Abstract
The Zingiberaceae family possess various phenolic compounds that have significant systemic bioactivities in the brain, including in age-related neurodegenerative diseases. Neurotrophins are growth factors that protect neurons from oxidative stress, and dysregulation of the neurotrophic system may result in neurocognitive disease. Phenolic compounds from the Zingiberaceae family have been used in traditional and complementary medicine (TCM) to improve cognitive functions. These compounds may affect the expression of neurotrophic agents, but their underlying molecular mechanisms require further investigation. Therefore, the goal of this review is to determine the expression and functional roles of phenolic compounds from the Zingiberaceae family in brain disorders and age-related neurodegenerative disorders. While previous studies have proposed various mechanisms for the neuroprotective activity of these compounds, their precise mechanism of action remains complex and poorly understood. Despite some promising findings, there are still shortcomings in the therapeutic use of these herbs, and current interventions involving the Zingiberaceae family appear to be clinically insufficient. This article aims to summarize recent discoveries of phenolic compounds from several Zingiberaceae family members and their use as neuroprotectants and provide the first review of evidence-linked neuroprotective activity of bioactive ingredients from prominent members of the Zingiberaceae family.
1. Introduction
Phenolic acids are a major group of polyphenols. Plant-based diets that are high in bioactive compounds including polyphenols have demonstrated potential in reducing the risk of developing neurodegenerative disorders. In the past, researchers primarily studied the antioxidant effects of phenolic acids, which are due to their ability to chelate metals and scavenge free radicals [1,2]. However, recent studies have reported that bioactive polyphenols such as quercetin and demethoxycurcumin (DMC) can help prevent age-related cognitive decline associated with neurodegenerative diseases [3,4]. Polyphenols obtained from dietary sources can also improve cognitive deficits [5], promote synaptic plasticity [6], and enhance neurogenesis [7].
Zingiberaceae is a family of plants that includes ginger, turmeric, and galangal, among others. Some studies have suggested that phenolic compounds found in these plants may have potential therapeutic benefits for neurological brain disorders and neurodegenerative diseases [4]. For example, curcumin, a compound found in turmeric, has been studied for its potential neuroprotective effects in conditions such as Alzheimer’s disease [8], Parkinson’s disease [9], and multiple sclerosis [10]. Some studies have suggested that curcumin may help to reduce neuroinflammation and improve cognitive function [11]. Similarly, ginger has been studied for its potential to reduce inflammation and oxidative stress [12], which are believed to contribute to the development of neurological diseases. Studies have suggested that ginger may have neuroprotective effects in conditions such as Parkinson’s disease [13], Alzheimer’s disease [13], and Huntington’s disease [14]. It is important to note that while these studies are promising, more research is needed to fully understand the nature of brain and neurological disorders and the role of neuroprotectants, including those from Zingiberaceae family plants, in ameliorating the disease. Therefore, this review aims to summarize the mechanisms and main triggers of neurological and neurodegeneration diseases, and the neuroprotective properties of commonly reported phenolic compounds from Zingiberaceae family plants. This review also discusses the mechanisms and pathways by which the main phenolic compounds can exert their roles in the management of neurological brain disorders and neurodegenerative diseases.
1.1. Zingiberaceae Family and Their Phenolic Compounds
The Zingiberaceae family has culinary and medicinal uses and is widely distributed in the Indo-Malayan region [15]. One thousand six hundred species have been found in this family, which has about 53 genera including Aframomum, Alpinia, Amomum, Boesenbergia, Curcuma, Elettaria, Etlingera, Hedychium, Hitchenia, Kaempferia, Renealmia, and Zingiber [16,17]. The largest genus in the Zingiberaceae family is called Alpinia [18]. Their rhizomes are a source of phytochemicals which are beneficial for bioactivities [19].
A wide range of chemical substances are naturally produced by plants. Through numerous metabolic pathways, primary metabolites provide the necessary components for photosynthesis, translocation, and respiration. Secondary metabolites, created by biosynthetic modifications such as methylation, glycosylation, and hydroxylation, are typically the by-product of primary metabolites from various metabolic pathways and do not directly contribute to growth and development. Plant metabolites can be categorized into several main groups (Figure 1): (i) phenolic groups, (ii) terpenes, (iii) nitrogen-containing compounds, and (iv) sulfurous compounds [20]. The aromatic ring of phenolic compounds contains one or more hydroxyl groups. Phenolic acid, flavonoids, stilbenes, coumarins, lignans, and tannins are the principal naturally occurring phenolic compounds or phenolic antioxidants [21]. Approximately half of the naturally occurring plants are phenolics, whether present as a free state or as glycosides [22]. Flavonoids include flavonols, flavononols, flavones, flavanol (catechin), flavanones, anthocyanidins, and isoflavonoids [21]. These phenolic phytochemicals contribute to various biochemical properties, including the ability to regulate gene expression, act as antioxidant agents, and have antimutagenic and anticarcinogenic properties [23]. In addition, they reduce the risk of cardiovascular diseases [24,25,26] and are utilized in many aesthetic applications [27]. Among various Zingiberaceae family members, Alpinia calcarata, A. malaccensis, A. nigra, Amomum aromaticum, A. maximum, A. koenigii, Curcuma amada, Curcumacaesia, C. picta, C. longa, Hedychiumcoronarium, Hedychium coccineum, and H. thyrsiforme are the sources of many aesthetic compounds [19] and provide sustainable ecosystem applications [28]. C. longa, C. xanthorrhiza, and Kaempferia pandurata shield the skin against the effects of UV-B damage and possible skin wrinkles, and can act as antimicrobial, antiobesity, and anticancer agents [29,30,31]. Extraction of Zingiber rhizomes yielded phenolic acids, diarylheptanoids, terpenoids, sesquiterpene hydrocarbons, gingerols, shogaols, paradols, and terpenes. Asamenew et al. (2019) reported a comprehensive library of phytochemicals from ginger rhizomes [17]. Gingerols, shogaols, and paradols contribute to its recognized biological activities [32,33]. Some Zingiberaceae members have been discovered to contain essential oils such as pinene, limonene, eugenol, and geraniol [34]. Another team of researchers also identified trace levels of wikstromol, carinol, methyl-D-glucopyranoside, pyranone, propionate, and furanone [35]. Table 1 shows phenolic compounds of Zingiberaceae and their associated pharmacological activities. Phenolic compounds are categorized as primary antioxidants due to their redox characteristics. Phenolic antioxidants prevent oxidation by acting as free radical terminators or metal chelators. Phenolic antioxidants hinder oxidation; their primary role as free radical scavengers lessens oxidative stress, slowing down or preventing lipid oxidation or interrupting the propagation process, thus reducing the development of volatile decomposition products such as aldehydes and ketones [36]. Over time, oxidative stress may influence cognitive function and the establishment of pathogenic phenotypes and neurodegenerative disorders [37,38].
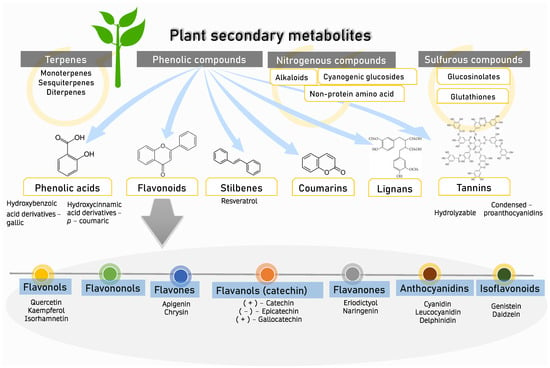
Figure 1.
Plant secondary metabolites. Four groups of major secondary metabolites which can be found in plants. The focus of this review is on phenolic compounds (phenolic acids, flavanoids, stilbenes, coumarins, lignans, and tannins) and their flavonoid subset (flavonols, flavononols, flavones, flavanols, flavanones, anthocyanidins, and isoflavonoids).

Table 1.
Zingiberaceae phenolic compounds and their associated pharmacological activities.
1.2. Zingiberaceae Family Plants Modulating Neurotrophic Pathways and Neuroinflammation
Neurological brain disorders and neurodegenerative diseases have complex and multifactorial causes and often involve a combination of genetic, environmental, and lifestyle factors. Imbalance of oxidative stress is also considered to be a potential trigger for neurological and neurodegenerative diseases [115]. Oxidative stress is harmful to healthy brain function and considered a causative factor for membrane hyperexcitability [116]. Accumulated reactive species can damage cellular macromolecules [117]. The production of O2−/H2O2 in neuronal brain redox signaling results in the formation of sulfenic acid [118]. Synaptic plasticity depends on Calcium (Ca2+) signaling. Therefore, the brain utilizes high amounts of adenosine triphosphate (ATP) to maintain its homeostasis. Due to the energy needs, the brain is susceptible to significant oxidative insults. The brain is high in lipid content and low in antioxidant capability [119]. There are two methods of antioxidant activity: prevention of reactive oxygen species (ROS) production and clearance of the damaged by-products of oxidative stress [120]. The first line of defense consists of enzymatic antioxidant disease, and the non-enzymatic antioxidant system serves as the second line of defense [121]. The repair systems engage by restoring oxidatively damaged macromolecules by stimulating the activities of phospholipases, peroxidases, or acryl transferases or eliminating them [122,123].
Nuclear factor erythroid 2-related factor 2 (NRF2) regulates antioxidant response. The actin-anchored protein Kelch-like ECH-associated protein 1 (Keap1), which is mostly located in the cytoplasm, and the transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2) interact when cells are dormant [124]. When exposed to oxidative stress or substances that affect the cysteine residues in Keap1, Nrf2 is freed from ongoing degradation by dissociating from Keap1 and translocating into the nucleus to promote the activity of antioxidant enzymes and reduction of intracellular ROS [125]. Previously, in experimental models of amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS), acetyl-11-keto-beta boswellic acid (AKBA) stimulated the Nrf2/HO-1 pathway and promoted neuroprotection [126,127]. Neurodegenerative diseases lead to irreversible neuronal degeneration [128] which can be manifested by an increase in protein aggregates that encourage glial activation and inflammation, mitochondrial dysfunction, imbalance of neurotransmitters, and autophagia [129,130] further associated with neuroinflammation [116].
Numerous inflammatory processes are linked to neurocognitive diseases affecting the central nervous system (CNS) [131,132,133]. Additionally, extracellular damage-associated molecular pattern molecules (DAMPs) and inflammatogenic molecules are produced from damaged brain cells by cerebral inflammation, exacerbating CNS pathologies [134]. By eradicating or suppressing various infections, neuroinflammation serves as a protective defense system for the brain [135]. By encouraging tissue healing and clearing away cellular waste, this inflammatory response may be beneficial yet detrimental as a persistent inflammatory response might prevent regeneration [136]. Due to exogenous factors such as infection or drugs, or endogenous factors such as genetic mutation and protein aggregation, inflammation can be stimulated persistently [137]. Chronic activation of microglia leads to sustained production of proinflammatory cytokines that contributes to progression of various neurodegenerative diseases [138]. Under neurotoxic and neurodegenerative circumstances, reactive astrocytes can be polarized into different phenotypes which are determined by microglia [139]. Tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) are examples of pro-inflammatory cytokines generated by activated microglia that induce A1 polarisation, while interleukin-10 (IL-10) promotes A2 polarisation [140]. Additionally, research has linked pathways associated with pain and inflammation [141,142]. Microglia and astrocytes are involved in persistent proinflammatory responses, which contribute to the development of neurodegenerative disorders [131,142]. Astrocyte polarisation has been reported in a number of neurodegenerative illnesses and neurotoxic circumstances, including ischemia [143], traumatic brain injury (TBI) [144], Alzheimer’s disease (AD) [145], and Parkinson’s disease (PD) [146]. Amyloid-β1-42 peptide (Aβ1-42) can activate microglial cells, which then in Alzheimer’s disease secrete pro-inflammatory chemokines and cytokines [147]. In addition, viral infections can stimulate astrocytes and microglia and subsequently trigger peripheral immune cells to invade the CNS, thus creating an inflammatory environment [148]. In mice with kainate-induced status epilepticus, microglia and astrocytes were sequentially activated [149]. Brain cells emit a variety of chemicals to deal with metabolic stressors. These chemicals including neurotrophic factors supporting neuronal survival and modulating the homeostasis of neuroinflammation [150].
Neurotrophic factors are proteins secreted by different cell types. They support neuronal cell development, survival, and neurite outgrowth. They perform crucial functions in the differentiation and proliferation of neurites and apoptosis [151]. There are three families of neurotrophic factors: The first is the NGF family, also known as neurotrophins. Several examples include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4) also known as neurotrophins 5 (NT-5) (NT-4/5) [152]. Second are the glial cell line-derived neurotrophic factor (GDNF) family ligands including GDNF and neurturin (NRTN). The third group is a heterogeneous group of molecules that belong to the cytokine family [153]. In vivo study hypothesized that dysregulation of neurotrophic factors may be involved in the etiology of autism [154], anxiety [155], major depressive disorder (MDD) [156], post-traumatic stress disorder (PTSD) [157], schizophrenia [158], and various neurodegenerative disorders such as PD [159], AD [160], and bipolar disorder (BD) [161]. The stimulation of motor neurons with BDNF may improve motor function in an amyotrophic lateral sclerosis (ALS) model [162]. Another study verified the association of neurotrophic factors with neuroinflammation. They established that combined injury to mouse hippocampal neurons can release BDNF and speed up neuronal apoptosis [163]. In a proteomic profile, neurotrophic factors (BDNF and epidermal growth factor, EGF) were also reportedly implicated in neuroinflammation and blood-brain barrier (BBB) permeability in familial and sporadic ALS [164]. Depression and AD have been connected to polymorphisms in genes that change the level of neurotrophins [165]. In diabetic polyneuropathy, neuropathic pain and obesity are modulated by TrkB signaling. Thus, patients with diabetic polyneuropathy have significantly greater serum levels of TrkB and BDNF than healthy individuals [166]. NGF and PGE2 serum levels in the brain were positively associated with headache frequency in adult migraine patients, whereas BDNF and VEGF serum levels were not [167]. Moreover, a four-week period of persistent overexpression of BDNF, TrkB, and GDNF was associated with anticonvulsant effects [168]. The extent of the injury and genetic variation influence the expression of neurotrophins after traumatic brain injury (TBI) [152]. Furthermore, reduction in NGF-expressing neurons increases the risk of developing autism spectrum disorder (ASD) [169].
Neurotrophins are produced in the nervous system, gastrointestinal tract, and urinary tract [170,171]. They mediate the differentiation and survival of neurons by activating downstream signaling pathways [172,173]. Neurotrophins are initially produced in a pro-form, then go through proteolytic cleavage to create mature neurotrophins. Neurotrophins possess stronger affinity for binding with Trk receptors compared with p75 neurotrophin receptors (p75NTR) [174]. With the capacity to transmit signals, the phosphorylated tyrosine residues then induce the recruitment of intracellular proteins. NGF is regulated by tropomyosin receptor kinase receptor A (TrkA), BDNF and NT-4/5 are primarily regulated by tropomyosin receptor kinase receptor B (TrkB), and NT-3 is primarily regulated by tropomyosin receptor kinase receptor C (TrkC) [175]. There is some overlap in cross-activations due to significant structural homology shared by Trk receptors and neurotrophins. Additionally, TrkB and TrkC have truncated isoforms. However, these truncated variants cannot evoke the same reaction since they lack cytoplasmic tyrosine kinase catalytic domains [176]. Upon binding with neurotrophins, Trk receptors then activate several intracellular signaling pathways (Figure 2).
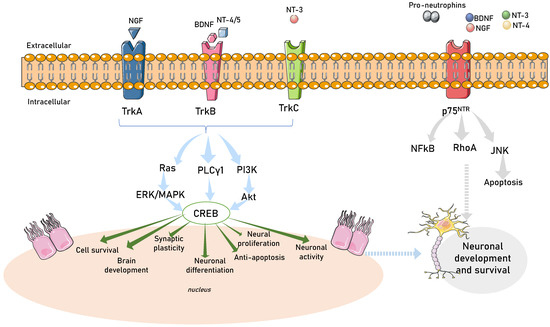
Figure 2.
Neurotrophin intracellular signaling pathways. A schematic view of TRK receptor tyrosine kinases and major signal transduction pathways involved in cell survival, development, synaptic plasticity, neuronal differentiation, neural proliferation, and neuronal activity. Activity-dependent mechanisms act through activation of transcription mediated by CREB (calcium responsive element binding protein) in the nucleus of the cell. TRKA is activated by nerve growth factor (NGF), TRKB is activated by brain-derived neurotrophic factor (BDNF), neurotrophin 4/5 (NT-4/5) and TRKC are activated by neurotrophin-3 (NT3). RAS, rat sarcoma oncogene; ERK, extracellular-signal-regulated kinase; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; Ak strain transforming (Akt); tropomyosin-related kinase A (TrkA); tropomyosin-related kinase B (TrkB); tropomyosin-related kinase C (TrkC); phospholipase C-γ1 (PLCγ1); extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK); p75 neurotrophin receptor (P75NTR); nuclear factor-κB (NFKB); ras homolog family member A (RhoA); Jun N-terminal kinases (JNK).
