Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases
Abstract
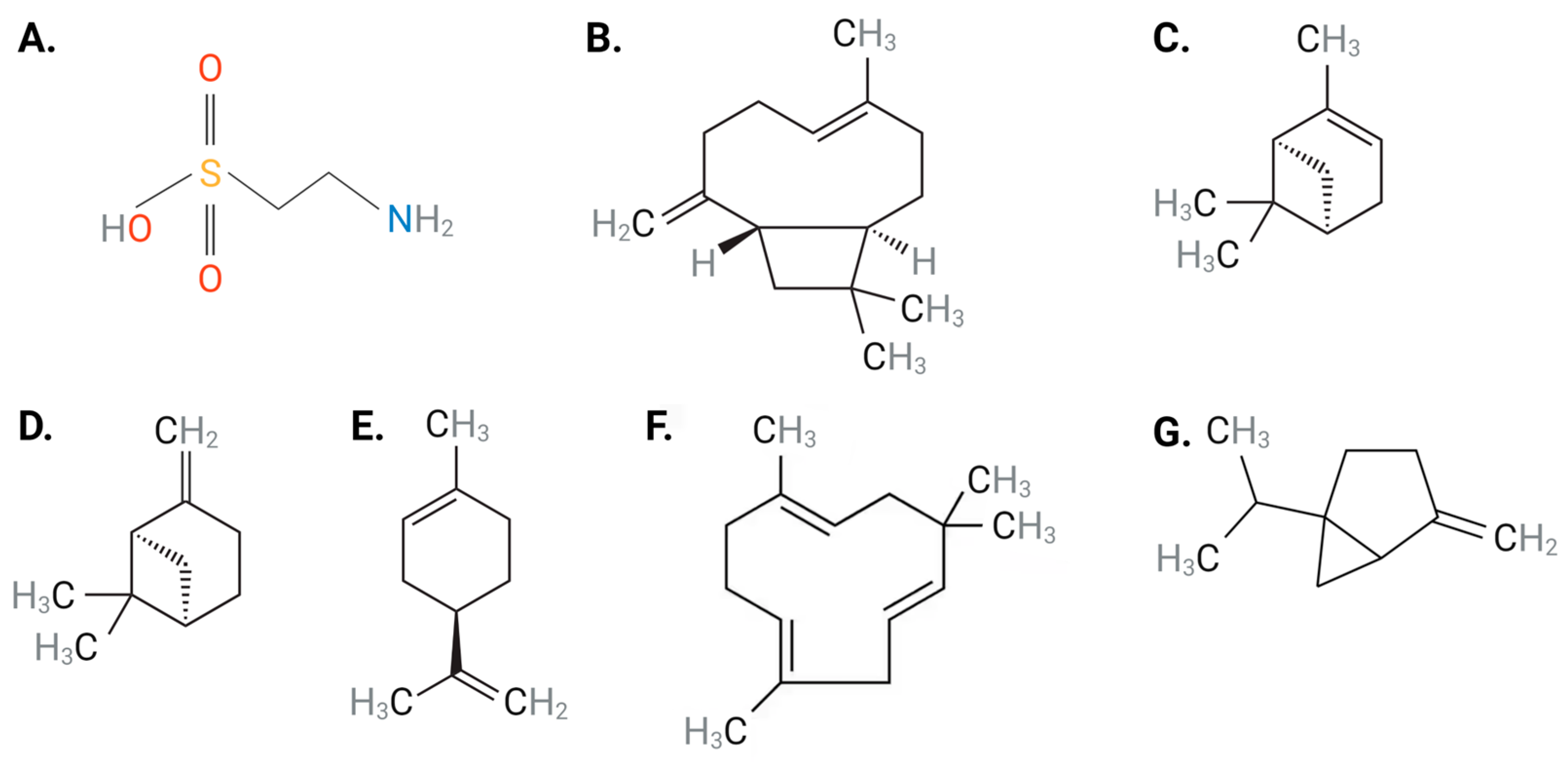
1. Introduction
2. Anti-Inflammatory Effects of Taurine and Terpenes
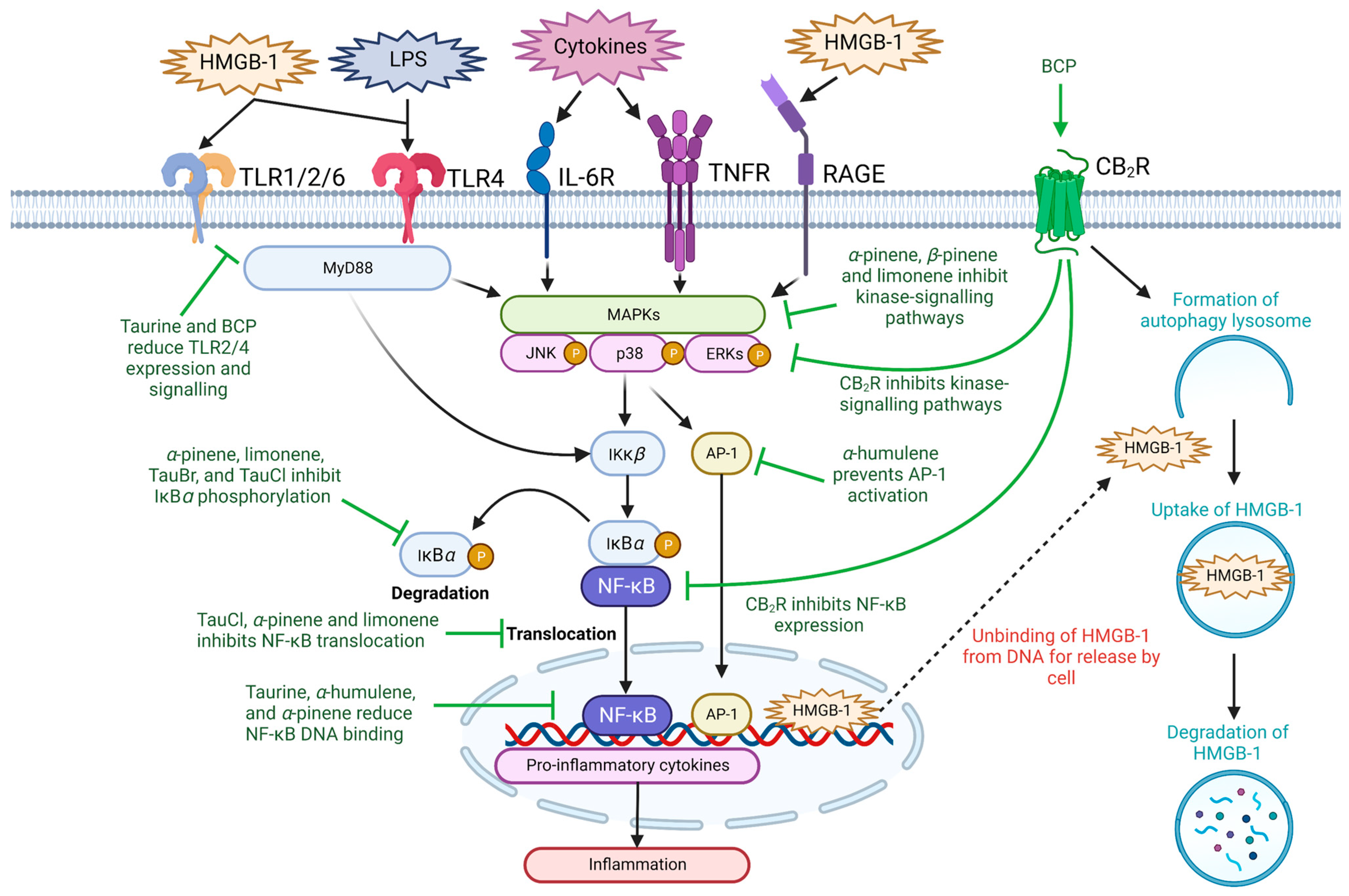
2.1. Inflammatory Signaling Pathways
2.2. High-Mobility Group Box-1
2.3. Toll-like Receptors
| Compound | Dosage | Experimental Model | Outcome | Ref |
|---|---|---|---|---|
| α-humulene | 50 mg/kg | In vivo LPS-induced inflammation in the paw of Wistar rats | ↓ neutrophil migration, ↓ IL-5 ↓ NF-κB DNA binding ↓ IL-1β and TNF-α expression. | [106] |
| α-humulene | 50 mg/kg, 22 days | In vivo female BALB/c mice | ↓ Eosinophil recruitment ↓ NF-κB and activator protein-1 activation | [107] |
| α-pinene | 50 mg/kg, 7 days | In vivo ISO-induced myocardial inflammation in Wistar albino rats | ↓ cardiac injury biomarkers ↓ NF-κB signaling ↓ IL-6 and TNF-α expression | [108] |
| BCP | 50–200 µg/mL | In vitro Mouse RAW267.4 macrophages | ↓ ERK/p38-MAPK signaling ↓ COX-1 and COX-2 | [109] |
| BCP | (0.2–25 µM) | In vitro LPS-induced inflammation in C57BL/6 mouse microglial cells | ↓ IL-1β, TNF-α, PGE2, iNOS expression ↓ ROS inflammatory biomarkers | [110] |
| BCP | 10 mg/kg | In vivo cisplatin-induced nephropathy in C57BL/6J mice | CB2R-dependant decrease in MCP-1, IL-1β, TNF-α, ICAM-1, neutrophil and macrophage infiltration | [111] |
| Limonene | 50 mg/kg, 21 days | In vivo ISO-induced inflammation in male Wister rats | ↓ MAPK/JNK/ERK/NF-κB signaling ↓ IL-1β, IL-6, and TNF-α expression | [112] |
| Limonene | 20, 50, and 100 mg/kg | In vivo gastritis-induced male Sprague-Dawley rats | ↓ NF-κB nuclear translocation ↓ intracellular Ca2+, IL-1β, IL-6, TNF | [113] |
| Sabinene | 0.32–1.25 µL/mL, 1 h | In vitro LPS-induced inflammation in mouse Raw 264.7 leukemic macrophage cell line | Strong anti-inflammatory activity through potent NO scavenging and inhibition of iNOS | [114] |
| Taurine | 100 mM, 30 days | In vivo myocardial ischemia-induced male albino Wister rats | ↓ myocardial infarct size ↑ superoxide dismutase ↓ IL-6 and TNF-α expression | [44] |
| Taurine | 3000 mg/day, 8 weeks | Clinical study of 50 patients with type-2 diabetes | ↓ TNF-α ↑ superoxide dismutase ↑ catalase | [115] |
3. Anti-Oxidative Effects of Taurine and Terpenes
| Compound | Dosage | Experimental Model | Outcome | Ref |
|---|---|---|---|---|
| α-pinene | 10–400 µM, 24 h | In vitro H2O2-induced oxidative stress in U373-MG cell line | ↓ H2O2-induced ROS production and decreased lipid peroxidation | [130] |
| BCP | 200 mg/kg, 45 days | In vivo male albino Wistar rats | ↑ SOD, CAT and GPx ↓ IL-6 and TNF-α | [162] |
| β-pinene | 10 μM | In vitro Arsenic-induced oxidative stress in O. sativa seeds | ↓ H2O2 | [163] |
| Limonene | 5–1000 μg/mL | In vitro H2O2 BALB/c mice lymphoid cells | ↓ H2O2 | [164] |
| Sabinene | 0.08–0.16 μL/ml | In vitro RAW 264.7 murine macrophage cells | ↑ N62O scavenging | [114] |
| Taurine | 80 mM | In vitro ROS-induced oxidative stress in Rat H9c2 Cardiomyocyte cells | ↑ Cell viability ↓ apoptosis ↓ intracellular Ca2+ | [165] |
| Taurine | 100 mg/kg/day, 10 days | In vivo tamoxifen-induced mitochondrial oxidative stress in Swiss albino rats | ↓ mitochondrial lipid peroxidation ↓ O2− ↑ mitochondrial antioxidants | [166] |
4. Anti-Hypertensive Effects of Taurine and Terpenes
| Compound | Dosage | Experimental Model | Outcome | Ref |
|---|---|---|---|---|
| α-pinene | 50 and 100 mg/kg I. V | In vivo ISO-induced myocardial infarction in male Wistar rats | ↓ SBP, DBP and heart rate | [113] |
| Citrus aurantium (9.6% ß-pinene and 8.54% limonene) | 0.05–0.2% | Ex vivo isolated thoracic aorta of C57BL/6 mice | ↑ vasorelaxation and ↓ Ca2+ influx | [204] |
| Lamiaceae (α-pinene and BCP) | In vivo, 5,10, 20, and 40 mg/kg I.V. Ex vivo, 1–1000µg/mL | In vivo male Wistar rats and isolated mesenteric artery | ↓ blood pressure, ↑ vasorelaxation and caused tachycardia | [205] |
| Limonene | (0.01, 0.1, and 0.01% v/v) | Ex vivo isolated thoracic aorta of C57BL/6 mice | ↑ vasorelaxation and ↓ Ca2+ influx | [206] |
| Taurine | 10 µM–10 mM | Ex vivo isolated human radial artery | ↑ vasorelaxation and ↓ Ca2+ influx | [179] |
| Taurine | 1–2% (w/v) ad libitum, 3 weeks | In vivo L-NAME-induced hypertension in male Sprague-Dawley rats | ↑ endothelial NO and ↓ blood pressure, AngI and AngII | [207] |
| Taurine | 2.5%, ad libitum | In vivo male Wistar rats | ↓ SBP, DBP, and mean arterial pressure, and ↑ SOD and eNOS | [208] |
5. Abilities of Taurine to Modulate HHcy Pathology
6. Anti-Atherosclerotic Effects of Taurine and Terpenes
| Compound | Dosage | Experimental Model | Outcome | Ref |
|---|---|---|---|---|
| α-pinene | 25, 50, and 100 mg/kg, 7 days | In vivo alloxan-induced diabetes in male Wistar rats | ↓ plasma, TC, TGA VLDL, and LDL | [271] |
| β-pinene | 25, 50, and 100 mg/kg, 7 days | In vivo alloxan-induced hyperlipidemia in male Wister rats | ↓ plasma TGA, VLDL, and LDL levels | [272] |
| BCP | 1 mL/kg, 3 days | In vivo Triton WR-1339-induced hypercholesterolemia in female Wistar rats | ↓ cardiac TC and TG levels ↓ atherogenic and coronary risk index ↓ ROS | [158] |
