Can Dietary Patterns Impact Fertility Outcomes? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration
2.2. Selection Criteria
2.3. Outcomes
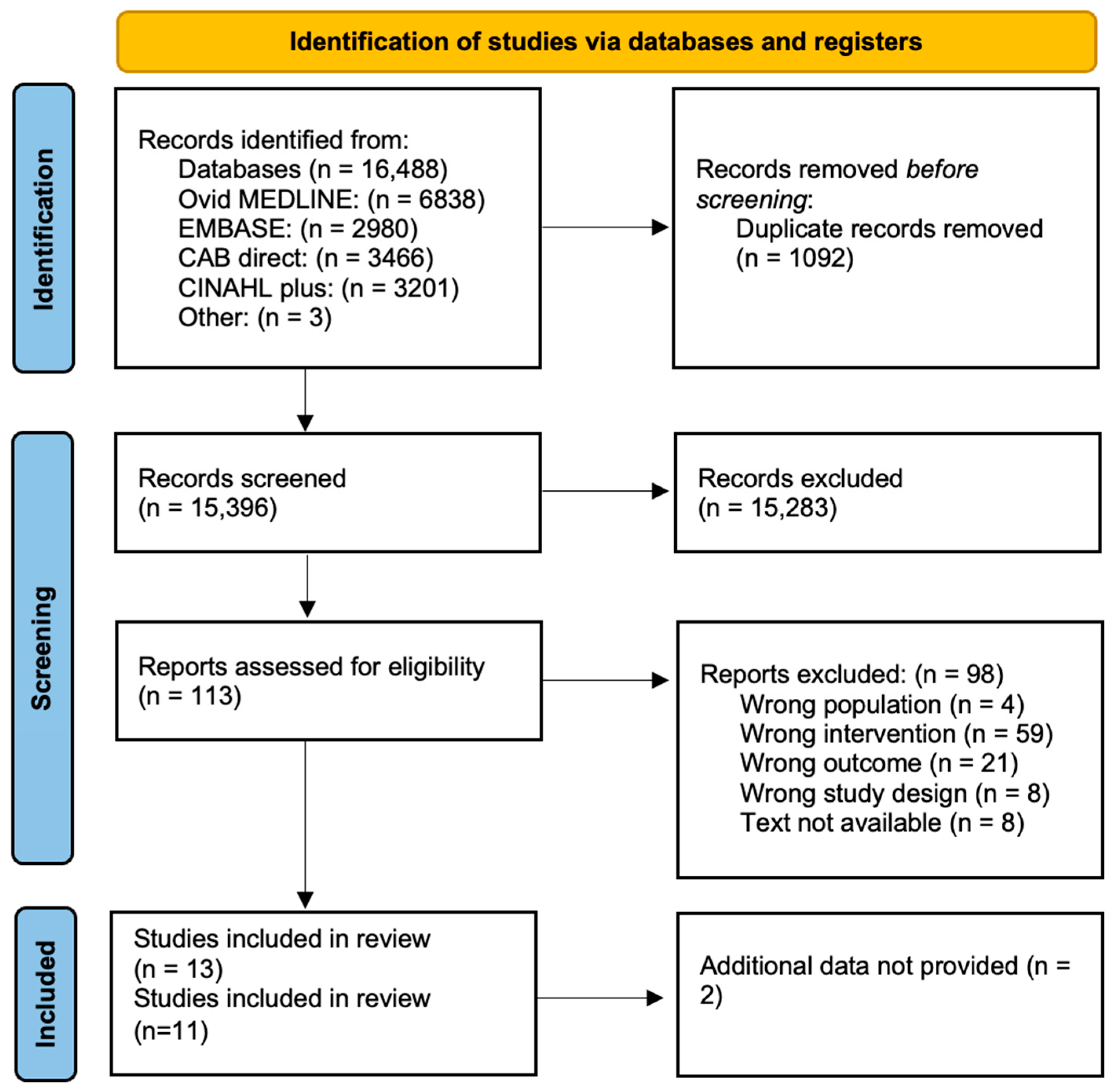
2.4. Search Strategy
2.5. Data Extraction and Quality Assessment
2.6. Classification of Dietary Intake
2.7. Statistical Analysis
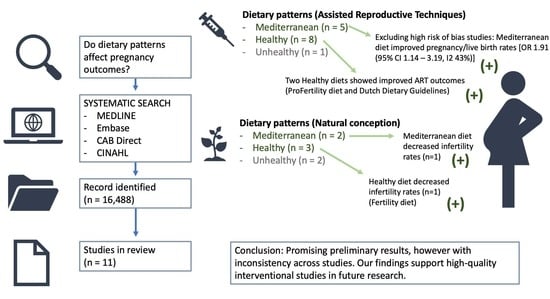
3. Results
3.1. Description of Studies
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Primary Outcomes
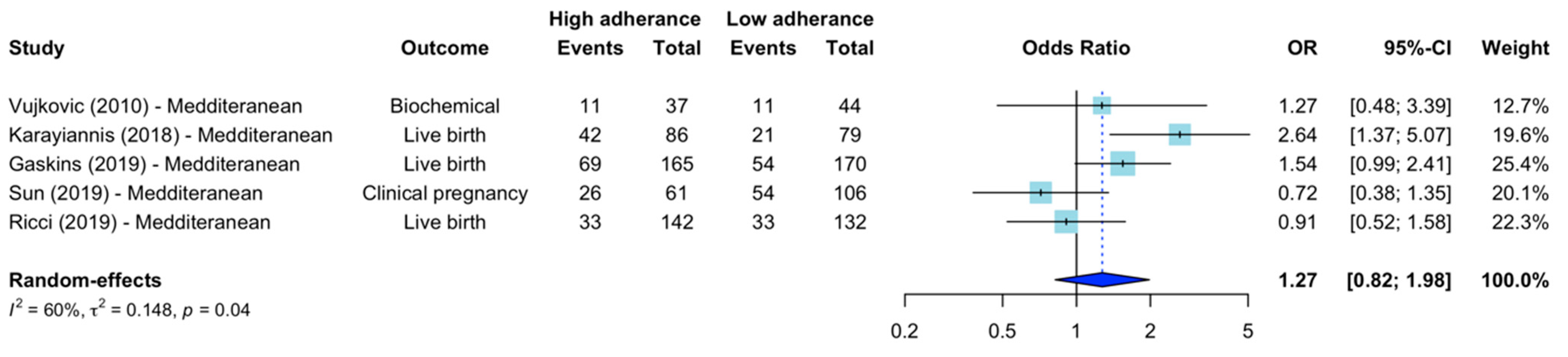
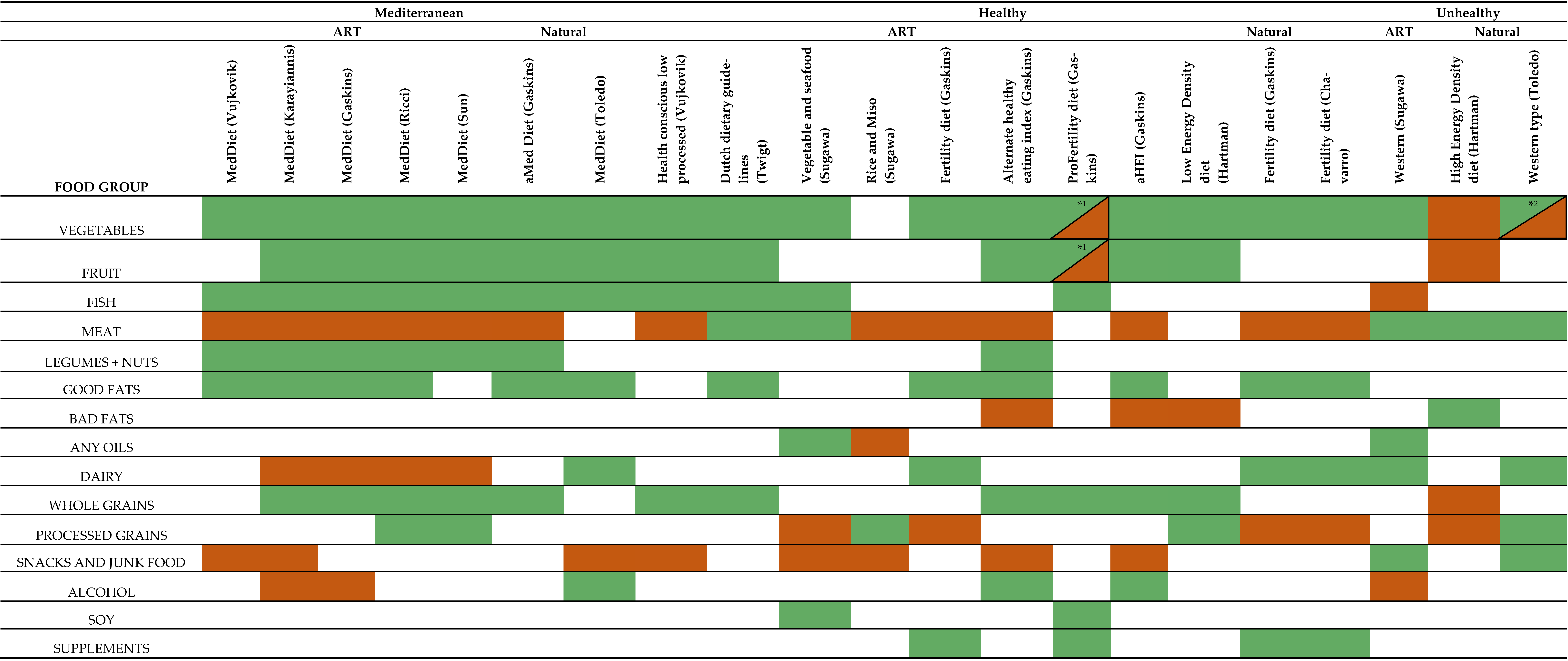
3.5. Mediterranean Diets
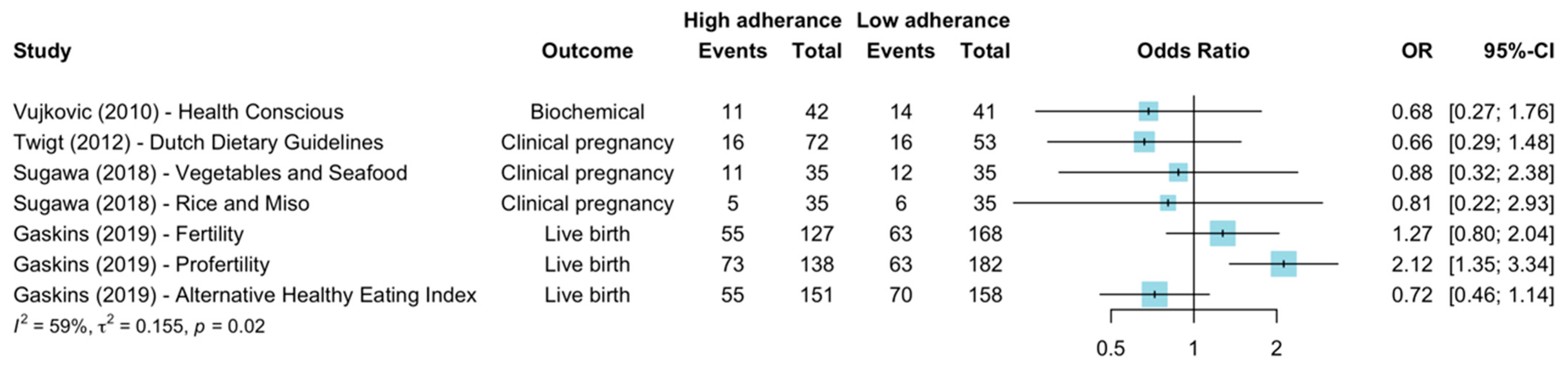
3.6. Healthy Diets
3.7. Unhealthy Diets
3.8. Secondary Outcomes
3.9. Publication Bias
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
4.3. Clinical and Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009*. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Factsheet: Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 3 August 2021).