1.2.1. The Ras/ERK (MAPK) Pathway
The small, membrane-associated Ras proteins’ activity controls the Ras/ERK pathway. Shc binds to the phosphorylated tyrosine 490 and induces the signal activation cascade. Ras is then activated through guanosine triphosphate (GTP) binding [177]. Raf further activates MEK to phosphorylate ERKs [178]. ERK activation regulates neuronal enzymes and ion channels [179], neuritic process lengthening, and neuronal survival [180]. Depression can affect the activation of ERKs [181] and chronic pain is caused by hypersensitization of NGF [182]. Moreover, activation of JNK and p38 MAPK induced cerebral angiopathy [183].
1.2.2. Phospholipase C, Gamma 1 (PLCγ1) Pathways
PLCγ1 mediates NT/Trk receptor interactions [184]. Tyrosine phosphorylation controls the PLC-beta protein. The SH-2 and SH-3 domains are two of the domains found in PLC. Once activated, lipid phosphatidylinositol 4,5-bisphosphate is cleaved by PLC-1 to produce inositol trisphosphate (IP3) and diacylglycerol (DAG) [185]. Since IP3 is soluble, it can diffuse and cause Ca2+ to be released into the cytoplasm [186]. Protein kinase C may be activated to compensate for the increase in Ca2+ or DAG concentration in the cells [187].
1.2.3. The PI3K/Akt-mTOR Pathway
Trk receptor phosphorylation triggers PI3K activation. TrkA phosphorylates Shc, which then forms a complex with Grb2 to phosphorylate the Gab1 adaptor protein [188]. It also induces insulin receptor substrate 1 (IRS1) to become phosphorylated [189]. Mechanistic target rapamycin complex 1 or 2 (mTORC1 or mTORC2) is triggered by PI3K/Akt activation [190]. Promoting protein translation and synthesis, ribosomal biogenesis, and autophagy, the mTORC1 protein kinase complex is important for metabolism. It also controls mRNA translation by phosphorylating downstream effectors including P70 ribosomal S6 protein kinase (p70S6K) [191]. In addition, protein kinase B (Akt), which is linked to a number of clinical diseases, is one of the AGC kinases that are phosphorylated by mTORC2 to promote cell proliferation and survival and promote cytoskeleton remodeling [192].
1.2.4. The p75NTR-Mediated Signaling Pathway
All neurotrophins have low affinity for the p75 neurotrophin receptor (p75NTR). Neurotrophins and each of their proforms are bound by the extracellular domain. The outcomes of p75NTR activation are influenced by interactions with other receptors. Due to the absence of enzymatic activity in its cytoplasmic domain, p75NTR does not signal via conventional channels. Therefore, interactors of the intracellular domain or interacting proteins that are attracted to or connected with the receptor contribute to the signaling of the p75NTR including apoptosis [193,194], p53 activation via the Jun kinase signaling cascade, and activation of NF-κB for neuronal survival [195] and axonal development [196]. p75NTR activation is also influenced by interactions with other receptors and transmembrane proteins [197,198]. p75NTR has a beneficial effect on cell survival by promoting ceramide production [199]. Ceramide controls ERK/MAPK, PI3K/Akt, Jun kinase, and NFkB signaling pathways [200].
Ginger’s 6-shogaol has been shown to have neuroprotective effects against H2O2-induced neuronal death in astrocytes by inhibiting ROS, Bax, and caspase 3 while increasing BDNF, GDNF, NGF, Bcl-2, and Bcl-xL via ERK1/2-mediated signaling [201]. In a mouse model of systemic neuroinflammation, a botanical mixture consisting of Zingiber officinale (150 mg kg−1), Echinacea purpurea (20 mg kg−1), and Centella asiatica (200 mg kg−1) demonstrated significant potential as an anti-inflammatory agent. Specifically, the study found that the density of the complement component 3 (C3) in the prefrontal cortex of lipopolysaccharides (LPS)-treated mice was significantly reduced following administration of the botanical mixture. This reduction in C3 density is indicative of anti-inflammatory activity and may contribute to the observed improvement in cognitive function seen in in vivo studies. Notably, the botanical mixture was found to be effective despite the persistently high level of C1q, which can modulate gene expression critical for neuronal survival when over-expressed in the absence of other complement components [202].
Several pathways associated with neuroinflammation and neurodegeneration can be activated by matrix metalloproteinases (MMP)-2 and MMP-9. Natural compounds have been found to regulate signal transduction pathways, resulting in the downregulation of both MMP-2 and MMP-9 gene and protein expression. Although these effects may be attributed to their general anti-inflammatory and antioxidant properties, certain compounds have been directly shown to have anti-proteolytic effects on MMP-2 and/or MMP-9. Examples of such compounds include Ala–Thr–Pro–Gly–Asp–Glu–Gly (ATPGDEG), Leu-Ser-Gly-Tyr-Gly-Pro (LSGYGP), a naturally occurring N-farnesylated dibenzodiazepinone-BU-4664L, ageladine A, quercetin, and myricetin [203]. Phenolic compounds such as 6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, 6-paradol, zerumbone, curcumin, α-terpinyl acetate, curcumenol, and methoxyflavone have demonstrated beneficial effects on neurogenesis and neuroprotection, improved clinical symptoms in animal models, and reduced inflammatory mediators such as IL-1β, TNF-α, and IL-6 release [204,205,206,207,208]. Pre-treatment of SIM-A9 microglial cells (CRL-3265) with ginger root ethanol extract at a dose of 200 µg/mL showed neuroprotective effects by inhibiting the expression of cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS), which reduced the release of prostaglandin E2 (PGE2) and nitric oxide (NO). Additionally, ginger root ethanol extract has been shown to decrease the production of TNF-α and IL-6 induced by LPS. Ginger root ethanol extract also improved microglia-mediated neuronal damage by decreasing the expression of Bcl-2 and increasing Bax. As a result, ginger root ethanol extract was able to reduce neuroinflammation in LPS-stimulated mouse microglia by modulating serine-threonine protein kinase (Akt)-signal transducer and activator of transcription 3 (STAT3), Akt/STAT3, mitogen-activated protein kinases (MAPK), and NF-κB signaling pathways in the neuroinflammatory response [209]. Treatment with 6-gingerol at a dose of 5 mg/kg in male C57/BL6J mice and doses of 10, 20 and 30 μM of primary microglia from 1-day-old C57/BL6J mice showed improvements in cerebral ischemia injury by suppressing microglia-mediated neuroinflammation by downregulating the Akt-mammalian target of the rapamycin (mTOR)-STAT3 pathway [204]. On the other hand, oral administration of 10 or 25 mg/kg/day of 6-gingerol was reported to upregulate the protein levels of neurotrophic factor BDNF, apparently mediated by activation of the Akt-cAMP response element-binding protein (CREB) pathway [210]. Their crosstalk is summarized in Figure 3. Zerumbone and curcumin were found to reduce inflammation and provide neuroprotection by inhibiting the translocation of NF-κB into the nucleus, thereby reducing the activation of reactive oxygen species. Moreover, activation of the pathways of phosphatidylinositol-3/protein kinase B/glycogen synthase kinase-3 and phosphatidylinositol-3/protein kinase B/cAMP response element-binding protein/brain-derived neurotrophic factor was found to reduce neurodegeneration [211].
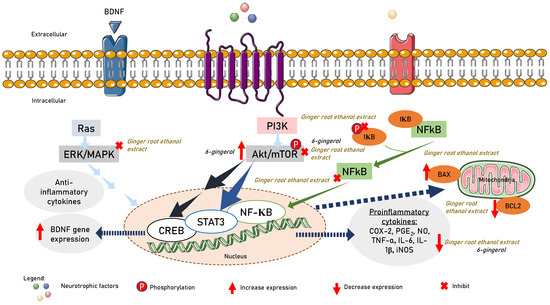
Figure 3.
6-gingerol suppression and activation of Akt/STAT3 to suppress proinflammatory cytokine release and Akt/CREB to promote BDNF gene expression. Ginger root extract ameliorated microglia-mediated neuronal insults via upregulating the expression of Bax and reducing the expression of Bcl-2. Ginger root extract suppressed NF-κB and AKT/STAT3, and the MAPK pathway in the neuroinflammatory response.
The basic fibroblast growth factor (bFGF)/NGF/TrkA/heat shock protein 70 (Hsp70) signaling pathway was reportedly modulated by Afromomum extracts [212], while the BDNF/TrkB/Akt signaling pathway was activated by Alpinia extract [213]. The CREB pathway [214] and the peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha (PGC1)/fibronectin type III domain-containing protein 5 (FNDC5)/BDNF pathway can both be activated by curcumin [215]. Additionally, Kaempferia spp. blocks autocrine IL-6/STAT3 signaling and decreases EGF-induced IL-6 synthesis [216]. A summary of beneficial effects of Zingiberaceae family members on neurogenesis and neuroprotection is presented in Table 2 and Figure 4.

Table 2.
Zingiberaceae modulating neurotrophic pathways.
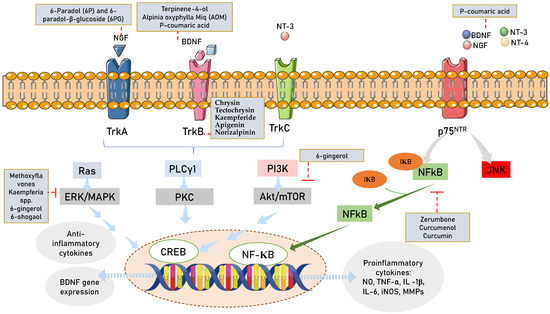
Figure 4.
Zingiberaceae targeting neurotrophin intracellular signaling pathways. Legends for figure: Akt—Ak strain transforming; NGF—nerve growth factor; BDNF—brain derived neurotrophic nerve factor; TrkA—tropomyosin-related kinase A; TrkB—tropomyosin-related kinase B; TrkC—tropomyosin-related kinase C; NT3-neurotrophin 3; NT4/5—neurotrophin 4/5; PLCγ1—phospholipase C-γ1; ERK/MAPK—extracellular signal-regulated kinase/mitogen-activated protein kinase; CREB-cAMP—response element binding protein; P75NTR—p75 neurotrophin receptor; NFKB—nuclear factor-κB; RhoA—ras homolog family member A; JNK—c-Jun N-terminal kinases.
1.3. Brain Disorders and Brain Insults
Brain disorders are medical conditions that can interfere with the normal functioning of the brain and can result in a variety of symptoms that impair cognitive, emotional, and physical abilities, depending on which areas of the brain or nervous system are affected. Some examples of brain disorders include neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, mood disorders such as depression and bipolar disorder, anxiety disorders such as generalized anxiety disorder and panic disorder, schizophrenia, substance use disorders, developmental disorders such as autism spectrum disorder and attention deficit hyperactivity disorder (ADHD), stroke (caused by a blockage or bleeding in the brain), and traumatic brain injury. Stroke, ischemia, and traumatic brain injury are brain insults that are considered risk factors for developing neurodegenerative disorders. The degeneration of higher-order structural brain networks is commonly observed in cases of sub-acute ischemic stroke, and this breakdown of structural brain networks might play a role in the development of domain-specific cognitive impairments over time [237] contributing to age-related neurodegenerative disease.
1.4. Role of Zingiberaceae Family Plants in Managing Age-Related Neurodegenerative Disease
Specific brain regions were impacted more frequently in cases of cognitive impairment. Age-related alterations in neuroanatomical structures have been connected to cognitive function. A previous study indicated that geriatric cognitive performance is determined as early as the age of 20 [238]. Even though some cognitive abilities are relatively stable with ageing, those that depend on mental functioning deteriorate as people grow older [239]. The dentate gyrus (DG) of the hippocampal formation has the capacity to produce new neurons throughout the human lifetime. While pathological ageing, which is characterized by memory deficits, is linked to neurogenesis exhaustion, successful ageing, when it can preserve memory functions, is associated with the maintenance of a relatively high neurogenesis level [240,241,242]. The phenotypic age of the human brain, as revealed via deep learning of anatomic magnetic resonance images (MRI) reflects patterns of structural change related to cognitive decline, and the prefrontal cortex is the region of the greatest age-related vulnerability [243]. Whole ethanol extract of fresh Zingiber cassumunar rhizomes reduced neuronal cell loss in the hippocampus while suppressing the inflammatory response by reducing the expression of glial fibrillary acidic protein, GFAP (a marker of astrocyte activation) and IL-1ß in the hippocampus [244]. Another group of researchers reported that Zingiber purpureum Roscoe extract promotes neuronal differentiation of hfNSCs and enhances neurite outgrowth of immature neurons while promoting the expression of genes involved with forebrain development and neuronal differentiation, such as eomesodermin (EOMES, or T-box brain protein 2(TBR2)), doublecortin (DCX), and distal-less homeobox 2(DLX2) [206].
Neurodegenerative illnesses are fatal conditions that result in gradual deterioration of memory and motor skills [245]. There are several types of neurodegenerative disorders, including Pick’s disease, transmissible spongiform encephalopathies (TSEs), and Lou Gehrig’s disease (LGM). In various neurodegenerative diseases, age-related pathological changes in multiple brain regions have been associated with cognitive impairments [246]. In Alzheimer’s disease (AD) research, the hippocampus is the most affected area, with a reduction in its volume [247]. Parkinson’s disease (PD) patients with cognitive impairments have lower global and nodal phase linearity measurement (PLM) results in various temporal regions [248]. Amyotrophic lateral sclerosis (ALS) patients show a reduction in cortical volumes through voxel-based morphometry [249]. Due to the loss of specific neuronal populations, various neuropathological changes in neurodegenerative disorders are related to pain and decline in function [250]. Abnormal protein dynamics are also characteristic of neurodegenerative diseases, where disease-specific proteins can accumulate, mislocalize, or multimerize into fibrils and become toxic [251]. Several pathways, such as mTOR, MAPK, Ca2+, apolipoprotein E (APOE)-cholesterol, and high mobility group box 1 (HMGB1), are involved in the progression of these diseases, regulating the apoptosis, regeneration, and plasticity of neurons and microglia (Table 3). Although neurodegenerative diseases are typically sporadic, they can also be induced by mutations in proteins that are prone to aggregation. Mutations in the amyloid precursor protein (APP) gene cause familial Alzheimer’s disease [252]. In addition to genetic factors, ischemic conditions that cause damage to neuronal tissue can lead to secondary injury or progressive degeneration. Stroke is an example of an injury-triggered neurodegenerative disease [253]. Apart from abnormal protein and gene dynamics, these diseases share common features such as imbalanced antioxidant systems, mitochondrial dysfunction, neuroinflammation, impaired neurotrophin function, oxidative stress, and ineffective antioxidant defense [252,254,255].
Zingiberaceae plants and their active components have potential therapeutic applications in the treatment of neurodegenerative disorders. Previous studies have used a variety of extraction techniques to increase the yield of active compounds from ginger and curcumin, which can then be tested via in vitro and in vivo models of neurodegenerative diseases. These techniques include methods such as solvent extraction, steam distillation, and Soxhlet extraction, among others [256]. By using these extraction methods, researchers have been able to obtain higher concentrations of active compounds such as 6-gingerol and curcuminoids. Testing of these compounds in relevant disease models provides insights into their potential therapeutic applications in the treatment of neurodegenerative disorders. In some cases, standardized commercial formulations of the extracts have also been used. One such extract, curcumin, was found to promote neuronal regeneration in a rat model of parkinsonism induced by 6-hydroxydopamine (6-OHDA) by modulating the bFGF/NGF/TrkA/Hsp70 pathway. It increased the levels of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), as well as the expressions of bFGF, NGF, and TrkA, while reducing the concentration of malonaldehyde (MDA) [212]. Another extract, the methanol extract of 6-paradol (6P) and 6-paradol-β-glucoside (6PG) from Aframomum meleguet seed, showed significant neurite outgrowth activity in a scopolamine-induced dementia mouse model of Alzheimer’s disease by increasing Ca2+ influx into the cells without activating ERK and CREB in the NGF-TrkA pathway [233]. The efficacy of A. oxyphylla (AO) in the treatment of Alzheimer’s disease (AD) has been demonstrated through research; the study identified 26 bioactive phytochemicals in AO that target 168 key molecules involved in the pathogenesis of neurodegenerative dementia. Yakuchinone B, 5-HYD, oxyhylladiketone, oxyphyllacinol, butyl-β-D-fructopyranoside, dibutyl phthalate, chrysin, yakuchinone A, rhamnetin, and rhamnocitrin were found to be the key phytochemicals that regulate the pathogenesis of neurodegenerative dementia in a multitargeted manner. Protein–protein interaction (PPI) analysis showed that the core targets, including AKT1, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), TNF, IL6, catenin beta–1 (CTNNB1), mitogen-activated protein kinase 3 (MAPK3), vascular endothelial growth factor A (VEGFA), caspase-3 (CASP3), heat shock protein HSP 90-alpha (HSP90AA1), STAT3, estrogen receptor–1 (ESR1), and MTOR were highly ranked in the PPI network formed by all 662 AO targets [257]. Pharmacology and molecular docking approaches were used to investigate the mechanism of AO’s anti-AD activity, revealing that AO, particularly in its terpenes, possesses neuroprotective effects that regulate the synthesis, release, and transmission of neurotransmitters, as well as the formation and plasticity of dendritic spines and synapses in the nervous system [258]. Studies have demonstrated the significant neuroprotective activity effects of chloroform (CF) extract from the fruits of Alpinia oxyphylla. The CF extract was found to enhance cognitive performance, increase activities of glutathione peroxidase (GSH-px), and decrease levels of malondialdehyde (MDA), acetylcholinesterase (AChE), and amyloid-β (Aβ) in mice injected with Aβ1−42. The long-term treatment of CF also reversed the activation of microglia, degeneration of neuronal acidophilia, and nuclear condensation in the cortex and hippocampus. These results showed that CF ameliorates learning and memory deficits by attenuating oxidative stress and regulating the activation of microglia and degeneration of neuronal acidophilia to reinforce cholinergic functions [259].
