The Role of Protein Intake on the Total Milk Protein in Lead-Exposed Lactating Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessment of Maternal Diet
2.3. Blood Sample Collection and Measurement
2.4. Breast Milk Sample Collection and Measurement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
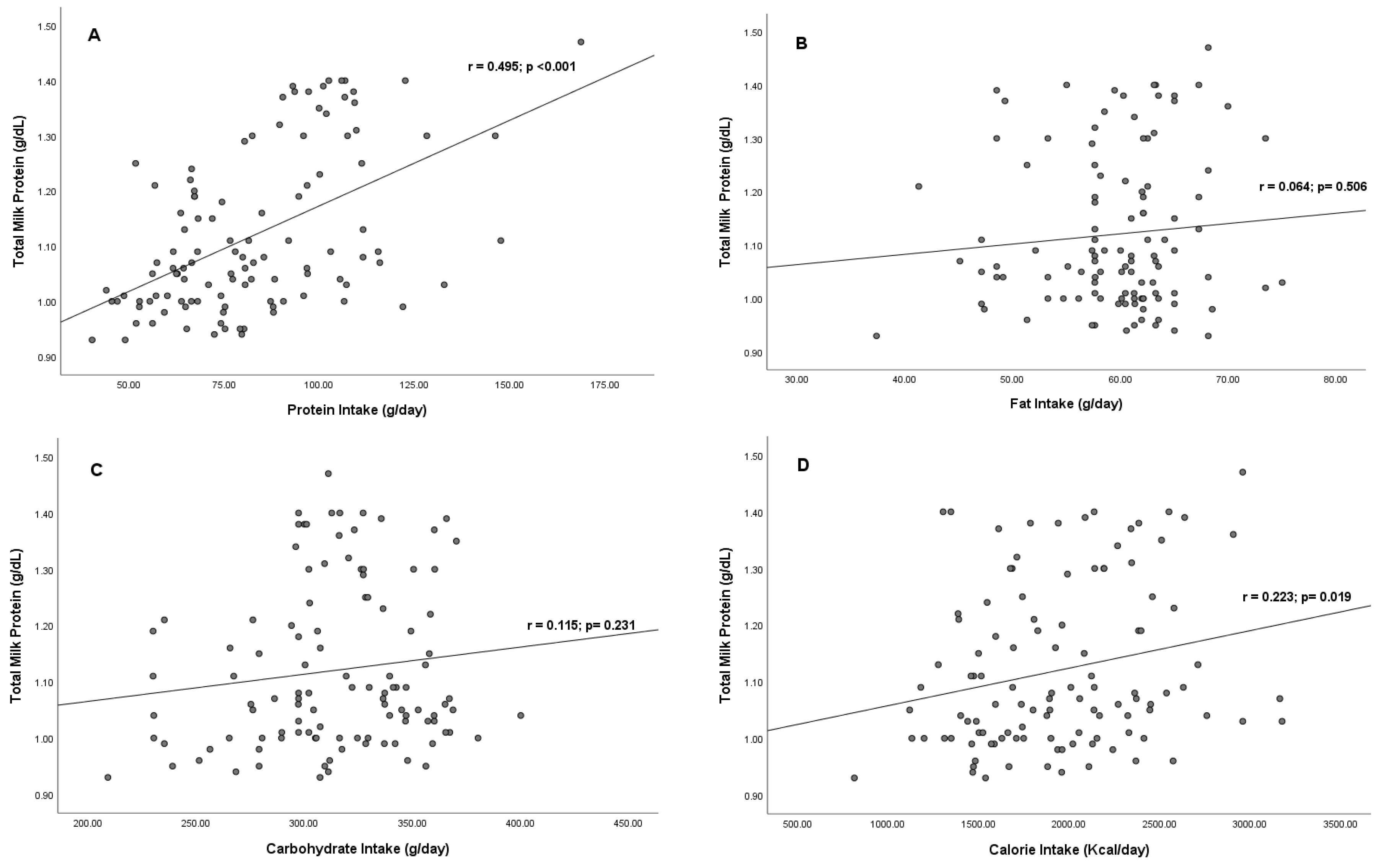
3.2. Correlation between Maternal Macronutrient Intake and Total Milk Protein
3.3. Correlation between Maternal Blood Lead Levels and Total Milk Protein
3.4. Correlation between Maternal Protein Intake and Total Milk Protein in Each Lead Exposure Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, O.; Marrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Andersons, J.; Volkovs, V.; Ceļmalniece, K. Impact of Maternal Diet on Human Milk Composition among Lactating Women in Latvia. Medicina 2019, 55, 173. [Google Scholar] [CrossRef]
- Donovan, S.M. Human Milk Proteins: Composition and Physiological Significance. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 93–101. [Google Scholar] [CrossRef]
- Zhang, S.; Heng, J.; Song, H.; Zhang, Y.; Lin, X.; Tian, M.; Chen, F.; Guan, W. Role of Maternal Dietary Protein and Amino Acids on Fetal Programming, Early Neonatal Development, and Lactation in Swine. Animals 2019, 9, 19. [Google Scholar] [CrossRef]
- Semba, R.D.; Trehan, I.; Gonzalez-Friere, M.; Kraemer, K.; Moaddel, R.; Ordiz, M.I.; Ferrucci, L.; Manary, M.J. Perspective: The Potential Role of Essential Amino Acids and the Mechanistic Target of Rapamycin Complex 1. Adv. Nutr. 2016, 7, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Esfarjani, F.; Roustaee, R.; Mohammadi, F.; Esmaillzadeh, A. Determinants of Stunting in School-Aged Children of Tehran, Iran. Int. J. Prev. Med. 2013, 4, 173–179. [Google Scholar] [PubMed]
- Hodson, N.; Philp, A. The Importance of MTOR Trafficking for Human Skeletal Muscle Translational Control. Exerc. Sport Sci. Rev. 2019, 47, 46–53. [Google Scholar] [CrossRef]
- Daniels, L.; Gibson, R.S.; Diana, A.; Haszard, J.J.; Rahmannia, S.; Luftimas, D.E.; Hampel, D.; Shahab-Ferdows, S.; Reid, M.; Melo, L.; et al. Micronutrient Intakes of Lactating Mothers and Their Association with Breast Milk Concentrations and Micronutrient Adequacy of Exclusively Breastfed Indonesian Infants. Am. J. Clin. Nutr. 2019, 110, 391–400. [Google Scholar] [CrossRef]
- De Souza Mesquita, L.M.; Mennitti, L.V.; De Rosso, V.V.; Pisani, L.P. The Role of Vitamin A and Its Pro-Vitamin Carotenoids in Fetal and Neonatal Programming: Gaps in Knowledge and Metabolic Pathways. Nutr. Rev. 2021, 79, 76–87. [Google Scholar] [CrossRef]
- Kementrian Kesehatan RI. Laporan Nasional RISKESDAS. 2018. Available online: http://repository.bkpk.kemkes.go.id/3514/1/Laporan%20Riskesdas%202018%20Nasional.pdf (accessed on 6 August 2021).
