Abstract
Exposure to early life stress (ELS), prenatal or postnatal during childhood and adolescence, can significantly impact mental and physical health. The role of the intestinal microbiome in human health, and particularly mental health, is becoming increasingly evident. This systematic review aims to summarize the clinical data evaluating the effect of ELS on the human intestinal microbiome. The systematic review (CRD42022351092) was performed following PRISMA guidelines, with ELS considered as exposure to psychological stressors prenatally and during early life (childhood and adolescence). Thirteen articles met all inclusion criteria, and all studies reviewed found a link between ELS and the gut microbiome in both prenatal and postnatal periods. However, we failed to find consensus microbiome signatures associated with pre- or postnatal stress, or both. The inconsistency of results is likely attributed to various factors such as different experimental designs, ages examined, questionnaires, timing of sample collection and analysis methods, small population sizes, and the type of stressors. Additional studies using similar stressors and validated stress measures, as well as higher-resolution microbiome analytical approaches, are needed to draw definitive conclusions about the links between stress and the human gut microbiome.
1. Introduction
Early life stress (ELS) is associated with myriad negative neuropsychiatric and physical health outcomes in later life. The concept of ELS is broad and includes both pre- and postnatal stress. Prenatal stress (or prenatal maternal stress) refers to any psychological or physical stress experienced by the mother during pregnancy that impacts fetal health [1]. Postnatal stress is related to any psychological/physical stress, including emotional, physical and sexual, childhood neglect, parental psychopathology and separation, and adolescent bullying, victimization, or violence [2]. Children exposed to ELS show alterations in brain development and are at increased risk of developing mental illness [3]. Indeed, it has been suggested that an adverse environment may contribute up to 45% of the development of mental illness in children and up to 30% in adults [4,5].
Chronic psychological stress is associated with the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, manifesting as elevated cortisol levels and alterations in the immune system and the gut microbiome [6,7]. While the acute stress response is regulated through physiological changes in the endocrine and nervous system, ultimately returning to the basal state, chronic and/or severe stress, particularly during childhood, can lead to long-lasting activation of stress pathways. This has detrimental physical and psychological consequences for major biological systems, including metabolic, cardiovascular, endocrine, immune, and nervous systems [8,9]. Many studies have investigated the mechanisms linking ELS to these systems and, more recently, several have examined the relationship between stress and the gut microbiome specifically in early life [10,11]. Some of these human studies, which are included in this systematic review, are summarized in Figure 1.
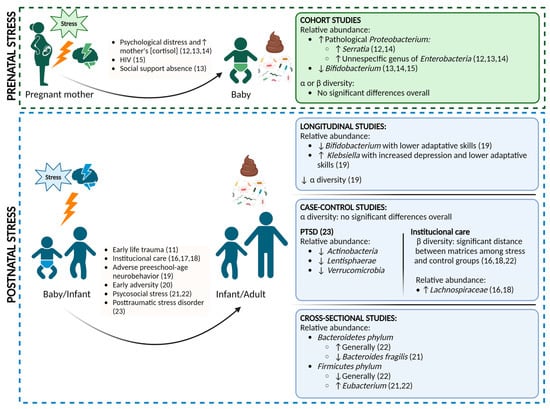
Figure 1.
Overview of the main results compiled in the systematic review of prenatal [12,13,14,15] and postnatal stress studies [11,16,17,18,19,20,21,22,23].
The gut microbiome is a complex ecosystem consisting of trillions of microorganisms including bacteria (representing the majority), viruses, protozoa, helminths, fungi, and archaea [24]. The term microbiome refers to the collective genome and activity associated with a specific host habitat or environment. The gut microbiome is involved in nutrient and xenobiotic metabolism, and in the development and function of the endocrine and immune systems and the gut barrier [25,26]. The gut microbiome participates in bidirectional communication with the brain to modulate central nervous system function [27], and preclinical studies indicate that gut microbes can influence brain behavior [28,29]. Alterations in the gut microbiome have been linked to mental health conditions such as depression or anxiety in pre-clinical and clinical studies [29,30,31,32].
The colonization and development of the gut microbiome is critical during the first years of life and may have long-lasting consequences owing to its intimate interaction with the immune system [33]. While there is debate over when the first contact with microbes begins, pre- or postnatally, it is clear that massive colonization of the infant gut by different microorganisms starts at birth. The extent of this is contingent on several factors such as the mode of delivery (vaginal or cesarean), prematurity, antibiotic treatments, feeding practices (breastfeeding or formula feeding), introduction of complementary food, exposure to animals (pets), number of siblings, or psychological stress [3,16]. The development of the gut microbiome from childhood to adulthood rests mainly on lifestyle and diet factors [16] and is characterized by an increase in the diversity of microbial species. Adolescence is also a critical developmental period when the individual is exposed to many challenges and stressors and the brain is developing. Accordingly, the richness of the gut microbiome also changes during this period, which can influence brain development and function [34].
Recent studies have shown that maternal prenatal stress is associated with infant development outcomes on several levels, emotional, behavioral, and cognitive [35,36], and with increased risk of developing somatic conditions such as asthma [37]. Indeed, even moderate stress exposure may have an influence if it occurs chronically [38]. Although the precise molecular mechanisms underlying the adverse effects of prenatal stress on offspring remain enigmatic, studies in animal models suggest that it can impact the microbiome [39,40]. Moreover, the few studies in humans point to a role for the gut microbiome in mediating prenatal stress and associated outcomes [12,13,14,15]. Hantsoo and Zemel [41] recently reviewed the impact of ELS on the gut microbiome and the role of dietary interventions to moderate its impact, but not systematically. The present systematic review aims to compile and discuss the existing scientific evidence on the link between ELS (vs. no ELS) and changes to the human gut microbiome. We sought to clarify whether ELS influences the gut microbiome and whether this can be a biological predictor and/or causative factor for the development of somatic or mental disorders. We review the literature evaluating how ELS shapes the gut microbiome, especially pre- and postnatal stress.