In a mouse model of Alzheimer’s disease displaying cerebral amyloidosis and neuroinflammation, zerumbone, a sesquiterpene, was found effectively to improve behavioral impairments, reduce β-amyloid deposition, and attenuate neuroinflammation. The anti-inflammatory activity of zerumbone was observed in microglial cells, and it induced a phenotypic switch in microglia from a pro-inflammatory to an anti-inflammatory phenotype by inhibiting the MAPK signaling pathway. These findings suggest that the neuroprotective effects of zerumbone may be attributed to its ability to support the survival of neurons [260].

Table 3.
Common pathways associated with neurodegenerative diseases.
Table 3.
Common pathways associated with neurodegenerative diseases.
| Disease | Manifestation | Pathogenesis | Canonical Pathway | Reference |
|---|---|---|---|---|
| Age-related macular degeneration (AMD) | ↑ level of complement components (C3, C3d, Bb, and C5, C5a) | Dysregulated metabolites in glycerophospholipid metabolism | mTOR | [261,262] |
| Transmissible spongiform encephalopathies (TSEs) | Presence of PrPSc and/or pathognomonic histopathological characteristics | The C-terminal region of the protein can cause amyloid formation by increasing the propensity of huPrP to aggregate due to the mutation T183A variant | Insulin signaling pathway | [263,264] |
| Pick’s disease | Atrophy of the frontal and anterior temporal lobes caused by an intraneuronal accumulation of aberrant protein inclusion bodies | ↓ N-acetyl aspartate and glutamate | mTOR or Class III-PI3K/Beclin-1 complex | [265,266] |
| AD | ↑ of β secretase expression causes, ↑ in amyloidogenesis↑ LPO under oxidative stress is significantly linked to neurotoxicity in AD Triggered by mitochondrial malfunction and oxidative stress | Mitochondria ↑ 4-HNE generated by damaged mitochondria Oxidative stress ↑ Ca2+ and ROS levels ↑ p-tau aggregates ↓ activities of antioxidants | APOE-cholesterol pathway | [253,267,268] |
| PD | α-Synuclein aggregation and build-up in the nervous system | ↑ Protein aggregation of-α synuclein was accelerated by oxidative stress which causes the brain cells to die | Autophagy-lysosomal pathway (ALP) Ca2+ signaling MAPK mTOR | [269] |
| Progressive supranuclear palsy | Intracerebral accumulation of microtubule | Hyperphosphorylation of Tau protein | Insulin and neurotrophic factor pathways, Fyn kinase pathways in myelinating oligodendrocytes, PP1 pathway | [270,271,272] |
| ALS | Motor neuron degeneration | ↑ β–catenin protein | Ca2+ pathway HMGB1 pathway | [273,274] |
Common pathways associated with neurodegenerative diseases. (↑) denotes increment; (↓) denotes reduction.
1.5. Other Brain Disorders and the Role of Zingiberaceae Family Plants
Cerebral ischemia (lack of oxygen and blood flow to the brain) and brain trauma can cause loss of cellular function and tissue death. These conditions can result from various initial insults, including metabolic stress, biochemical and molecular events, and ionic perturbations [275]. Brain ischemic insult can be caused by factors such as severe reductions in cerebral blood flow, occlusion of cerebral and extracerebral vessel tissues due to thrombosis or embolism, or prolonged systemic hypotension [276]. Mechanisms of brain ischemic insult include glutamate toxicity, calcium toxicity, free radicals, nitric oxide, inflammatory responses, and endoplasmic reticulum or mitochondrion dysfunction [277]. Since one of the pathological characteristics of cerebral ischemia is represented by the neuroinflammation following microglia activation, it is necessary to find useful therapeutic strategies to reduce the neuroinflammatory responses in activated microglia. Kaempferia parviflora Wall. ex Baker provides neuroprotection for HT-22 neuronal cells with glutamate-induced cytotoxicity by increasing BDNF expression and reducing p-ERK levels [100]. If ischemia persists long enough, irreversible neuronal loss can occur, leading to an ischemic stroke. Neurons are unable to maintain their function without enough energy (ATP), which causes them to release their contents uncontrollably. This pathological release leads to an excessive amount of glutamate that binds to ionotropic glutamate receptors [-amino-3-hydroxy 5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors], causing over-excitation and allowing calcium to enter the cells. The influx of calcium activates downstream signaling pathways, leading to cellular damage and death [278].
Traumatic brain injury (TBI) is a brain insult caused by an external mechanical force, resulting in temporary or permanent impairment of cognitive, physical, and psychological functions, often accompanied by loss of or change in consciousness [279]. There are two types of neuronal tissue damage associated with TBI: primary and secondary injury. Primary injury occurs during the initial insult and affects localized areas, causing damage to brain tissues and vessels, and may lead to skull fractures or hematomas. Secondary injury, which follows primary insult, causes further tissue and cellular damage and progresses slowly over months to years [280]. The events during secondary damage include axonal degeneration, mitochondrial dysfunction, excitotoxicity, oxidative stress, and apoptotic cell death of neurons and glia [280]. This can lead to neurodegeneration and cognitive impairment. Patients with a history of TBI are at an increased risk of developing proteinopathy, which is a pathological feature of neurodegenerative disorders. Moderate to severe TBI also increases the long-term risk of stroke [281], particularly in older adults [282]. There is a need to find treatment to improve brain plasticity which could allow greater recovery after cerebral ischemia and injury. Pre-treatment with 6-gingerol from ginger reduced middle cerebral artery occlusion (MCAO)-induced iNOS, IL-6, and IL-1β protein production in microglia of C57/BL6J mice in an ischemia brain injury model [204]. Zingiber officinale Roscoe extract had a positive effect on inflammation-impaired SH-SY5Y cell viability by reduction of pERK levels and HDAC1 protein levels, inhibiting NF-κB signaling activation and reducing release of IL-1β, TNF-α, and IL-6 [283]. Increase in neurogenesis in a traumatic brain injury model using Sprague–Dawley rat brains by using curcumin powder was reported by Sun et al. (2020) [229]. P-coumaric acid from whole Alpinia oxyphylla Miq. ethanol extract improved poststroke cognitive impairment of adult hippocampal neurogenesis, and improved spatial learning, memory, and cognitive functions in post-MCAO ischemic rats [213]. Furthermore, repeated oral administration of Alpinia katsumadai extract protected neurons from ischemic damage in the hippocampus in a gerbil model of transient cerebral ischemia [235].
In an amnesia model, treatment of C57BL/6 mice with 6-gingerol increased the protein expression of BDNF, which was mediated via the activation of protein kinase B/Akt- and cAMP-response element binding protein (CREB) signaling pathway [210]. While amnesia is not necessarily considered a mental health problem in and of itself, it can be a symptom of underlying mental health conditions or brain disorders. For example, amnesia can be a symptom of depression, anxiety, dissociative disorders, or post-traumatic stress disorder (PTSD). Moreover, depression has been associated with combined frontal lobe and corpus callosum abnormalities [284]. The compound curcumin found in Zingiberaceae plants may also have antidepressant-like effects by modulating the PGC1α/FNDC5/BDNF pathway. It reversed depression and pseudodementia by reducing corticosterone levels and increasing hippocampal BDNF, 5-hydroxytryptamine (5-HT), dopamine (DA), and acetylcholine (ACh) levels in male Wistar rats [285]. Zingiber purpureum significantly induced neurite sprouting in PC12 cells, increasing the neurite length and number of neurites in primary cultured rat cortical neurons [286]. Alpinia oxyphylla Miq (fruits, ethanol extraction) demonstrated antidepressant-like effects on chronic unpredictable mild stress protocol on male Kunming mice. Another Zingiberaceae plant product, ginger-degraded collagen hydrolysate (GDCH) from ginger rhizomes reduced immobility time in the forced swim test while increasing GDNF and ciliary neurotrophic factor (CNTF) mRNA expression in the hippocampus of male ddY mice. This might have induced a neurotrophic effect on neural stem cells, astrocytes, and neurons in the stress model, exerting antidepressant effects [287].
On the other hand, volume loss in specific temporal and occipital regions has been linked to higher annual rates of memory deterioration [288]. Reductions in the tract integrity of white matter associated with ageing influence capacity for mental tasks [289]. An abnormal cortex is linked to schizophrenia [290] whereas abnormalities in perisylvian magnetoencephalography have also been observed in people with autism spectrum disorders [291]. For autistic neurodevelopmental disorders, 6-shogaol of ginger provides neuroprotection by reducing 4-hydroxy-2-nonenal (4-HNE) and myeloperoxidase, the biomarkers for autism [292]. Essential oil of Alpinia zerumbet leaves (EOAZ) reversed schizophrenia-like symptoms in male Swiss mice by increasing the release of IL6 and BDNF [208].
A polyherbal preparation from the Zingiberaceae family boosted the levels of expression of BDNF in a murine model of scopolamine-induced dementia [179]. It was reported that 6-shogaol increased choline acetyltransferase, choline transporter, and BDNF expression and decreased ROS production thereby decreasing ROS production through the BDNF/TrkB-mediated signaling pathway in H2O2-treated HT22 hippocampal neuronal cells [293] and exhibited anti-inflammatory activity by inhibiting NO, iNOS, PGE2, IL-1, TNF-, Cox-2, P38 MAPK, and NF-KB in BV2 and primary microglial cells that had been exposed to LPS [173,294]. These findings indicate the promising outcomes of 6-shogaol as a phytotherapeutic agent for the treatment of neurodegenerative illnesses. Zingiber purpureum Rosc., or bangle, exhibited neurotrophic effects in a murine model by increasing the number of Ki67-positive cells in the dentate gyrus of SAMP8 mice, showing neurotrophin-like activity by inducing neurite sprouting in PC12 cells [217]. Bangle extract increased the rate of neurite outgrowth from developing neurons and improved neuronal differentiation. Bangle extract also promoted the expression of genes involved in neurogenesis and the targets of WNT signaling. It also changed the histone modifications in human fetal neural stem cells (hfNSCs), making it easier for β-catenin to accumulate in their nuclei. To lessen the symptoms of neurological diseases and aid in neurorehabilitation, bangle may therefore be a compelling candidate [206].
Inhibition of Erk1/2 and Pkc had no effect on the (-) trans-banglene potentiation of NGF-induced neurogenesis [295]. Furthermore, the combined use of ginger and gabapentin demonstrated a neuroprotective effect as shown by overall improvement in the examined fetal brain tissues [296]. Gingerol-enriched ginger supplementation has also shown to be able to reduce neuropathic pain via the gut–brain axis [297]. Additionally, Alpinia zerumbet and Alpinia oxyphylla Miq. extracts alleviated schizophrenia-like symptoms and improved post-stroke cognitive impairment [208,213].
1.6. Zingiberaceae Family in Clinical and Toxicity Study
Although only a limited number of natural products have been tested in clinical trials, many compounds have shown promising properties in preclinical studies [298]. Ginger is listed as “Generally Recognized as Safe” (GRAS) by the United States Food and Drug Administration (FDA) and has been shown to possess significant potential for improving and preventing memory impairments. In preclinical and clinical studies [299], the use of ginger has shown potential as a cognitive enhancer in middle-aged women. A study involving 60 participants found that standardized ginger extract at a dose of 800 mg once daily for 2 months improved working memory and resulted in increased N100 and P300 amplitudes, decreased P300 latencies, and enhanced working memory, evaluated using computerized battery tests and the auditory oddball paradigm of event-related potentials [300]. However, for the prophylactic treatment of migraines, administering 200 mg of dry ginger extract (5% active ingredient) for three months did not provide significant benefits [301]. In a clinical trial involving children with generalized epilepsies, the addition of ginger to antiepileptic drugs (AEDs) such as sodium valproate or carbamazepine resulted in 87% of participants becoming seizure-free and all experiencing reductions in seizure duration and frequency [302].
Targeting the brain with drug delivery systems (DDS) is a promising strategy for improving the bioavailability and transport of compounds across the blood–brain barrier (BBB). However, despite the potential benefits, only a small number of natural compounds have been encapsulated in DDS for brain targeting. These include curcumin, which has been studied for its potential therapeutic effects on neurodegenerative disorders [298]. Several strategies have been proposed to overcome the challenges of curcumin’s low bioavailability, including the use of solid self-emulsifying drug delivery systems, solid dispersions, cyclodextrin inclusion complexes, prodrug synthesis, manipulation of the solid-state crystal structure, and micronization to increase the surface area of the drug for improved dissolution. Other approaches include the use of P-glycoprotein inhibitors such as piperine or quercetin, as well as nanoformulations. These strategies aim to enhance curcumin’s absorption and transport across the blood–brain barrier to improve its efficacy as a therapeutic agent for various diseases [303]. According to a meta-analysis study, curcumin was found to improve working memory performance more effectively than the placebo, although some gastrointestinal adverse events were reported. In the trials, six different curcumin formulations were tested to improve its bioavailability, including the use of turmeric essential oil, submicron curcumin particles, and solid lipid curcumin particles. However, there was no systematic comparison of these different formulations in terms of their clinical effectiveness on cognitive function [211]. Curcumin interventions have shown beneficial associations with gastrointestinal, neurological, and oral diseases. It was shown that 180 mg/day of Theracurmin, a highly absorbable curcumin formulation, improved memory and attention in non-demented people, as well as providing stabilization of cognitive functions in patients with Alzheimer’s disease (AD) and mild cognitive impairment (MCI) [304]. However, the interpretation of curcumin trial data is limited by considerable variation in the studies and small sample sizes (range: 34–96) [211,305]. It is also worth noting that the pharmacological and toxicological effects of curcumin are dose-dependent, and high doses may produce toxic and carcinogenic effects [306] as well as pro-oxidant effects [307]. Other reported side effects include mild nausea and diarrhea, and it can chelate iron and suppress hepcidin, leading to subclinical iron deficiency [308,309]. Additionally, a study using human hepatoma G2 cells found that curcumin had no mutagenic effect at a low concentration of 2.5 μg/mL, but it caused DNA damage in a dose-dependent manner at higher concentrations (10–40 μg/mL) [13]. However, human clinical trials using doses of 1125–8000 mg/day reported no toxic or adverse effects. These results indicate that synthetic curcumin is not mutagenic and is not toxic at doses up to 1000 mg/kg bw/day. Bacterial reverse mutation testing and mammalian micronucleus testing showed no evidence of synthetic curcumin causing mutations. Additionally, repeated oral dose studies lasting 14 to 90 days, with the highest dose tested being 1000 mg/kg bw/day, showed no toxicological concerns related to curcumin [310]. The toxicity of curcumin-loaded nanocomplexes (CNCs) was also found to be very low, at 0.27 and 0.54 g/kg bodyweight/day in mice and hamsters. In conclusion, the toxicity of high-dose CNC treatment was graded as very low, possibly due to the components of the nanocomplex [311]. Curcumin is generally considered safe and has demonstrated positive effects on multiple health outcomes in humans, with the advantages outweighing the disadvantages [312]. Combining curcumin with other dietary supplements, including piperine, α-lipoic acid, N-acetylcysteine, B vitamins, vitamin C, and folate, has been suggested to have a synergistic effect on cognitive function [313].
Alpinia galanga has demonstrated the ability to enhance cognitive performance in animals, although its psychostimulant properties and potential benefits for cognitive function in humans have not been extensively investigated. However, recent research suggests that Alpinia galanga (E-AG-01) may have a positive effect on mental alertness, and when combined with caffeine, it may improve sustained attention up to three hours after consumption [314]. These findings could potentially be relevant for elderly individuals, who commonly experience deficits in attention and cognitive function.
