Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
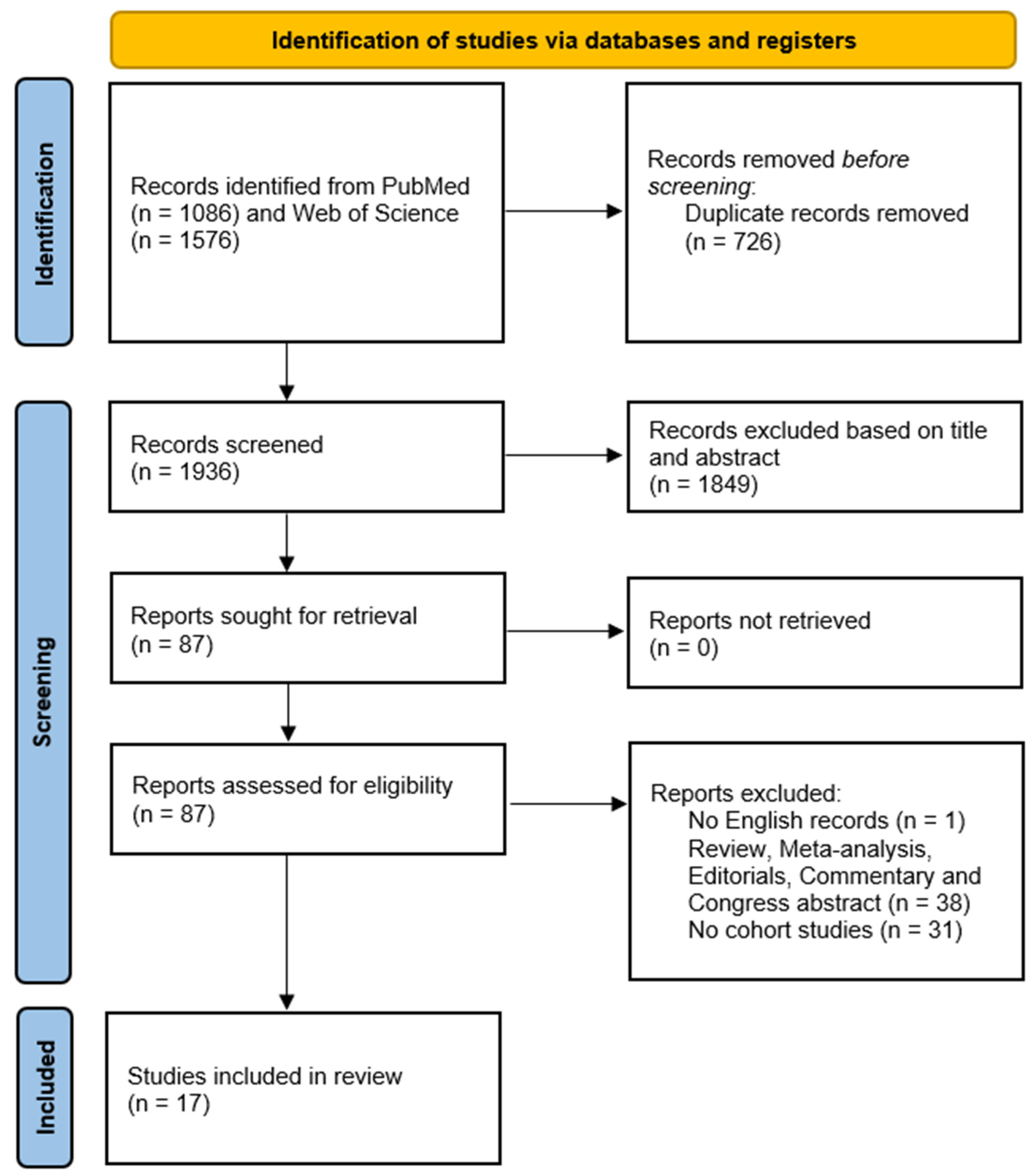
2.2. Study Selection, Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Consumption of Ultra-Processed Food, Excess Body Weight, and Abdominal Obesity
3.3. Consumption of Ultra-Processed Food, Impaired Fasting Glucose, and Diabetes Mellitus
3.4. Consumption of Ultra-Processed Food and Hypertension
3.5. Consumption of Ultra-Processed Food and Lipid Profile
3.6. Consumption of Ultra-Processed Food and Metabolic Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. World Obesity Day 2022—Accelerating Action to Stop Obesity; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 25 January 2023).