2. Materials and Methods
2.1. Search Strategy and Inclusion/Exclusion Criteria
The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [42]. The protocol was registered in PROSPERO under the Registration Number CRD42022351092.
We searched for published articles in Medline (Pubmed), EMBASE, Web of Science and Scopus (via Elsevier), in which the title, abstract, and keywords contained terms related with child abuse, adverse child experiences, maternal separation, ELS, gut microbiome, and human. Searches were performed on 30 April 2022 using a combination of keywords of subject and free text terms, with no date limit or language restriction. The strategy was developed for Medline and then adapted for other databases. The complete Medline strategy, including keywords, is shown in Supplementary Table S1.
Eligibility criteria included reports comparing ELS versus no ELS on gut microbiome outcomes in humans in the following types of study: case report, clinical trial, clinical trial protocol, clinical trial phase I–IV, controlled clinical trial, evaluation study, multicenter study, observational study, twin studies, and validation study. In vitro and animal studies were excluded. Different exposures and/or interventions were reviewed including prenatal exposure, such as in utero exposure to clinically significant depression, exposure to precarious situations, or serious illness during pregnancy; and postnatal exposure, such as emotional, physical, and sexual abuse or neglect in childhood, parental psychopathology and separation, prepubertal bullying, in addition to victimization or violence and serious physical illness during childhood or adolescence.
Titles, abstracts, and full texts of articles were screened independently by two reviewers (Agusti, A. and Lamers, F.) for eligibility using Abstract r free software (Brown University, Providence, RI, USA, http://abstrackr.cebm.brown.edu, accessed on 30 April 2022). Disagreement was resolved by discussion and by a third senior reviewer (Sanz, Y.) when needed.
2.2. Data Extraction
Data extracted included: (a) study data (journal, authors, publication year, study design, type of sample collected (feces), methods of gut microbiome analysis, measures of ELS and methods of assessing ELS, (b) sample description (sex, age, and ethnicity), and (c) effect sizes (analyses used to compare the gut microbiome between cases and controls, or association studies between ELS and gut microbiome changes). Data were extracted by two independent authors (Lamers, F. and Agusti, A.).
2.3. Assessment of Risk of Bias
Quality assessment of risk of bias of non-randomized studies, including cohort studies and case-control studies, was performed with the Newcastle-Ottawa Scale (NOS) [43], which follows the “star system” and evaluates 8 items grouped into 3 categories: selection of participants (maximum 4 stars), comparability of the groups (maximum 2 stars) and ascertainment of the outcomes (for cohort studies) or exposure (for case-control studies) of interest (maximum 3 stars). For quality assessment of risk of bias of cross-sectional studies, we used an adapted NOS scale created by Herzog et al. [44], which evaluates 7 items grouped into the same 3 categories: participants (maximum 5), comparability (maximum 2), and ascertainment of the outcomes (maximum score 3). Each study can be awarded a maximum of 10 stars, with a higher score indicating better methodology quality. Two authors (Agusti, A., Tamayo, M.) independently assessed the risk of bias of individual studies and any differences were resolved through consensus.
2.4. Strategy for Data Synthesis and Analysis of Subgroups
Because study designs and outcome assessments varied, the results are presented in a narrative manner with tables. Studies are presented based on the population, intervention (or exposure), comparison (if applicable), outcome criteria. We considered the subgroups pre- and postnatal stress.
3. Results
3.1. Overview
Of 202 articles found, 50 were duplicates and were removed before screening. Subsequently, 132 articles were excluded based on title/abstract. Seven of the remaining 20 full-text articles assessed for eligibility were also excluded, resulting in a total of 13 studies included. Figure 2 describes the screening and selection process in full. Studies were grouped into two categories: prenatal and postnatal stress. All 13 studies were observational. The four prenatal studies were prospective in design [12,13,14,15]. The nine postnatal studies included one longitudinal study [19], five case-control studies [16,17,18,20,23], and three cross-sectional studies [11,21,22]. All 13 studies were published between 2015 and 2022. Geographically, the distribution of the studies was: five in the U.S.A. [11,16,18,19,21], five in Europe [12,14,17,20,22], two in Africa [15,23], and one in South America [13].
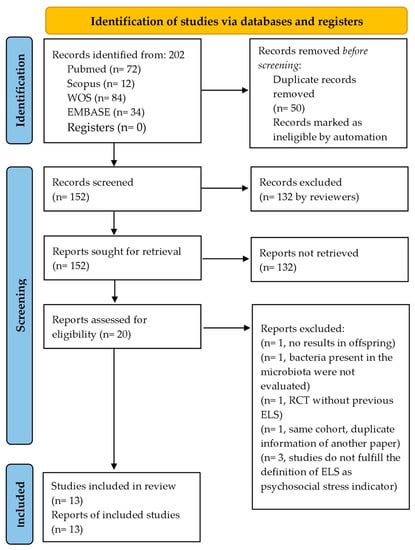
Figure 2.
PRISMA flow diagram.
Prenatal stress included not only studies in which the pregnant mother experienced psychological stress (depression and/or anxiety [12,14]) but also in which the mother’s lifestyle and physical circumstances may have caused stress to the baby, such as being exposed to precarious situations [13,15] or suffering from a serious illness such as HIV [15]. The postnatal studies included children and/or adolescents experiencing ELS; for example, studies on children/adolescents in institutional care [16,17,18], a study on adults with post-traumatic stress disorder (PTSD) in whom the impact of stress during their childhood had been assessed [23], a case-control study evaluating the impact of maternal prenatal psychological stress on the stress response of babies [20] and finally, studies investigating ELS reported by parents via questionnaire [11,19,21], sex-specific associations, and preschool-age neurobehavior [19].