- Rizky Maulidiana, A.; Sutjiati, E. Low Intake of Essential Amino Acids and Other Risk Factors of Stunting among Under-Five Children in Malang City, East Java, Indonesia. J. Public Health Res. 2021, 10. [Google Scholar] [CrossRef]
- Bzikowska, A.; Czerwonogrodzka-Senczyna, A.; Weker, H.; Wesołowska, A. Correlation between Human Milk Composition and Maternal Nutritional Status. Rocz. Panstw. Zakl. Hig. 2018, 69, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, A.; Lai, S.; Yuan, Q.; Jia, X.; Wang, P.; Zhang, Y. Longitudinal Changes in the Concentration of Major Human Milk Proteins in the First Six Months of Lactation and Their Effects on Infant Growth. Nutrients 2021, 13, 1476. [Google Scholar] [CrossRef]
- Velázquez-Villegas, L.A.; López-Barradas, A.M.; Torres, N.; Hernández-Pando, R.; León-Contreras, J.C.; Granados, O.; Ortíz, V.; Tovar, A.R. Prolactin and the Dietary Protein/Carbohydrate Ratio Regulate the Expression of SNAT2 Amino Acid Transporter in the Mammary Gland during Lactation. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 1157–1164. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. N. Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Lee, H.-G. Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows. Animals 2021, 11, 2118. [Google Scholar] [CrossRef]
- Habibian, A.; Abyadeh, M.; Abyareh, M.; Rahimi Kakavandi, N.; Habibian, A.; Khakpash, M.; Ghazi-Khansari, M. Association of Maternal Lead Exposure with the Risk of Preterm: A Meta-Analysis. J. Matern.-Fetal Neonatal Med. 2022, 35, 7222–7230. [Google Scholar] [CrossRef]
- Wang, G.; Dibari, J.; Bind, E.; Steffens, A.M.; Mukherjee, J.; Azuine, R.E.; Singh, G.K.; Hong, X.; Ji, Y.; Ji, H.; et al. Association between Maternal Exposure to Lead, Maternal Folate Status, and Intergenerational Risk of Childhood Overweight and Obesity. JAMA Netw. Open 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Santoso, M.; Dwiana Lestari, D.; Mukhtar, R.; Hamonangan, E. Peringatan Dini Status Kualitas Udara Melalui Karakterisasi Kandungan Timbal Pada Pm2.5 Di Beberapa Kota Di Indonesia. J. Ecolab 2015, 9, 1–8. [Google Scholar] [CrossRef]
- Gulson, B.L.; Mahaffey, K.R.; Jameson, C.W.; Mizon, K.J.; Korsch, M.J.; Cameron, M.A.; Eisman, J.A. Mobilization of Lead from the Skeleton during the Postnatal Period Is Larger than during Pregnancy. J. Lab. Clin. Med. 1998, 131, 324–329. [Google Scholar] [CrossRef]