| BCP | 100 mg/kg/day, 2 days | In vivo ISO-induced myocardial infarction in rats | ↓ IL-1β, IL-6, iNOS, COX-2 and TNF-α expression ↓ HMGB-1 expression | [69] |
| Taurine | 0.5–10 g/kg, 2 weeks | In vivo high-cholesterol-fed male rats | ↓ plasma TC, TG, LDL, and hepatic TG levels | [273] |
| Taurine | 0.3% (w/v) ad libitum, 24 weeks | In vivo Watanabe heritable hyperlipidemic rabbits | ↓ aortic lesions and cholesterol ester in arteries and macrophage migration | [274] |
| Taurine | 2.5% to diet ad libitum, 4 weeks | Male New Zealand White rabbits on high-cholesterol and Hcy diet | ↓ endothelial cell apoptosis and left main coronary artery atherosclerosis. | [222] |
| Taurine | 1% (w/v) ad libitum, 14 days | Buthionine sulfoximine-induced oxidative stress in New Zealand white rabbits | ↓ blood pressure, plasma ROS and LOX-1 expression | [275] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Parashar, S.; Singh, S.; Sood, G. Examining the role of health consciousness, environmental awareness and intention on purchase of organic food: A moderated model of attitude. J. Clean. Prod. 2023, 386, 135553. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Chan, K.G.; Goh, B.H.; Yap, W.H. The Role of Natural Products in Targeting Cardiovascular Diseases via Nrf2 Pathway: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1308. [Google Scholar] [CrossRef]
- Prakash, M.D.; Fraser, S.; Boer, J.C.; Plebanski, M.; de Courten, B.; Apostolopoulos, V. Anti-cancer effects of carnosine—A dipeptide molecule. Molecules 2021, 26, 1644. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef]
- Lusis, A.J.; Fogelman, A.M.; Fonarow, G.C. Genetic basis of atherosclerosis: Part I: New genes and pathways. Circulation 2004, 110, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S. Cardiovascular disease review series. EMBO Mol. Med. 2011, 3, 697. [Google Scholar] [CrossRef]
- Bosevski, M.; Nikolovski, P.; Stojanovska, L.; Apostolopoulos, V. Progression of carotid artery disease could stratify a risk of coronary artery disease patients with type 2 diabetes. Acta Biochim. Biophys. Sin. 2019, 51, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Lee, Y.S. Recent Molecular Mechanisms and Beneficial Effects of Phytochemicals and Plant-Based Whole Foods in Reducing LDL-C and Preventing Cardiovascular Disease. Antioxidants 2021, 10, 784. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Siddharth, V.; Singh, S.I.; Narang, R. Cost analysis of treating cardiovascular diseases in a super-specialty hospital. PLoS ONE 2022, 17, e0262190. [Google Scholar] [CrossRef]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Shakoor, H.; Platat, C.; Ali, H.I.; Ismail, L.C.; Al Dhaheri, A.S.; Bosevski, M.; Apostolopoulos, V.; Stojanovska, L. The benefits of physical activity in middle-aged individuals for cardiovascular disease outcomes. Maturitas 2023, 168, 49–52. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 2014, 6, 1145–1157. [Google Scholar] [CrossRef]
- Bkaily, G.; Jazzar, A.; Normand, A.; Simon, Y.; Al-Khoury, J.; Jacques, D. Taurine and cardiac disease: State of the art and perspectives. Can. J. Physiol. Pharmacol. 2020, 98, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Arneja, A.S.; Tappia, P.S.; Dhalla, N.S. The potential health benefits of taurine in cardiovascular disease. Exp. Clin. Cardiol. 2008, 13, 57–65. [Google Scholar] [PubMed]
- Obeid, O.A.; Johnston, K.; Emery, P.W. Plasma taurine and cysteine levels following an oral methionine load: Relationship with coronary heart disease. Eur. J. Clin. Nutr. 2004, 58, 105–109. [Google Scholar] [CrossRef]
- Zulli, A. Taurine in cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 57–60. [Google Scholar] [CrossRef]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, L.; Huang, J.; Himabindu, K.; Tewari, D.; Horbańczuk, J.O.; Xu, S.; Chen, Z.; Atanasov, A.G. Cardiovascular protective effect of black pepper (Piper nigrum L.) and its major bioactive constituent piperine. Trends Food Sci. Technol. 2021, 117, 34–45. [Google Scholar] [CrossRef]
- Musenga, A.; Mandrioli, R.; Ferranti, A.; D’Orazio, G.; Fanali, S.; Raggi, M.A. Analysis of aromatic and terpenic constituents of pepper extracts by capillary electrochromatography. J. Sep. Sci. 2007, 30, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of black pepper fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef]
- Steffens, S.; Pacher, P. Targeting cannabinoid receptor CB2 in cardiovascular disorders: Promises and controversies. Br. J. Pharmacol. 2012, 167, 313–323. [Google Scholar] [CrossRef]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of entourage: Terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Donkor, P.O.; Chen, Y.; Ding, L.; Qiu, F. Locally and traditionally used Ligusticum species—A review of their phytochemistry, pharmacology and pharmacokinetics. J. Ethnopharmacol. 2016, 194, 530–548. [Google Scholar] [CrossRef]
- Suroowan, S.; Mahomoodally, F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clin. Phytosci. 2015, 1, 1. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Ahn, G.; Ham, Y.M.; Song, S.M.; Ko, E.Y.; Cho, S.H.; Yoon, W.J.; Kim, K.N. Anti-inflammatory effect and mechanism of action of Lindera erythrocarpa essential oil in lipopolysaccharide-stimulated RAW264.7 cells. EXCLI J. 2017, 16, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Lin, M.; Su, X.; Li, C.; Wang, H.; Li, B.; Chen, R.; Kang, J. Anti-inflammatory terpenes from Schefflera rubriflora C. J. Tseng & G. Hoo with their TNF-alpha and IL-6 inhibitory activities. Phytochemistry 2019, 163, 23–32. [Google Scholar] [CrossRef]