- Waldby, C. The Business of IVF: How Human Eggs Went from Simple Cells to a Valuable Commodity; The Conversation: Melbourne, Australia, 2019. [Google Scholar]
- Wang, Y.A.; Chambers, G.M.; Sullivan, E.A. Assisted Reproductive Technology in Australia and New Zealand 2008; Australian Institute of Health and Welfare: Canberra, Australia, 2010.
- Newman, J.; Paul, R.; Chambers, G. Assisted Reproductive Technology in Australia and New Zealand 2018; University of New South Wales: Sydney, Australia, 2020. [Google Scholar]
- Chiware, T.M.; Vermeulen, N.; Blondeel, K.; Farquharson, R.; Kiarie, J.; Lundin, K.; Matsaseng, T.C.; Ombelet, W.; Toskin, I. IVF and other ART in low- and middle-income countries: A systematic landscape analysis. Hum. Reprod. Update 2021, 27, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C. Towards the global coverage of a unified registry of IVF outcomes. Reprod. Biomed. Online 2019, 38, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Fertility Problems: Assessment and Treatment. Available online: https://www.nice.org.uk/guidance/cg156/chapter/Recommendations#prediction-of-ivf-success (accessed on 22 December 2021).
- Rao, M.; Zeng, Z.; Tang, L. Maternal physical activity before IVF/ICSI cycles improves clinical pregnancy rate and live birth rate: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 11. [Google Scholar] [CrossRef]
- Teede, H.; Misso, M.; Costello, M.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. 2018. Available online: https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence-Based-Guidelines_20181009.pdf (accessed on 3 August 2021).