3.2. Risk of Bias
3.2.1. Quality Assessment of Longitudinal Cohort Studies Based on the Newcastle-Ottawa Scale
Five longitudinal studies were evaluated following NOS methodology [43] (Table 1). In the category of “selection”, two studies received a rating of three because one used a self-report questionnaire rather than a structured interview, failing in the “ascertainment of the exposure”, and the other study failed to report information on the “outcome of interest at start of study”. One study received a rating of two, as information was only collected with a self-report questionnaire and no information was provided on “outcome of interest at start of study”. Two studies received a maximum score of four as they satisfied all the conditions in the selection of participants for a high-quality longitudinal study. In the “comparability” category, one study received a rating of one because it did not include appropriate confounders in the analysis. Five studies were awarded a rating of four because they included different confounders in the adjustment of the analysis, such as peripartum antibiotic exposure, exclusively or mixed breastfed, vaginal delivery, infant sex, or infant age during sampling. In the “outcome” category, only one study achieved the maximum score of three because it met all stipulated requirements. Four studies received two stars because they did not specify the “adequacy of follow-up of cohorts”. Four studies received a total score of seven [12,13,14,15] and one study had a total of eight [38].

Table 1.
Quality assessment for the selected studies based on Newcastle-Ottawa Quality Assessment Scale for longitudinal cohort studies. A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. Total score ranges from 0 to 9. Higher scores indicate better methodological quality.
3.2.2. Quality Assessment of Case-Control Studies Based on the Newcastle-Ottawa Scale
Five case-control studies were evaluated based on NOS methodology [43] (Table 2). In the category of “selection”, three studies received the maximum score of four because all adequately defined cases with good representativeness and suitable selection and definition of controls. The two remaining studies received three stars because none of them reported details about the participants (e.g., where they were recruited, hospital, clinic, city); therefore, the “representativeness of the cases” was rated 0. In the “comparability” category, one study received a rating of one because it did not adjust the analysis for appropriate confounders. Contrastingly, the remaining four studies included several confounders in the analysis, including birth mode, antibiotics, age, presence of siblings, country of origin, sex, diet, breastfeeding, and ethnicity, and received the maximum score of two. In the “exposure” category, all five studies received a rating of two. All used a diagnosis or a structured interview to assess the exposure and used the same method for control participants. Moreover, none of the studies clearly described the non-response rate in each group. In sum, three of the five studies received a final rating of seven [16,18,20] and two received a rating of eight [17,23].

Table 2.
Quality assessment for the selected studies based on Newcastle-Ottawa Quality Assessment Scale for case-control studies. A study can be awarded a maximum of one star for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability. Total score ranges from 0 to 9. Higher scores indicate better methodological quality.
3.2.3. Quality Assessment of Cross-Sectional Studies Based on the Newcastle-Ottawa Scale
Three cross-sectional studies [11,21,22] were evaluated with the adapted NOS [44] (Table 3). One study received a score of two in the “selection” category, as it failed to specify the selection of the control group, only used self-report questionnaires to evaluate the stressor, and provided no data about non-response. The second study received a rating of three, as it failed to report on non-response, and the third study received four stars. In the “comparability” category, one study received one star as it only included one confounder (diet) in the adjustment of the analysis. The remaining two studies received the maximum score of two as they included several confounders in the analysis, such as age, gender, socio-economic status, food intake frequency of fiber-rich food, protein-rich food, sweet food and fatty food, and day sleep duration. The last category evaluated was “outcome”, and all three studies received the maximum score of three. In sum, one study was rated six [21], one was rated eight [22], and the third study was rated nine [11].

Table 3.
Quality assessment for the selected studies based on a scale adapted from the Newcastle-Ottawa Quality Assessment Scale for cohort studies and an adapted Newcastle-Ottawa scale created for cross-sectional studies by Herzog et al., 2013 [44]. A study can be awarded a maximum of five stars in the Selection, two stars in the Comparability, three in the Outcomes and one in the Statistical test categories. Total score ranges from 0 to 9. Higher scores indicate better methodological quality.
3.3. Prenatal Stress
We reviewed the four human longitudinal studies published at the time the bibliographic search was conducted (Supplementary Table S1).
Longitudinal Studies
In a large prospective study in Finland (n = 446) [12], maternal prenatal psychological distress and hair cortisol levels were both associated with changes in the gut microbiome of 2.5-month-old infants. Chronic maternal prenatal psychological distress evaluated by different standardized questionnaires was negatively associated with Akkermansia, Phascolarctobacterium, and Megamonas abundance and positively with Veillonella, Finegoldia, Dialister, Dorea, and Coprococcus abundance. Likewise, maternal hair cortisol levels were related to the infant gut microbiome, and were negatively associated with Lactobacillus (phylum Firmicutes), Slackia and Actinobaculum (phylum Actinobacteria), Butyricimonas (phylum Bacteroidetes), and Citrobacter (phylum Proteobacteria). The second prospective longitudinal cohort study compared 272 mother–infant pairs from Nigeria [15] during the first 18 months of life exposed to maternal HIV infection but not infected themselves versus infants unexposed to maternal HIV. We assumed that experiencing a serious illness such as HIV generates physical and possibly psychological stress in the mother. Although a direct relationship between maternal HIV and the infant microbiome was not reported, antiretroviral drugs in the breast milk of HIV mothers was associated with a lower relative abundance of Bifidobacterium longum in non-infected infants. The third longitudinal cohort study, performed in 25 mother–infant dyads, revealed associations between exposure to precarity and alterations in the HPA axis during peripartum, and alterations in the gut microbiome of the offspring [13]. Measures of maternal precarity were obtained during and after pregnancy using validated questionnaires, and the HPA axis was evaluated through cortisol measurements in saliva from the mother during/after pregnancy. Saliva and stool samples were also obtained from the newborns at 3 days and 2 months of life. The authors reported that both measures of precarity exposure and HPA dysregulation were consistently associated with gut microbiome alterations in the infants, characterized by decreased species diversity and abundance of Bifidobacterium at the genus level and increased abundance of potential pathogens of the family Enterobacteriaceae. In the final prospective longitudinal study in Dutch children followed from the 3rd trimester of pregnancy until 110 days after birth [14], the authors found that prenatal stress assessed by questionnaires or by measures of basal cortisol in saliva, or both, was associated with the gut microbiome composition of the offspring during at least 3 months. Infants with high cumulative prenatal stress (high reported stress and high cortisol) had a higher relative abundance of Proteobacteria (Escherichia, Serratia, and Enterobacter), lower relative abundance of lactic acid bacteria (Lactobacillus, Lactoccus, and Aerococcus) and Actinobacteria (Bifidobacterium, Collinsella, and Eggerthella) and more gastrointestinal symptoms and allergic reactions reported by the mothers, suggesting an association with the dysbiosis.