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, H.; Wang, W.; Jin, H.; Feng, G.; Jia, N. Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem. Biophys. Res. Commun. 2014, 447, 485–489. [Google Scholar] [CrossRef]
- Su, Y.; Fan, W.; Ma, Z.; Wen, X.; Wang, W.; Wu, Q.; Huang, H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 2014, 266, 56–65. [Google Scholar] [CrossRef]
- Niu, X.; Zheng, S.; Liu, H.; Li, S. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol. Med. Rep. 2018, 18, 4516–4522. [Google Scholar] [CrossRef]
- Hou, X.; Sun, G.; Guo, L.; Gong, Z.; Han, Y.; Bai, X. Cardioprotective effect of taurine and β-alanine against cardiac disease in myocardial ischemia and reperfusion-induced rats. Electron. J. Biotechnol. 2020, 45, 46–52. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Solt, L.A.; May, M.J. The IkappaB kinase complex: Master regulator of NF-kappaB signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology 2008, 123, 348–357. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Tobon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals targeting NF-kappaB signaling: Potential anti-cancer interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Hsieh, I.N.; Chang, A.S.; Teng, C.M.; Chen, C.C.; Yang, C.R. Aciculatin inhibits lipopolysaccharide-mediated inducible nitric oxide synthase and cyclooxygenase-2 expression via suppressing NF-kappaB and JNK/p38 MAPK activation pathways. J. Biomed. Sci. 2011, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.-M.; Ko, Y.-J.; Song, S.-M.; Kim, J.; Kim, K.-N.; Yun, J.-H.; Cho, J.-H.; Ahn, G.; Yoon, W.-J. Anti-inflammatory effect of litsenolide B2 isolated from Litsea japonica fruit via suppressing NF-κB and MAPK pathways in LPS-induced RAW264.7 cells. J. Funct. Foods 2015, 13, 80–88. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortes, H.; Caballero-Floran, I.H.; Gonzalez-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chavez, S.A.; Giraldo-Gomez, D.M.; Magana, J.J.; Leyva-Gomez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Barua, M.; Liu, Y.; Quinn, M.R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: Decreased NF-kappaB activation and IkappaB kinase activity. J. Immunol. 2001, 167, 2275–2281. [Google Scholar] [CrossRef]
- Kim, B.S.; Cho, I.S.; Park, S.Y.; Schuller-Levis, G.; Levis, W.; Park, E. Taurine chloramine inhibits NO and TNF-alpha production in zymosan plus interferon-gamma activated RAW 264.7 cells. J. Drugs Dermatol. 2011, 10, 659–665. [Google Scholar] [PubMed]
- Kim, J.W.; Kim, C. Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated though Ras-ERK-NF-kappaB. Biochem. Pharmacol. 2005, 70, 1352–1360. [Google Scholar] [CrossRef]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-kappaB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef]
- Nam, S.Y.; Chung, C.K.; Seo, J.H.; Rah, S.Y.; Kim, H.M.; Jeong, H.J. The therapeutic efficacy of alpha-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-alpha-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Yang, J.; Choi, W.S.; Kim, K.J.; Eom, C.D.; Park, M.J. Investigation of Active Anti-Inflammatory Constituents of Essential Oil from Pinus koraiensis (Sieb. et Zucc.) Wood in LPS-Stimulated RBL-2H3 Cells. Biomolecules 2021, 11, 817. [Google Scholar] [CrossRef]
- Aly, E.; Khajah, M.A.; Masocha, W. beta-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2019, 25, 106. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB(2) receptor and its role as a regulator of inflammation. Cell Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Benicky, J.; Sanchez-Lemus, E.; Pavel, J.; Saavedra, J.M. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol. Neurobiol. 2009, 29, 781–792. [Google Scholar] [CrossRef]
- Parlar, A.; Arslan, S.O.; Dogan, M.F.; Cam, S.A.; Yalcin, A.; Elibol, E.; Ozer, M.K.; Uckardes, F.; Kara, H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018, 16, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-kappaB activation in microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.N.; Laham, F.; Azimullah, S.; Sharma, C.; Al Kaabi, A.J.; Tariq, S.; Adeghate, E.; Goyal, S.N.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates β-adrenergic agonist-induced myocardial injury in a cannabinoid receptor-2 dependent and independent manner. Free Radic Biol. Med. 2021, 167, 348–366. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-gamma receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Dai, X.; Ao, Y.; Li, Y. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol. Appl. Pharmacol. 2017, 329, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Cai, L.; Lin, L.; Liu, J.; Cheng, J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid Med. Cell Longev. 2020, 2020, 4293206. [Google Scholar] [CrossRef] [PubMed]
