Assessment of the Efficacy of a Low-Dose Iron Supplement in Restoring Iron Levels to Normal Range among Healthy Premenopausal Women with Iron Deficiency without Anemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
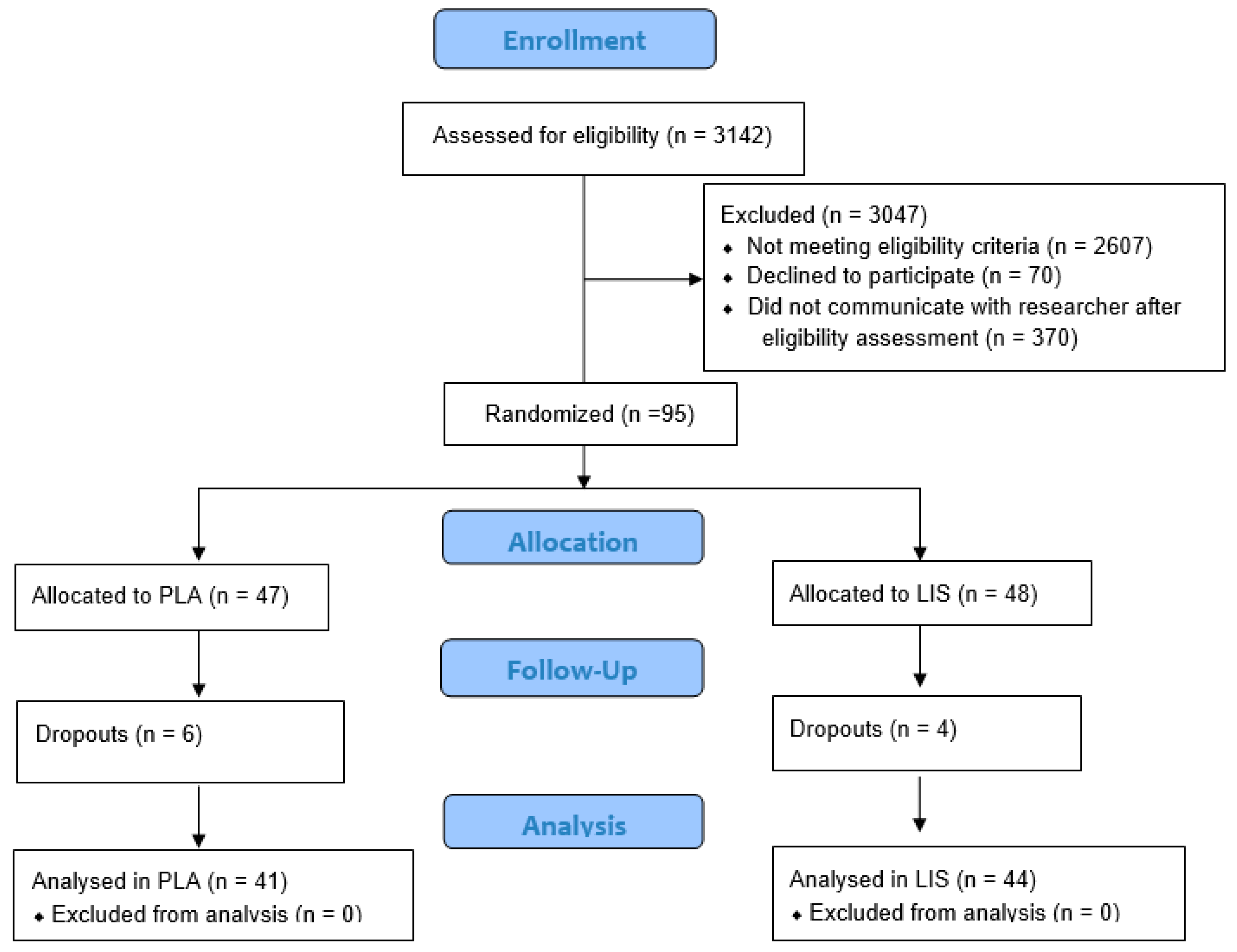
2.2. Participants
2.3. Supplement Protocol, Blinding, and Compliance
2.4. Blood Sampling
2.5. Gastrointestinal Symptom Rating Scale (GSRS)
2.6. Patient Assessment of Constipation-Symptoms (PAC-SYM)
2.7. Abbreviated Profile of Mood States (POMS)
2.8. Adverse Events
2.9. Statistical Analysis
3. Results
3.1. Hematological Outcomes
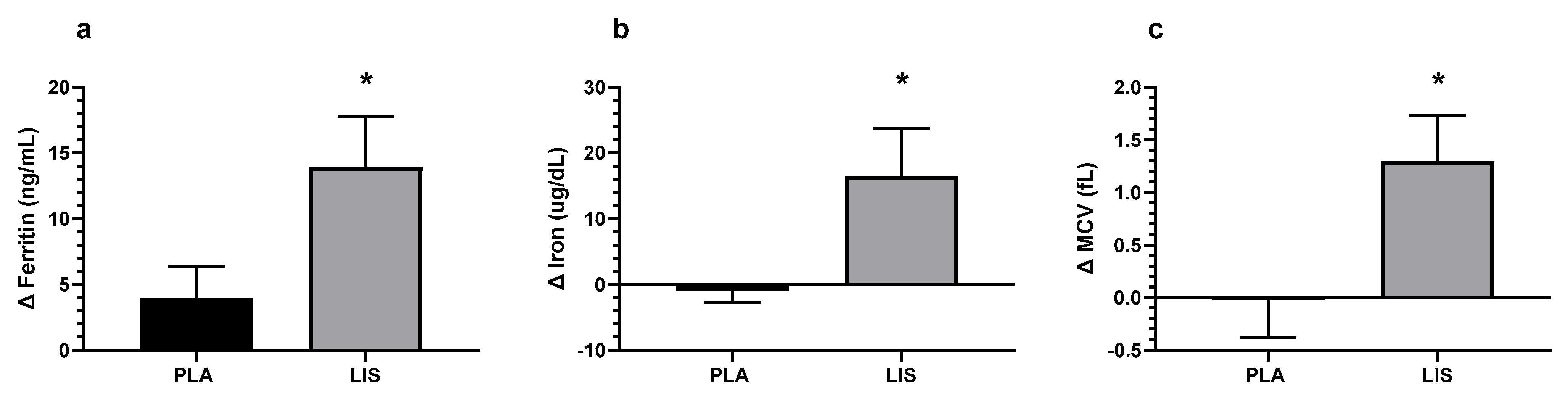
3.1.1. Blood-Iron Status
3.1.2. Complete Blood Count
3.2. Gastrointestinal Symptom Rating Scale (GSRS) and the Patient Assessment of Constipation-Symptoms Questionnaire (PAC-SYM)
3.3. Profile of Mood States (POMS)
3.4. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balendran, S.; Forsyth, C. Non-Anaemic Iron Deficiency. Aust. Prescr. 2021, 44, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron Deficiency without Anaemia: A Diagnosis That Matters. Clin. Med. 2021, 21, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Soppi, E.T. Iron Deficiency without Anemia—A Clinical Challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.W.; Hinchliffe, R.F.; Briggs, C.; Macdougall, I.C.; Littlewood, T.; Cavill, I. British Committee for Standards in Haematology Guideline for the Laboratory Diagnosis of Functional Iron Deficiency. Br. J. Haematol. 2013, 161, 639–648. [Google Scholar] [CrossRef]
- Chatard, J.C.; Mujika, I.; Guy, C.; Lacour, J.R. Anaemia and Iron Deficiency in Athletes. Practical Recommendations for Treatment. Sports Med. 1999, 27, 229–240. [Google Scholar] [CrossRef]
- Punnonen, K.; Irjala, K.; Rajamäki, A. Serum Transferrin Receptor and Its Ratio to Serum Ferritin in the Diagnosis of Iron Deficiency. Blood 1997, 89, 1052–1057. [Google Scholar] [CrossRef]