Methoxyflavones found in Kaempferia parviflora (KP) and Curcuma longa are known for their anti-inflammatory properties. However, due to their low bioavailability and first-pass metabolism, their efficacy in humans has not been fully established. Although some clinical trials have reported positive effects of Kaempferia parviflora, the evidence remains inconclusive due to the small size of the studies. Moreover, there were no reported harmful effects when a dose of 1.35 g/d of KP was administered.
Despite preclinical studies suggesting potential cognitive benefits of Zingiberaceae plants, limited clinical studies have been inconclusive due to various factors such as differences in plant origin, extraction methods, active compound strength, and low bioavailability of the herbal preparation. Future research should focus on improving the bioavailability of herbal preparations and their ability to cross the blood–brain barrier.
2. Conclusions
As evidenced by the literature review, Zingiberaceae family plants have an impact on neurotrophins and downstream signaling targets for neurocognitive function. The phenolic compounds from the Zingiberaceae family provide neuroprotection by preventing the NF-KB pathway from being phosphorylated (p-P65 and p-IκBα) and thus inhibiting NF-κB signaling activation and further reducing the release of proinflammatory cytokines. They might also prevent neuronal loss. Certain Zingiberaceae family members have demonstrated the potential to regulate neurotrophin levels by functioning as a modulator targeting the tropomyosin-related kinase (Trk) receptor or p75 neurotrophin receptor (p75NTR) to stop neurotrophin loss. They also enable neurons to renew and create a suitable environment for maintaining mature neurons. The Zingiberaceae family stimulates neurotrophin expression by modulating various pathways, including the mTOR, phospholipase C, Gamma 1 (PLC1), and p75NTR-mediated signaling pathways. The protective effect may occur through several pathways, mainly the P13KAKT-mTOR/STAT3 pathway, NF-κB, MAPK pathway, and cAMP-PKA-CREB signaling pathway. Neuronal regeneration promotion occurs via modulating the bFGF/NGF/TrkA/Hsp70, NGF-TrkA pathway and BDNF/TrkB/AKT signaling pathway, and the antidepressant-like effects of curcumin involve the PGC1α/FNDC5/BDNF pathway. Therefore, it can stop or slow the progression of dangerous and complicated neurodegenerative disorders. Additionally, based on their chemical makeup, plant phenolic compounds do not seem to be harmful. The BBB was found to be permeable to gingerol and shogaol, two of the bioactive compounds found in ginger, through passive diffusion [315].
3. Limitations and Future Direction
These studies should be interpreted considering their limitations. First, this is a narrative review and not a systematic review, hence it poses potential bias. Zingiberaceae plants are typically safe and exhibit potent neuroprotective effects in various neurodegenerative diseases and brain insults, even though they still have very few clinical therapeutic applications. Numerous studies indicate that dietary plant polyphenols from Zingiberaceae family plants are safe. While natural phytochemicals might be less harmful than newly developed synthetic drugs, their usage in traditional herbal medicines raises concerns regarding their reproducibility, specific medicinal effects, mechanism of action, and the identification of active ingredients, because such formulations are often prepared from crude materials, which makes it difficult to assess their effectiveness and identify the key components responsible for their medicinal properties. Despite various instances in the field of ethnopharmacology, preclinical in vivo investigations on phenolic substances that control neurodegenerative illnesses are still underrepresented. Knowledge of the mechanism remains in its infancy and the literature is scarce. There is a need to perform in-depth research to shed more light on the molecular interactions and crosstalk between the pathways involved, given the limited evidence on neurotrophic factor potentiation effects and the numerous pathways affected by Zingiberaceae family phenolic compounds. Low bioavailability is the biggest obstacle for phenolic compounds and largely limits their evidence-based adoption into clinical practice. Encouragingly, results from human studies, although limited in number, appear to support this preclinical basis, with improvements in cognitive performance and disease risk observed across healthy and disease states. By further understanding the pharmacokinetics and thereby improving delivery and bioavailability, researchers can exploit these phytochemicals’ abilities to halt age-related decline in cognitive function, brain diseases, and neurodegenerative disorders. Phenolic compounds that augment neurotrophins may not be a perfect treatment, but they may help to halt the progression of neurodegenerative illnesses or at least delay their development. A range of phenolic compounds from the Zingiberaceae family has been identified in this review and, as a result, they may offer interesting prospects for the treatment and symptom relief of brain illnesses and neurodegenerative diseases. For the observed relationships to be supported, nevertheless, more high-quality research is required.
Author Contributions
Conceptualization, A.M.R. and S.M.; methodology, A.M.R. and S.M.; writing—original draft preparation, A.M.R. and S.M.; writing—review and editing, S.M., J.K.T. and M.M.S.; visualization, A.M.R.; supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education, Malaysia, grant number PRGS/1/2021/SKK0/UKM/01/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in kandelia obovata under cadmium and zinc stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Q.; Yu, Q. Quercetin enrich diet during the early-middle not middle-late stage of alzheimer’s disease ameliorates cognitive dysfunction. Am. J. Transl. Res. 2018, 10, 1237–1246. [Google Scholar] [PubMed]
- Ramkumar, M.; Rajasankar, S.; Swaminathan Johnson, W.M.; Prabu, K.; Venkatesh Gobi, V. Demethoxycurcumin ameliorates rotenone-induced toxicity in rats. Front. Biosci. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Lamport, D.J.; Christodoulou, E.; Achilleos, C. Beneficial Effects of Dark Chocolate for Episodic Memory in Healthy Young Adults: A Parallel-Groups Acute Intervention with a White Chocolate Control. Nutrients 2020, 12, 483. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Zhang, L.; Li, X.; Dong, R.; Song, C.; Cheng, L.; Shi, M.; Zhao, H. Combination of tea polyphenols and proanthocyanidins prevents menopause-related memory decline in rats via increased hippocampal synaptic plasticity by inhibiting p38 MAPK and TNF-α pathway. Nutr. Neurosci. 2022, 25, 1909–1927. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Modi, P.; Sharma, S.; Deep, S. Quercetin and Baicalein Act as Potent Antiamyloidogenic and Fibril Destabilizing Agents for SOD1 Fibrils. ACS Chem. Neurosci. 2020, 11, 1129–1138. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.-P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Rizzo, G.; Fileccia, E.; Ventruto, F.; Riva, A.; Tiso, D.; Recchia, M.; Vacchiano, V.; Infante, R.; et al. The Effect of Curcumin on Idiopathic Parkinson Disease: A Clinical and Skin Biopsy Study. J. Neuropathol. Exp. Neurol. 2022, 81, 545–552. [Google Scholar] [CrossRef]
- Khadka, S.; Omura, S.; Sato, F.; Nishio, K.; Kakeya, H.; Tsunoda, I. Curcumin β-D-Glucuronide Modulates an Autoimmune Model of Multiple Sclerosis with Altered Gut Microbiota in the Ileum and Feces. Front. Cell. Infect. Microbiol. 2021, 11, 1192. [Google Scholar] [CrossRef]
- Kou, J.; Wang, M.; Shi, J.; Zhang, H.; Pu, X.; Song, S.; Yang, C.; Yan, Y.; Döring, Y.; Xie, X.; et al. Curcumin Reduces Cognitive Deficits by Inhibiting Neuroinflammation through the Endoplasmic Reticulum Stress Pathway in Apolipoprotein E4 Transgenic Mice. ACS Omega 2021, 6, 6654–6662. [Google Scholar] [CrossRef]
- Razak, A.M.; Zakaria, S.N.A.; Abdul Sani, N.F.; Ab Rani, N.; Hakimi, N.H.; Mohd Said, M.; Tan, J.K.; Gan, H.K.; Mad Nordin, M.F.; Makpol, S. A subcritical water extract of soil grown Zingiber officinale Roscoe: Comparative analysis of antioxidant and anti-inflammatory effects and evaluation of bioactive metabolites. Front. Pharmacol. 2023, 14, 1006265. [Google Scholar] [CrossRef]
- Park, G.; Kim, H.G.; Ju, M.S.; Ha, S.K.; Park, Y.; Kim, S.Y.; Oh, M.S. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson’s disease models via anti-neuroinflammation. Acta Pharmacol. Sin. 2013, 34, 1131–1139. [Google Scholar] [CrossRef]
- Ramanathan, A.; Shaji, D. Ginger Loaded Chitosan Nanoparticles for The Management of 3–Nitropropionic Acid-Induced Huntington’s Disease-like Symptoms In Male Wistar Rats. Int. J. Pharm. Pharm. Sci. 2022, 14, 28–36. [Google Scholar] [CrossRef]
- Adekoya, A.A.; Ahmad, S.; Maziah, M. Assessment of total phenolic compounds and in vitro free radical scavenging potentials of water extracts of ten selected species of Zingiberaceae rhizomes use in folkloric medicine. Pak. J. Pharm. Sci. 2016, 29, 979–984. [Google Scholar]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Asamenew, G.; Kim, H.-W.; Lee, M.-K.; Lee, S.-H.; Kim, Y.J.; Cha, Y.-S.; Yoo, S.M.; Kim, J.-B. Characterization of phenolic compounds from normal ginger (Zingiber officinale Rosc.) and black ginger (Kaempferia parviflora Wall.) using UPLC–DAD–QToF–MS. Eur. Food Res. Technol. 2019, 245, 653–665. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, L. Zingiberaceae. In Identification and Control of Common Weeds: Volume 3; Xu, Z., Chang, L., Eds.; Springer: Singapore, 2017; pp. 909–911. [Google Scholar]
- Saha, K.; Sinha, R.; Sinha, S. Distribution, Cytology, Genetic Diversity and Molecular phylogeny of selected species of Zingiberaceae—A Review. Feddes Repert. 2019, 131, 58–68. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J. Pharm. Sci. 2012, 74, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Zam, W.; Kumar, M.; Cardoso, S.M.; Pereira, O.R.; Ademiluyi, A.O.; Adeleke, O.; Moreira, A.C.; Živković, J.; et al. Phenolic Bioactives as Antiplatelet Aggregation Factors: The Pivotal Ingredients in Maintaining Cardiovascular Health. Oxidative Med. Cell. Longev. 2021, 2021, 2195902. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Musa, A.; Almalki, A.H.; Alzarea, S.I.; Mostafa, E.M.; Hegazy, M.M.; Mostafa-Hedeab, G.; Ghoneim, M.M.; Parambi, D.G.T.; Bakr, R.B.; et al. Novel Phenolic Compounds as Potential Dual EGFR and COX-2 Inhibitors: Design, Semisynthesis, in vitro Biological Evaluation and in silico Insights. Drug Des. Dev. Ther. 2021, 15, 2325–2337. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Abdelbassat, H.; Bourkhis, B.; Khouya, T.; Ramchoun, M.; Zegzouti, Y.; Alem, C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 2020, 6, e03436. [Google Scholar] [CrossRef]
- Cherubim, D.J.d.L.; Martins, C.V.B.; Fariña, L.O.d.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Menezes, B.; Pires, D.; Ferreira, E.; Cruvinel, K. Efficiency of pollutant removal in a hybrid constructed wetland with Hedychiumcoronarium J. König: A sustainable alternative for poor communities. Rev. Monogr. Ambient. 2021, 20, e6. [Google Scholar] [CrossRef]
- Bajaj, S.; Fuloria, S.; Porwal, O.; Subramaniyan, V.; Sharma, p.; Ozdemir, M.; Dhana Lekshmi, U.M.; Kishore, N.; Fuloria, N. Ethnomedicinal and Pharmacological Uses of Curcuma Caesia. NVEO-Nat. Volatiles Essent. Oils J. NVEO 2022, 8, 14902–14910. [Google Scholar]
- Koser, H.; Abbas, R.; Rizwan, B.; Sultan, H.; Islam, Z.; Jawad, M.; Jawad, M.; Waheed, M.; Basharat, S. Pharmacological Effects of Curcuma Longa and Its Bioactive Constitute Curcumin: Curcuma Longa and Its Bioactive Constitute Curcumin. Pak. BioMed. J. 2022, 5, 22–27. [Google Scholar] [CrossRef]
- Cristie Edina, B.; Fadilah, F.; Rahmawanti, R.; Wiyono, L.; Paramita, R.I. Phytochemical Analysis and In Vitro Toxicity of N-Hexane Extract of Kaempferia pandurata and Its Nanoparticle to Breast Cancer MCF-7 Cells. J. Glob. Pharma Technol. 2019, 11, 591–596. [Google Scholar]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]
- Van, T.; Pham, T.V.; Nguyen, H.; Nguyen, A.; Duy, T. A review on chemical constituents of essential oils of Aframomum genus. J. Phytol. 2021, 13, 161–170. [Google Scholar] [CrossRef]
- Idris, N.A.; Yasin, H.M.; Usman, A. Voltammetric and spectroscopic determination of polyphenols and antioxidants in ginger (Zingiber officinale Roscoe). Heliyon 2019, 5, e01717. [Google Scholar] [CrossRef]
- Santos Sánchez, N.; Salas-Coronado, R.; Villanueva, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; Intechopen: London, UK, 2019. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef]
- Han, A.R.; Kim, H.; Piao, D.; Jung, C.H.; Seo, E.K. Phytochemicals and Bioactivities of Zingiber cassumunar Roxb. Molecules 2021, 26, 2377. [Google Scholar] [CrossRef]
- Koontongkaew, S.; Poachanukoon, O.; Sireeratawong, S.; Dechatiwongse Na Ayudhya, T.; Khonsung, P.; Jaijoy, K.; Soawakontha, R.; Chanchai, M. Safety Evaluation of Zingiber cassumunar Roxb. Rhizome Extract: Acute and Chronic Toxicity Studies in Rats. Int. Sch. Res. Not. 2014, 2014, 632608. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Verma, S.K.; Iqbal, H.; Chanda, D.; Verma, R.K.; Darokar, M.P.; et al. Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. J. Sci. Food Agric. 2018, 98, 321–327. [Google Scholar] [CrossRef]
- Ghazalee, N.S.; Jantan, I.; Arshad, L.; Haque, M.A. Immunosuppressive effects of the standardized extract of Zingiber zerumbet on innate immune responses in Wistar rats. Phytother. Res. 2019, 33, 929–938. [Google Scholar] [CrossRef]
- Aji, N.; Kumala, S.; Mumpuni, E.; Rahmat, D. Antibacterial Activity and Active Fraction of Zingiber officinale Roscoe, Zingiber montanum (J. Koenig) Link ex A., and Zingiber zerumbet (L.) Roscoe ex Sm. Against Propionibacterium acnes. Pharmacogn. J. 2022, 14, 103–111. [Google Scholar] [CrossRef]
- Tandirogang, N.; Anitasari, S.; Arung, E.T.; Paramita, S.; Shen, Y.K. Evaluations of Antibacterial Properties of Zingiber purpureum Essential Oil Against 13 Different Gram-positive and Gram-negative Bacteria. Indones. Biomed. J. 2022, 14, 303–308. [Google Scholar] [CrossRef]