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ 2014, 186, E649–E657. [Google Scholar] [CrossRef]
- Leone, A.; De Amicis, R.; Battezzati, A.; Bertoli, S. Adherence to the Mediterranean Diet and Risk of Metabolically Unhealthy Obesity in Women: A Cross-Sectional Study. Front. Nutr. 2022, 9, 858206. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Bertoli, S.; Bedogni, G.; Vignati, L.; Pellizzari, M.; Battezzati, A. Association between Mediterranean Diet and Fatty Liver in Women with Overweight and Obesity. Nutrients 2022, 14, 3771. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-processed foods and obesity and adiposity parameters among children and adolescents: A systematic review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef]
- Leone, A.; Martínez-González, M.; Craig, W.; Fresán, U.; Gómez-Donoso, C.; Bes-Rastrollo, M. Pre-Gestational Consumption of Ultra-Processed Foods and Risk of Gestational Diabetes in a Mediterranean Cohort. The SUN Project. Nutrients 2021, 13, 2202. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Juul, F.; Neri, D.; Rauber, F.; Monteiro, C.A. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev. Med. 2019, 125, 40–48. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Batal, M.; Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017, 108, 512–520. [Google Scholar] [CrossRef]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk; Aromataris, E., Munn, Z., Eds.; JBI Manual for Evidence Synthesis: Adelaide, Australia, 2020. [Google Scholar]
- Canhada, S.L.; Luft, V.C.; Giatti, L.; Duncan, B.B.; Chor, D.; Fonseca, M.; Matos, S.M.A.; Molina, M.; Barreto, S.M.; Levy, R.B.; et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2020, 23, 1076–1086. [Google Scholar] [CrossRef]
- Scaranni, P.; Cardoso, L.O.; Chor, D.; Melo, E.C.P.; Matos, S.M.A.; Giatti, L.; Barreto, S.M.; da Fonseca, M.J.M. Ultra-processed foods, changes in blood pressure and incidence of hy.ypertension: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2021, 24, 3352–3360. [Google Scholar] [CrossRef]
- Scaranni, P.; de Oliveira Cardoso, L.; Griep, R.H.; Lotufo, P.A.; Barreto, S.M.; da Fonseca, M.J.M. Consumption of ultra-processed foods and incidence of dyslipidemias: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Br. J. Nutr. 2022, 129, 336–344. [Google Scholar] [CrossRef]
- Magalhães, E.; de Oliveira, B.R.; Rudakoff, L.C.S.; de Carvalho, V.A.; Viola, P.; Arruda, S.P.M.; de Carvalho, C.A.; Coelho, C.; Bragança, M.; Bettiol, H.; et al. Sex-Dependent Effects of the Intake of NOVA Classified Ultra-Processed Foods on Syndrome Metabolic Components in Brazilian Adults. Nutrients 2022, 14, 3126. [Google Scholar] [CrossRef]
- Beslay, M.; Srour, B.; Méjean, C.; Allès, B.; Fiolet, T.; Debras, C.; Chazelas, E.; Deschasaux, M.; Wendeu-Foyet, M.G.; Hercberg, S.; et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: A prospective analysis of the French NutriNet-Santé cohort. PLoS Med. 2020, 17, e1003256. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Alles, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Sante Prospective Cohort. JAMA Intern. Med. 2019, 180, 283–291. [Google Scholar] [CrossRef]