- Hortelano, S.; Gonzalez-Cofrade, L.; Cuadrado, I.; de Las Heras, B. Current status of terpenoids as inflammasome inhibitors. Biochem. Pharmacol. 2020, 172, 113739. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, W.T.; Chen, S.Y.; Lee, T.M. Taurine Alleviates Sympathetic Innervation by Inhibiting NLRP3 Inflammasome in Postinfarcted Rats. J. Cardiovasc. Pharmacol. 2021, 77, 745–755. [Google Scholar] [CrossRef]
- Qiu, T.; Pei, P.; Yao, X.; Jiang, L.; Wei, S.; Wang, Z.; Bai, J.; Yang, G.; Gao, N.; Yang, L.; et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018, 9, 946. [Google Scholar] [CrossRef]
- Zou, X.S.; Xie, L.; Wang, W.Y.; Zhao, G.Y.; Tian, X.Y.; Chen, M.H. Pomelo peel oil alleviates cerebral NLRP3 inflammasome activation in a cardiopulmonary resuscitation rat model. Exp. Ther. Med. 2021, 21, 233. [Google Scholar] [CrossRef]
- Wong, W.T.; Wu, C.H.; Li, L.H.; Hung, D.Y.; Chiu, H.W.; Hsu, H.T.; Ho, C.L.; Chernikov, O.V.; Cheng, S.M.; Yang, S.P.; et al. The leaves of the seasoning plant Litsea cubeba inhibit the NLRP3 inflammasome and ameliorate dextran sulfate sodium-induced colitis in mice. Front. Nutr. 2022, 9, 871325. [Google Scholar] [CrossRef]
- Yu, W.; Jin, G.; Zhang, J.; Wei, W. Selective Activation of Cannabinoid Receptor 2 Attenuates Myocardial Infarction via Suppressing NLRP3 Inflammasome. Inflammation 2019, 42, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Livesey, K.M.; Kroemer, G.; Billiar, T.R.; Van Houten, B.; Zeh Iii, H.J.; Lotze, M.T. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011, 13, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, M.; Stylianou, E.; Griffiths, K.; Lang, Z.; Meyer, J.; Harris, S.A.; Rowland, R.; Minassian, A.M.; Pathan, A.A.; Fletcher, H. Roles for Treg expansion and HMGB1 signaling through the TLR1-2-6 axis in determining the magnitude of the antigen-specific immune response to MVA85A. PLoS ONE 2013, 8, e67922. [Google Scholar] [CrossRef]
- Andersson, U.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 2002, 72, 1084–1091. [Google Scholar] [CrossRef]
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed. Pharmacother. 2021, 139, 111555. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Lu, L.; Peng, W.H.; Wang, L.J.; Zhang, Q.; Zhang, R.Y.; Chen, Q.J.; Shen, W.F. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis 2009, 205, 544–548. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, H.; Bai, Q.; Zhou, X.; Xu, C.; Lu, Z.; Cui, B.; Wen, H. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin. Chim. Acta 2009, 406, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Tinelli, G.; Biscetti, F.; Straface, G.; Angelini, F.; Pitocco, D.; Mucci, L.; Landolfi, R.; Flex, A. Serum high mobility group box-1 and osteoprotegerin levels are associated with peripheral arterial disease and critical limb ischemia in type 2 diabetic subjects. Cardiovasc. Diabetol. 2017, 16, 99. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Chen, R.; Shi, R.; Zeng, P.; Chen, R.; Leng, Y.; Chen, A.F. NRP1 regulates HMGB1 in vascular endothelial cells under high homocysteine condition. Am. J. Physiol.—Heart Circ. Physiol. 2019, 316, H1039–H1046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, X.; Zhang, H.; Li, W. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat. Oncol. 2017, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, H.; Ge, X.; Desert, R.; Das, S.; Han, H.; Lantvit, D.; Guzman, G.; Nieto, N. Ablation of Hmgb1 in intestinal epithelial cells causes intestinal lipid accumulation and reduces NASH in mice. Hepatol. Commun. 2020, 4, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Sun, Y.; Luo, Z.-M.; Su, D.-F.; Dai, S.-M.; Liu, X. Cannabinoid receptor 2 protects against acute experimental sepsis in mice. Mediat. Inflamm. 2013, 2013, 741303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Du, R.; Li, G.; Bai, Z.; Ma, J.; Mao, C.; Wang, J.; Gui, H. Cannabinoid receptor 2 promotes the intracellular degradation of HMGB1 via the autophagy-lysosome pathway in macrophage. Int. Immunopharmacol. 2020, 78, 106007. [Google Scholar] [CrossRef]
- Cho, H.-I.; Hong, J.-M.; Choi, J.-W.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Frantz, S.; Ertl, G.; Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Jaén, R.I.; Val-Blasco, A.; Prieto, P.; Gil-Fernández, M.; Smani, T.; López-Sendón, J.L.; Delgado, C.; Boscá, L.; Fernández-Velasco, M. Innate immune receptors, key actors in cardiovascular diseases. Basic Transl. Sci. 2020, 5, 735–749. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Feng, Y.; Bian, Y.; Fu, Z.; Wu, Y.; Ma, Y.; Li, C.; Wang, J.; Dai, J. Taurine Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR-4/NF-κB Pathway. In Taurine 12: A Conditionally Essential Amino Acid; Springer: Berlin/Heidelberg, Germany, 2022; pp. 63–72. [Google Scholar]
- Lin, C.-J.; Chiu, C.-C.; Chen, Y.-C.; Chen, M.-L.; Hsu, T.-C.; Tzang, B.-S. Taurine attenuates hepatic inflammation in chronic alcohol-fed rats through inhibition of TLR4/MyD88 signaling. J. Med. Food 2015, 18, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S.; Ghanim, A.M.; Elmorsy, M.A.; Metwaly, H.A. Taurine ameliorates thioacetamide induced liver fibrosis in rats via modulation of toll like receptor 4/nuclear factor kappa B signaling pathway. Sci. Rep. 2021, 11, 12296. [Google Scholar] [CrossRef]