- Zhou, F.; Yin, G.; Gao, Y.; Liu, D.; Xie, J.; Ouyang, L.; Fan, Y.; Yu, H.; Zha, Z.; Wang, K.; et al. Toxicity Assessment Due to Prenatal and Lactational Exposure to Lead, Cadmium and Mercury Mixtures. Environ. Int. 2019, 133, 105192. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Venkareddy, L.K.; Muralidhara. Potential of Casein as a Nutrient Intervention to Alleviate Lead (Pb) Acetate-Mediated Oxidative Stress and Neurotoxicity: First Evidence in Drosophila melanogaster. NeuroToxicology 2015, 48, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Zheng, Y.; Shi, X.; Zhang, J.; Ma, C.; Guan, B.; Peng, Y.; Yang, M.; Yue, X. New Insights into the Alterations of Full Spectrum Amino Acids in Human Colostrum and Mature Milk between Different Domains Based on Metabolomics. Eur. Food Res. Technol. 2020, 246, 1119–1128. [Google Scholar] [CrossRef]
- Ghosh, A.; Shcherbik, N. Effects of Oxidative Stress on Protein Translation: Implications for Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 2661. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Sinkiewicz-Darol, E.; Gadzała-Kopciuch, R. The Impact of Environmental Pollution on the Quality of Mother’s Milk. Environ. Sci. Pollut. Res. 2019, 26, 7405–7427. [Google Scholar] [CrossRef]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant Properties and Potential Mechanisms of Hydrolyzed Proteins and Peptides from Cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef]
- Doumouchtsis, K.K.; Doumouchtsis, S.K.; Doumouchtsis, E.K.; Perrea, D.N. The Effect of Lead Intoxication on Endocrine Functions. J. Endocrinol. Investig. 2009, 32, 175–183. [Google Scholar] [CrossRef]
- Dobrakowski, M.; Boroń, M.; Birkner, E.; Kasperczyk, A.; Chwalińska, E.; Lisowska, G.; Kasperczyk, S. The Effect of a Short-Term Exposure to Lead on the Levels of Essential Metal Ions, Selected Proteins Related to Them, and Oxidative Stress Parameters in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 8763793. [Google Scholar] [CrossRef]
- Takser, L.; Mergler, D.; Lafond, J. Very Low Level Environmental Exposure to Lead and Prolactin Levels during Pregnancy. Neurotoxico. Teratol. 2005, 27, 505–508. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tsugami, Y.; Matsunaga, K.; Oyama, S.; Kuki, C.; Kumura, H. Prolactin and Glucocorticoid Signaling Induces Lactation-Specific Tight Junctions Concurrent with β-Casein Expression in Mammary Epithelial Cells. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2006–2016. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, N.; Su, C.; Wang, J.; Soyeurt, H. Relationships between Pb, As, Cr, and Cd in Individual Cows’ Milk and Milk Composition and Heavy Metal Contents in Water, Silage, and Soil. Environ. Pollut. 2019, 255, 113322. [Google Scholar] [CrossRef]
- Saleem, S.M.; Jan, S.S. Modified Kuppuswamy Socioeconomic Scale Updated for the Year 2021. Indian J. Forensic Commun. Med. 2021, 8, 113322. [Google Scholar] [CrossRef]
- Kementrian Kesehatan RI. Tabel Komposisi Pangan Indonesia. 2017. Available online: https://kesmas.kemkes.go.id/konten/133/0/020419-update-data-komposisi-pangan-indonesia# (accessed on 2 June 2021).