- Monge, A.; Silva Canella, D.; López-Olmedo, N.; Lajous, M.; Cortés-Valencia, A.; Stern, D. Ultraprocessed beverages and processed meats increase the incidence of hypertension in Mexican women. Br. J. Nutr. 2021, 126, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.J.; Vinke, P.C.; Navis, G.; Corpeleijn, E.; Dekker, L.H. Ultra-processed food and incident type 2 diabetes: Studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. 2022, 20, 7. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Jiménez-Onsurbe, M.; Donat-Vargas, C.; Rey-García, J.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed Food Consumption Is Associated with Abdominal Obesity: A Prospective Cohort Study in Older Adults. Nutrients 2020, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, R.D.; Pimenta, A.M.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.; Bes-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, R.D.; Lopes, A.C.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens 2017, 30, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Llavero-Valero, M.; Escalada-San Martín, J.; Martínez-González, M.A.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M. Ultra-processed foods and type-2 diabetes risk in the SUN project: A prospective cohort study. Clin. Nutr. 2021, 40, 2817–2824. [Google Scholar] [CrossRef]
- Donat-Vargas, C.; Sandoval-Insausti, H.; Rey-García, J.; Moreno-Franco, B.; Åkesson, A.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. High Consumption of Ultra-Processed Food is Associated with Incident Dyslipidemia: A Prospective Study of Older Adults. J. Nutr. 2021, 151, 2390–2398. [Google Scholar] [CrossRef]
- Levy, R.B.; Rauber, F.; Chang, K.; Louzada, M.L.D.C.; Monteiro, C.A.; Millett, C.; Vamos, E.P. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin. Nutr. 2021, 40, 3608–3614. [Google Scholar] [CrossRef]
- Rauber, F.; Chang, K.; Vamos, E.P.; da Costa Louzada, M.L.; Monteiro, C.A.; Millett, C.; Levy, R.B. Â Ultra-processed food consumption and risk of obesity: A prospective cohort study of UK Biobank. Eur. J. Nutr. 2021, 60, 2169–2180. [Google Scholar] [CrossRef]
- Li, M.; Shi, Z. Ultra-Processed Food Consumption Associated with Overweight/Obesity among Chinese Adults-Results from China Health and Nutrition Survey 1997-2011. Nutrients 2021, 13, 2796. [Google Scholar] [CrossRef]
- Cordova, R.; Kliemann, N.; Huybrechts, I.; Rauber, F.; Vamos, E.P.; Levy, R.B.; Wagner, K.H.; Viallon, V.; Casagrande, C.; Nicolas, G.; et al. Consumption of ultra-processed foods associated with weight gain and obesity in adults: A multi-national cohort study. Clin. Nutr. 2021, 40, 5079–5088. [Google Scholar] [CrossRef]
- Rauber, F.; Steele, E.M.; Louzada, M.L.d.C.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008–2016). PLoS ONE 2020, 15, e0232676. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Alles, B.; Mejean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Sante). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Sadeghirad, B.; Duhaney, T.; Motaghipisheh, S.; Campbell, N.R.; Johnston, B.C. Influence of unhealthy food and beverage marketing on children’s dietary intake and preference: A systematic review and meta-analysis of randomized trials. Obes. Rev. 2016, 17, 945–959. [Google Scholar] [CrossRef]
- Pérez-Escamilla, R.; Obbagy, J.E.; Altman, J.M.; Essery, E.V.; McGrane, M.M.; Wong, Y.P.; Spahn, J.M.; Williams, C.L. Dietary energy density and body weight in adults and children: A systematic review. J. Acad. Nutr. Diet. 2012, 112, 671–684. [Google Scholar] [CrossRef]
- Canella, D.S.; Levy, R.B.; Martins, A.P.; Claro, R.M.; Moubarac, J.C.; Baraldi, L.G.; Cannon, G.; Monteiro, C.A. Ultra-processed food products and obesity in Brazilian households (2008–2009). PLoS ONE 2014, 9, e92752. [Google Scholar] [CrossRef]
- Lam, M.C.L.; Adams, J. Association between home food preparation skills and behaviour, and consumption of ultra-processed foods: Cross-sectional analysis of the UK National Diet and nutrition survey (2008–2009). Int. J. Behav. Nutr. Phys. Act. 2017, 14, 68. [Google Scholar] [CrossRef]