In sum, although the four prenatal studies reported different results, there are some similarities; for example, in the association of stress symptoms/conditions with a lower abundance of Bifidobacterium and increased abundance of the Enterobacter genus (see Table 4 for additional information).

Table 4.
Characteristics and results of prenatal stress studies.
3.4. Postnatal Stress
3.4.1. Longitudinal Studies
Laue et al. [38] designed a 3-year follow-up prospective study to test for sex-specific associations between the gut microbiome and behavior, and collected stool samples from 260 children at 6 weeks, 1 year, and 2 years postpartum. When the children were ~3 years old, their parents completed a behavior assessment questionnaire to capture different types of phenotypes. Results showed that most outcomes were not related to beta diversity changes; however, higher microbiome diversity at the youngest age (6 weeks) was related to lower depression in the overall sample and with lower anxiety and better internalizing behavior, especially in boys. Specifically, better composite scores for adaptive skills in boys associated positively with Bifidobacterium abundance and negatively with Klebsiella abundance. In girls, Granulicatella was associated with worse anxiety scores at 6 weeks of age, whereas at 1 year Streptocuccus peroris was associated with better internalizing problems. Finally, some Blautia species were linked to worse hyperactivity scores with stronger associations among girls (Table 5).

Table 5.
Characteristics and results of postnatal stress studies.
3.4.2. Case-Control Studies
One study of 344 children aged 3–18 years investigated whether early adversity (EA), specifically parental deprivation, international adoption, or institutional care, was related to gastrointestinal and mental disorders and to changes in microbiome diversity (115 with EA and 229 controls) [16]. A positive association was found between EA experiences and gastrointestinal symptoms and anxiety. A sub-sample of children was then used to analyze the microbiome in stool samples and its relationship with functional magnetic resonance imaging of the brain (5–11 years old). Despite the low sample size (N = 8 EA; N = 8 control), the authors found changes in alpha (richness) and beta (uniqueness) diversity as well as decreases in Lachnospiraceae and an unknown bacterium in the EA group compared with controls, which associated with brain reactivity within emotion networks and with the frequency of diarrhea. A second case-control study performed by Malan-Muller et al. [23] investigated the associations between the gut microbiome and mental health outcomes in adults with PTSD and trauma-exposed controls (TE controls). The authors also reported on several questionnaires to evaluate potentially traumatic lifetime events, major depressive disorder (MDD) and anxiety disorders. A fecal sample was collected within the same week as the clinical assessment. The results showed that a consortium of four genera (Mitsuokella and Odoribacter, Catenibacterium, Olsenella) was positively associated with PTSD status and MDD was associated with a higher relative abundance of the phylum Bacteroidetes. Individuals using psychotropic medication (PTSD and TE controls) at the time of sampling showed an increase in the relative abundance of Ruminococcus and a decrease in Akkermansia. Treatment was also positively associated with Bacteroidetes, Firmicutes, and negatively with Verrucomicrobia. Keskitalo et al. [20] studied the link between gut microbiome composition and the cortisol releasing response to a stressor. Children from the FinnBrain Birth Cohort with an available fecal microbiome profile and a salivary test of cortisol at 2.5 months of age were included in this nested case-control study. Cortisol levels were evaluated 0, 15, 25, and 35 min after exposing the children to a mild acute stressor. Results showed that a blunted cortisol stress response was weakly associated with gut microbiome diversity, but associations were found between cortisol levels and the taxonomic composition of the fecal microbiome. Another case-control study associated ELS in adolescents adopted internationally from orphanages into the United States [18] with alterations in gut microbiome and inflammatory markers in a case-control study as compared with youth reared in birth families (controls). Results showed that the abundance of several bacterial taxa was significantly higher in the stressed group than in the control group, including the genera Prevotella, Bacteroides (Bacteroidaceae), Coprococcus, Streptococcus, and Escherichia. Cytomegalovirus was also higher in the stressed group and there were also differences in bacterial abundance in viral seropositive groups compared with seronegative groups: Escherichia, Ruminococcaceae, Lachnospiraceae, and Catabacteriaceae. Moreover, Escherichia was related to seropositive individuals in the stressed group (see Table 5 for additional information).
