the most? Front. Nutr. 2022, 9, 865497. [Google Scholar] [CrossRef]
- Adamczak, M.; Surma, S.; Więcek, A. Vitamin D and arterial hypertension: Facts and myths. Curr. Hypertens. Rep. 2020, 22, 57. [Google Scholar] [CrossRef]
- Nishizawa, M.; Hara, T.; Miura, T.; Fujita, S.; Yoshigai, E.; Ue, H.; Hayashi, Y.; Kwon, A.H.; Okumura, T.; Isaka, T. Supplementation with a flavanol-rich lychee fruit extract influences the inflammatory status of young athletes. Phytother. Res. 2011, 25, 1486–1493. [Google Scholar] [CrossRef]
- Surma, S.; Sahebkar, A.; Banach, M. Coffee or tea: Anti-inflammatory properties in the context of atherosclerotic cardiovascular disease prevention. Pharmacol. Res. 2023, 187, 106596. [Google Scholar] [CrossRef]
- Surma, S.; Kokot, F. Influence of chronic coffee consumption on the risk of kidney and other organ diseases. Review of the literature and clinical studies. Ren. Dis. Transplant. Forum 2022, 15, 1–18. [Google Scholar]
- Ingenbleek, Y. Revisiting PINI scoring in light of recent biological advances. Nutrients 2023, 15, 1846. [Google Scholar] [CrossRef] [PubMed]
| Nutraceuticals | Class of Evidence | Level of Evidence |
|---|---|---|
| Omega-3 fatty acids * | I | A |
| Red yeast rice | I | A |
| Flavonoids | IIa | B |
| Soy | IIa | A |
| Curcumin | IIa | B |
| Omega-6 fatty acids | IIb | B |
| Berberine | IIb | B |
| Garlic | IIb | B |
| Bergamot | III | C |
| Lupin | III | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surma, S.; Sahebkar, A.; Banach, M. Nutrition, Nutraceuticals and Bioactive Compounds in the Prevention and Fight against Inflammation. Nutrients 2023, 15, 2629. https://doi.org/10.3390/nu15112629
Surma S, Sahebkar A, Banach M. Nutrition, Nutraceuticals and Bioactive Compounds in the Prevention and Fight against Inflammation. Nutrients. 2023; 15(11):2629. https://doi.org/10.3390/nu15112629
Chicago/Turabian StyleSurma, Stanisław, Amirhossein Sahebkar, and Maciej Banach. 2023. "Nutrition, Nutraceuticals and Bioactive Compounds in the Prevention and Fight against Inflammation" Nutrients 15, no. 11: 2629. https://doi.org/10.3390/nu15112629
APA StyleSurma, S., Sahebkar, A., & Banach, M. (2023). Nutrition, Nutraceuticals and Bioactive Compounds in the Prevention and Fight against Inflammation. Nutrients, 15(11), 2629. https://doi.org/10.3390/nu15112629