- Robinson, E.; Aveyard, P.; Daley, A.; Jolly, K.; Lewis, A.; Lycett, D.; Higgs, S. Eating attentively: A systematic review and meta-analysis of the effect of food intake memory and awareness on eating. Am. J. Clin. Nutr. 2013, 97, 728–742. [Google Scholar] [CrossRef]
- Robinson, E.; Almiron-Roig, E.; Rutters, F.; de Graaf, C.; Forde, C.G.; Tudur Smith, C.; Nolan, S.J.; Jebb, S.A. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am. J. Clin. Nutr. 2014, 100, 123–151. [Google Scholar] [CrossRef]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Carter, A.; Hendrikse, J.; Lee, N.; Yücel, M.; Verdejo-Garcia, A.; Andrews, Z.B.; Hall, W. The Neurobiology of “Food Addiction” and Its Implications for Obesity Treatment and Policy. Annu. Rev. Nutr. 2016, 36, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. A review of the carbohydrate-insulin model of obesity. Eur. J. Clin. Nutr. 2017, 71, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Vinceti, M. Sodium Intake and Risk of Hypertension: A Systematic Review and Dose-Response Meta-analysis of Observational Cohort Studies. Curr. Hypertens Rep. 2022, 24, 133–144. [Google Scholar] [CrossRef]
- Lustig, R.H. Fructose: Metabolic, hedonic, and societal parallels with ethanol. J. Am. Diet. Assoc. 2010, 110, 1307–1321. [Google Scholar] [CrossRef]
- Zakim, D. The effect of fructose on hepatic synthesis of fatty acids. Acta Med. Scand. Suppl. 1972, 542, 205–214. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef]
- Matthan, N.R.; Welty, F.K.; Barrett, P.H.; Harausz, C.; Dolnikowski, G.G.; Parks, J.S.; Eckel, R.H.; Schaefer, E.J.; Lichtenstein, A.H. Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1092–1097. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar]
- El-Ezaby, M.M.; Abd-El Hamide, N.-A.H.; El-Maksoud, M.A.E.; Shaheen, E.M.; Embashi, M.M.R. Effect of some food additives on lipid profile, kidney function and liver function of adult male albino rats. J. Bas Environ. Sci. 2018, 5, 52–59. [Google Scholar]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Carrageenan Inhibits Insulin Signaling through GRB10-mediated Decrease in Tyr(P)-IRS1 and through Inflammation-induced Increase in Ser(P)307-IRS1. J. Biol. Chem. 2015, 290, 10764–10774. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Bertoli, S.; Leone, A.; Battezzati, A. Human Bisphenol A Exposure and the “Diabesity Phenotype”. Dose Response 2015, 13, 1559325815599173. [Google Scholar] [CrossRef]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal-Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef]
- Tonini, C.; Segatto, M.; Bertoli, S.; Leone, A.; Mazzoli, A.; Cigliano, L.; Barberio, L.; Mandalà, M.; Pallottini, V. Prenatal Exposure to BPA: The Effects on Hepatic Lipid Metabolism in Male and Female Rat Fetuses. Nutrients 2021, 13, 1970. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Toward a new philosophy of preventive nutrition: From a reductionist to a holistic paradigm to improve nutritional recommendations. Adv. Nutr. 2014, 5, 430–446. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F. Chapter 1 Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 5–48. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Lorenzoni, G.; Di Benedetto, R.; Silano, M.; Gregori, D. What Is the Nutritional Composition of Ultra-Processed Food Marketed in Italy? Nutrients 2021, 13, 2364. [Google Scholar] [CrossRef]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Country (Cohort) | Subjects (n) and Baseline Characteristics | Outcome | Follow-Up Time | Dietary Assessment | Covariates Included in the Fully Adjusted Model | Type of Exposure | Results |