- Vaucher, P.; Druais, P.-L.; Waldvogel, S.; Favrat, B. Effect of Iron Supplementation on Fatigue in Nonanemic Menstruating Women with Low Ferritin: A Randomized Controlled Trial. CMAJ 2012, 184, 1247–1254. [Google Scholar] [CrossRef]
- Soppi, E. Iron Deficiency in the Absence of Anemia—A Common, Complex and Challenging Disease to Treat. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Verdon, F.; Burnand, B.; Stubi, C.-L.F.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; de Vevey, M.; Studer, J.-P.; Herzig, L.; et al. Iron Supplementation for Unexplained Fatigue in Non-Anaemic Women: Double Blind Randomised Placebo Controlled Trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef]
- Baird-Gunning, J.; Bromley, J. Correcting Iron Deficiency. Aust. Prescr. 2016, 39, 193–199. [Google Scholar] [CrossRef]
- Mawani, M.; Aziz Ali, S. Iron Deficiency Anemia among Women of Reproductive Age, an Important Public Health Problem: Situation Analysis. Reprod. Syst. Sex. Disord. 2016, 5, 1. [Google Scholar] [CrossRef]
- Leblanc, V.; Bégin, C.; Corneau, L.; Dodin, S.; Lemieux, S. Gender Differences in Dietary Intakes: What Is the Contribution of Motivational Variables? J. Hum. Nutr. Diet. 2015, 28, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron Absorption from Supplements Is Greater with Alternate Day than with Consecutive Day Dosing in Iron-Deficient Anemic Women. Haematologica 2020, 105, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral Iron Supplementation in Iron-Deficient Women: How Much and How Often? Mol. Asp. Med. 2020, 75, 100865. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [PubMed]
- Duque, X.; Martinez, H.; Vilchis-Gil, J.; Mendoza, E.; Flores-Hernández, S.; Morán, S.; Navarro, F.; Roque-Evangelista, V.; Serrano, A.; Mera, R.M. Effect of Supplementation with Ferrous Sulfate or Iron Bis-Glycinate Chelate on Ferritin Concentration in Mexican Schoolchildren: A Randomized Controlled Trial. Nutr. J. 2014, 13, 71. [Google Scholar] [CrossRef]