- Food and Agriculture Organization of the United Nations; Food Policy and Food Science Service. Amino-Acid Content of Foods and Biological Data on Proteins; Food and Agriculture Organization of the United Nations: Rome, Italy, 1981; pp. 1–285. [Google Scholar]
- Kordas, K.; Burganowski, R.; Roy, A.; Peregalli, F.; Baccino, V.; Barcia, E.; Mangieri, S.; Ocampo, V.; Manay, N.; Gabriela, M.; et al. Nutritional Status and Diet as Predictors of Children’s Lead Concentrations. Environ. Int. 2018, 111, 43–51. [Google Scholar] [CrossRef]
- Hayes, M. Measuring Protein Content Method. Foods 2020, 9, 1–4. [Google Scholar]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy Metal Toxicity: An Update of Chelating Therapeutic Strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Soegianto, A.; Putranto, T.W.C.; Lutfi, W.; Almirani, F.N.; Hidayat, A.R.; Muhammad, A.; Firdaus, R.A.; Rahmadhani, Y.S.; Fadila, D.A.N.; Hidayati, D. Concentrations of Metals in Tissues of Cockle Anadara granosa (Linnaeus, 1758) from East Java Coast, Indonesia, and Potential Risks to Human Health. Int. J. Food Sci. 2020, 2020, 5345162. [Google Scholar] [CrossRef]
- Soyata, A.; Hasanah, A.N.; Rusdiana, T. Isoflavones in Soybean as a Daily Nutrient: The Mechanisms of Action and How They Alter the Pharmacokinetics of Drugs. Turk. J. Pharm. Sci. 2021, 18, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Cao, W.; Huang, Y.; Fang, Y.; Cheng, Y.; Pan, S.; Xu, X. Isoflavone Biochanin A, a Novel Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-Antioxidant Response Element Activator, Protects against Oxidative Damage in HepG2 Cells. BioFactors 2019, 45, 563–574. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-Derived Bioactive Peptides on Inflammation and Oxidative Stress. BioMed Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef]
- Rísová, V. The Pathway of Lead through the Mother’s Body to the Child. Interdiscip. Toxicol. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Perng, W.; Tamayo-Ortiz, M.; Tang, L.; Sánchez, B.N.; Cantoral, A.; Meeker, J.D.; Dolinoy, D.C.; Roberts, E.F.; Martinez-Mier, E.A.; Lamadrid-Figueroa, H.; et al. Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) Project. BMJ Open 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Q.Y.; Cao, X.; Wang, K.; Wang, Y.H.; Wu, Y.; Yang, Z. Lead Exposure Promotes the Inflammation via the CircRNA-05280/MiR-146a/IRAK1 Axis in Mammary Gland. Ecotoxicol. Environ. Saf. 2022, 247, 114204. [Google Scholar] [CrossRef]
- Erliana, U.D.; Fly, A.D. The Function and Alteration of Immunological Properties in Human Milk of Obese Mothers. Nutrients 2019, 11, 1284. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food Proteins from Animals and Plants: Differences in the Nutritional and Functional Properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of Maternal Nutrition on Breast-Milk Composition: A Systematic Review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Nwachoko, N.; Akuru, U.B.; Odejayi, F.M.; Tetam, J.G. Amino Acids and Carbohydrates Composition in Breast Milk of Lactating Mothers of Different Age Group from Aleto Health Center, Rivers State. Afr. J. Biochem. Res. 2022, 16, 19–23. [Google Scholar]
- Lestari, N.P.Y.S.; Wardani, W.K.; Ratnawati, L.; Nurseta, T.; Rahardjo, B. Correlation of Leucine Intake with Serum Prolactin and Breast Milk Protein Levels in Breastfeeding Women. Devotion 2022, 3, 926–933. [Google Scholar] [CrossRef]