3.4.3. Cross-Sectional Studies
A cross-sectional study [11] explored the relationship between the gut microbiome, its metabolites, and brain alterations in adults with ELA, measured by the Early Traumatic Inventory-Self Report (ETI-SR), which includes 27 questions about general trauma, physical punishment, emotional abuse, and sexual abuse. The study enrolled 128 adults without psychiatric conditions as assessed by the modified MINI questionnaire. They also collected stool samples and performed cerebral structural and functional magnetic resonance. Results showed that higher scores in ETI-SR (>4) versus lower scores (≥4) were not associated with differences in microbial diversity or abundance of specific taxa. They also found that symptoms of anxiety, depression, and body mass index correlated significantly with several feces metabolites such as urate, glutamate gamma-methyl ester, and 5-oxoproline. These scales as well as current stress related significantly to brain functional connectivity of sensorimotor, central executive, default mode, and central autonomic regions, and subsets of these networks, in addition to salience, emotion regulation and occipital correlated significantly with the metabolites. A cross-sectional study performed by Michels et al. [22] with children and adolescents investigated the link between the gut microbiome in feces and psychosocial stress. Stress was reported with different questionnaires assessing negative events, negative versus positive emotions and emotional problems. Moreover, cortisol was measured as a stress biomarker and heart rate variability (pnn50) was used as a measure of parasympathetic nervous system activity. The results showed that high levels of stress (low pnn50 and elevated negative events) were associated with a decrease in alpha diversity. Adjusted and unadjusted taxonomic differences were also more pronounced for happiness and pnn50 (as a measure of parasympathetic nervous system activity), being associated with two abundant observed taxonomic units (OTUs), respectively (24 OTUs representing 11.8% of bacterial counts and 31 OTUs representing 13.0%). Overall, high stress was related to higher levels of Bacteroides, Parabacteroides, Rhodococcus, Methanobrevibacter, and Roseburia but lower Phascolarctobacterium at genus level as well as with lower Firmicutes at the phylum level. However, conflicting results were reported between different stress measures as well as differences between preadolescents and adolescents. In another cross-sectional study [21], the role of the psychosocial environment during childhood and caregivers behavior on the gut microbiome was evaluated in 40 early school-age (5–7 years) children in the Pacific Northwest of the U.S.A. Stool samples were analyzed (16S and metagenomics) in addition to a wide range of socioeconomic factors (socioeconomic risk, behavioral dysregulation, caregiver behavior, demography, gut-related history, and diet). Specifically, the microbial taxon that varied with behavioral disturbances was Bacteroides fragilis, which was associated with reduced levels of aggressivity, emotional reactivity, sadness, and impulsivity, as well as lower family incidents. Contrastingly, two butyrate-producing bacteria (Coprococcus comes and Eubacterium rectale) were associated with more anxious and depressive problems and less inhibitory control. However, Roseburia inulinivorans, also a butyrate-producing bacterium, was associated with a decrease in depressive problems. The authors further found associations between individual taxa (e.g., B. fragilis) and functional groups (e.g., monoamine metabolism) within the microbiome and metrics of socioeconomic risk and behavioral dysregulation. More details of case-control studies are compiled in Table 5.
4. Discussion
In the present systematic review, we sought to clarify the effect of ELS on the human gut microbiome by assessing the strengths and weaknesses of the existing literature. Although the knowledge on this subject is by no means extensive, with the majority of studies conducted in animal models, we compiled and critically reviewed human studies investigating both pre- and postnatal stress. Overall, the quality of the studies as assessed with the NOS scales was satisfactory, with most scoring seven or eight out of nine stars (only one study scored six). Figure 1 provides an overview of the main results of the systematic review.
The four studies on prenatal stress [12,13,14,15] were longitudinal in design and analyzed the gut microbiome at the genus level using the same sequencing platform. Three of the four studies [12,13,14] also reported maternal cortisol levels during and after pregnancy. While a detailed comparison of studies is difficult because of the different questionnaires used to assess stress, similar results were reported in some studies with respect to microbiome analysis. For example, in the Zijlmans et al. study [14], infants with high cumulative stress (high reported mother stress and high cortisol) had an increased relative abundance of Proteobacteria groups. Likewise, in the study by Janhke et al. [13], the stress perceived by pregnant mothers was associated with a high abundance of Proteobacteria in the newborn gut microbiome, in particular an unspecific genus of Enterobacteriaceae. The same study showed that this genus was also increased in infants with high basal cortisol levels 3 days postpartum. These results suggest that prenatal stress might be associated with an increase in the relative abundance of Proteobacteria phylum. Of note, gamma-proteobacteria have been linked to human infant necrotizing enterocolitis [45]. There were also similarities in the negative association between stress and the relative abundance of the genus Bifidobacterium in at least three of the four prenatal studies. Janhke et al. [13] found that the stress perceived by pregnant women in items including “postpartum depression” and “low family support in the postpartum” was negatively associated with Bifidobacterium. The same genus was also negatively associated with high infant basal cortisol levels at 3 days postpartum. Zijlmans et al. [14] also found that infants with high cumulative stress showed a negative association with the abundance of Bifidobacterium and lactic-acid bacteria (i.e., Lactobacillus, Lactoccus) and Aerococcus.
In their study on pregnant women with and without HIV, Grant-Beurmann et al. [15] found that breastfeeding positively associated with Bifidobacterium and Collinsella in children both unexposed and exposed to the virus. They also demonstrated that the abundance of the genus Bifidobacterium was significantly greater in unexposed children than in exposed children. That being said, antiretroviral therapy was associated with the reduction in the abundance of this genus. Bifidobacterium is associated with a variety of beneficial effects for health, with an important role in the barrier effect or regulation of immune system [46], but also in mental health-related diseases such as depression [47,48]. These results might suggest that prenatal stress is linked to a decrease in the relative abundance of Bifidobaterium, which theoretically may impact infant health. Nevertheless, we believe that the results are inconclusive and further studies are needed.
The 10 postnatal stress studies addressed diverse ELS stressors, including childhood trauma, psychiatric disorders, perceived stress, and care situations (institutionalized vs. not and childcare vs. home care). While exemplifying the diverse nature of ELS stressors, this scope hampered our ability to compare results. Indeed, the studies did not have the same end goal, although all met the stated requirements for ELS. For instance, in three of the nine studies, the impact of stress on the gut microbiome was not the final objective, but we included them in the analysis since they assessed whether institutional care for a prolonged period impacted the microbiome [16,17,18]. We assumed that these children would be under enormous stress; however, no cortisol measurements were conducted and no assessment of emotional state was made. All nine reviewed studies analyzed the microbiome at least at one time point, but only two studies included cortisol analysis.