- Cornbluth Szarfarc, S.; Núñez de Cassana, L.M.; Fujimori, E.; Guerra-Shinohara, E.M.; Vianna de ’Oliveira, I.M. Relative effectiveness of iron bis-glycinate chelate (Ferrochel) and ferrous sulfate in the control of iron deficiency in pregnant women. Arch. Latinoam. De. Nutr. 2001, 51, 42–47. [Google Scholar]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron Bioavailability from Commercially Available Iron Supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef]
- Institute of Medicine Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Casgrain, A.; Collings, R.; Harvey, L.J.; Hooper, L.; Fairweather-Tait, S.J. Effect of Iron Intake on Iron Status: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2012, 96, 768–780. [Google Scholar] [CrossRef]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Újszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and Validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) Questionnaire in Dyspepsia: A Six-Country Study. Health Qual. Life Outcomes 2008, 6, 12. [Google Scholar] [CrossRef]
- Yiannakou, Y.; Tack, J.; Piessevaux, H.; Dubois, D.; Quigley, E.M.M.; Ke, M.Y.; Da Silva, S.; Joseph, A.; Kerstens, R. The PAC-SYM Questionnaire for Chronic Constipation: Defining the Minimal Important Difference. Aliment. Pharmacol. Ther. 2017, 46, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Grove, R.; Prapavessis, H. Preliminary Evidence for the Reliability and Validity of an Abbreviated Profile of Mood States. Int. J. Sport Psychol. 1992, 23, 93–109. [Google Scholar]
- Cumming, G. Understanding The New Statistics | Effect Sizes, Confidence Intervals. Available online: https://www.taylorfrancis.com/books/mono/10.4324/9780203807002/understanding-new-statistics-geoff-cumming (accessed on 28 December 2022).