- Premkumar, V.; Dey, M.; Dorn, R.; Raskin, I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem. Biol. 2010, 10, 3. [Google Scholar] [CrossRef]
- Triantafilou, M.; Gamper, F.G.; Haston, R.M.; Mouratis, M.A.; Morath, S.; Hartung, T.; Triantafilou, K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006, 281, 31002–31011. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Wang, Z.; Wu, B.; Zhang, J.; Xu, Y.; Han, X.; Phouthapane, V.; Miao, J. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front. Immunol. 2022, 13, 927215. [Google Scholar] [CrossRef]
- Miao, J.; Zheng, L.; Zhang, J.; Ma, Z.; Zhu, W.; Zou, S. The effect of taurine on the toll-like receptors/nuclear factor kappa B (TLRs/NF-kappaB) signaling pathway in Streptococcus uberis-induced mastitis in rats. Int. Immunopharmacol. 2011, 11, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a potential protective agent against myocardial injury: The role of toll-like receptors. Molecules 2019, 24, 1929. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Yang, Z.; Cao, M.; Wang, K.; Wang, G.; Zhao, Y. Protective effect of alpha-pinene against isoproterenol-induced myocardial infarction through NF-kappaB signaling pathway. Hum. Exp. Toxicol. 2020, 39, 1596–1606. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Levy, R.M. The combination of beta-caryophyllene, baicalin and catechin synergistically suppresses the proliferation and promotes the death of RAW267.4 macrophages in vitro. Int. J. Mol. Med. 2016, 38, 1940–1946. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. The protective effects of beta-caryophyllene on LPS-induced primary microglia M(1)/M(2) imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Horvath, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. beta-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic. Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S. D-Limonene mitigate myocardial injury in rats through MAPK/ERK/NF-kappaB pathway inhibition. Korean J. Physiol. Pharmacol. 2020, 24, 259–266. [Google Scholar] [CrossRef]
- Yeo, D.; Hwang, S.J.; Song, Y.S.; Lee, H.J. Humulene Inhibits Acute Gastric Mucosal Injury by Enhancing Mucosal Integrity. Antioxidants 2021, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Goncalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Mahdavi, R.; Hajizadeh-Sharafabad, F.; Alizadeh, M. The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol. Metab. Syndr. 2020, 12, 9. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Bayer, G.; Shayganpour, A.; Bayer, I.S. Antioxidant activity of limonene modified cellulose pulp fiber-polylactic acid (PLA) composites. Cellulose 2022, 30, 1599–1622. [Google Scholar] [CrossRef]
- Himed, L.; Merniz, S.; Monteagudo-Olivan, R.; Barkat, M.; Coronas, J. Antioxidant activity of the essential oil of citrus limon before and after its encapsulation in amorphous SiO2. Sci. Afr. 2019, 6, e00181. [Google Scholar] [CrossRef]
- Amiri, H. Chemical Composition and Antioxidant Activity of Essential Oil and Methanolic Extracts of Ferula microcolea (Boiss.) Boiss (Apiaceae). Int. J. Food Prop. 2014, 17, 722–730. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef]
- Yamauchi-Takihara, K.; Azuma, J.; Kishimoto, S.; Onishi, S.; Sperelakis, N. Taurine prevention of calcium paradox-related damage in cardiac muscle. Its regulatory action on intracellular cation contents. Biochem. Pharmacol. 1988, 37, 2651–2658. [Google Scholar] [CrossRef]
- El Idrissi, A.; Trenkner, E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J. Neurosci. 1999, 19, 9459–9468. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef]
- Jong, C.J.; Ito, T.; Mozaffari, M.; Azuma, J.; Schaffer, S. Effect of beta-alanine treatment on mitochondrial taurine level and 5-taurinomethyluridine content. J. Biomed. Sci. 2010, 17 (Suppl. S1), S25. [Google Scholar] [CrossRef]
- Chang, C.Y.; Shen, C.Y.; Kang, C.K.; Sher, Y.P.; Sheu, W.H.; Chang, C.C.; Lee, T.H. Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol. Appl. Pharmacol. 2014, 279, 351–363. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Cuvelliez, M.; Fiedler, J.; Charrier, H.; Mulder, P.; Hebbar, E.; Pfanne, A.; Beseme, O.; Chwastyniak, M.; Amouyel, P.; et al. MicroRNAs regulating superoxide dismutase 2 are new circulating biomarkers of heart failure. Sci. Rep. 2017, 7, 14747. [Google Scholar] [CrossRef] [PubMed]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Major selected monoterpenes alpha-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Elmann, A.; Mordechay, S.; Rindner, M.; Larkov, O.; Elkabetz, M.; Ravid, U. Protective Effects of the Essential Oil of Salvia fruticosa and Its Constituents on Astrocytic Susceptibility to Hydrogen Peroxide-Induced Cell Death. J. Agric. Food Chem. 2009, 57, 6636–6641. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M. Taurine modulation of hypochlorous acid-induced lung epithelial cell injury in vitro. Role of anion transport. J. Clin. Investig. 1994, 93, 606–614. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Walczewska, M. Neutrophils as Sentinel Cells of the Immune System: A Role of the MPO-halide-system in Innate and Adaptive Immunity. Curr. Med. Chem. 2020, 27, 2840–2851. [Google Scholar] [CrossRef]