We reviewed only one postnatal longitudinal stress study [38], in which the main objective was to test for sex-specific associations between gut microbiome and behavioral development. The authors reported that an increase in alpha diversity at 6 weeks was associated with lower levels of depression, anxiety, and internalizing problems and, at 2 years, with better social and adaptive skills, but only in boys. They also found associations between gut microbiome and adaptive skills behavior and hyperactivity at 6 weeks, 1 year, and 2 years, but no associations with domains related to ELS. We believe that the study was limited by the non-inclusion of dietary factors (with the exception of breastfeeding) or antibiotics as covariates. Moreover, most findings were identified in the sex-specific analyses and not in the unstratified analyses. This would suggest that future studies on gut microbiome and ELS should consider the sex influence.
With respect to the case-control studies on postnatal stress and gut microbiome, these varied widely in design, including participant age (ranging from newborns to adults) and the timing of fecal collection, which makes comparative analyses challenging. The type of ELS in the five studies also varied widely, including the stress of institutional care [16,17,18], PTSD as a possible childhood trauma [23] and ELS evaluated using questionnaires [20]. One of the five studies, which compared infants in institutional childcare from 3 months of age with home-cared peers, failed to find significant differences in the gut microbiome [17]. In two studies, no differences were found in the alpha diversity of the gut microbiome between cases and controls [18,23]. One of these studies [23] was performed within the “Shared Roots” parent study with patients with PTSD and TE controls [23]. Five years earlier, Hemmings et al. [49] analyzed the same cohort and although they also failed to find differences in alpha diversity between the PTSD and TE groups, they identified three phyla that were decreased and potentially important to classify PTSD vs. TE with an error rate of 30.7%: Actinobacteria, Lentisphaerae, and Verrucomicrobia. Interestingly, the relative abundance of Actinobacteria and Verrucomicrobia was associated with childhood trauma. In the study performed 5 years later by Malan-Muller et al. [23], the authors closely examined the impact of psychotropic medication on the gut microbiome, finding that medication in both groups associated positively with Bacteroidetes, Firmicutes, and negatively with Verrucomicrobia, in particular with its only genus Akkermansia. Accordingly, changes in the abundance of Verrucomicrobia (and Akkermansia) can be the result of the medication rather than PTSD, as discussed. Furthermore, the authors of the more recent study identified a consortium of four genera (Mitsuokella, Odoribacter, Catenibacterium, and Olsenella) to discriminate individuals with moderate PTSD from TE controls. We consider that this is a more robust study not only because of the increased taxonomic resolution to at least genus level, but also because medication was considered as a modulating factor, unlike the 2017 study. Because of this, we elected to include only the study of Malan-Muller et al. [23] in the review. Regarding the three studies reporting on children cared for in institutions or in foster care, followed by international adoption, one study failed to find any significant effect on the gut microbiome between cases and controls [17]; however, some interesting results were found in the other two studies. The Callahan et al. study [16] found that alpha diversity was lower in children exposed to adversity than in controls, whereas the study by Reid et al. [18] reported no differences in alpha diversity, and both studies found alterations in beta diversity. The Callahan study identified two biomarkers for those children cared for in institutions, but both belonged to unknown genera (one from the family Lachnospiraceae and the other was an unknown genus and family). The Reid study also identified changes in the Lachnospiraceae family between CMV serotypes, but not between the cases and controls. The role of Lachnospiraceae in the gut is contentious: it is considered as a main producer of beneficial short-chain fatty acids, but some members of the family are associated with intestinal diseases [50]. It is evident that a greater taxonomic resolution is needed to better interpret the potential biological meaning of these findings. The latter study also found differences in Prevotella, Bacteroides, Coprococcus, Streptococcus, and Escherichia at the genus level between cases and controls. The study by Keskitalo et al. [20] attempted to identify a link between cortisol measured in infant saliva and the gut microbiome at the age of 2.5 months, based on a previous study suggesting a positive association between gut microbiome alpha diversity and saliva cortisol reactivity in 1-month-old babies [51]. However, the authors failed to confirm the earlier findings.
Three cross-sectional studies related to postnatal stress were reviewed. The study by Coley et al. [11] assessed whether ELS-related changes in gut microbial metabolites are associated with alterations in brain connectivity and increased vulnerability to stress in adulthood, but did not obtain conclusive results. Nevertheless, the authors found associations between anxiety symptoms and fecal metabolites such as glutamate gamma-methyl ester, but they did not determine the responsible gut bacteria. A comprehensive study by Michels et al. [22] with children and adolescents evaluated both self-reported levels of stress as well as biomarkers (hair cortisol and pnn50) and their association with gut microbiome. They found that high stress was associated with a lower abundance of Firmicutes at the phylum level, and higher Bacteroides, Parabacteroides, Rhodococcus, Methanobrevibacter, and Roseburia but lower Phascolarctobacterium at the genus level. Some of these results are in line with the published literature. For example, Firmicutes is under-represented in major depression [52]. Nevertheless, the associations at this high taxonomic level (phylum) are of limited value. Finally, the Flannery et al. [21] study focused more on the triad of caregiver behavior, social risks, and child behavior, finding an association between Bacteroides fragilis and less aggressiveness, sadness, impulsivity, and lower family incidents, and an association between Eubacterium rectale with the increase in anxious depression. These findings contrast with Michels et al. [22], who associated the increased abundance of the genus Bacteroides with high levels of stress, and Eubacterium coprostanoligenes was associated with stress albeit with inconsistent results. Moreover, both Eubacterium species differ and, therefore, the findings are not consistent. Flannery et al. [21] also identified two butyrate-producing bacteria, Coprococcus comes and Eubacterium rectale, which were associated with more anxious depression and less inhibitory control. Conflictingly, Coprococcus spp. and Dialister have been shown to be depleted in depressed patients in the literature [30].