- Ding, M.; Li, W.; Zhang, Y.; Wang, X.; Zhao, A.; Zhao, X.; Wang, P.; Sheng, Q.H. Amino Acid Composition of Lactating Mothers’ Milk and Confinement Diet in Rural North China. Asia Pac. J. Clin. Nutr. 2010, 19, 344–349. [Google Scholar]
- Appuhamy, J.A.D.R.N.; Knoebel, N.A.; Nayananjalie, W.A.D.; Escobar, J.; Hanigan, M.D. Isoleucine and Leucine Independently Regulate MTOR Signaling and Protein Synthesis in MAC-T Cells and Bovine Mammary Tissue Slices. J. Nutr. 2012, 142, 484–491. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Zou, Y.; Zhao, F.-Q.; Liu, J.; Liu, H. Lysine Stimulates Protein Synthesis by Promoting the Expression of ATB0,+ and Activating the MTOR Pathway in Bovine Mammary Epithelial Cells. J. Nutr. 2018, 148, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Wickramasinghe, J.; Dooley, B.; McCarthy, C.; Branstad, E.; Grilli, E.; Baumgard, L.; Appuhamy, R. Effects of Microencapsulated Methionine on Milk Production and Manure Nitrogen Excretions of Lactating Dairy Cows. Animals 2021, 11, 3545. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Sequestration of Heavy Metal Ions by Methionine Modified Bentonite/Alginate (Meth-Bent/Alg): A Bionanocomposite. Groundw. Sustain. Dev. 2015, 1, 50–58. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Sunehag, A.L.; Haymond, M.W. Effect of Dietary Macronutrient Composition under Moderate Hypocaloric Intake on Maternal Adaptation during Lactation. Am. J. Clin. Nutr. 2009, 89, 1821–1827. [Google Scholar] [CrossRef]
- Morrison, B.; Cutler, M.L. The Contribution of Adhesion Signaling to Lactogenesis. J. Cell Commun. Signal. 2010, 4, 131–139. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Matos, A.; Valente, A.; Alonso, I.; Ribeiro, R.; Bicho, M. Body Composition Assesment and Nutritional Status Evaluation in Men and Women Portuguese Centenarians. J. Nutr. Health Aging 2016, 20, 256–266. [Google Scholar]
- Daniel, A.L.; Shama, S.; Ismail, S.; Bourdon, C.; Kiss, A.; Mwangome, M.; Bandsma, R.H.J.; O’Connor, D.L. Maternal Bmi Is Positively Associated with Human Milk Fat: A Systematic Review and Meta-Regression Analysis. Am. J. Clin. Nutr. 2021, 113, 1009–1022. [Google Scholar] [CrossRef]
- Chang, N.; Jung, J.A.; Kim, H.; Jo, A.; Kang, S.; Lee, S.W.; Yi, H.; Kim, J.; Yim, J.G.; Jung, B.M. Macronutrient Composition of Human Milk from Korean Mothers of Full Term Infants Born at 37–42 Gestational Weeks. Nutr. Res. Pract. 2015, 9, 433–438. [Google Scholar] [CrossRef]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between Maternal Body Composition and Appetite Hormones and Macronutrients in Human Milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. The Impact of Maternal Obesity on Human Milk Macronutrient Composition: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The Need to Study Human Milk as a Biological System. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.R.; Lipsmeyer, M.E.; Turner, D.E.; Andres, A. Human Milk Composition Differs by Maternal BMI in the First 9 Months Postpartum. Am. J. Clin. Nutr. 2020, 112, 548–557. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Frequency | % | Maternal BLLs (Mean ± SD) | p-Value *** |
|---|---|---|---|---|
| Maternal age (year) | ||||
| <20 | 2 | 1.82 | 3.75 ± 1.48 | 0.811 |