|---|---|---|---|---|---|---|---|---|
| Mendonça et al. (2016) [26] | Spain (SUN cohort) | 8451 participants 35.1% men 64.9% women Age: 37.6 ± 11.0 years | Overweight/obesity | Median follow-up: 8.9 years | Semi-quantitative FFQ (136 items) | Sex, age, baseline BMI, educational status, marital status, physical activity, smoking status, siesta sleep, television watching, following a special diet at baseline, snacking between meals, and consumption of fruit and vegetables. | servings/d | Participants in the fourth quartile of UPF consumption had a higher risk of developing overweight or obesity (HR = 1.26, 95% CI: 1.10, 1.45, Ptrend = 0.001) than participants in the first quartile. |
| Canhada et al. (2019) [17] | Brazil (ELSA cohort) | 11,827 participants 45% men 55% women Age: 51.3 ± 8.7 years | Overweight/obesity | Mean follow-up: 3.8 years | FFQ (114 items) | Age, sex, school achievement, center, and color/race, as well as smoking and physical activity, waist/weight gain, incidence of overweight/obesity, baseline BMI, and baseline waist circumference. | %UPFenergy | Participants in the fourth quartile of UPF consumption (>30.8 %) presented 20% greater risk (RR:1.20; 95% CI: 1.03, 1–40) of incident overweight and obesity than participants in the first quartile (<17.8%). No association between UPF quartiles and risk of incident obesity among overweight participants was observed (RR:1.02; 95% CI: 0.85, 1.21). |
| Beslay et al. (2020) [21] | France (French NutriNet-Santè cohort) | 110,260 participants 22.8% men 78.2% women Age: 43.1 ± 14.6 years | Overweight/obesity | Median follow-up: 4.1 years | 24 h dietary record | Age, sex, marital status, BMI, educational level, physical activity, smoking status, alcohol intake, number of 24 h dietary records, energy intake, health, and Western dietary pattern. | %UPFintake | Normal-weight participants with low UPF consumption had a lower risk of developing overweight or obesity during follow-up (HRQ4 vs.Q1 = 1.22, 95% CI: 1.14, 1.31, Ptrend < 0.001) than those with a higher intake. Moreover, a 10% increment of UPF intake was associated with a higher risk of developing overweight or obesity (HR = 1.10, 95% CI: 1.07, 1.13; P < 0.001). Non-obese subjects with low UPF consumption had a lower risk of developing obesity during follow-up (HRQ4 vs.Q1 = 1.20, 95% CI: 1.08, 1.33, Ptrend < 0.001) than those with a higher intake. Moreover, a 10% increment of UPFs intake was associated with a higher risk of developing obesity (HR = 1.11, 95% CI: 1.07, 1.15; P < 0.001). |
| Sandoval-Insausti et al. (2020) [25] | Spain (Seniors-ENRICA-1) | 652 participants 55.7% men 44.3% women Age: 67.08 ± 5.8 years | Abdominal obesity | Median follow-up: 6 years | Dietary history (DH-ENRICA) record | Age, sex, educational level, marital status, ex-drinker status, smoking, physical activity in the household, physical activity during leisure time, prevalence of chronic disease, number of medications consumed daily, and adherence to Mediterranean diet. | %UPFenergy | Participants in the first tertiles of UPF consumption had a higher risk of developing abdominal obesity (RR: 1.61; 95% CI: 1.01, 2.56, Ptrend=0.048) than participants in the first tertile. |