- Kim, S.H.; Yum, H.W.; Kim, S.H.; Kim, W.; Kim, S.J.; Kim, C.; Kim, K.; Suh, Y.G.; Surh, Y.J. Protective Effects of Taurine Chloramine on Experimentally Induced Colitis: NFkappaB, STAT3, and Nrf2 as Potential Targets. Antioxidants 2021, 10, 479. [Google Scholar] [CrossRef]
- Khanh Hoang, N.; Maegawa, E.; Murakami, S.; Schaffer, S.W.; Ito, T. N-Chlorotaurine Reduces the Lung and Systemic Inflammation in LPS-Induced Pneumonia in High Fat Diet-Induced Obese Mice. Metabolites 2022, 12, 349. [Google Scholar] [CrossRef]
- Kim, C.; Cha, Y.N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Roth Flach, R.J.; Su, C.; Bollinger, E.; Cortes, C.; Robertson, A.W.; Opsahl, A.C.; Coskran, T.M.; Maresca, K.P.; Keliher, E.J.; Yates, P.D.; et al. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS ONE 2019, 14, e0214150. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Azuma, J. Taurine is a possible anti-atherosclerotic agent. Nihon Yakurigaku Zasshi 2004, 123, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.N.; Seenipandi, A.; Javed, H.; Sharma, C.; Hashiesh, H.M.; Goyal, S.N.; Jha, N.K.; Ojha, S. Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19? Cell Press 2021, 7, e05703. [Google Scholar] [CrossRef]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Oday, O.H.; Lateef, A.; Hassan, S.K.; Rashid, S.; Ali, N.; Zeeshan, M.; et al. D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFkappaB in kidneys of Wistar rats. Exp. Biol. Med. 2014, 239, 465–476. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.Y.; Bach, F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Lee, D.S.; Cheong, S.H. Taurine Have Neuroprotective Activity against Oxidative Damage-Induced HT22 Cell Death through Heme Oxygenase-1 Pathway. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 159–171. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, N.; Yang, J.; Chen, G. Nrf2 Signaling Pathway Mediates the Antioxidative Effects of Taurine Against Corticosterone-Induced Cell Death in HUMAN SK-N-SH Cells. Neurochem. Res. 2018, 43, 276–286. [Google Scholar] [CrossRef]
- Siraj, A.; Islam, A.; Al Fahad, A.; Kheya, H.R.; Xiao, J.; Simal-Gandara, J. Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update. Appl. Sci. 2021, 11, 10806. [Google Scholar] [CrossRef]
- Xu, M.; Che, L.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Li, M.; Jiang, Z. Taurine alleviates oxidative stress in porcine mammary epithelial cells by stimulating the Nrf2-MAPK signaling pathway. Food Sci. Nutr. 2023, 11, 1736–1746. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Chain, B.; Nowak, B.; Grabowska, A.; Bryniarski, K.; Baran, J. Antimicrobial and cytotoxic activity of hypochlorous acid: Interactions with taurine and nitrite. Inflamm. Res. 2000, 49, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Olszanecki, R.; Marcinkiewicz, J. Taurine chloramine and taurine bromamine induce heme oxygenase-1 in resting and LPS-stimulated J774.2 macrophages. Amino Acids 2004, 27, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Kanayama, N.; Kamasawa, N.; Osumi, M.; Tanaka, A. Up-regulation of the peroxisomal beta-oxidation system occurs in butyrate-grown Candida tropicalis following disruption of the gene encoding peroxisomal 3-ketoacyl-CoA thiolase. Biochim. Biophys. Acta 2003, 1631, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and alpha-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
- Lou, J.; Cao, G.; Li, R.; Liu, J.; Dong, Z.; Xu, L. beta-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016, 41, 1291–1304. [Google Scholar] [CrossRef]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. beta-Caryophyllene protects the C6 glioma cells against glutamate-induced excitotoxicity through the Nrf2 pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Stefani, L.M.; Monteiro, S.G. beta-caryophyllene reduces atherogenic index and coronary risk index in hypercholesterolemic rats: The involvement of cardiac oxidative damage. Chem. Biol. Interact. 2017, 270, 9–14. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Chegodaev, Y.S.; Wu, W.K.; Orekhov, A.N. Oxidative Stress and Antioxidants in Atherosclerosis Development and Treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Schnabel, R.; Genth-Zotz, S.; Torzewski, M.; Lackner, K.; Munzel, T.; Blankenberg, S.; AtheroGene Investigators. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am. J. Cardiol. 2007, 99, 808–812. [Google Scholar] [CrossRef]
- Cheng, F.; Torzewski, M.; Degreif, A.; Rossmann, H.; Canisius, A.; Lackner, K.J. Impact of glutathione peroxidase-1 deficiency on macrophage foam cell formation and proliferation: Implications for atherogenesis. PLoS ONE 2013, 8, e72063. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. beta-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Chowhan, N.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R. beta-Pinene alleviates arsenic (As)-induced oxidative stress by modulating enzymatic antioxidant activities in roots of Oryza sativa. Ecotoxicol. Environ. Saf. 2022, 229, 113080. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Liu, X.; Zuo, J.; Wang, K.; Liu, W.; Ge, J. Exogenous taurine attenuates mitochondrial oxidative stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta Biochim. Biophys. Sin. 2013, 45, 359–367. [Google Scholar] [CrossRef]