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Key, T.J.; Allen, N.E.; Spencer, E.A.; Travis, R.C. The effect of diet on risk of cancer. Lancet 2002, 360, 861–868. [Google Scholar] [CrossRef]
- Agnoli, C.; Pounis, G.; Krogh, V. Chapter 4—Dietary Pattern Analysis. In Analysis in Nutrition Research; Pounis, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 75–101. [Google Scholar]
- Vujkovic, M.; de Vries, J.H.; Lindemans, J.; Macklon, N.S.; van der Spek, P.J.; Steegers, E.A.; Steegers-Theunissen, R.P. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil. Steril. 2010, 94, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- Kellow, N.J.; Le Cerf, J.; Horta, F.; Dordevic, A.L.; Bennett, C.J. The Effect of Dietary Patterns on Clinical Pregnancy and Live Birth Outcomes in Men and Women Receiving Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 857–874. [Google Scholar] [CrossRef]
- Huang, J.; Xie, L.; Lin, J.; Lu, X.; Song, N.; Cai, R.; Kuang, Y. Adherence to healthy dietary patterns and outcomes of assisted reproduction: A systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2020, 72, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Alesi, S. Effects of Preconception Diet on Reproduction: A Systematic Scoping Review Protocol; Open Sciences Framework: Charlottesville, VA, USA, 2021. [Google Scholar]
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E. Dietary patterns and outcomes of assisted reproduction. Am. J. Obstet. Gynecol. 2019, 220, e561–e567.e518. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.M.N.; AlAhwany, H.; Bhattacharya, S.; Collura, B.; Curtis, C.; Evers, J.L.H.; Farquharson, R.G.; Franik, S.; Giudice, L.C.; Khalaf, Y.; et al. Developing a core outcome set for future infertility research: An international consensus development study. Hum. Reprod. 2020, 35, 2725–2734. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, J.J.; Nabu, S.; Yeung, Q.S.; Li, Y.; Tan, J.H.; Suksalak, W.; Chanchamroen, S.; Quangkananurug, W.; Wong, P.S.; et al. The Pregnancy Outcome of Mosaic Embryo Transfer: A Prospective Multicenter Study and Meta-Analysis. Genes 2020, 11, 973. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R; 2022.7.1.554; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Twigt, J.M.; Bolhuis, M.E.; Steegers, E.A.; Hammiche, F.; van Inzen, W.G.; Laven, J.S.; Steegers-Theunissen, R.P. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum. Reprod. 2012, 27, 2526–2531. [Google Scholar] [CrossRef]
- Gaskins, A.J.; (Harvard T.H. Chan School of Public Health, Boston, MA, USA). Personal communication, 2021. Additional data: Crude Pregnancy Outcomes for High and Low Adherence Groups.
- Steegers-Theunissen, R.P.; (University Medical Centre Rotterdam, Rotterdam, The Netherlands); Twigt, J.M.; (University Medical Centre Rotterdam, Rotterdam, The Netherlands). Personal communication, 2021. Additional Information: Crude and Adjusted Pregnancy Outcomes for High and Low Adherance Groups.
- Jahangirifar, M.; Taebi, M.; Nasr-Esfahani, M.H.; Askari, G.H. Dietary Patterns and The Outcomes of Assisted Reproductive Techniques in Women with Primary Infertility: A Prospective Cohort Study. Int. J. Fertil. Steril. 2019, 12, 316–323. [Google Scholar] [CrossRef]
- Diba-Bagtash, F.; Shahnazi, M.; Ghasemzadeh, A.; Jahanjoo, F.; Dolatkhah, N.; Farshbaf-Khalili, A. Association between dietary inflammatory index and inflammatory biomarkers with outcomes of in vitro fertilization treatment. J. Obstet. Gynaecol. Res. 2021, 47, 287–295. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef]
- Sugawa, M.; Okubo, H.; Sasaki, S.; Nakagawa, Y.; Kobayashi, T.; Kato, K. Lack of a meaningful association between dietary patterns and in vitro fertilization outcome among Japanese women. Reprod. Med. Biol. 2018, 17, 466–473. [Google Scholar] [CrossRef]
- Sun, H.; Lin, Y.; Lin, D.; Zou, C.; Zou, X.; Fu, L.; Meng, F.; Qian, W. Mediterranean diet improves embryo yield in IVF: A prospective cohort study. Reprod. Biol. Endocrinol. 2019, 17, 73. [Google Scholar] [CrossRef]
- Ricci, E.; Bravi, F.; Noli, S.; Somigliana, E.; Cipriani, S.; Castiglioni, M.; Chiaffarino, F.; Vignali, M.; Gallotti, B.; Parazzini, F. Mediterranean diet and outcomes of assisted reproduction: An Italian cohort study. Am. J. Obstet. Gynecol. 2019, 221, e621–e627.e614. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Penzias, A.; Missmer, S.A.; Chavarro, J.E. Prepregnancy dietary patterns and risk of pregnancy loss. Am. J. Clin. Nutr. 2014, 100, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.J.; Fung, J.L.; Hsiao, P.Y.; Fan, W.; Mitchell, D.C.; Goldman, M.B. Dietary Energy Density and Fertility: Results from the Lifestyle and Fertility Study. Curr. Dev. Nutr. 2021, 5, nzab075. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Lopez-del Burgo, C.; Ruiz-Zambrana, A.; Donazar, M.; Navarro-Blasco, I.; Martínez-González, M.A.; de Irala, J. Dietary patterns and difficulty conceiving: A nested case-control study. Fertil. Steril. 2011, 96, 1149–1153. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 12 June 2021).