| Cordova et al. (2021) [33] | Denmark, Germany, Italy, France, Greece, the Netherlands, Spain, Norway, Sweden and the UK (EPIC cohort) | 348,748 participants 26.6% men 73.4% women Age: 51.7 ± 9.0 years | Overweight/obesity | Median follow-up: 5 years | (a) Quantitative FFQ (Italy, Spain, the Netherlands, Germany, and France) (b) Semi-quantitative FFQ (Denmark, Naples (Italy), Norway, and Umeå (Sweden), (c) A combination of semi-quantitative FFQ and 7- and 14-day records in the UK and Malmo (Sweden). | Age, sex, BMI baseline, education level, smoking history, physical activity, alcohol intake, Mediterranean diet score, and plausibility of dietary energy reporting. | g/day | Normal-weight participants in the fifth quintile of UPF consumption had a 15% higher risk (RR = 1.15, 95% CI: 1.11, 1.19, Ptrend <0.001) of becoming overweight or obese during follow-up than participants in the first quintile. Similarly, participants with overweight in the highest quintile of UPF consumption had a 16% higher risk (RR = 1.16; 95% CI: 1.09, 1.23, Ptrend <0.001) of becoming obese during follow-up than participants in the lowest quintile. |
| Li et al. (2021) [32] | China (CNHS cohort) | 12,451 participants 48.7% men 51.3% women Age: 43.7 ± 14.7 years | Overweight/obesity and abdominal obesity | 10 years | 3-day 24 h dietary recall | Age, sex, income, urbanization, education, smoking, alcohol drinking, and physical activity, energy intake, fat intake, and dietary patterns. | g/day | Participants consuming 1–19 g/day, 20–49 g/day, or ≥ 50 g/day of UPF were at a higher risk of developing overweight and obesity and abdominal obesity than non-consumers. Adjusted ORs for overweight and obesity were 1.45 (95% CI: 1.26, 1.65), 1.34 (95% CI: 1.15–1.57), and 1.45 (95% CI: 1.21–1.74), respectively. Adjusted ORs for abdominal obesity were 1.54 (95% CI: 1.38, 1.72), 1.35 (95% CI: 1.19, 1.54), and 1.50 (95% CI: 1.29, 1.74), respectively. |
| Rauber et al. (2021) [31] | England, Scotland and Wales (UK Biobank) | 22,659 participants 47.9% men 52.1% women Age: 55.9 ± 7.4 years | General and abdominal obesity | Median follow-up: 5 years | 24 h dietary recall | Sex, BMI, waist circumference or body fat at baseline, smoking status, level of physical activity, sleep duration, Index of Multiple Deprivation (IMD). | %UPFenergy | Non-obese participants in the uppermost quartile of UPF consumption were at a higher risk of developing obesity (HR = 1.79, 95% CI: 1.06, 3.03) than participants in the lowest quartile. Similarly, participants with normal waist circumference at baseline but in the first quartile of UPF consumption were at a higher risk of developing abdominal obesity (HR = 1.30, 95% CI: 1.14, 1.48) than participants in the lowest quartile. |
| DaSilva Magalhães et al. (2022) [20] | Brazil (Ribeirão Preto cohort) | 896 particpants 44.3% men 55.7% women Age: 23–25 years | MetS and its components | 14–16 years | Semi-quantitative FFQ (83 items) | Sex, age, education, marital status, skin color, family income, smoking, level of physical activity, and alcohol consumption. In the analyses with the consumption of UPF in %g, total energy intake was additionally included. | %UPFenergy and %UPFintake | UPF consumption was not associated with the risk of metabolic syndrome (%kcal PR: 1.00; 95% CI: 0.99–1.01; %g PR: 1.00; 95% CI: 0.99–1.01). However, women with higher UPF consumption were at a higher risk of developing abdominal obesity (%kcal: RR = 1.01, 95% CI: 1.00, 1.02, p = 0.030; %g: RR = 1.01, 95% CI: 1.00, 1.02, p = 0.003) and low HDL-cholesterol (%kcal: RR = 1.02, 95% CI: 1.01, 1.04, p = 0.041). No significant associations between UPF consumption and other metabolic syndrome components were observed. |