- Parvez, S.; Tabassum, H.; Banerjee, B.D.; Raisuddin, S. Taurine prevents tamoxifen-induced mitochondrial oxidative damage in mice. Basic Clin. Pharmacol. Toxicol. 2008, 102, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, N.C.D.R.F. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Chahine, R.; Hanna, J.; Bassil, C.; Rihana, N.; Mounayar, A.; Greige, H. Beneficial effect of taurine in spontaneous hypertensive rats: Implication of its antioxidant activity. Afr. J. Pharm. Pharmacol. 2010, 4, 874–877. [Google Scholar]
- Wang, B.; Sun, Q.; Li, Y.; Li, P.; Xia, W.; Liu, D.; Zhu, Z. OS 12-04 Taurine supplementation lowers blood pressure and reduces carotid intima-media thickness in prehypertension. J. Hypertens. 2016, 34, e76–e77. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J. Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: Randomized, double-blind, placebo-controlled study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef]
- Waldron, M.; Patterson, S.D.; Tallent, J.; Jeffries, O. The effects of oral taurine on resting blood pressure in humans: A meta-analysis. Curr. Hypertens. Rep. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Militante, J.D.; Lombardini, J.B. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids 2002, 23, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, N.; Katori, R. Decrease of urinary taurine in essential hypertension. Jpn. Heart J. 1983, 24, 91–102. [Google Scholar] [CrossRef]
- Fujita, T.; Ando, K.; Noda, H.; Ito, Y.; Sato, Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987, 75, 525–532. [Google Scholar] [CrossRef]
- Niu, L.G.; Zhang, M.S.; Liu, Y.; Xue, W.X.; Liu, D.B.; Zhang, J.; Liang, Y.Q. Vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. Eur. J. Pharmacol. 2008, 580, 169–174. [Google Scholar] [CrossRef]
- Ulusoy, K.G.; Kaya, E.; Karabacak, K.; Seyrek, M.; Duvan, İ.; Yildirim, V.; Yildiz, O. Taurine relaxes human radial artery through potassium channel opening action. Korean J. Physiol. Pharmacol. 2017, 21, 617–623. [Google Scholar] [CrossRef]
- Abebe, W.; Mozaffari, M.S. Effects of chronic taurine treatment on reactivity of the rat aorta. Amino Acids 2000, 19, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, J.L.K.; Freehauf, C.L.; Ficicioglu, C.; Pena, L.D.M.; Moreau, K.L.; Henthorn, T.K.; Christians, U.; Jiang, H.; Cowan, T.M.; Young, S.P.; et al. Biomarkers of oxidative stress, inflammation, and vascular dysfunction in inherited cystathionine beta-synthase deficient homocystinuria and the impact of taurine treatment in a phase 1/2 human clinical trial. J. Inherit. Metab. Dis. 2019, 42, 424–437. [Google Scholar] [CrossRef]
- Moloney, M.A.; Casey, R.G.; O’Donnell, D.H.; Fitzgerald, P.; Thompson, C.; Bouchier-Hayes, D.J. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diab. Vasc. Dis. Res. 2010, 7, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Yu, Y.H.; Zhang, L.G.; Wang, Y.; Niu, N.; Li, Q.; Guo, L.M. Taurine rescues vascular endothelial dysfunction in streptozocin-induced diabetic rats: Correlated with downregulation of LOX-1 and ICAM-1 expression on aortas. Eur. J. Pharmacol. 2008, 597, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Ulusoy, K.G. Effects of taurine on vascular tone. Amino Acids 2022, 54, 1527–1540. [Google Scholar] [CrossRef]
- Leão, V.F.; Ferreira, L.L.; Melo, C.M.; Bonfleur, M.L.; da Silva, L.L.; Carneiro, E.M.; Raimundo, J.M.; Ribeiro, R.A. Taurine supplementation prevents endothelial dysfunction and attenuates structural changes in aortas from hypothalamic obese rats. Eur. J. Nutr. 2019, 58, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Guizoni, D.M.; Vettorazzi, J.F.; Carneiro, E.M.; Davel, A.P. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric. Oxide 2020, 94, 48–53. [Google Scholar] [CrossRef]
- Nehme, A.; Zouein, F.A.; Deris Zayeri, Z.; Zibara, K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Santos, R.A.; Bradford, C.N.; Mecca, A.P.; Sumners, C.; Katovich, M.J.; Raizada, M.K. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension 2010, 55, 207–213. [Google Scholar] [CrossRef]
- Takahashi, K.; Azuma, M.; Taira, K.; Baba, A.; Yamamoto, I.; Schaffer, S.W.; Azuma, J. Effect of taurine on angiotensin II-induced hypertrophy of neonatal rat cardiac cells. J. Cardiovasc. Pharmacol. 1997, 30, 725–730. [Google Scholar] [CrossRef]
- Lv, Q.; Yang, Q.; Cui, Y.; Yang, J.; Wu, G.; Liu, M.; Ning, Z.; Cao, S.; Dong, G.; Hu, J. Effects of taurine on ACE, ACE2 and HSP70 expression of hypothalamic-pituitary-adrenal axis in stress-induced hypertensive rats. In Taurine 10; Springer: Dordrecht, The Netherlands, 2017; pp. 871–886. [Google Scholar]
- Yamada, K.; Toyota, K.; Tsunoda, Y.; Matahira, Y.; Matsumura, S.; Yoshioka, Y.; Zaima, N.; Unno, N. Effects of inhaled β-caryophyllene on vascular stiffness in smokers: A randomized, double-blind, placebo-controlled trial. Exp. Ther. Med. 2023, 25, 57. [Google Scholar] [CrossRef]