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Stocking, K. Study design flaws and statistical challenges in evaluating fertility treatments. Reprod. Fertil. 2021, 2, C9–C21. [Google Scholar] [CrossRef] [PubMed]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chiu, Y.-H.; Williams, P.L.; Keller, M.G.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil. Steril. 2016, 105, 1503–1510.e1504. [Google Scholar] [CrossRef]
- Nassan, F.L.; Chiu, Y.-H.; Vanegas, J.C.; Gaskins, A.J.; Williams, P.L.; Ford, J.B.; Attaman, J.; Hauser, R.; Chavarro, J.E.; Team, E.S. Intake of protein-rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am. J. Clin. Nutr. 2018, 108, 1104–1112. [Google Scholar] [CrossRef]
- Vanegas, J.C.; Afeiche, M.C.; Gaskins, A.J.; Mínguez-Alarcón, L.; Williams, P.L.; Wright, D.L.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil. Steril. 2015, 103, 749–755.e742. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2017, 7, Cd007807. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Frolova, A.I.; Jiang, H.; Riley, J.K. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J. Clin. Endocrinol. Metab. 2013, 98, E1364–E1368. [Google Scholar] [CrossRef] [PubMed]
- Hammiche, F.; Vujkovic, M.; Wijburg, W.; de Vries, J.H.; Macklon, N.S.; Laven, J.S.; Steegers-Theunissen, R.P. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil. Steril. 2011, 95, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef]
- Unfer, V.; Casini, M.L.; Gerli, S.; Costabile, L.; Mignosa, M.; Di Renzo, G.C. Phytoestrogens may improve the pregnancy rate in in vitro fertilization-embryo transfer cycles: A prospective, controlled, randomized trial. Fertil. Steril. 2004, 82, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Sanderman, E.A.; Willis, S.K.; Wise, L.A. Female dietary patterns and outcomes of in vitro fertilization (IVF): A systematic literature review. Nutr. J. 2022, 21, 5. [Google Scholar] [CrossRef]
- Kermack, A.J.; Calder, P.C.; Houghton, F.D.; Godfrey, K.M.; Macklon, N.S. A randomised controlled trial of a preconceptional dietary intervention in women undergoing IVF treatment (PREPARE trial). BMC Women’s Health 2014, 14, 130. [Google Scholar] [CrossRef]
- Alibeigi, Z.; Jafari-Dehkordi, E.; Kheiri, S.; Nemati, M.; Mohammadi-Farsani, G.; Tansaz, M. The Impact of Traditional Medicine-Based Lifestyle and Diet on Infertility Treatment in Women Undergoing Assisted Reproduction: A Randomized Controlled Trial. Complement. Med. Res. 2020, 27, 230–241. [Google Scholar] [CrossRef]
- Kermack, A.J.; Lowen, P.; Wellstead, S.J.; Fisk, H.L.; Montag, M.; Cheong, Y.; Osmond, C.; Houghton, F.D.; Calder, P.C.; Macklon, N.S. Effect of a 6-week “Mediterranean” dietary intervention on in vitro human embryo development: The Preconception Dietary Supplements in Assisted Reproduction double-blinded randomized controlled trial. Fertil. Steril. 2020, 113, 260–269. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Casas, R.; Martín-Asuero, A.; Borràs, R.; Vieta, E.; Estruch, R.; Gratacós, E.; Investigators, I.B.T. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to At-Risk Pregnant Individuals: The IMPACT BCN Randomized Clinical Trial. JAMA 2021, 326, 2150–2160. [Google Scholar] [CrossRef]
- Firth, J.; Gangwisch, J.E.; Borsini, A.; Wootton, R.E.; Mayer, E.A. Food and mood: How do diet and nutrition affect mental wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]

| Author | Country | Study Design | Population | Diet Assessment Method | Diets Identified | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|
| Vujkovic et al., 2010 [14] | The Netherlands | Cohort study | 161 couples ART | Self-reported FFQ + baseline questionnaire Validated Posteriori | Health conscious–low processed Mediterranean | Biochemical pregnancy | RBC folate, folate, vitamin B6, vitamin B12, tHcy |