| Mendonca et al. (2017) [27] | Spain (SUN cohort) | 14,790 36.3% men 63.7% women Age: 36.3 ± 10.3 years | Hypertension | Mean follow-up: 9.1 years | Semi-quantitative FFQ (136 items) | Sex, age, baseline BMI, physical activity, hours of television watching, smoking status, following a special diet at baseline, use of analgesics, alcohol consumption, family history of hypertension, hypercholesterolemia, total energy intake, fruit and vegetable consumption, and olive oil intake. | servings/d | Participants in the third tertile of UPF consumption were at a higher risk of developing hypertension (HR = 1.21, 95% CI: 1.06, 1.37, Ptrend = 0.004) than participants in the first tertile. |
| Monge et al. (2021) [23] | Mexico (Mexican Teachers’ Cohort) | 64934 participants (only women) Age: 41.7 ± 7.2 years | Hypertension | Median follow-up: 2.2 years | Semi-quantitative FFQ (140 items) | Age, smoking status, physical activity, menopausal status, ethnicity, internet access and insurance for serious conditions, family history of hypertension, total energy intake, and multivitamin supplementation. | %UPFenergy | No association between categories of %UPFenergy (≤20%, 21–25%, 26–35%, 36–45% >45% energy/d) and incident hypertension was found. Compared with the first category, IRRs were 0.96 (95% CI: 0.86, 1.07), 0.92 (95% CI: 0.84, 1.02), 0.95 (95% CI: 0.85, 1.06), and 0.98 (95% CI: 0.84, 1.14). |
| Scaranni et al. (2021) [18] | Brazil (ELSA cohort) | 8754 participants 42% men 58% women Median age: 49.0 years | Hypertension | Mean follow-up: 3.9 years | 114-item FFQ | Sex, age, self-declared color/ race, education, smoking, alcohol consumption, antihypertensive drug use, Na consumption, physical activity, total daily energy intake, and BMI. | %UPFenergy | Participants with higher UPF consumption had a marginally significant greater risk of developing hypertension (OR = 1.17; 95% CI: 1.00, 1.37) than participants with lower UPF consumption. |
| Srour et al. (2019) [22] | France (French NutriNet-Santè cohort) | 1047,07 participants 20.8% men 79.2% women Age: 42.7 ± 14.5 years | Type 2 Diabetes | Median follow-up: 6 years | 24 h dietary record | Sex, age, BMI, weight change during follow-up, educational level, smoking status, physical activity level, number of 24 h dietary records, alcohol intake, energy intake without alcohol, overall diet quality, family history of diabetes, baseline dyslipidemia and hypertension, and treatments for these conditions. | g/day | An increment of 10% of UPFs in diet was associated with an increased risk of T2D (HR = 1.13, 95% CI: 1.03, 1.23, p = 0.04). Similarly, a 100g/day increment in UPF consumption was associated with the risk of T2D (HR = 1.05; 95% CI: 1.02, 1.08, p = 0.003). |
| Duan et al. (2022) [24] | Netherlands (Lifelines cohort) | 70,421 participants 41.4% men 58.6% women Age 49.1 ± 8.8 years | Type 2 Diabetes | Median follow-up: 3.4 years | Semi-quantitative FFQ (110 items) | Sex, age, BMI, educational level, energy intake, alcohol intake, Life diet score, smoking status, physical activity, and TV-watching time. | %UPFintake | An increment of 10% in UPF consumption was associated with a 25% higher risk of developing T2D (OR = 1.25; 95% CI: 1.16, 1.34). |
| Levy et al. (2021) [30] | England, Scotland and Wales (UK Biobank) | 21,730 participants 47.1% men 52.9% women Age: 55.8 ± 7.4 years | Type 2 Diabetes | Mean follow-up: 5.4 years | 24 h dietary recall | Sex, age, BMI, smoking, physical activity level, ethnicity, family history of T2D, Index of Multiple Deprivation (IMD), and total energy intake. | %UPFintake | Participants in the highest quartile of UPF consumption were at a higher risk for T2D (HR = 1.44; 95% CI: 1.04, 2.02, Ptrend < 0.028) than participants in the lowest quartile. Moreover, a 10%-point increment in UPF consumption was associated with a 12% increased risk of T2D (HR = 1.12, 95% CI: 1.04, 1.20). |