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Santiago, P. Ferrous versus Ferric Oral Iron Formulations for the Treatment of Iron Deficiency: A Clinical Overview. Sci. World J. 2012, 2012, 846824. [Google Scholar] [CrossRef] [PubMed]
- Maner, B.S.; Moosavi, L. Mean Corpuscular Volume. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kuragano, T.; Joki, N.; Hase, H.; Kitamura, K.; Murata, T.; Fujimoto, S.; Fukatsu, A.; Inoue, T.; Itakura, Y.; Nakanishi, T. Low Transferrin Saturation (TSAT) and High Ferritin Levels Are Significant Predictors for Cerebrovascular and Cardiovascular Disease and Death in Maintenance Hemodialysis Patients. PLoS ONE 2020, 15, e0236277. [Google Scholar] [CrossRef]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are We Giving Too Much Iron? Low-Dose Iron Therapy Is Effective in Octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic Galacto-Oligosaccharides Mitigate the Adverse Effects of Iron Fortification on the Gut Microbiome: A Randomised Controlled Study in Kenyan Infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron Fortification Adversely Affects the Gut Microbiome, Increases Pathogen Abundance and Induces Intestinal Inflammation in Kenyan Infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I.T. Oral Ferrous Sulfate Supplements Increase the Free Radical-Generating Capacity of Feces from Healthy Volunteers. Am. J. Clin. Nutr. 1999, 69, 250–255. [Google Scholar] [CrossRef]
- Lund, E.K.; Fairweather-Tait, S.J.; Wharf, S.G.; Johnson, I.T. Chronic Exposure to High Levels of Dietary Iron Fortification Increases Lipid Peroxidation in the Mucosa of the Rat Large Intestine. J. Nutr. 2001, 131, 2928–2931. [Google Scholar] [CrossRef]
- Stoltzfus, R.J. Iron Deficiency: Global Prevalence and Consequences. Food Nutr. Bull. 2003, 24, S99–S103. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group | N | Mean | ||
|---|---|---|---|---|---|
| Age (yrs) | PLA | 41 | 34.95 | ± | 8.58 |
| LIS | 44 | 35.00 | ± | 8.63 | |
| Total | 85 | 35.98 | ± | 8.56 | |
| Height (cm) | PLA | 41 | 166.22 | ± | 6.17 |
| LIS | 44 | 163.11 | ± | 5.66 | |
| Total | 85 | 164.66 | ± | 2.20 | |
| Weight (kg) | PLA | 41 | 66.81 | ± | 7.58 |
| LIS | 44 | 62.76 | ± | 8.27 | |
| Total | 85 | 64.79 | ± | 2.86 | |
| Body Mass Index (kg/m2) | PLA | 41 | 24.28 | ± | 3.22 |
| LIS | 44 | 23.65 | ± | 3.28 | |
| Total | 85 | 23.96 | ± | 0.45 | |
| Heart Rate (bpm) | PLA | 41 | 66.24 | ± | 9.69 |
| LIS | 44 | 68.91 | ± | 12.65 | |
| Total | 85 | 67.58 | ± | 1.89 | |
| Systolic Blood Pressure | PLA | 41 | 115.42 | ± | 10.26 |
| (mmHg) | LIS | 44 | 114.57 | ± | 10.54 |
| Total | 85 | 114.99 | ± | 0.60 | |
| Diastolic Blood Pressure | PLA | 41 | 71.22 | ± | 7.40 |