- Truong, V.L.; Manochai, B.; Pham, T.T.; Jeong, W.S. Antioxidant and Anti-Inflammatory Activities of Zingiber montanum Oil in HepG2 Cells and Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Med. Food 2021, 24, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Ma, Y.-P.; Zhang, H.-X.; Sun, H.-Z.; Su, H.-H.; Pei, S.-J.; Du, Z.-Z. Repellent, larvicidal and adulticidal activities of essential oil from Dai medicinal plant Zingiber cassumunar against Aedes albopictus. Plant Divers. 2021, 43, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Paramita, S.; Aminyoto, M.; Ismail, S.; Arung, E. Anti-hypercholesterolemic effect of Zingiber montanum extract. F1000Research 2018, 7, 1798. [Google Scholar] [CrossRef]
- Samia, E. Analgesic and Anti- Inflammatory Effect of Zingiber Officinale in Albino Mice. Sci. J. Univ. Benghazi 2021, 33, 5. [Google Scholar]
- Soltani, E.; Jangjoo, A.; Afzal Aghaei, M.; Dalili, A. Effects of preoperative administration of ginger (Zingiber officinale Roscoe) on postoperative nausea and vomiting after laparoscopic cholecystectomy. J. Tradit. Complement. Med. 2018, 8, 387–390. [Google Scholar] [CrossRef]
- Diki Prayugo, W.; Ria, M.; Siti Uswatun, H.; Diah Lia, A. Chemical Constituents, Antibacterial Activity and Mode of Action of Elephant Ginger (Zingiber officinale var. officinale) and Emprit Ginger Rhizome (Zingiber officinale var. Amarum) Essential Oils. Pharmacogn. J. 2020, 12, 404–409. [Google Scholar]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Seo, S.H.; Fang, F.; Kang, I. Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice. Int. J. Environ. Res. Public Health 2021, 18, 631. [Google Scholar] [CrossRef]
- Walstab, J.; Krüger, D.; Stark, T.; Hofmann, T.; Demir, I.; Ceyhan, G.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol. Motil. 2013, 25, 439–e302. [Google Scholar] [CrossRef]
- Hasanvand, A.; Ebrahimi, Y.; Mohamadi, A.; Nazari, A. Zingiber officinale Roscoe reduces chest pain on patients undergoing coronary angioplasty: A clinical trial. J. Herbmed Pharmacol. 2019, 8, 47–50. [Google Scholar] [CrossRef]
- Nurinda, E.; Kusumawardani, N.; Wulandari, A.S.; Fatmawati, A.; Emelda, E.; Nisa, H.; Hasan, N.A.; Iriyanti, W.F.; Rohmah, M.; Lestari, P.; et al. Pharmacological Study: Synergistic Antidiabetic Activity of Cinnamon Bark and Zingiber Extract in Streptozotocin-Induced Diabetic Rats. Open Access Maced. J. Med. Sci. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Tsai, Y.; Xia, C.; Sun, Z. The Inhibitory Effect of 6-Gingerol on Ubiquitin-Specific Peptidase 14 Enhances Autophagy-Dependent Ferroptosis and Anti-Tumor in vivo and in vitro. Front. Pharmacol. 2020, 11, 598555. [Google Scholar] [CrossRef]
- Prastiyanto, M.E.; Rohmah, N.; Efendi, L.; Arifin, R.; Wardoyo, F.A.; Wilson, W.; Mukaromah, A.H.; Dewi, S.S.; Darmawati, S. Antifungal activities of the rhizome extract of five member Zingiberaceae against candida albicans and trichophyton rubrum. Biodiversitas 2021, 22, 1509–1513. [Google Scholar] [CrossRef]
- Safitri, A.; Putri, A.S.; Octavianty, T.D.; Sari, D.R.T. Metabolomic Profiles of Curcuma longa L. and Cosmos caudatus Extracts and Their In-Silico Anti-cancer Activity. J. Phys. Conf. Ser. 2020, 1665, 012022. [Google Scholar] [CrossRef]
- Quirós-Fallas, M.I.; Vargas-Huertas, F.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A.; Navarro-Hoyos, M. Polyphenolic HRMS Characterization, Contents and Antioxidant Activity of Curcuma longa Rhizomes from Costa Rica. Antioxidants 2022, 11, 620. [Google Scholar] [CrossRef]
- Shenge, J.; Obi, R.; Salawu, K. Assessment of Antiviral Activity of Curcuma longa on Two RNA Viruses. Niger. J. Pure Appl. Sci. 2021, 34, 3915–3928. [Google Scholar] [CrossRef]
- Uchio, R.; Kawasaki, K.; Okuda-Hanafusa, C.; Saji, R.; Muroyama, K.; Murosaki, S.; Yamamoto, Y.; Hirose, Y. Curcuma longa extract improves serum inflammatory markers and mental health in healthy participants who are overweight: A randomized, double-blind, placebo-controlled trial. Nutr. J. 2021, 20, 91. [Google Scholar] [CrossRef]
- Handharyani, E.; Sutardi, L.N.; Mustika, A.A.; Andriani, A.; Yuliani, S. Antibacterial Activity of Curcuma longa (turmeric), Curcuma zedoaria (zedoary), and Allium sativum (garlic) Nanoparticle Extract on Chicken with Chronic Respiratory Disease Complex: In Vivo Study. E3S Web Conf. 2020, 151, 01054. [Google Scholar] [CrossRef]
- Vaithiyalingam, M.; Sumathi, D.L.; Sabarathinam, S. Isolation and In silico Study of Curcumin from Curcuma longa and Its Anti-Diabetic Activity. Appl. Biochem. Biotechnol. 2023, 195, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mohammed, S.J.; Eltayeb, I.M.; Elmosaad, Y.M.; Waggiallah, H.A. Comparative Study of the Anticoagulant Activity of Zingiber Officinale and Curcuma longa Rhizomes Extracts in Blood Samples of Normal Individuals. Pak. J. Med. Health Sci. 2022, 16, 348–351. [Google Scholar] [CrossRef]
- Cruz, J.D.d.; Mpalantinos, M.A.; Ramos, A.d.S.; Ferreira, J.L.P.; de Oliveira, A.A.; Júnior, N.L.N.; Silva, J.R.d.A.; Amaral, A.C.F. Chemical standardization, antioxidant activity and phenolic contents of cultivated Alpinia zerumbet preparations. Ind. Crops Prod. 2020, 151, 112495. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.-Y.; Peng, F.; Duan, W.-T.; Wu, C.-H.; Li, H.-T.; Zhang, X.-F.; Shi, Y.-S. Neolignans and Diarylheptanoids with Anti-Inflammatory Activity from the Rhizomes of Alpinia zerumbet. J. Agric. Food Chem. 2021, 69, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, S.; Tu, M.; He, L.; Xu, Y.; Gan, S.; Shen, X. Inhibitory Effect of Essential Oil From Fructus of Alpinia zerumbet on Endothelial-to-Mesenchymal Transformation Induced by TGF-β1 and Downregulation of KLF4. J. Cardiovasc. Pharmacol. 2022, 80, 82–94. [Google Scholar] [CrossRef]
- You, H.; He, M.; Pan, D.; Fang, G.; Chen, Y.; Zhang, X.; Shen, X.; Zhang, N. Kavalactones isolated from Alpinia zerumbet (Pers.) Burtt. et Smith with protective effects against human umbilical vein endothelial cell damage induced by high glucose. Nat. Prod. Res. 2022, 36, 5740–5746. [Google Scholar] [CrossRef]
- Taib, M.; Anuar, N.; Hanafiah, K.M.; Al-Shammary, A.A.K.; Saaid, M.; Awang, K. Chemicals Constituents Isolated from Cultivate Alpinia conchigera Griff. and Antimicrobial Activity. Trop. Life Sci. Res. 2020, 31, 159–178. [Google Scholar] [CrossRef]
- In, L.L.; Azmi, M.N.; Ibrahim, H.; Awang, K.; Nagoor, N.H. 1′S-1′-acetoxyeugenol acetate: A novel phenylpropanoid from Alpinia conchigera enhances the apoptotic effects of paclitaxel in MCF-7 cells through NF-κB inactivation. Anticancer Drugs 2011, 22, 424–434. [Google Scholar] [CrossRef]
- Ibrahim, M.; Hossain, M.A.; Shajib, M.S.; Rashid, M.A. Preliminary Phytochemical and Pharmacological Screenings of Plumbago indica L. and Alpinia conchigera Griff. Dhaka Univ. J. Pharm. Sci. 2018, 17, 73–79. [Google Scholar] [CrossRef][Green Version]
- Nag, A.; Banerjee, R.; Goswami, P.; Bandyopadhyay, M.; Mukherjee, A. Antioxidant and antigenotoxic properties of Alpinia galanga, Curcuma amada, and Curcuma caesia. Asian Pac. J. Trop. Biomed. 2021, 11, 363–374. [Google Scholar] [CrossRef]
- Ibrahim, S. Alpinia galanga Extract Inhibits MCF-7/HER2+ Cells by Inducing Apoptosis. J. Sci. Technol. Res. Pharm. 2022, 1, 31–36. [Google Scholar] [CrossRef]
- Nampoothiri, S.; Esakkidurai, T.; Pitchumani, K. Evaluation of antidiabetic, anti-inflammatory and LDL oxidation inhibitory potential of Alpinia galanga and Alpinia calcarata-An in vitro study. Trends Phytochem. Res. 2017, 1, 227–234. [Google Scholar]
- Lin, K.; Wang, Y.; Gong, J.; Tan, Y.; Deng, T.; Wei, N. Protective effects of total flavonoids from Alpinia officinarum rhizoma against ethanol-induced gastric ulcer in vivo and in vitro. Pharm. Biol. 2020, 58, 854–862. [Google Scholar] [CrossRef]
- Srividya, A.R.; Dhanabal, S.P.; Misra, V.K.; Suja, G. Antioxidant and Antimicrobial Activity of Alpinia officinarum. Indian J. Pharm. Sci. 2010, 72, 145–148. [Google Scholar] [CrossRef]
- Gamre, S.; Tyagi, M.; Chatterjee, S.; Patro, B.S.; Chattopadhyay, S.; Goswami, D. Synthesis of Bioactive Diarylheptanoids from Alpinia officinarum and Their Mechanism of Action for Anticancer Properties in Breast Cancer Cells. J. Nat. Prod. 2021, 84, 352–363. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crops Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Sahoo, A.; Kar, B.; Patnaik, J.; Panda, P.; Nayak, S.; Mahapatra, N. Hedychium coronarium extract arrests cell cycle progression, induces apoptosis, and impairs migration and invasion in HeLa cervical cancer cells. Cancer Manag. Res. 2019, 11, 483–500. [Google Scholar] [CrossRef]
- Yassin, M.T.; Abdel-Fattah Mostafa, A.; Al-Askar, A.A.; Alkhelaif, A.S. In vitro antimicrobial potency of Elettaria cardamomum ethanolic extract against multidrug resistant of food poisoning bacterial strains. J. King Saud Univ.-Sci. 2022, 34, 102167. [Google Scholar] [CrossRef]
- Almeer, R.S.; Alnasser, M.; Aljarba, N.; AlBasher, G.I. Effects of Green cardamom (Elettaria cardamomum Maton) and its combination with cyclophosphamide on Ehrlich solid tumors. BMC Complement. Med. Ther. 2021, 21, 133. [Google Scholar] [CrossRef]
- Al-Zereini, W.; Al-Trawneh, I.; Al-Qudah, M.; Tumallah, H.; Rawashdeh, H.; Abudayeh, Z. Essential oils from Elettaria cardamomum (L.) Maton grains and Cinnamomum verum J. Presl barks: Chemical examination and bioactivity studies. J. Pharm. Pharmacogn. Res. 2021, 10, 173–185. [Google Scholar] [CrossRef]
- Alkhalifah, E.A.R.; Alobaid, A.A.; Almajed, M.A.; Alomair, M.K.; Alabduladheem, L.S.; Al-Subaie, S.F.; Akbar, A.; Attimarad, M.V.; Younis, N.S.; Mohamed, M.E. Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway. Curr. Issues Mol. Biol. 2022, 44, 5390–5404. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rasoul, A.A.; Saleh, N.A.; Hosny, E.N.; El-Gizawy, M.M.; Ibrahim, E.A. Cardamom oil ameliorates behavioral and neuropathological disorders in a rat model of depression induced by reserpine. J. Ethnopharmacol. 2023, 308, 116254. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Balaraman, R.; Patel, P.; Buddhadev, M. Effects of Aqueous Extract of Tulsi and Cardamom on Elderly Depressive Subjects—A Preliminary Clinical Study. J. Nat. Remedies 2021, 21, 245–249. [Google Scholar] [CrossRef]
- Chan, E.; Lim, Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and Tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Juwita, T.; Puspitasari, M.; Levita, J. Anti-inflammatory Activity of Etlingera elatior (Jack) R.M. Smith Flower on Gastric Ulceration-induced Wistar Rats. Pak. J. Biol. Sci. 2020, 23, 1193–1200. [Google Scholar] [CrossRef]
- Noordin, L.; Wan Ahmad, W.A.N.; Muhamad Nor, N.A.; Abu Bakar, N.H.; Ugusman, A. Etlingera elatior Flower Aqueous Extract Protects against Oxidative Stress-Induced Nephropathy in a Rat Model of Type 2 Diabetes. Evid.-Based Complement. Altern. Med. 2022, 2022, 2814196. [Google Scholar] [CrossRef]
- Al-Mansoub, M.; Asif, M.; Revadigar, V.; Hammad, M.; Chear, N.; Hamdan, M.R.; Abdul Majid, A.M.S.; Abdullah, M.; Murugaiyah, V. Chemical composition, antiproliferative and antioxidant attributes of ethanolic extract of resinous sediment from Etlingera elatior (Jack.) inflorescence. Braz. J. Pharm. Sci. 2021, 57, e18954. [Google Scholar] [CrossRef]
- Fristiohady, A.; Wahyuni, W.; Bafadal, M.; Ode, L.; Julian Purnama, L.O.; Sangadji, F.; Malaka, M.; Malik, F.; Hong, J.; Wibawa, A.; et al. Nephroprotective effect of extract Etlingera elatior (Jack) R.M. Smith on CCl 4 -induced nephrotoxicity in rats. Curr. Res. Biosci. Biotechnol. 2020, 1, 62–65. [Google Scholar] [CrossRef]
- Zainin, N.S.; Lau, K.Y.; Zakaria, M.; Son, R.; Abdull Razis, A.F.; Rukayadi, Y. Antibacterial activity of Boesenbergia rotunda (L.) Mansf. A. extract against Escherichia coli. Int. Food Res. J. 2013, 20, 3319–3323. [Google Scholar]
- Taechowisan, T.; Chaisaeng, S.; Phutdhawong, W.S. Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07: An endophyte in Boesenbergia rotunda (L.) Mansf A. Food Agric. Immunol. 2017, 28, 1330–1346. [Google Scholar] [CrossRef]
- Chia, Y.; Teh, S.H.; Mohamed, Z. Isolation and Characterization of Chalcone Isomerase (CHI) Gene from Boesenbergia rotunda. S. Afr. J. Bot. 2020, 130, 475–482. [Google Scholar] [CrossRef]
- Galih, S.; Dina, F. Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Extract in Carrageenan-Induced Female Rats. In Proceedings of the 1st International Conference on Community Health (ICCH 2019), San Diego, CA, USA, 10 February 2020; pp. 13–17. [Google Scholar]
- Laksmitawati, D.R.; Widowati, W.; Kusuma, H.S.W.; Pratami, D.K.; Wijayanti, C.R.; Wahyuni, C.D.; Afifah, E.; Rizal, R. Antioxidant Potency of Kaempferia galanga Linn and Zingiber officinale var. Rubra rhizomes. In Proceedings of the 2021 IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce), Medan, Indonesia, 14–16 July 2021; pp. 1–6. [Google Scholar]
- Ichwan, S.; Sazeli, S.; Lestari, W. Analysis of the anti-cancer effect of ethyl-p-methoxycinnamate extracted cekur (Kaempferia galanga) on cancer cell lines with wild- type and null p53. IIUM J. Orofac. Health Sci. 2020, 1, 28–33. [Google Scholar] [CrossRef]
- Lallo, S.; Hardianti, B.; Sartini, S.; Ismail, I.; Laela, D.; Hayakawa, Y. Ethyl P-Methoxycinnamate: An Active Anti-Metastasis Agent and Chemosensitizer Targeting NFκB from Kaempferia galanga for Melanoma Cells. Life 2022, 12, 337. [Google Scholar] [CrossRef]
- Belgis, M.; Nafi, A.; Giyarto, G.; Wulandari, A. Antibacterial Activity of Kaempferia Galanga L. Hard Candy Against Streptococcus pyogenes and Staphylococcus aureus Bacteria Growth. J. Food Agric. Environ. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Ekowati, J.; Hardjono, S.; Hamid, I.S. Ethyl p-methoxycinnamate from Kaempferia galanga inhibits angiogenesis through tyrosine kinase. Universa Med. 2016, 34, 43. [Google Scholar] [CrossRef]
- Tonsomboon, A.; Mani Iyer, P.; Plaingam, W.; Tencomnao, T. Kaempferia parviflora Rhizome Extract Inhibits Glutamate-Induced Toxicity in HT-22 Mouse Hippocampal Neuronal Cells and Extends Longevity in Caenorhabditis elegans. Biology 2021, 10, 264. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Kwon, D.; Kim, M.-B.; Hwang, J.-K. Standardized Kaempferia parviflora Wall. ex Baker (Zingiberaceae) Extract Inhibits Fat Accumulation and Muscle Atrophy in ob/ob Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 8161042. [Google Scholar] [CrossRef]
- Ab Rahman, Z.; Abd Shukor, S.; Abbas, H.; Machap, C.; Alias, M.; Mirad, R.; Sofiyanand, S.; Othman, A. Optimization of Extraction Conditions for Total Phenolics and Total Flavonoids from Kaempferia parviflora Rhizomes. Adv. Biosci. Biotechnol. 2018, 09, 205–214. [Google Scholar] [CrossRef][Green Version]