| Llavero-Valero et al. (2021) [28] | Spain (SUN cohort) | 20,060 participants 38.5% men 61.5% women Age: 37.4 ± 12.2 years | Type 2 Diabetes | Median follow-up: 12 years | Semi-quantitative FFQ (136 items) | Age, sex, BMI, educational level, smoking status, 8-item active + sedentary lifestyle score, following a special diet at baseline, snacking, and family history of diabetes. | g/day | Participants in the highest tertile of UPF consumption were at a higher risk of T2D than participants in the lowest tertile (HR = 1.53, 95% CI: 1.06, 2.22, Ptrend = 0.024). After using repeated measurements of UPF consumption, the association remained significant (HR = 1.65, 95% CI: 1.14, 2.38). |
| Donat-Vargas et al. (2021) [29] | Spain (ENRICA cohort) | 1082 participants 48% men 52% women Age: 68 ± 6 years | Dyslipidemia | 5–7 years | Dietary history (DH-ENRICA) record | Sex, age, BMI, smoking status, physical activity, educational level, marital status, total energy intake, alcohol consumption, fiber intake, consumption of unprocessed or minimal processed foods, number of medications, and number of chronic diseases. | %UPFenergy | Participants in the uppermost tertile of UPF consumption were at a higher risk for incident low HDL cholesterol (OR = 2.23; 95% CI: 1.22, 4.05; Ptrend = 0.012) and hypertriglyceridemia (OR = 2.66, 95% CI: 1.20, 5.90; Ptrend = 0.011) than participants in the lowest tertile. However, the consumption of UPF was not associated with the incident risk of high LDL cholesterol. |
| Scaranni et al. (2022) [19] | Brazil (ELSA cohort) | 5275 participants 42.2% men 57.8% women Age: 50.6 ± 8.8 years | Dyslipidemia | 4 years | Semi-quantitative FFQ (114 items) | Sex, age, BMI, schooling, smoking, physical activity, alcohol consumption, total energy intake, diabetes and time since baseline, and Brazilian Healthy Eating Index—Revised (BHEI-R). | g/day | Individuals with medium and high consumption of UPF had higher risks of developing isolated hypertriacylglycerolemia (OR = 1.14, 95% CI: 1.03, 1.26 and OR = 1.30, 95% CI: 1.17, 1.45), isolated hypercholesterolemia (OR = 1.12, 95% CI: 1.00, 1.27 and OR = 1.28, 95% CI: 1.12, 1.47), mixed hyperlipidemia (OR = 1.21, 95% CI: 1.05, 1.39 and OR = 1.38, 95% CI: 1.18, 1.62), and low HDL (OR = 1.12, 95% CI: 1.00, 1.24 and OR = 1.18, 95% CI: 1.05, 1.32), respectively, than participants who consumed less UPF. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. https://doi.org/10.3390/nu15112583
Mambrini SP, Menichetti F, Ravella S, Pellizzari M, De Amicis R, Foppiani A, Battezzati A, Bertoli S, Leone A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients. 2023; 15(11):2583. https://doi.org/10.3390/nu15112583
Chicago/Turabian StyleMambrini, Sara Paola, Francesca Menichetti, Simone Ravella, Marta Pellizzari, Ramona De Amicis, Andrea Foppiani, Alberto Battezzati, Simona Bertoli, and Alessandro Leone. 2023. "Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies" Nutrients 15, no. 11: 2583. https://doi.org/10.3390/nu15112583
APA StyleMambrini, S. P., Menichetti, F., Ravella, S., Pellizzari, M., De Amicis, R., Foppiani, A., Battezzati, A., Bertoli, S., & Leone, A. (2023). Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients, 15(11), 2583. https://doi.org/10.3390/nu15112583