| (mmHg) | LIS | 44 | 68.36 | ± | 9.72 |
| Total | 85 | 69.79 | ± | 2.02 | |
| Ferritin (ng/mL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 29.49 ± 18.86 | 33.44 ± 23.03 | 0.033 * | 0.47 (0.04, 0.89) |
| LIS | 28.48 ± 15.75 | 42.43 ± 23.85 | ||
| Iron (ug/dL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 82.15 ± 38.85 | 77.51 ± 44.50 | 0.030 * | 0.47 (0.04, 0.90) |
| LIS | 81.29 ± 35.97 | 97.79 ± 36.94 | ||
| TIBC (ug/dL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 371.68 ± 51.64 | 360.59 ± 48.49 | 0.894 | −0.03 (−0.45, 0.39) |
| LIS | 351.46 ± 45.01 | 339.43 ± 43.94 | ||
| tSAT (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 22.91 ± 12.05 | 21.91 ± 12.96 | 0.067 | 0.40 (−0.03, 0.82) |
| LIS | 23.93 ± 11.61 | 28.24 ± 10.53 |
| WBC (K/uL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 6.44 ± 1.79 | 6.28 ± 1.89 | 0.773 | −0.06 (−0.48, 0.36) |
| LIS | 6.44 ± 1.90 | 6.17 ± 1.28 | ||
| RBC (M/uL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 4.39 ± 0.37 | 4.47 ± 0.33 | 0.900 | 0.02 (−0.40, 0.45) |
| LIS | 4.52 ± 0.49 | 4.61 ± 0.45 | ||
| Hemoglobin (g/dL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 12.82 ± 1.02 | 13.07 ± 1.11 | 0.122 | 0.34 (−0.09, 0.76) |
| LIS | 12.86 ± 1.26 | 13.39 ± 1.20 | ||
| Hematocrit (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 38.86 ± 2.75 | 39.57 ± 2.60 | 0.240 | 0.25 (−0.17, 0.68) |
| LIS | 39.02 ± 3.33 | 40.40 ± 3.07 | ||
| MCV (fL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 88.73 ± 4.77 | 88.71 ± 3.91 | 0.022 * | 0.50 (0.07, 0.93) |
| LIS | 86.93 ± 7.56 | 88.23 ± 6.99 | ||
| MCH (pg) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 29.24 ± 1.72 | 29.30 ± 2.22 | 0.209 | 0.27 (−0.15, 0.70) |
| LIS | 28.69 ± 3.31 | 29.23 ± 2.95 | ||
| MCHC (g/dL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 32.97 ± 0.97 | 33.07 ± 2.23 | 0.846 | 0.04 (−0.38, 0.46) |
| LIS | 32.95 ± 1.35 | 33.14 ± 1.58 | ||
| RDW (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 12.90 ± 1.02 | 12.90 ± 0.92 | 0.686 | 0.09 (−0.33, 0.51) |
| LIS | 13.09 ± 1.56 | 13.17 ± 1.38 | ||
| Platelet Count (K/uL) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 271.81 ± 49.51 | 274.39 ± 52.09 | 0.33 | −0.21 (−0.63, 0.21) |
| LIS | 295.75 ± 71.29 | 287.82 ± 65.12 | ||
| Neutrophils (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 56.46 ± 11.97 | 57.15 ± 9.10 | 0.817 | −0.05 (−0.47, 0.37) |
| LIS | 57.29 ± 10.95 | 57.50 ± 10.13 | ||
| Lymphs (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 30.34 ± 8.20 | 31.24 ± 7.55 | 0.462 | −0.16 (−0.58, 0.26) |
| LIS | 31.86 ± 9.53 | 31.80 ± 9.30 | ||
| Monocytes (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 8.15 ± 2.55 | 7.90 ± 1.95 | 0.445 | −0.16 (−0.59, 0.26) |
| LIS | 7.66 ± 1.77 | 7.11 ± 1.93 | ||