5. Limitations
Human studies in general are complicated to design, as it is difficult to recruit patients who meet all inclusion and exclusion criteria, often resulting in a small sample [13,14,18,21]. In the case of longitudinal studies (especially if they last for some years) the main limitation is loss to follow-up, which again considerably reduces the sample size, as was the case in some reviewed studies [19,20]. There are two important key limitations to comparing gut microbiome studies: (i) variations in stool sample collection, preservation, DNA extraction, and bioinformatic analysis methods can impact the results; and (ii) the majority of studies only identify bacteria at the genus level, with very few analyses at the species level.
6. Conclusions and Recommendations
Here, we summarized the human literature to date, investigating the link between ELS and gut microbiome. We identified and described 13 studies of both pre- and postnatal stressors. An important observation is that few studies have used biomarkers of stress systems to complement self- or interview-reported stress. Furthermore, the types of early life stressors are very diverse, and results are generally weak (e.g., the level of phylogenetic resolution is family or at most genus), and so no firm conclusions can be drawn. Nonetheless, the review does demonstrate links between ELS and gut microbiome changes, with only 2 of the 13 studies not finding associations. Further research will be necessary to draw more robust conclusions. Moreover, to ensure consistency and comparability across studies, it would be beneficial to use the same validated questionnaires. Regarding the gut microbiome, future studies using standard procedures and species and strain resolution shotgun metagenomics sequencing are needed for analyses with a sufficient level of specificity. Finally, more attention should be paid to the influence of environmental variables (diet, physical activity, etc.) and sex on gut microbiome analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15112566/s1. Table S1. Medline search strategy.
Author Contributions
Conceptualization, A.A., B.W.J.H.P., F.L. and Y.S.; Methodology, A.A., F.L., M.T., G.V.M.-M. and C.B.-A.; Validation, A.A., B.W.J.H.P., F.L. and Y.S.; Formal Analysis, A.A., C.B.-A. and F.L.; Investigation, A.A., M.T., G.V.M.-M. and F.L.; Resources, A.A. and C.B.-A.; Data Curation, B.W.J.H.P. and Y.S.; Writing—Original Draft Preparation, A.A.; Writing—Review and Editing, A.A., B.W.J.H.P., F.L. and Y.S.; Visualization, A.A., B.W.J.H.P., F.L. and Y.S.; Supervision, B.W.J.H.P. and Y.S.; Project Administration, Y.S.; Funding Acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the European Union’s Horizon 2020 research and innovation programme (EarlyCause, grant n° 848158) and the by the European Commission—NextGeneration EU funds through CSIC Interdisciplinary Thematic Platform Neuroaging+. The FPI contract to GM is supported by the Spanish Ministry of Science and Innovation (PRE2018-083895). MT is supported by Margarita Salas contract (CA1/RSUE/2021-00467).
Acknowledgments
A grant from the Spanish Ministry of Science and Innovation (MCIN/AEI) to IATA-CSIC as an Accredited Center of Excellence (CEX2021-001189-S/MCIN/AEI/10.13039/501100011033) is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coussons-Read, M.E. Effects of prenatal stress on pregnancy and human development: Mechanisms and pathways. Obstet. Med. 2013, 6, 52–57. [Google Scholar] [CrossRef]
- Leeb, R.T. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2008; pp. 131–135. [Google Scholar]
- Vogel, S.C.; Brito, N.H.; Callaghan, B.L. Early Life Stress and the Development of the Infant Gut Microbiota: Implications for Mental Health and Neurocognitive Development. Curr. Psychiatry Rep. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Green, J.G.; McLaughlin, K.A.; Berglund, P.A.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry 2010, 67, 113–123. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: Associations with persistence of DSM-IV disorders. Arch. Gen. Psychiatry 2010, 67, 124–132. [Google Scholar] [CrossRef]
- Knowles, S.R.; Nelson, E.A.; Palombo, E. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biol. Psychol. 2008, 77, 132–137. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented milk con taining lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef]
- Lupien, S.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Mariani, N.; Borsini, A.; Cecil, C.A.M.; Felix, J.F.; Sebert, S.; Cattaneo, A.; Walton, E.; Milaneschi, Y.; Cochrane, G.; Amid, C.; et al. Identifying causative mechanisms linking early-life stress to psycho-cardio-metabolic multi-morbidity: The EarlyCause project. PLoS ONE 2021, 16, e0245475. [Google Scholar] [CrossRef]
- Coley, E.J.; Mayer, E.A.; Osadchiy, V.; Chen, Z.; Subramanyam, V.; Zhang, Y.; Hsiao, E.Y.; Gao, K.; Bhatt, R.; Dong, T.; et al. Early life adversity predicts brain-gut alterations associated with increased stress and mood. Neurobiol. Stress 2021, 15, 100348. [Google Scholar] [CrossRef]