| Twigt et al., 2012 [35] | The Netherlands | Cohort study | 199 women ART | Self-reported FFQ + baseline questionnaire, professionally checked a priori | Dutch Dietary Guidelines | Clinical pregnancy | - |
| Karayiannis et al., 2018 [40] | Greece | Cohort study | 244 women ART | Self-reported FFQ + baseline questionnaire Validated a priori | Mediterranean | Biochemical pregnancy Clinical pregnancy Live birth | Total oocyte, mature oocyte M2, Fertilized embryo, day 3 FSH, day 3 LH, day 3 oestradiol, baseline AMH, fertilization rate, embryos produced, high quality embryos, number of embryos transferred |
| Sugawa et al., 2018 [41] | Japan | Cohort study | 140 women ART | Self-reported FFQ + baseline questionnaire Validated posteriori | Vegetables and Seafood Western Rice and Miso | Clinical pregnancy | Total oocyte yield, Veeck’s criteria |
| Gaskins et al., 2019 [21] | USA | Cohort study | 357 women ART | Self-reported FFQ + baseline questionnaire Validated a priori | Mediterranean diet Alterative Healthy Eating Index 2010 Fertility diet ProFertility diet | Biochemical pregnancy Clinical pregnancy Live birth | Oestradiol trigger levels, endometrial thickness, total oocyte yield, mature oocytes M2, fertilized embryos |
| Sun et al., 2019 [42] | China | Cohort study | 590 women 166 in final analysis ART | Self-reported FFQ + baseline questionnaire Not validated a priori | Mediterranean diet | Clinical pregnancy | Endometrial thickness, Gn duration, total oocyte yield, mature oocytes M2, fertilization rate, high quality embryos, number of embryos transferred, basal FSH, basal LH/oestradiol/progesterone, HCG, day 3 LH/oestradiol/progesterone, AFC, number embryos available, number of ET cycles. |
| Ricci et al., 2019 [43] | Italy | Cohort study | 414 women ART | Self-reported FFQ + baseline questionnaire Validated a priori | Mediterranean diet | Clinical pregnancy Live birth | FSH level, AMH level, good quality oocytes, good quality embryos, embryo transfer |
| Toledo et al., 2011 [46] | Spain | Nested case-control study | 485 cases 1669 controls Natural Conception | Self-reported FFQ + baseline questionnaire Validated posteriori | Mediterranean diet Western diet | Consulted a doctor for difficulty conceiving | - |
| Chavarro et al., 2007 [15] | USA | Cohort study | 17,544 women Natural Conception | Self-reported FFQ + baseline questionnaire Validated a priori | Fertility diet | Rate of female factor infertility/ovulatory disorder infertility | - |
| Gaskins et al., 2014 [44] | USA | Cohort study | 11,072 women 15,950 pregnancies Natural Conception | Self-reported FFQ + baseline questionnaire Validated a priori | Alternative Healthy Eating Index 2010 Alternative Mediterranean diet Fertility diet | Pregnancy loss (miscarriages and stillbirths) | - |
| Hartman et al., 2021 [45] | USA | Cohort study | 131 couples Natural Conception | Three separate telephone 24-h dietary recalls a priori | Low energy density diet | Clinical pregnancy | - |
| ROBINS-I Observational Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias Domains | |||||||||
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overrall | ||
| Study | Vujkovic (2010) [14] |  |  |  |  |  |  |  |  |
| Twigt (2012) [35] |  |  |  |  |  |  |  |  | |
| Karayiannis (2018) [40] |  |  |  |  |  |  |  |  | |
| Sugawa (2018) [41] |  |  |  |  |  |  |  |  | |
| Gaskins (2019) [21] |  |  |  |  |  |  |  |  | |
| Sun (2019) [42] |  |  |  |  |  |  |  |  | |
| Ricci (2019) [43] |  |  |  |  |  |  |  |  | |
| Chavarro (2007) [15] |  |  |  |  |  |  |  |  | |
| Toledo (2011) [46] |  |  |  |  |  |  |  |  | |
| Gaskins (2014) [44] |  |  |  |  |  |  |  |  | |
| Hartman (2021) [45] |  |  |  |  |  |  |  |  | |
 Serious.