- Sitthichai, P.; Chanpirom, S.; Maneerat, T.; Charoensup, R.; Tree-Udom, T.; Pintathong, P.; Laphookhieo, S.; Sripisut, T. Kaempferia parviflora Rhizome Extract as Potential Anti-Acne Ingredient. Molecules 2022, 27, 4401. [Google Scholar] [CrossRef]
- Nemidkanam, V.; Kato, Y.; Kubota, T.; Chaichanawongsaroj, N. Ethyl acetate extract of Kaempferia parviflora inhibits Helicobacter pylori-associated mammalian cell inflammation by regulating proinflammatory cytokine expression and leukocyte chemotaxis. BMC Complement. Med. Ther. 2020, 20, 124. [Google Scholar] [CrossRef]
- Zohmachhuana, A.; Lalnunmawia, F.; Mathipi, V.; Lalrinzuali, K.; Kumar, N.S. Curcuma aeruginosa Roxb. exhibits cytotoxicity in A-549 and HeLa cells by inducing apoptosis through caspase-dependent pathways. Biomed. Pharmacother. 2022, 150, 113039. [Google Scholar] [CrossRef] [PubMed]
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef]
- Suwal, N.; Subba, R.K.; Paudyal, P.; Khanal, D.P.; Panthi, M.; Suwal, N.; Nassan, M.A.; Alqarni, M.; Batiha, G.E.; Koirala, N. Antimicrobial and antibiofilm potential of Curcuma longa Linn. Rhizome extract against biofilm producing Staphylococcus aureus and Pseudomonas aeruginosa isolates. Cell. Mol. Biol. 2021, 67, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antivir. Res. 2019, 162, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Anggriani, L.; Yasmin, A.; Wulandari, A.; Leksono, G.; Ikawati, M.; Meiyanto, E. Extract of Temu Ireng (Curcuma aeruginosa Roxb.) Rhizome Reduces Doxorubicin-Induced Immunosuppressive Effects; AIP Publishing LLC.: Melville, NY, USA, 2019; Volume 2099, p. 020001. [Google Scholar]
- Vanda, H.; Parindra, R.; Hambal, M.; Athaillah, F. Anthelmintic Activity of Curcuma Aeruginosa Roxb Extract on Fasciola gigantica in Vitro. E3S Web Conf. 2020, 151, 01046. [Google Scholar] [CrossRef]
- Triastuti, A. Investigation of Anti-Inflammatory Activities of Curcuma aeruginosa Roxb. in Experimental Animals. Int. J. Pharm. Clin. Res. 2016, 8, 471–475. [Google Scholar]
- Srivilai, J.; Kanjana, N.; Nutuan, T.; Waranuch, N.; Khorana, N.; Wisuthiprot, W.; Scholfield, C.N.; Champachaisri, K.; Ingkaninan, K. Sesquiterpene-Enriched Extract of Curcuma aeruginosa Roxb. Retards Axillary Hair Growth: A Randomised, Placebo-Controlled, Double-Blind Study. Ski. Pharmacol. Physiol. 2018, 31, 99–106. [Google Scholar] [CrossRef]
- Reanmongkol, W.; Subhadhirasakul, S.; Khaisombat, N.; Fuengnawakit, P.; Jantasila, S.; Khamjun, A. Investigation the antinociceptive, antipyretic and anti-inflammatory activities of Curcuma aeruginosa Roxb. extracts in experimental animals. Songklanakarin J. Sci. Technol. 2006, 28, 999–1008. [Google Scholar]
- Manvizhi, S.S.; Sabitha, V.; Kumar, A.; Margaret Shanthi, F.X. Effect of curcumin on the contraction of isolated goat uterus. Int. J. Basic Clin. Pharmacol. 2020, 10, 81–84. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Pardillo-Díaz, R.; Pérez-García, P.; Castro, C.; Nunez-Abades, P.; Carrascal, L. Oxidative Stress as a Potential Mechanism Underlying Membrane Hyperexcitability in Neurodegenerative Diseases. Antioxidants 2022, 11, 1511. [Google Scholar] [CrossRef]
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K.U. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2021, 23, 106. [Google Scholar] [CrossRef]
- Conway, M.E.; Lee, C. The redox switch that regulates molecular chaperones. Biomol. Concepts 2015, 6, 269–284. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Chenyang, Z.; Xin, W.; Jiangfeng, D.; Zhanjun, G.; Yuliang, Z. Reactive Oxygen Species-Regulating Strategies Based on Nanomaterials for Disease Treatment. Adv. Sci. 2021, 8, 2002797. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J. Oxidative DNA damage & repair: An introduction. Free. Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar]
- Hamezah, H.S.; Durani, L.W.; Ibrahim, N.F.; Yanagisawa, D.; Kato, T.; Shiino, A.; Tanaka, S.; Damanhuri, H.A.; Ngah, W.Z.W.; Tooyama, I. Volumetric changes in the aging rat brain and its impact on cognitive and locomotor functions. Exp. Gerontol. 2017, 99, 69–79. [Google Scholar] [CrossRef]
- Dikovskaya, D.; Appleton, P.L.; Bento-Pereira, C.; Dinkova-Kostova, A.T. Measuring the Interaction of Transcription Factor Nrf2 with Its Negative Regulator Keap1 in Single Live Cells by an Improved FRET/FLIM Analysis. Chem. Res. Toxicol. 2019, 32, 500–512. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Minj, E.; Upadhayay, S.; Mehan, S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem. Res. 2021, 46, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Michalska, P.; León, R. When It Comes to an End: Oxidative Stress Crosstalk with Protein Aggregation and Neuroinflammation Induce Neurodegeneration. Antioxidants 2020, 9, 740. [Google Scholar] [CrossRef]
- Mulica, P.; Grünewald, A.; Pereira, S.L. Astrocyte-Neuron Metabolic Crosstalk in Neurodegeneration: A Mitochondrial Perspective. Front. Endocrinol. 2021, 12, 668517. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X.; Li, Y.; Chen, S.; Liu, S.; Yu, Z.; Wang, W. Transforming growth factor-β1 protects against LPC-induced cognitive deficit by attenuating pyroptosis of microglia via NF-κB/ERK1/2 pathways. J. Neuroinflamm. 2022, 19, 194. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef]
- Gerganova, G.; Riddell, A.; Miller, A.A. CNS border-associated macrophages in the homeostatic and ischaemic brain. Pharmacol. Ther. 2022, 240, 108220. [Google Scholar] [CrossRef]
- Otani, K.; Shichita, T. Cerebral sterile inflammation in neurodegenerative diseases. Inflamm. Regen. 2020, 40, 28. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Inflammatory neuroprotection following traumatic brain injury. Science 2016, 353, 783–785. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, H.-C.; Shin, E.-J. Effect of rottlerin on astrocyte phenotype polarization after trimethyltin insult in the dentate gyrus of mice. J. Neuroinflamm. 2022, 19, 142. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Tan, P.H.; Ji, J.; Yeh, C.C.; Ji, R.R. Interferons in Pain and Infections: Emerging Roles in Neuro-Immune and Neuro-Glial Interactions. Front. Immunol. 2021, 12, 783725. [Google Scholar] [CrossRef]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef]
- Miyamoto, N.; Magami, S.; Inaba, T.; Ueno, Y.; Hira, K.; Kijima, C.; Nakajima, S.; Yamashiro, K.; Urabe, T.; Hattori, N. The effects of A1/A2 astrocytes on oligodendrocyte linage cells against white matter injury under prolonged cerebral hypoperfusion. Glia 2020, 68, 1910–1924. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen attenuates traumatic brain injury by inhibiting the activation of microglia and astrocyte-mediated neuroinflammatory responses. Mol. Neurobiol. 2021, 58, 1052–1061. [Google Scholar] [CrossRef]
- Acioglu, C.; Li, L.; Elkabes, S. Contribution of astrocytes to neuropathology of neurodegenerative diseases. Brain Res. 2021, 1758, 147291. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Chang, S.-C.; Lee, J. Significant roles of neuroinflammation in Parkinson’s disease: Therapeutic targets for PD prevention. Arch. Pharmacal Res. 2019, 42, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Watters, J.J.; Ulland, T.K. Transcriptional response of murine microglia in Alzheimer’s disease and inflammation. BMC Genom. 2022, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Yujia, W.; Tingjun, C.; Bosco, D.S.; Manling, X.; Jiaying, Z.; Aastha, D.; Yanlu, Y.; Qian, W.; Lennon, V.A.; Long-Jun, W. The complement C3-C3aR pathway mediates microglia–astrocyte interaction following status epilepticus. Glia 2021, 69, 1155–1169. [Google Scholar]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; De Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Skaper, S.D. Neurotrophic Factors: An Overview. Methods Mol. Biol. 2018, 1727, 1–17. [Google Scholar] [CrossRef]
- Lin, P.-H.; Kuo, L.-T.; Luh, H.-T. The Roles of Neurotrophins in Traumatic Brain Injury. Life 2021, 12, 26. [Google Scholar] [CrossRef]
- Anna, S.; Bogusław, M. Trophic Factors in the Therapeutic Challenge Against ALS: Current Research Directions. In Update on Amyotrophic Lateral Sclerosis; Humberto Foyaca, S., Lourdes de Fatima Ibañez, V., Eds.; IntechOpen: Rijeka, Croatia, 2016; Chapter 11. [Google Scholar]
- Masmudi-Martín, M.; Navarro-Lobato, I.; Khan, Z.U. Implication of 14-3-3ζ-BDNF pathway in long-lasting memory enhancement and the rescue from memory deficits. Neural Regen. Res. 2022, 17, 2685–2686. [Google Scholar] [CrossRef]
- Casagrande, B.P.; Ribeiro, A.M.; Pisani, L.P.; Estadella, D. Hippocampal BDNF mediated anxiety-like behaviours induced by obesogenic diet withdrawal. Behav. Brain Res. 2023, 436, 114077. [Google Scholar] [CrossRef]
- Liu, X.; Li, P.; Ma, X.; Zhang, J.; Sun, X.; Luo, X.; Zhang, Y. Association between plasma levels of BDNF and GDNF and the diagnosis, treatment response in first-episode MDD. J. Affect. Disord. 2022, 315, 190–197. [Google Scholar] [CrossRef]
- Domitrovic Spudic, S.; Nikolac Perkovic, M.; Uzun, S.; Nedic Erjavec, G.; Kozumplik, O.; Svob Strac, D.; Mimica, N.; Pivac, N. Reduced plasma BDNF concentration and cognitive decline in veterans with PTSD. Psychiatry Res. 2022, 316, 114772. [Google Scholar] [CrossRef]
- Lucaci, A.G.; Notaras, M.J.; Kosakovsky Pond, S.L.; Colak, D. The evolution of BDNF is defined by strict purifying selection and prodomain spatial coevolution, but what does it mean for human brain disease? Transl. Psychiatry 2022, 12, 258. [Google Scholar] [CrossRef]
- Camuso, S.; Canterini, S. Brain-derived neurotrophic factor in main neurodegenerative diseases. Neural Regen. Res. 2023, 18, 554–555. [Google Scholar] [CrossRef]
- Zyuz’kov, G.N.; Miroshnichenko, L.A.; Kotlovskaya, L.Y.; Chayikovskyi, A.V. Prospect of Using ERK1/2 and p38 in Regeneration-Competent Cells of Nervous Tissue as a Drug Targets for Treating Alzheimer’s Disease. Biointerface Res. Appl. Chem. 2023, 13, 6508338421. [Google Scholar] [CrossRef]
- Shi, A. Effects of BDNF and ANK3 in bipolar disorder. In Proceedings of the AIP Conference, Adelaide, Australia, 11–16 December 2022. [Google Scholar]
- Tosolini, A.P.; Sleigh, J.N.; Surana, S.; Rhymes, E.R.; Cahalan, S.D.; Schiavo, G. BDNF-dependent modulation of axonal transport is selectively impaired in ALS. Acta Neuropathol. Commun. 2022, 10, 121. [Google Scholar] [CrossRef]
- Zhou, T.; He, S.; Ye, X.; Wei, Z.; Wan, J.; Zhang, H.; Ding, S. Exposure to dibutyl phthalate adsorbed to multi-walled carbon nanotubes causes neurotoxicity in mice by inducing the release of BDNF. Sci. Total Environ. 2022, 852, 158319. [Google Scholar] [CrossRef]
- Cao, M.C.; Cawston, E.E.; Chen, G.; Brooks, C.; Douwes, J.; McLean, D.; Graham, E.S.; Dragunow, M.; Scotter, E.L. Serum biomarkers of neuroinflammation and blood-brain barrier leakage in amyotrophic lateral sclerosis. BMC Neurol. 2022, 22, 216. [Google Scholar] [CrossRef]
- Nordvall, G.; Forsell, P.; Sandin, J. Neurotrophin-targeted therapeutics: A gateway to cognition and more? Drug Discov. Today 2022, 27, 103318. [Google Scholar] [CrossRef]
- Filimonova, T.; Karakulova, Y. Tropomyosin receptor kinase B-mediated signaling in integration of neuropathic pain and obesity in diabetic polyneuropathy. Einstein 2021, 19, eAO6256. [Google Scholar] [CrossRef]
- Mozafarihashjin, M.; Togha, M.; Ghorbani, Z.; Farbod, A.; Rafiee, P.; Martami, F. Assessment of peripheral biomarkers potentially involved in episodic and chronic migraine: A case-control study with a focus on NGF, BDNF, VEGF, and PGE2. J. Headache Pain 2022, 23, 3. [Google Scholar] [CrossRef]
- Kustova, A.O.; Gavrish, M.S.; Sergeeva, M.A.; Avlasenko, D.A.; Kiseleva, A.O.; Epifanova, E.A.; Babaev, A.A.; Mishchenko, T.A.; Vedunova, M.V. The Influence of Neurotrophic Factors BDNF and GDNF Overexpression on the Functional State of Mice and Their Adaptation to Audiogenic Seizures. Brain Sci. 2022, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Sivasangari, K.; Emmanuvel Rajan, K. Prenatal exposure to valproic acid alters Reelin, NGF expressing neuron architecture and impairs social interaction in their autistic-like phenotype male offspring. Exp. Brain Res. 2022, 240, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol. Motil. 2018, 30, e13446. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.P.; Roberts, C.; Waseem, M.; Tyagi, P. Drug Targets in Neurotrophin Signaling in the Central and Peripheral Nervous System. Mol. Neurobiol. 2018, 55, 6939–6955. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. BioMed Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef]
- Mohamed, R.; El-Remessy, A.B. Imbalance of the Nerve Growth Factor and Its Precursor: Implication in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2015, 6, 483. [Google Scholar] [CrossRef]
- Chang, H.-M.; Wu, H.-C.; Sun, Z.-G.; Lian, F.; Leung, P. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: Physiological and pathophysiological implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Proenca, C.C.; Song, M.; Lee, F.S. Differential effects of BDNF and neurotrophin 4 (NT4) on endocytic sorting of TrkB receptors. J. Neurochem. 2016, 138, 397–406. [Google Scholar] [CrossRef]
- Tian, X.; Yan, H.; Li, J.; Wu, S.; Wang, J.; Fan, L. Neurotrophin Promotes Neurite Outgrowth by Inhibiting Rif GTPase Activation Downstream of MAPKs and PI3K Signaling. Int. J. Mol. Sci. 2017, 18, 148. [Google Scholar] [CrossRef]
- Zou, J.; Lei, T.; Guo, P.; Yu, J.; Xu, Q.; Luo, Y.; Ke, R.; Huang, D. Mechanisms shaping the role of ERK1/2 in cellular senescence (Review). Mol. Med. Rep. 2019, 19, 759–770. [Google Scholar] [CrossRef]
- Wang, S.; Wang, B.; Shang, D.; Zhang, K.; Yan, X.; Zhang, X. Ion Channel Dysfunction in Astrocytes in Neurodegenerative Diseases. Front. Physiol. 2022, 13, 11. [Google Scholar] [CrossRef]
- Bennison, S.A.; Blazejewski, S.M.; Smith, T.H.; Toyo-Oka, K. Protein kinases: Master regulators of neuritogenesis and therapeutic targets for axon regeneration. Cell. Mol. Life Sci. 2020, 77, 1511–1530. [Google Scholar] [CrossRef]
- Wang, J.Q.; Mao, L. The ERK Pathway: Molecular Mechanisms and Treatment of Depression. Mol. Neurobiol. 2019, 56, 6197–6205. [Google Scholar] [CrossRef]
- Fang, J.; Wang, S.; Zhou, J.; Shao, X.; Sun, H.; Liang, Y.; He, X.; Jiang, Y.; Liu, B.; Jin, X.; et al. Electroacupuncture Regulates Pain Transition Through Inhibiting PKCε and TRPV1 Expression in Dorsal Root Ganglion. Front. Neurosci. 2021, 15, 685715. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Stamatelopoulos, K.; Bampatsias, D.; Sachse, M.; Zormpas, E.; Vlachogiannis, N.I.; Tual-Chalot, S.; Stellos, K. The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 952–967. [Google Scholar] [CrossRef]