| 20–35 | 92 | 83.63 | 3.61 ± 1.41 | |
| >35 | 16 | 14.55 | 3.82 ± 1.28 | |
| Parity | ||||
| Primiparous | 36 | 32.73 | 3.48 ± 1.40 | 0.402 |
| Multiparous | 74 | 67.27 | 3.73 ± 1.37 | |
| Education level | ||||
| Primary * | 22 | 20 | 4.21 ± 1.32 | 0.083 |
| Secondary ** | 57 | 51.82 | 3.52 ± 1.41 | |
| University | 31 | 28.18 | 3.48 ± 1.28 | |
| Maternal Occupation | ||||
| Employed | 38 | 34.55 | 3.45 ± 1.37 | 0.366 |
| Unemployed | 72 | 65.45 | 3.73 ± 1.38 | |
| Current BMI (kg/m2) | ||||
| Underweight (<18.5) | 5 | 4.54 | 4.02 ± 1.71 | 0.904 |
| Normal (18.5–24.9) | 58 | 52.73 | 3.63 ± 1.45 | |
| Overweight (25.0–29.9) | 37 | 33.64 | 3.58 ± 1.24 | |
| Obese (≥30) | 10 | 9.09 | 3.80 ± 1.51 | |
| Seafood Consumption (times/week) | ||||
| Never-1 | 23 | 20.91 | 2.92 ± 1.07 | <0.001 |
| 2–3 | 64 | 58.18 | 3.53 ± 1.32 | |
| >3 | 23 | 20.91 | 4.79 ± 1.19 |
| Variables | Coefficients Correlation | T | p-Value | R2 |
|---|---|---|---|---|
| Protein Intake | 0.554 | 5.796 | <0.001 | 0.330 |
| Fat Intake | 0.051 | 0.600 | 0.550 | |
| Carbohydrate Intake | 0.076 | 0.918 | 0.361 | |
| Calorie intake | −0.128 | −1.285 | 0.202 | |
| Current BMI | 0.216 | 2.619 | 0.010 |
| Mean ± SD | p-Value * | |
|---|---|---|
| Animal protein (g/day) | 45.47 ± 20.10 | <0.001 |
| Plant protein (g/day) | 37.59 ± 16.45 | 0.012 |
| Food source of protein (g/day) | - | |
| Fish and sea products | 168 ± 14.53 | |
| Eggs | 120 ± 17.21 | |
| Meat | 23 ± 5.73 | |
| Chicken | 80 ± 18.34 | |
| Soy (tempeh, tofu) | 180 ± 20.33 | |
| Grains | 580 ± 23.17 | |
| Essential amino acid (g/day) | ||
| Isoleucine | 3.26 ± 0.93 | <0.001 |
| Leucine | 5.57 ± 1.99 | 0.03 |
| Lysine | 5.22 ± 1.58 | 0.003 |
| Phenilalanine | 4.03 ± 1.21 | 0.01 |
| Methionine | 1.45 ± 0.47 | 0.001 |
| Threonine | 2.99 ± 0.88 | 0.167 |
| Tryptopan | 1.14 ± 0.69 | 0.053 |
| Valine | 3.13 ± 0.91 | 0.018 |
| Maternal BLLs (µg/dL) | Maternal Protein Intake | Frequency | Total Milk Protein (g/dL) | ||
|---|---|---|---|---|---|
| Mean (SD) | β (SE) | p-Value | |||
| <2.0 | Low | 3 | 1.09 (0.09) | 0.943 (0.001) | 0.018 |
| Adequate | 1 | 1.16 (NA) | |||
| Hight | 4 | 1.19 (0.19) | |||
| 2.0 to <5.0 | Low | 24 | 1.05 (0.10) | 0.460 (0.001) | <0.001 |
| Adequate | 12 | 1.05 (0.07) | |||
| Hight | 45 | 1.21 (0.15) | |||
| ≥5.0 | Low | 12 | 1.03 (0.08) | 0.474 (0.001) | 0.088 |
| Adequate | 3 | 1.05 (0.06) | |||
| Hight | 6 | 1.07 (0.13) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wati, L.R.; Sargowo, D.; Nurseta, T.; Zuhriyah, L. The Role of Protein Intake on the Total Milk Protein in Lead-Exposed Lactating Mothers. Nutrients 2023, 15, 2584. https://doi.org/10.3390/nu15112584
Wati LR, Sargowo D, Nurseta T, Zuhriyah L. The Role of Protein Intake on the Total Milk Protein in Lead-Exposed Lactating Mothers. Nutrients. 2023; 15(11):2584. https://doi.org/10.3390/nu15112584
Chicago/Turabian StyleWati, Linda Ratna, Djanggan Sargowo, Tatit Nurseta, and Lilik Zuhriyah. 2023. "The Role of Protein Intake on the Total Milk Protein in Lead-Exposed Lactating Mothers" Nutrients 15, no. 11: 2584. https://doi.org/10.3390/nu15112584
APA StyleWati, L. R., Sargowo, D., Nurseta, T., & Zuhriyah, L. (2023). The Role of Protein Intake on the Total Milk Protein in Lead-Exposed Lactating Mothers. Nutrients, 15(11), 2584. https://doi.org/10.3390/nu15112584