Serious.  Moderate.
Moderate.  Low.
Low.| Author | Diet | Biochemical Pregnancy | Clinical Pregnancy | Live Births | Infertility Rates | Pregnancy Loss | Adjusted for Confounders | Confounders Controlled (Confounders Measured but Not Controlled) |
|---|---|---|---|---|---|---|---|---|
| Vujkovic et al., 2010 [14] | Mediterranean | OR 1.27 (95% CI 0.48–3.39) a | - | - | - | - | OR 1.4 (95% CI 1.0–1.9) (Biochemical pregnancy) (Couple adherence) a | Age, BMI, smoking, alcohol, IVF/ICSI treatment, stimulation scheme |
| Health conscious–low processed | OR 0.68 (95% CI 0.27–1.76) a | - | - | - | - | OR 0.8 (95% CI 0.6–1.0) (Biochemical pregnancy) (women adherence) a | ||
| Twigt et al., 2012 [35] | Dutch dietary guidelines | - | OR 0.66 (95% CI 0.29–1.48) b | - | - | - | OR 2.94 (95% CI 0.88–9.76) (Clinical pregnancy) b | Age, smoking, BMI, partner diet, treatment indication (Alcohol, exercise, stress, ethnicity, education) |
| Karayiannis et al., 2018 [40] | Mediterranean | OR 1.98 (95% CI 1.05–3.78) a | OR 2.43 (95% CI 1.28–4.63) a | OR 2.64 (95% CI 1.37–5.07) a | - | - | RR 3.12 (1.40–7.10) (Live births) a | Age, stimulation scheme, BMI, energy intake, treatment indication, anxiety, supplements |
| Sugawa et al., 2018 [41] | Vegetables and seafood | - | OR 0.88 (95%CI 0.32–2.38) a | - | - | - | OR 0.90 (95% CI 0.3–2.69) (Clinical pregnancy) a | Age, BMI, parity, education, smoking, alcohol, folate (Number of oocytes retrieved, Veeck’s criteria) |
| Rice and Miso | - | OR 0.81 (95%CI 0.22–2.93) a | - | - | - | OR 0.72 (95% CI0.18–2.93) (Clinical pregnancy) a | ||
| Western | - | OR 0.70 (95% CI 0.21–2.28) a | - | - | - | OR 0.84 (95% CI 0.23–3.11) (Clinical pregnancy) a | ||
| Gaskins et al., 2019 [21] | Mediterranean diet | RR 1.12 p = 0.17 a, c OR 1.26 d | RR 1.12 p = 0.25 a, c OR 1.24 d | RR 1.32 p = 0.06 a, c OR 1.55 (95% CI 0.99–2.42) b | - | - | OR 1.54 (95% CI 0.85–2.80) (Live births) b | Age, BMI, calorie intake, smoking, vigorous exercise |
| Alterative Healthy Eating Index 2010 | RR 0.87 p = 0.12 a, c OR 0.72 d | RR 0.82 p = 0.08 a, c OR 0.67 d | RR 0.84 p = 0.19 a, c OR 0.72 (95% CI 0.45–1.14) b | - | - | OR 0.72 (95% CI 0.40–1.30) (Live births) b | ||
| Fertility diet | RR 1.00 p = 0.83 a, c OR 1.00 d | RR 0.98 p = 0.89 a, c OR 0.96 d | RR 1.16 p = 0.37 a, c OR 1.27 (95% CI 0.79–2.05) b | - | - | OR 1.36 (95% CI 0.75–2.50) (Live births) b | ||
| ProFertility diet | RR 1.48 p =< 0.001 a, c OR 2.51 d | RR 1.53 p =< 0.001 a, c OR 2.37 d | RR 1.70 p =< 0.001 a, c OR 2.14 (95% CI 1.35–3.40) b | - | - | OR 2.56 (95% CI 1.42–4.63) (Live births) b | ||
| Sun et al., 2019 [42] | Mediterranean | - | OR 0.72 (95% CI 0.38–1.35) a | - | - | - | None provided | Age, BMI, duration of infertility (Infertility diagnosis, sperm concentration, total motile sperm basal FSH, Gn duration, dosage Gn) |
| Ricci et al., 2019 [43] | Mediterranean | - | - | OR 1.00 (95% CI 0.58–1.73) a | - | - | OR 0.91 (95% CI 1.63–0.54) (Live births) d | Age, BMI, previous ART, leisure physical activity, smoking, caloric intake, calorie restriction |