- Aatsinki, A.-K.; Keskitalo, A.; Laitinen, V.; Munukka, E.; Uusitupa, H.-M.; Lahti, L.; Kortesluoma, S.; Mustonen, P.; Rodrigues, A.J.; Coimbra, B.; et al. Maternal prenatal psychological distress and hair cortisol levels associate with infant fecal microbiota composition at 2.5 months of age. Psychoneuroendocrinology 2020, 119, 104754. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.R.; Roach, J.; Azcarate-Peril, M.A.; Thompson, A.L. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS ONE 2021, 16, e0251782. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Grant-Beurmann, S.; Jumare, J.; Ndembi, N.; Matthew, O.; Shutt, A.; Omoigberale, A.; Martin, O.A.; Fraser, C.M.; Charurat, M. Dynamics of the infant gut microbiota in the first 18 months of life: The impact of maternal HIV infection and breastfeeding. Microbiome 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.L.; Fields, A.; Gee, D.G.; Gabard-Durnam, L.; Caldera, C.; Humphreys, K.L.; Goff, B.; Flannery, J.; Telzer, E.H.; Shapiro, M.; et al. Mind and gut: Associations between mood and gastrointestinal distress in children exposed to adversity. Dev. Psychopathol. 2020, 32, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Hermes, G.D.A.; Eckermann, H.A.; de Vos, W.M.; de Weerth, C. Does entry to center-based childcare affect gut microbial colonization in young infants? Sci. Rep. 2020, 24, 10235. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Horne, R.; Donzella, B.; Szamosi, J.C.; Coe, C.L.; Foster, J.A.; Gunnar, M.R. Microbiota-immune alterations in adolescents following early life adversity: A proof of concept study. Dev. Psychobiol. 2021, 63, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Laue, H.E.; Karagas, M.R.; Coker, M.O.; Bellinger, D.C.; Baker, E.R.; Korrick, S.A.; Madan, J.C. Sex-specific relationships of the infant microbiome and early-childhood behavioral outcomes. Pediatr. Res. 2022, 92, 580–591. [Google Scholar] [CrossRef]
- Keskitalo, A.; Aatsinki, A.-K.; Kortesluoma, S.; Pelto, J.; Korhonen, L.; Lahti, L.; Lukkarinen, M.; Munukka, E.; Karlsson, H.; Karlsson, L. Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress 2021, 24, 551–560. [Google Scholar] [CrossRef]
- Flannery, J.E.; Stagaman, K.; Burns, A.R.; Hickey, R.J.; Roos, L.E.; Giuliano, R.J.; Fisher, P.A.; Sharpton, T.J. Gut Feelings Begin in Childhood: The Gut Metagenome Correlates with Early Environment, Caregiving, and Behavior. mBio 2020, 11, e02780-19. [Google Scholar] [CrossRef]
- Michels, N.; Van de Wiele, T.; Fouhy, F.; O’Mahony, S.; Clarke, G.; Keane, J. Gut microbiome patterns depending on children’s psychosocial stress: Reports versus biomarkers. Brain, Behav. Immun. 2019, 80, 751–762. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; Heuvel, L.L.v.D.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.M.; Threadgill, D.; Threadgill, D.S. The gastrointestinal microbiome: A malleable, third genome of mammals. Mamm. Genome 2009, 20, 395–403. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Campillo, I.; Balzano, T.; Benítez-Páez, A.; López-Almela, I.; Romaní-Pérez, M.; Forteza, J.; Felipo, V.; Avena, N.M.; Sanz, Y. Bacteroides uniformis CECT 7771 Modulates the Brain Reward Response to Reduce Binge Eating and Anxiety-Like Behavior in Rat. Mol. Neurobiol. 2021, 58, 4959–4979. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Doll, J.P.; Vázquez-Castellanos, J.F.; Schaub, A.C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression-Case Report. Front. Psychiatry 2022, 17, 815422. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F. Programming of host metabolism by the gut microbiota. Ann. Nutr. Metab. 2011, 58 (Suppl. 2), 44–52. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.-A.M.; Luczynski, P.; Dinan, T.G.; Cryan, J.F. Reframing the Teenage Wasteland: Adolescent Microbiota-Gut-Brain Axis. Can. J. Psychiatry 2016, 61, 214–221. [Google Scholar] [CrossRef]
- Monk, C.; Lugo-Candelas, C.; Trumpff, C. Prenatal Developmental Origins of Future Psychopathology: Mechanisms and Pathways. Annu. Rev. Clin. Psychol. 2019, 15, 317–344. [Google Scholar] [CrossRef]
- Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008, 32, 1073–1086. [Google Scholar] [CrossRef]
- van de Loo, K.F.; van Gelder, M.M.; Roukema, J.; Roeleveld, N.; Merkus, P.J.; Verhaak, C.M. Prenatal maternal psychological stress and childhood asthma and wheezing: A meta-analysis. Eur. Respir. J. 2016, 47, 133–146. [Google Scholar] [CrossRef]
- Soares-Cunha, C.; Coimbra, B.; Borges, S.; Domingues, A.V.; Silva, D.; Sousa, N.; Rodrigues, A.J. Mild prenatal stress causes emotional and brain structuralmodifications in rats of both sexes. Front. Behav. Neurosci. 2018, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, N.; Chen, R.; Lee, T.; Gao, Y.; Yuan, Z.; Nie, Y.; Sun, T. Prenatal stress leads to deficits in brain development, mood related behaviors and gut microbiota in offspring. Neurobiol. Stress 2021, 15, 100333. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Zemel, B.S. Stress gets into the belly: Early life stress and the gut microbiome. Behav. Brain Res. 2021, 414, 113474. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis; The Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. [Google Scholar]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health 2013, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.A.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: A prospective case-control study. Lancet 2016, 387, 1928–1936. [Google Scholar] [CrossRef]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, S.M.; Malan-Muller, S.; van den Heuvel, L.L.; Demmitt, B.A.; Stanislawski, M.A.; Smith, D.G.; Bohr, A.D.; Stamper, C.E.; Hyde, E.R.; Morton, J.T.; et al. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosom. Med. 2017, 79, 936–946. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Rosin, S.; Xia, K.; Azcarate-Peril, M.A.; Carlson, A.L.; Propper, C.B.; Thompson, A.L.; Grewen, K.; Knickmeyer, R.C. A preliminary study of gut microbiome variation and HPA axis reactivity in healthy infants. Psychoneuroendocrinology 2021, 124, 105046. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).