| Eos (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 2.63 ± 1.97 | 2.78 ± 2.15 | 0.668 | 0.09 (−0.33, 0.51) |
| LIS | 2.27 ± 1.78 | 2.55 ± 1.61 | ||
| Basos (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.90 ± 0.37 | 0.90 ± 0.49 | 0.267 | 0.24 (−0.18, 0.66) |
| LIS | 0.86 ± 0.51 | 0.96 ± 0.43 | ||
| Neutrophils (abs) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 3.83 ± 1.52 | 3.68 ± 1.53 | 0.830 | −0.05 (−0.47, 0.38) |
| LIS | 3.82 ± 1.63 | 3.61 ± 1.15 | ||
| Lymphs (abs) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 1.90 ± 0.51 | 1.89 ± 0.52 | 0.736 | −0.07 (−0.49, 0.35) |
| LIS | 1.96 ± 0.52 | 1.91 ± 0.51 | ||
| Monocytes (abs) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.51 ± 0.18 | 0.49 ± 0.17 | 0.435 | −0.17 (−0.59, 0.25) |
| LIS | 0.48 ± 0.13 | 0.44 ± 0.13 | ||
| Eos (abs) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.16 ± 0.13 | 0.17 ± 0.15 | 0.708 | 0.09 (−0.33, 0.51) |
| LIS | 0.15 ± 0.13 | 0.16 ± 0.11 | ||
| Basos (abs) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.04 ± 0.05 | 0.04 ± 0.05 | 0.815 | −0.04 (−0.46, 0.38) |
| LIS | 0.05 ± 0.05 | 0.05 ± 0.05 | ||
| Immature Granulocytes (%) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.05 ± 0.22 | 0.00 ± 0.00 | 0.419 | 0.17 (−0.25, 0.60) |
| LIS | 0.05 ± 0.21 | 0.05 ± 0.21 | ||
| Immature Granulocytes (abs) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.00 ± 0.02 | 0.00 ± 0.00 | 0.986 | −0.06 (−0.49, 0.36) |
| LIS | 0.00 ± 0.02 | 0.00 ± 0.00 | ||
| hs-CRP (mg/L) | Wk0 | Wk8 | G × T p | d (95% CI) |
| PLA | 0.96 ± 0.79 | 1.18 ± 1.47 | 0.155 | −0.31 (−0.73, 0.12) |
| LIS | 1.17 ± 0.97 | 1.08 ± 0.88 |
| GSRS Total Score | Wk0 | Wk4 | Wk8 | Friedman p | M.W.Δ4-0 | M.W.Δ8-0 | d (95% CI) |
| PLA | 1.56 ± 0.48 | 1.45 ± 0.39 | 1.60 ± 0.45 a | 0.008 | 0.695 | 0.533 | 0.11 (−0.31, 0.53) |
| LIS | 1.76 ± 0.57 | 1.62 ± 0.408 | 1.74 ± 0.55 | 0.451 | |||
| PAC-SYM Total Score | Wk0 | Wk4 | Wk8 | Friedman p | M.W.Δ4-0 | M.W.Δ8-0 | d (95% CI) |
| PLA | 0.47 ± 0.49 | 0.45 ± 0.42 | 0.64 ± 0.49 | 0.123 | 0.479 | 0.049 * | −0.37 (−0.80, 0.05) |
| LIS | 0.69 ± 0.60 | 0.57 ± 0.43 | 0.66 ± 0.58 | 0.475 |
| Total Score | Wk0 | Wk8 | Wilcoxon p | M.W. p Δ8-0 | d (95% CI) |
| PLA | 86.61 ± 12.90 | 86.42 ± 11.89 | 0.702 | 0.429 | 0.05 (−0.37, 0.48) |
| LIS | 93.50 ± 17.36 | 94.05 ± 17.33 | 0.452 |
| Frequency | Severity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 0 | 1 | 2 | χ2 | Group | 0 | 1 | 2 | 3 | χ2 | |
| Constipation | PLA | 37 | 3 | 1 | 0.639 | PLA | 37 | 2 | 1 | 2 | 0.182 |
| LIS | 37 | 6 | 1 | LIS | 37 | 1 | 6 | 1 | |||
| Total | 74 | 9 | 2 | Total | 74 | 3 | 7 | 3 | |||
| Bloating | PLA | 41 | 0 | 0 | 0.084 | PLA | 41 | 0 | 0 | 0 | 0.118 |