| Toledo et al., 2011 [46] | Mediterranean | - | - | - | OR 0.56 (95% CI 0.35–0.95) a | - | OR 0.74 (95% CI 0.55–1.00) (Infertility) a | Age, BMI, smoking, physical activity, alcohol, total energy intake, supplement use, intake of plant proteins, animal proteins, trans fat, fibre |
| Western | - | - | - | OR 0.83 (95% CI 0.64–1.08) a | - | OR 0.91 (95% CI 0.66–1.24) (Infertility) a | ||
| Chavarro et al., 2007 [15] | Fertility diet | - | - | - | - | - | RR 0.32 (95% CI 0.23–0.48) (Ovulatory infertility) RR 0.73 (95% CI 0.57–0.95) (All cause infertility) | Age, BMI, alcohol, coffee, smoking, physical activity, parity, COCP use |
| Gaskins et al., 2014 [44] | Alternative Mediterranean diet | - | - | - | - | RR 1.11 (95% CI 1.01–1.23) a | RR 1.02 (95% CI 0.98, 1.05) (Pregnancy loss) a | Age, BMI, total energy, smoking, physical activity, history of infertility, marital status, race, nulliparity |
| Alternative Healthy Eating Index 2010 | - | - | - | - | RR 1.23 (95% CI 1.13–1.36) a | RR 1.01 (95% CI 0.98–1.05) (Pregnancy loss) a | ||
| Fertility diet | - | - | - | - | RR 0.91 (95% CI 0.82–1.00) a | RR 0.98 (95% CI 95–1.01) (Pregnancy loss) a | ||
| Hartman et al., 2021 [45] | Low energy density diet | - | OR 1.44 (95% CI 0.62–3.36) a | OR 1.44 (95% CI 0.62–3.34) a | - | - | OR 2.56 (live birth) a, e | Race, partner diet, physical activity, BMI, total energy intake (Educational status, income, female smoking, total trans fats, total protein, alcohol) |
| High energy density diet | - | OR 0.69 (95% CI 0.30–1.61) a | OR 0.69 (95% CI 0.30–1.61) a | - | - | OR 0.39 (Live birth) a, e |
| Subgroup | Number of Studies | OR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | p | |||
| Primary Outcome | ||||
| Biochemical pregnancy | 1 | 1.27 (0.48–3.39) | - | - |
| Clinical pregnancy | 1 | 0.72 (0.38–1.35) | - | - |
| Live birth | 3 | 1.51 (0.88–2.62) | 67 | 0.05 |
| Dietary measurement method | ||||
| a priori | 4 | 1.27 (0.76–2.14) | 70 | 0.02 |
| posteriori | 1 | 1.27 (0.48–3.39) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, H.G.; Rolnik, D.L.; Mol, B.W.J.; Torkel, S.; Alesi, S.; Mousa, A.; Habibi, N.; Silva, T.R.; Oi Cheung, T.; Thien Tay, C.; et al. Can Dietary Patterns Impact Fertility Outcomes? A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2589. https://doi.org/10.3390/nu15112589
Winter HG, Rolnik DL, Mol BWJ, Torkel S, Alesi S, Mousa A, Habibi N, Silva TR, Oi Cheung T, Thien Tay C, et al. Can Dietary Patterns Impact Fertility Outcomes? A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(11):2589. https://doi.org/10.3390/nu15112589
Chicago/Turabian StyleWinter, Hugo G., Daniel L. Rolnik, Ben W. J. Mol, Sophia Torkel, Simon Alesi, Aya Mousa, Nahal Habibi, Thais R. Silva, Tin Oi Cheung, Chau Thien Tay, and et al. 2023. "Can Dietary Patterns Impact Fertility Outcomes? A Systematic Review and Meta-Analysis" Nutrients 15, no. 11: 2589. https://doi.org/10.3390/nu15112589
APA StyleWinter, H. G., Rolnik, D. L., Mol, B. W. J., Torkel, S., Alesi, S., Mousa, A., Habibi, N., Silva, T. R., Oi Cheung, T., Thien Tay, C., Quinteros, A., Grieger, J. A., & Moran, L. J. (2023). Can Dietary Patterns Impact Fertility Outcomes? A Systematic Review and Meta-Analysis. Nutrients, 15(11), 2589. https://doi.org/10.3390/nu15112589