- Zeng, L.; Palaia, I.; Šarić, A.; Su, X. PLCγ1 promotes phase separation of T cell signaling components. J. Cell Biol. 2021, 220, e202009154. [Google Scholar] [CrossRef]
- Bill, C.A.; Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [CrossRef]
- Lock, J.T.; Parker, I. IP3 mediated global Ca2+ signals arise through two temporally and spatially distinct modes of Ca2+ release. eLife 2020, 9, e55008. [Google Scholar] [CrossRef]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Cuesta, C.; Arévalo-Alameda, C.; Castellano, E. The Importance of Being PI3K in the RAS Signaling Network. Genes 2021, 12, 1094. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, P. Role of insulin receptor substance-1 modulating PI3K/Akt insulin signaling pathway in Alzheimer’s disease. 3 Biotech 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, Q. mTOR pathway: A potential therapeutic target for spinal cord injury. Biomed. Pharmacother. 2022, 145, 112430. [Google Scholar] [CrossRef] [PubMed]
- Baffi, T.R.; Newton, A.C. mTOR Regulation of AGC Kinases: New Twist to an Old Tail. Mol. Pharmacol. 2022, 101, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Cana, A.; Baumgärtner, W.; Spitzbarth, I. p75 Neurotrophin Receptor: A Double-Edged Sword in Pathology and Regeneration of the Central Nervous System. Veter Pathol. 2018, 55, 786–801. [Google Scholar] [CrossRef]
- Qin, T.; Yuan, Z.; Yu, J.; Fu, X.; Deng, X.; Fu, Q.; Ma, Z.; Ma, S. Saikosaponin-d impedes hippocampal neurogenesis and causes cognitive deficits by inhibiting the survival of neural stem/progenitor cells via neurotrophin receptor signaling in mice. Clin. Transl. Med. 2020, 10, e243. [Google Scholar] [CrossRef]
- Kuhn, K.D.; Edamura, K.; Bhatia, N.; Cheng, I.; Clark, S.A.; Haynes, C.V.; Heffner, D.L.; Kabir, F.; Velasquez, J.; Spano, A.J.; et al. Molecular dissection of TNFR-TNFα bidirectional signaling reveals both cooperative and antagonistic interactions with p75 neurotrophic factor receptor in axon patterning. Mol. Cell Neurosci. 2020, 103, 103467. [Google Scholar] [CrossRef]
- Yamashita, T.; Tucker, K.L.; Barde, Y.-A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef]
- Sajanti, A.; Lyne, S.B.; Girard, R.; Frantzén, J.; Rantamäki, T.; Heino, I.; Cao, Y.; Diniz, C.; Umemori, J.; Li, Y.; et al. A comprehensive p75 neurotrophin receptor gene network and pathway analyses identifying new target genes. Sci. Rep. 2020, 10, 14984. [Google Scholar] [CrossRef]
- Iobbi, C.; Korte, M.; Zagrebelsky, M. Nogo-66 Restricts Synaptic Strengthening via Lingo1 and the ROCK2–Cofilin Pathway to Control Actin Dynamics. Cereb. Cortex 2017, 27, 2779–2792. [Google Scholar] [CrossRef]
- Dobrowsky, R.T.; Carter, B.D. Coupling of the p75 Neurotrophin Receptor to Sphingolipid Signalinga. Ann. N. Y. Acad. Sci. 1998, 845, 32–45. [Google Scholar] [CrossRef]
- Kagan, T.; Stoyanova, G.; Lockshin, R.A.; Zakeri, Z. Ceramide from sphingomyelin hydrolysis induces neuronal differentiation, whereas de novo ceramide synthesis and sphingomyelin hydrolysis initiate apoptosis after NGF withdrawal in PC12 Cells. Cell Commun. Signal. 2022, 20, 15. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, J. [6]-Shogaol Attenuates Neuronal Apoptosis in Hydrogen Peroxide-Treated Astrocytes Through the Up-Regulation of Neurotrophic Factors. Phytother. Res. 2013, 27, 1795–1799. [Google Scholar] [CrossRef]
- Micheli, L.; Toti, A.; Lucarini, E.; Ferrara, V.; Ciampi, C.; Olivero, G.; Pittaluga, A.; Mattoli, L.; Pelucchini, C.; Burico, M.; et al. Efficacy of a vegetal mixture composed of Zingiber officinale, Echinacea purpurea, and Centella asiatica in a mouse model of neuroinflammation: In vivo and ex vivo analysis. Front. Nutr. 2022, 9, 887378. [Google Scholar] [CrossRef]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, S.; Zhang, Z.; Gu, Y.; Xia, S.; Bao, X.; Cao, X.; Xu, Y. 6-Gingerol attenuates microglia-mediated neuroinflammation and ischemic brain injuries through Akt-mTOR-STAT3 signaling pathway. Eur. J. Pharmacol. 2020, 883, 173294. [Google Scholar] [CrossRef]
- Gu, M.; Lee, P.; Ha, S.K.; Hur, J. Zerumbone attenuates lipopolysaccharide-induced activation of BV-2 microglial cells via NF-κB signaling. Appl. Biol. Chem. 2020, 63, 46. [Google Scholar] [CrossRef]
- Hirano, K.; Kubo, M.; Fukuyama, Y.; Namihira, M. Indonesian Ginger (Bangle) Extract Promotes Neurogenesis of Human Neural Stem Cells through WNT Pathway Activation. Int. J. Mol. Sci. 2020, 21, 4772. [Google Scholar] [CrossRef]
- Cárdenas Garza, G.R.; Elizondo Luévano, J.H.; Bazaldúa Rodríguez, A.F.; Chávez Montes, A.; Pérez Hernández, R.A.; Martínez Delgado, A.J.; López Villarreal, S.M.; Rodríguez Rodríguez, J.; Sánchez Casas, R.M.; Castillo Velázquez, U.; et al. Benefits of Cardamom (Elettaria cardamomum (L.) Maton) and Turmeric (Curcuma longa L.) Extracts for Their Applications as Natural Anti-Inflammatory Adjuvants. Plants 2021, 10, 1908. [Google Scholar] [CrossRef]
- Araújo, F.; Chaves-Filho, A.; Nunes, A.; Oliveira, G.; Vasconcelos, G.; Carletti, J.; Moraes, M.; Moraes, E.; Vasconcelos, S.; Sousa, F.; et al. Involvement of Anti-Inflammatory, Antioxidant, and BDNF Up-Regulating Properties in the Antipsychotic-like Effect of the Essential Oil of Alpinia Zerumbet in Mice: A Comparative Study With Olanzapine. Metab. Brain Dis. 2021, 36, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Cho, B.O.; Wang, F.; Shin, J.Y.; Shin, D.; Jang, S.I. Zingiber officinale attenuates neuroinflammation in LPS-stimulated mouse microglia by AKT/STAT3, MAPK, and NF-κB signaling. Food Sci. Technol. 2022, 42, e104221. [Google Scholar] [CrossRef]
- Kim, C.Y.; Seo, Y.; Lee, C.; Park, G.H.; Jang, J.H. Neuroprotective Effect and Molecular Mechanism of [6]-Gingerol against Scopolamine-Induced Amnesia in C57BL/6 Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 8941564. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.C.; Hsu, C.W.; Chang, C.H.; Tseng, P.T.; Chang, K.V. The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Nie, Q.; Li, Z.; Du, G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol.-Res. Pract. 2016, 212, 247–251. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, S.; Tsoi, B.; Qi, S.; Gu, B.; Wang, Z.; Peng, C.; Shen, J. Alpinia oxyphylla Miq. and Its Active Compound P-Coumaric Acid Promote Brain-Derived Neurotrophic Factor Signaling for Inducing Hippocampal Neurogenesis and Improving Post-cerebral Ischemic Spatial Cognitive Functions. Front. Cell Dev. Biol. 2021, 8, 577790. [Google Scholar] [CrossRef]
- Wei, W.; Qiuying, D.; Wenbo, J.; Yue, W.; Yingying, C.; Tianshu, H.; Changhao, S. Dichloroacetic acid-induced dysfunction in rat hippocampus and the protective effect of curcumin. Metab. Brain Dis. 2021, 36, 545–556. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, F.; Guo, Y.; Zhang, Y.; Li, L.; Dang, R.; Jiang, P. Curcumin Relieves Chronic Unpredictable Mild Stress-Induced Depression-Like Behavior through the PGC-1α/FNDC5/BDNF Pathway. Behav. Neurol. 2021, 2021, 2630445. [Google Scholar] [CrossRef]
- Benjamart, S.; Siriwoot, S.; Jukreera, P.; Pitchaya, M.; Nitwara, W.; Smith, D.R.; Saranyapin, P.; Wutigri, N. Kaempferia parviflora Extract Inhibits STAT3 Activation and Interleukin-6 Production in HeLa Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, E4226. [Google Scholar] [CrossRef]
- Nakai, M.; Iizuka, M.; Matsui, N.; Hosogi, K.; Imai, A.; Abe, N.; Shiraishi, H.; Hirata, A.; Yagi, Y.; Jobu, K.; et al. Bangle (Zingiber purpureum) Improves Spatial Learning, Reduces Deficits in Memory, and Promotes Neurogenesis in the Dentate Gyrus of Senescence-Accelerated Mouse P8. J. Med. Food 2016, 19, 435–441. [Google Scholar] [CrossRef]
- Kubo, M.; Gima, M.; Baba, K.; Nakai, M.; Harada, K.; Suenaga, M.; Matsunaga, Y.; Kato, E.; Hosoda, S.; Fukuyama, Y. Novel neurotrophic phenylbutenoids from Indonesian ginger Bangle, Zingiber purpureum. Bioorg. Med. Chem. Lett. 2015, 25, 1586–1591. [Google Scholar] [CrossRef]
- Kim, H.G.; Lim, S.; Hong, J.; Kim, A.J.; Oh, M.S. Effects of Myoga on Memory and Synaptic Plasticity by Regulating Nerve Growth Factor-Mediated Signaling. Phytother. Res. 2016, 30, 208–213. [Google Scholar] [CrossRef]
- Tang, H.; Shao, C.; Wang, X.; Cao, Y.; Li, Z.; Luo, X.; Yang, X.; Zhang, Y. 6-Gingerol attenuates subarachnoid hemorrhage-induced early brain injury via GBP2/PI3K/AKT pathway in the rat model. Front. Pharmacol. 2022, 13, 3. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.-G.; Yang, W.; Xu, P.; Xiao, Y.-L.; Zhang, H.-T. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed. Pharmacother. 2018, 107, 1523–1529. [Google Scholar] [CrossRef]
- Majdi Yazdi, G.; Vaezi, G.; Hojati, V.; Mohammad-Zadeh, M. The Effect of 6-gingerol on Growth Factors and Apoptosis Indices in Rats Exposed to Gold Nanoparticles. Basic Clin. Neurosci. 2021, 12, 301–307. [Google Scholar] [CrossRef]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.K.J.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014, 449, 8–13. [Google Scholar] [CrossRef]
- Shim, S.; Kwon, J. Effects of [6]-shogaol on cholinergic signaling in HT22 cells following neuronal damage induced by hydrogen peroxide. Food Chem. Toxicol. 2012, 50, 1454–1459. [Google Scholar] [CrossRef]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef]
- Han, Q.; Yuan, Q.; Meng, X.; Huo, J.; Bao, Y.; Xie, G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget 2017, 8, 42001–42006. [Google Scholar] [CrossRef]
- Sapkota, A.; Park, S.J.; Choi, J.W. Neuroprotective Effects of 6-Shogaol and Its Metabolite, 6-Paradol, in a Mouse Model of Multiple Sclerosis. Biomol. Ther. 2019, 27, 152–159. [Google Scholar] [CrossRef]
- Yang, X.; Li, B.; Tian, H.; Cheng, X.; Zhou, T.; Zhao, J. Curcumenol Mitigates the Inflammation and Ameliorates the Catabolism Status of the Intervertebral Discs In Vivo and In Vitro via Inhibiting the TNFα/NFκB Pathway. Front. Pharmacol. 2022, 13, 905966. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhao, P.; Fan, L.; Bao, Z.; Tu, Y.; Li, C.; Chao, H.; Xu, X.; Ji, J. Curcumin alleviates neuroinflammation, enhances hippocampal neurogenesis, and improves spatial memory after traumatic brain injury. Brain Res. Bull. 2020, 162, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, N.; Ikawati, Z.; Kertia, I. Antiinflamatory and antidepressive activities of Extract Curcuma xanthorrhiza Roxb in Systemic Lupus Erythematosus. Indones. J. Pharm. 2017, 28, 185. [Google Scholar] [CrossRef]
- Busca Guerzoni, L.P.; Valérie, N.; Angelova, A. In Vitro Modulation of TrkB Receptor Signaling upon Sequential Delivery of Curcumin-DHA Loaded Carriers Towards Promoting Neuronal Survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinosa, J.; Arce-Aceves, M.; López Torres, M.; Lozano-Ordaz, V.; Mata-Espinosa, D.; Payán, J.A.; Silva-Islas, C.; Maldonado, P.; Marquina, B.; Hernández-Pando, R. Effect of Curcumin in Experimental Pulmonary Tuberculosis: Antimycobacterial Activity in the Lungs and Anti-Inflammatory Effect in the Brain. Int. J. Mol. Sci. 2022, 23, 1964. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mitsunaga, T.; Yamauchi, K. 6-Paradol and its glucoside improve memory disorder in mice. Food Funct. 2020, 11, 9892–9902. [Google Scholar] [CrossRef]
- Yan, T.; Wu, B.; Liao, Z.Z.; Liu, B.; Zhao, X.; Bi, K.S.; Jia, Y. Brain-derived Neurotrophic Factor Signaling Mediates the Antidepressant-like Effect of the Total Flavonoids of Alpiniae Oxyphyllae Fructus in Chronic Unpredictable Mild Stress Mice. Phytother. Res. 2016, 30, 1493–1502. [Google Scholar] [CrossRef]
- Li, H.; Park, J.H.; Yan, B.; Yoo, K.-Y.; Lee, C.; Choi, J.; Hwang, I.; Won, M.-H. Neuroprotection of Alpinia katsumadai Seed Extract against Neuronal Damage in the Ischemic Gerbil Hippocampus is Linked to Altered Brain-Derived Neurotrophic Factor. Lab. Anim. Res. 2011, 27, 67–71. [Google Scholar] [CrossRef]
- Natsume, N.; Yamano, A.; Watanabe, A.; Yonezawa, T.; Woo, J.T.; Yamakuni, T.; Teruya, T. Effect of methoxyflavones contained in Kaempferia parviflora on CRE-mediated transcription in PC12D cells. Bioorg. Med. Chem. Lett. 2020, 30, 127606. [Google Scholar] [CrossRef]
- Veldsman, M.; Cheng, H.-J.; Ji, F.; Werden, E.; Khlif, M.S.; Ng, K.K.; Lim, J.K.W.; Qian, X.; Yu, H.; Zhou, J.H.; et al. Degeneration of structural brain networks is associated with cognitive decline after ischaemic stroke. Brain Commun. 2020, 2, fcaa155. [Google Scholar] [CrossRef]
- Park, D.C. Cognitive ability in old age is predetermined by age 20 y. Proc. Natl. Acad. Sci. USA 2019, 116, 1832–1833. [Google Scholar] [CrossRef]
- McCabe, D.P.; Roediger, H.L.; McDaniel, M.A.; Balota, D.A.; Hambrick, D.Z. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef]
- Tamnes, C.K.; Walhovd, K.B.; Dale, A.M.; Østby, Y.; Grydeland, H.; Richardson, G.; Westlye, L.T.; Roddey, J.C.; Hagler, D.J., Jr.; Due-Tønnessen, P.; et al. Brain development and aging: Overlapping and unique patterns of change. Neuroimage 2013, 68, 63–74. [Google Scholar] [CrossRef]
- McDonald, W.M. Overview of Neurocognitive Disorders. Focus (Am. Psychiatr. Publ.) 2017, 15, 4–12. [Google Scholar] [CrossRef]
- Montaron, M.-F.; Garcia, P.; Abrous, D.N. Responsiveness of Dentate Neurons Generated Throughout Adult Life Determines Spatial Memory Ability of Aged Rats. bioRxiv 2018. [Google Scholar] [CrossRef]
- Yin, C.; Imms, P.; Cheng, M.; Amgalan, A.; Chowdhury, N.F.; Massett, R.J.; Chaudhari, N.N.; Chen, X.; Thompson, P.M.; Bogdan, P.; et al. Anatomically interpretable deep learning of brain age captures domain-specific cognitive impairment. Proc. Natl. Acad. Sci. USA 2023, 120, e2214634120. [Google Scholar] [CrossRef]
- Kongsui, R.; Sriraksa, N.; Thongrong, S. The Neuroprotective Effect of Zingiber cassumunar Roxb. Extract on LPS-Induced Neuronal Cell Loss and Astroglial Activation within the Hippocampus. BioMed Res. Int. 2020, 2020, 4259316. [Google Scholar] [CrossRef]
- Ou, G.Y.; Lin, W.W.; Zhao, W.J. Neuregulins in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 662474. [Google Scholar] [CrossRef]
- Kijonka, M.; Borys, D.; Psiuk-Maksymowicz, K.; Gorczewski, K.; Wojcieszek, P.; Kossowski, B.; Marchewka, A.; Swierniak, A.; Sokol, M.; Bobek-Billewicz, B. Whole Brain and Cranial Size Adjustments in Volumetric Brain Analyses of Sex- and Age-Related Trends. Front. Neurosci. 2020, 14, 278. [Google Scholar] [CrossRef]
- Gokce, U.; Mahmut, O. Classifying Early and Late Mild Cognitive Impairment Stages of Alzheimer’s Disease by Analyzing Different Brain Areas. In Proceedings of the 2020 Medical Technologies Congress (TIPTEKNO), Antalya, Turkey, 19–20 November 2020; pp. 1–4. [Google Scholar]