| LIS | 39 | 4 | 1 | LIS | 39 | 2 | 2 | 2 | |||
| Total | 80 | 4 | 1 | Total | 80 | 2 | 2 | 2 | |||
| Nausea | PLA | 36 | 5 | 0 | 0.905 | PLA | 36 | 3 | 2 | 0 | 0.466 |
| LIS | 39 | 5 | 0 | LIS | 39 | 2 | 1 | 2 | |||
| Total | 75 | 10 | 0 | Total | 75 | 5 | 3 | 2 | |||
| Upset Stomach | PLA | 39 | 2 | 0 | 0.277 | PLA | 39 | 2 | 0 | 0 | 0.161 |
| LIS | 39 | 5 | 0 | LIS | 39 | 1 | 3 | 2 | |||
| Total | 78 | 7 | 0 | Total | 78 | 3 | 3 | 2 | |||
| Abdominal Discomfort | PLA | 39 | 1 | 1 | 0.580 | PLA | 39 | 2 | 0 | 1 | 0.246 |
| LIS | 43 | 1 | 0 | LIS | 43 | 0 | 1 | 0 | |||
| Total | 82 | 2 | 1 | Total | 82 | 2 | 1 | 1 | |||
| Diarrhea | PLA | 39 | 2 | 0 | 0.515 | PLA | 39 | 0 | 0 | 2 | 0.213 |
| LIS | 43 | 1 | 0 | LIS | 43 | 0 | 1 | 0 | |||
| Total | 82 | 3 | 0 | Total | 82 | 0 | 1 | 2 | |||
| Headache | PLA | 41 | 0 | 0 | 0.167 | PLA | 41 | 0 | 0 | 0 | 0.385 |
| LIS | 42 | 2 | 0 | LIS | 42 | 0 | 1 | 1 | |||
| Total | 83 | 2 | 0 | Total | 83 | 0 | 1 | 1 | |||
| Heartburn | PLA | 41 | 0 | 0 | 0.332 | PLA | 41 | 0 | 0 | 0 | 0.332 |
| LIS | 43 | 1 | 0 | LIS | 43 | 1 | 0 | 0 | |||
| Total | 84 | 1 | 0 | Total | 84 | 1 | 0 | 0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, M.W.; Gundermann, D.M.; Sharp, M.H.; Jennings, B.A.; Gheith, R.H.; Lowery, R.P.; LowDog, T.; Ghatak, S.B.; Barbosa, J.; Wilson, J.M. Assessment of the Efficacy of a Low-Dose Iron Supplement in Restoring Iron Levels to Normal Range among Healthy Premenopausal Women with Iron Deficiency without Anemia. Nutrients 2023, 15, 2620. https://doi.org/10.3390/nu15112620
Stefan MW, Gundermann DM, Sharp MH, Jennings BA, Gheith RH, Lowery RP, LowDog T, Ghatak SB, Barbosa J, Wilson JM. Assessment of the Efficacy of a Low-Dose Iron Supplement in Restoring Iron Levels to Normal Range among Healthy Premenopausal Women with Iron Deficiency without Anemia. Nutrients. 2023; 15(11):2620. https://doi.org/10.3390/nu15112620
Chicago/Turabian StyleStefan, Matthew W., David M. Gundermann, Matthew H. Sharp, Brooke A. Jennings, Raad H. Gheith, Ryan P. Lowery, Tieraona LowDog, Somsuvra B. Ghatak, Jose Barbosa, and Jacob M. Wilson. 2023. "Assessment of the Efficacy of a Low-Dose Iron Supplement in Restoring Iron Levels to Normal Range among Healthy Premenopausal Women with Iron Deficiency without Anemia" Nutrients 15, no. 11: 2620. https://doi.org/10.3390/nu15112620
APA StyleStefan, M. W., Gundermann, D. M., Sharp, M. H., Jennings, B. A., Gheith, R. H., Lowery, R. P., LowDog, T., Ghatak, S. B., Barbosa, J., & Wilson, J. M. (2023). Assessment of the Efficacy of a Low-Dose Iron Supplement in Restoring Iron Levels to Normal Range among Healthy Premenopausal Women with Iron Deficiency without Anemia. Nutrients, 15(11), 2620. https://doi.org/10.3390/nu15112620