- Rucco, R.; Lardone, A.; Liparoti, M.; Lopez, E.T.; De Micco, R.; Tessitore, A.; Granata, C.; Mandolesi, L.; Sorrentino, G.; Sorrentino, P. Brain Networks and Cognitive Impairment in Parkinson’s Disease. Brain Connect. 2021, 12, 465–475. [Google Scholar] [CrossRef]
- Ogura, A.; Kawabata, K.; Watanabe, H.; Choy, S.W.; Bagarinao, E.; Kato, T.; Imai, K.; Masuda, M.; Ohdake, R.; Hara, K.; et al. Fiber-specific white matter analysis reflects upper motor neuron impairment in amyotrophic lateral sclerosis. Eur. J. Neurol. 2022, 29, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.; Garcia-Rendueles, M.E.R. Oncogenic Pathways in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 3223. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.G.; Lee, H.; Lee, S.B. Mechanisms of protein toxicity in neurodegenerative diseases. Cell. Mol. Life Sci. 2018, 75, 3159–3180. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J. Neuroinflamm. 2022, 19, 223. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Thompson, T.B.; Chaggar, P.; Kuhl, E.; Goriely, A.; Alzheimer’s Disease Neuroimaging Initiative. Protein-protein interactions in neurodegenerative diseases: A conspiracy theory. PLoS Comput. Biol. 2020, 16, e1008267. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.A.; Akinduti, P.A.; Ajanaku, K.O. Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Zeng, P.; Liu, Y.-C.; Wang, X.-M.; Ye, C.-Y.; Sun, Y.-W.; Su, H.-F.; Qiu, S.-W.; Li, Y.-N.; Wang, Y.; Wang, Y.-C.; et al. Targets and mechanisms of Alpinia oxyphylla Miquel fruits in treating neurodegenerative dementia. Front. Aging Neurosci. 2022, 14, 1013891. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Guo, J.; Xu, C.; Cao, Y.; Fang, Z.; Wang, Q. Pharmacological Mechanisms Underlying the Neuroprotective Effects of Alpinia oxyphylla Miq. on Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2071. [Google Scholar] [CrossRef]
- Shi, S.-H.; Zhao, X.; Liu, B.; Li, H.; Liu, A.-J.; Wu, B.; Bi, K.-S.; Jia, Y. The Effects of Sesquiterpenes-Rich Extract of Alpinia oxyphylla Miq. on Amyloid-β-Induced Cognitive Impairment and Neuronal Abnormalities in the Cortex and Hippocampus of Mice. Oxidative Med. Cell. Longev. 2014, 2014, 451802. [Google Scholar] [CrossRef]
- Li, L.; Wu, X.-H.; Zhao, X.-J.; Xu, L.; Pan, C.-L.; Zhang, Z.-Y. Zerumbone ameliorates behavioral impairments and neuropathology in transgenic APP/PS1 mice by suppressing MAPK signaling. J. Neuroinflamm. 2020, 17, 61. [Google Scholar] [CrossRef]
- Park, Y.G.; Park, Y.S.; Kim, I.-B. Complement system and potential therapeutics in age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, N.; Chu, Y.; Postnikova, O.; Varghese, R.; Horvath, A.; Cheema, A.K.; Golestaneh, N. Dysregulated metabolic pathways in age-related macular degeneration. Sci. Rep. 2020, 10, 2464. [Google Scholar] [CrossRef]
- Sanz-Hernández, M.; Barritt, J.D.; Sobek, J.; Hornemann, S.; Aguzzi, A.; De Simone, A. Mechanism of misfolding of the human prion protein revealed by a pathological mutation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019631118. [Google Scholar] [CrossRef]
- Piccardo, P.; Asher, D.M. Complex proteinopathies and neurodegeneration: Insights from the study of transmissible spongiform encephalopathies. Arq. Neuropsiquiatr. 2018, 76, 705–712. [Google Scholar] [CrossRef]
- Murley, A.G.; Tsvetanov, K.A.; Rouse, M.A.; Jones, P.S.; Sværke, K.; Li, W.; Carpenter, A.; Rowe, J.B. Proton magnetic resonance spectroscopy in frontotemporal lobar degeneration-related syndromes. Neurobiol. Aging 2022, 111, 64–70. [Google Scholar] [CrossRef]
- Pacheco, C.D.; Lieberman, A.P. The pathogenesis of Niemann-Pick type C disease: A role for autophagy? Expert Rev. Mol. Med. 2008, 10, e26. [Google Scholar] [CrossRef]
- Adav, S.S.; Park, J.E.; Sze, S.K. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in Alzheimer’s disease. Mol. Brain 2019, 12, 8. [Google Scholar] [CrossRef]
- Gauba, E.; Chen, H.; Guo, L.; Du, H. Cyclophilin D deficiency attenuates mitochondrial F1Fo ATP synthase dysfunction via OSCP in Alzheimer’s disease. Neurobiol. Dis. 2019, 121, 138–147. [Google Scholar] [CrossRef]
- Lang, M.; Pramstaller, P.P.; Pichler, I. Crosstalk of organelles in Parkinson’s disease—MiT family transcription factors as central players in signaling pathways connecting mitochondria and lysosomes. Mol. Neurodegener. 2022, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Gilbert, R. Progressive Supranuclear Palsy. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rampello, L.; Buttà, V.; Raffaele, R.; Vecchio, I.; Battaglia, G.; Cormaci, G.; Alvano, A. Progressive supranuclear palsy: A systematic review. Neurobiol. Dis. 2005, 20, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.L.; Combs, B.; Alhadidy, M.M.; Brady, S.T.; Morfini, G.A.; Kanaan, N.M. Tau: A Signaling Hub Protein. Front. Mol. Neurosci. 2021, 14, 647054. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guan, Y.; Zhao, Z.; Meng, F.; Wang, X.; Gao, X.; Liu, J.; Chen, Y.; Zhou, F.; Zhou, S.; et al. Potential Roles of the WNT Signaling Pathway in Amyotrophic Lateral Sclerosis. Cells 2021, 10, 839. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M.F. Implication of HMGB1 signaling pathways in Amyotrophic lateral sclerosis (ALS): From molecular mechanisms to pre-clinical results. Pharmacol. Res. 2020, 156, 104792. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Pathophysiology of Cerebral Ischemia and Brain Trauma: Similarities and Differences. J. Cereb. Blood Flow Metab. 2004, 24, 133–150. [Google Scholar] [CrossRef]
- Pluta, R.; Ulamek-Koziol, M.; Januszewski, S.; Czuczwar, S. From Brain Ischemia to Alzheimer-Like Neurodegeneration. Neuropsychiatry 2018, 8, 1708–1714. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Post-Ischemic Neurodegeneration of the Hippocampus Resembling Alzheimer’s Disease Proteinopathy. Int. J. Mol. Sci. 2022, 23, 306. [Google Scholar]
- Stradecki-Cohan, H.M.; Cohan, C.H.; Raval, A.P.; Dave, K.R.; Reginensi, D.; Gittens, R.A.; Youbi, M.; Perez-Pinzon, M.A. Cognitive Deficits after Cerebral Ischemia and Underlying Dysfunctional Plasticity: Potential Targets for Recovery of Cognition. J. Alzheimers Dis. 2017, 60, S87–S105. [Google Scholar] [CrossRef]
- Teegala, R. Chapter 5—Role of nutraceuticals in the management of severe traumatic brain injury. In Nutraceuticals in Brain Health and Beyond; Ghosh, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 47–56. [Google Scholar]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Sperl, M.A.; Esterov, D.; Ransom, J.E.; Mielke, M.M.; Witkowski, J.E.; Brown, A.W. Long-Term Risk of Stroke after Traumatic Brain Injury: A Population-Based Medical Record Review Study. Neuroepidemiology 2022, 56, 283–290. [Google Scholar] [CrossRef]
- Albrecht, J.S.; Liu, X.; Smith, G.S.; Baumgarten, M.; Rattinger, G.B.; Gambert, S.R.; Langenberg, P.; Zuckerman, I.H. Stroke incidence following traumatic brain injury in older adults. J. Head Trauma Rehabil. 2015, 30, E62–E67. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Pellati, F.; Galeotti, N. Zingiber officinale Roscoe rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine 2020, 78, 153307. [Google Scholar] [CrossRef]
- Kopchak, O.O.; Odintsova, T.A. Cognitive impairment and depression in patients with relapsing–remitting multiple sclerosis depending on age and neuroimaging findings. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 119. [Google Scholar] [CrossRef]
- Afzal, A.; Batool, Z.; Sadir, S.; Liaquat, L.; Shahzad, S.; Tabassum, S.; Ahmad, S.; Kamil, N.; Perveen, T.; Haider, S. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem. Res. 2021, 46, 3273–3285. [Google Scholar] [CrossRef]
- Matsui, N.; Kido, Y.; Okada, H.; Kubo, M.; Nakai, M.; Fukuishi, N.; Fukuyama, Y.; Akagi, M. Phenylbutenoid dimers isolated from Zingiber purpureum exert neurotrophic effects on cultured neurons and enhance hippocampal neurogenesis in olfactory bulbectomized mice. Neurosci. Lett. 2012, 513, 72–77. [Google Scholar] [CrossRef]
- Mizushige, T.; Nogimura, D.; Nagai, A.; Mitsuhashi, H.; Taga, Y.; Kusubata, M.; Hattori, S.; Kabuyama, Y. Ginger-degraded collagen hydrolysate exhibits antidepressant activity in mice. J. Nutr. Sci. Vitaminol. 2019, 65, 251–257. [Google Scholar] [CrossRef]
- Armstrong, N.M.; An, Y.; Shin, J.J.; Williams, O.A.; Doshi, J.; Erus, G.; Davatzikos, C.; Ferrucci, L.; Beason-Held, L.L.; Resnick, S.M. Associations between cognitive and brain volume changes in cognitively normal older adults. NeuroImage 2020, 223, 117289. [Google Scholar] [CrossRef]
- Cheng, S.T. Cognitive Reserve and the Prevention of Dementia: The Role of Physical and Cognitive Activities. Curr. Psychiatry Rep. 2016, 18, 85. [Google Scholar] [CrossRef]
- Yu, L.; Guo, L.; Fang, X.; Yang, F.; Chen, Y.; Wang, Y.; Wang, D.; Wu, Z.; Liu, R.; Tian, X.; et al. Altered brain activity in the bilateral frontal cortices and neural correlation with cognitive impairment in schizophrenia. Brain Imaging Behav. 2022, 16, 415–423. [Google Scholar] [CrossRef]
- Palau-Baduell, M.; Salvado-Salvado, B.; Idiazabal-Alecha, M.A.; Fernandez-Teruel, A.; Ortiz, T. Perisylvian magnetoencephalografic impairments in patients with autism spectrum disorders. Rev. Neurol. 2018, 66, S45–S49. [Google Scholar] [PubMed]
- da Rosa, N.; de Medeiros, F.D.; de Oliveira, J.; Laurentino, A.O.M.; Peretti, E.M.; Machado, R.S.; Lourenço, M.P.; da Silva, T.I.; Fernandes, T.C.; Reis, P.A.; et al. 6-Shogaol Exerts a Neuroprotective Factor in Offspring after Maternal Immune Activation in Rats. Dev. Neurosci. 2022, 44, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Liu, B.-G.; Mo, X.-N.; Zou, M.; Mei, X.-P.; Chen, W.; Huang, G.-D.; Wu, L. Gingerol ameliorates neuronal damage induced by hypoxia-reoxygenation via the miR-210/brain-derived neurotrophic factor axis. Kaohsiung J. Med. Sci. 2022, 38, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L.A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; et al. Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. J. Pers. Med. 2022, 12, 1515. [Google Scholar] [PubMed]
- Gohil, K.; Kazmi, M.Z.H.; Williams, F.J. Structure-activity relationship and bioactivity studies of neurotrophic trans-banglene††Electronic supplementary information (ESI) available: Synthetic protocols, characterization data for all compounds (including purity and enantio-enrichment data), HPLC traces, PC 12 cell assay protocols, dose response study of (–) t-BG and all relevant spectra. See. Org. Biomol. Chem. 2022, 20, 2187–2193. [Google Scholar] [CrossRef]
- Badawy, G.M.; Atallah, M.N.; Sakr, S.A. Effect of gabapentin on fetal rat brain and its amelioration by ginger. Heliyon 2019, 5, e02387. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, R.; Yakhnitsa, V.; Santos, J.M.; Watson, C.; Kiritoshi, T.; Ji, G.; Hamood, A.N.; Neugebauer, V. Gingerol-Enriched Ginger Supplementation Mitigates Neuropathic Pain via Mitigating Intestinal Permeability and Neuroinflammation: Gut-Brain Connection. Front. Pharmacol. 2022, 13, 912609. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.d.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Talebi, M.; İlgün, S.; Ebrahimi, V.; Talebi, M.; Farkhondeh, T.; Ebrahimi, H.; Samarghandian, S. Zingiber officinale ameliorates Alzheimer’s disease and Cognitive Impairments: Lessons from preclinical studies. Biomed. Pharmacother. 2021, 133, 111088. [Google Scholar] [CrossRef]
- Saenghong, N.; Wattanathorn, J.; Muchimapura, S.; Tongun, T.; Piyavhatkul, N.; Banchonglikitkul, C.; Kajsongkram, T. Zingiber officinale Improves Cognitive Function of the Middle-Aged Healthy Women. Evid.-Based Complement. Altern. Med. 2012, 2012, 383062. [Google Scholar] [CrossRef]
- Martins, L.B.; Rodrigues, A.; Monteze, N.M.; Tibaes, J.R.B.; Amaral, M.H.A.; Gomez, R.S.; Teixeira, A.L.; Ferreira, A.V.M. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia 2020, 40, 88–95. [Google Scholar] [CrossRef]
- Ahmed, A. Preclinical Studies of Ginger (Zingiber officinale) Rhizome and Its Clinical Trial as an Add-on Antiepileptic Therapy in Children with Generalized Epilepsies. Ph.D. Thesis, University of Gezira, Wad Madani, Sudan, 2018. [Google Scholar]
- Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants 2022, 11, 1281. [Google Scholar] [CrossRef]
- Dost, F.S.; Kaya, D.; Ontan, M.S.; Erken, N.; Bulut, E.A.; Aydin, A.E.; Isik, A.T. Theracurmin Supplementation May be a Therapeutic Option for Older Patients with Alzheimer’s Disease: A 6-Month Retrospective Follow-Up Study. Curr. Alzheimer Res. 2021, 18, 1087–1092. [Google Scholar] [CrossRef]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scirè, A.; Armeni, T. Side Effects of Curcumin: Epigenetic and Antiproliferative Implications for Normal Dermal Fibroblast and Breast Cancer Cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S.; Murata, M. Evaluation for Safety of Antioxidant Chemopreventive Agents. Antioxid. Redox Signal. 2005, 7, 1728–1739. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J., IV; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef]
- Servello, A.; Leccese, V.; Ettorre, E. Natural Products for Neurocognitive Disorders; Exon Publications: Brisbane City, Australia, 2020; pp. 205–220. [Google Scholar]
- Damarla, S.R.; Komma, R.; Bhatnagar, U.; Rajesh, N.; Mulla, S.M.A. An Evaluation of the Genotoxicity and Subchronic Oral Toxicity of Synthetic Curcumin. J. Toxicol. 2018, 2018, 6872753. [Google Scholar] [CrossRef]
- Jantawong, C.; Priprem, A.; Intuyod, K.; Pairojkul, C.; Pinlaor, P.; Waraasawapati, S.; Mongkon, I.; Chamgramol, Y.; Pinlaor, S. Curcumin-loaded nanocomplexes: Acute and chronic toxicity studies in mice and hamsters. Toxicol. Rep. 2021, 8, 1346–1357. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Tang, Y.; Guan, X.; Chen, X.; Fan, J. Zingiberaceae plants/curcumin consumption and multiple health outcomes: An umbrella review of systematic reviews and meta-analyses of randomized controlled trials in humans. Phytother. Res. 2022, 36, 3080–3101. [Google Scholar] [CrossRef]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mennemeier, M.; Pimple, S. Effect of Alpinia galanga on Mental Alertness and Sustained Attention With or Without Caffeine: A Randomized Placebo-Controlled Study. J. Am. Coll. Nutr. 2017, 36, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Darcsi, A.; Kéry, Á.; Riethmüller, E. Blood-Brain Barrier Permeability Study of Ginger Constituents. J. Pharm. Biomed. Anal. 2019, 177, 112820. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).