- Menezes, I.A.; Barreto, C.M.; Antoniolli, Â.R.; Santos, M.R.; de Sousa, D.P. Hypotensive activity of terpenes found in essential oils. Z. Für Nat. C 2010, 65, 562–566. [Google Scholar] [CrossRef]
- Tsunetsugu, Y.; Morikawa, T.; Miyazaki, Y. The relaxing effect of the smell of wood. Wood Ind. 2005, 60, 598–602. [Google Scholar]
- Sadraei, H.; Asghari, G.; Hajhashemi, V.; Kolagar, A.; Ebrahimi, M. Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine 2001, 8, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Câmara, C.C.; Nascimento, N.R.; Macêdo-Filho, C.L.; Almeida, F.B.; Fonteles, M.C. Antispasmodic effect of the essential oil of Plectranthus barbatus and some major constituents on the guinea-pig ileum. Planta Med. 2003, 69, 1080–1085. [Google Scholar] [CrossRef]
- JA Moreira, I.; Menezes, P.P.; Serafini, M.R.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Bonjardim, L.R.; Filho, V.J.S.; Júnior, D.B.P.; Santos, S.L.; Júnior, W.L. Characterization and antihypertensive effect of the complex of (-)-β-pinene in β-cyclodextrin. Curr. Pharm. Biotechnol. 2016, 17, 837–845. [Google Scholar] [CrossRef]
- Thaina, P.; Tungcharoen, P.; Wongnawa, M.; Reanmongkol, W.; Subhadhirasakul, S. Uterine relaxant effects of Curcuma aeruginosa Roxb. rhizome extracts. J. Ethnopharmacol. 2009, 121, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.V.A.; Jayachitra, J.; Shenbagam, M.; Nalini, N. d-limonene attenuates blood pressure and improves the lipid and antioxidant status in high fat diet and L-NAME treated rats. J. Pharm. Sci. Res. 2010, 2, 752. [Google Scholar]
- Cardoso-Teixeira, A.C.; Ferreira-da-Silva, F.W.; Peixoto-Neves, D.; Oliveira-Abreu, K.; Pereira-Goncalves, A.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats. Molecules 2018, 23, 1430. [Google Scholar] [CrossRef] [PubMed]
- Rhana, P.; Barros, G.M.; Santos, V.C.O.; Costa, A.D.; Santos, D.M.D.; Fernandes-Braga, W.; Durco, A.O.; Santos, M.R.V.; Roman-Campos, D.; Vasconcelos, C.M.L.; et al. S-limonene protects the heart in an experimental model of myocardial infarction induced by isoproterenol: Possible involvement of mitochondrial reactive oxygen species. Eur. J. Pharmacol. 2022, 930, 175134. [Google Scholar] [CrossRef]
- Hajagos-Tóth, J.; Hódi, Á.; Seres, A.B.; Gáspár, R. Effects of d-and l-limonene on the pregnant rat myometrium in vitro. Croat. Med. J. 2015, 56, 431–438. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Gokila Vani, M.; Wang, C.-S.; Chen, C.-C.; Chen, Y.-C.; Lu, L.-P.; Huang, C.-H.; Lai, C.-S.; Wang, S.-Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef]
- Kang, P.; Ryu, K.H.; Lee, J.M.; Kim, H.K.; Seol, G.H. Endothelium- and smooth muscle-dependent vasodilator effects of Citrus aurantium L. var. amara: Focus on Ca2+ modulation. Biomed. Pharmacother. 2016, 82, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.; Carvalho, A.A.; Medeiros, I.A.; Alves, P.B.; Marchioro, M.; Antoniolli, A.R. Cardiovascular effects of Hyptis fruticosa essential oil in rats. Fitoterapia 2007, 78, 186–191. [Google Scholar] [CrossRef]
- Kang, P.; Suh, S.H.; Min, S.S.; Seol, G.H. The essential oil of Citrus bergamia Risso induces vasorelaxation of the mouse aorta by activating K+ channels and inhibiting Ca2+ influx. J. Pharm. Pharmacol. 2013, 65, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, X.; Yang, J.; Wu, G.; Sun, C.; Lv, Q. Antihypertensive effect of taurine in rat. Adv. Exp. Med. Biol. 2009, 643, 75–84. [Google Scholar] [CrossRef]
- Maia, A.R.; Batista, T.M.; Victorio, J.A.; Clerici, S.P.; Delbin, M.A.; Carneiro, E.M.; Davel, A.P. Taurine supplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS ONE 2014, 9, e105851. [Google Scholar] [CrossRef] [PubMed]
- Balint, B.; Jepchumba, V.K.; Guéant, J.-L.; Guéant-Rodriguez, R.-M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef]
- Shen, W.; Gao, C.; Cueto, R.; Liu, L.; Fu, H.; Shao, Y.; Yang, W.Y.; Fang, P.; Choi, E.T.; Wu, Q. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol. 2020, 28, 101322. [Google Scholar] [CrossRef]
- Sreckovic, B.; Sreckovic, V.D.; Soldatovic, I.; Colak, E.; Sumarac-Dumanovic, M.; Janeski, H.; Janeski, N.; Gacic, J.; Mrdovic, I. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Sukla, K.; Raman, R. Association of MTHFR and RFC1 gene polymorphism with hyperhomocysteinemia and its modulation by vitamin B12 and folic acid in an Indian population. Eur. J. Clin. Nutr. 2012, 66, 111–118. [Google Scholar] [CrossRef]
- Xu, R.; Huang, F.; Wang, Y.; Liu, Q.; Lv, Y.; Zhang, Q. Gender-and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci. Rep. 2020, 10, 17401. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Widdop, R.E.; Hare, D.L.; Buxton, B.F.; Black, M.J. High methionine and cholesterol diet abolishes endothelial relaxation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1358–1363. [Google Scholar] [CrossRef]
- Zulli, A.; Hare, D.L. High dietary methionine plus cholesterol stimulates early atherosclerosis and late fibrous cap development which is associated with a decrease in GRP78 positive plaque cells. Int. J. Exp. Pathol. 2009, 90, 311–320. [Google Scholar] [CrossRef]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef]
- Debreceni, B.; Debreceni, L. The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovasc. Ther. 2014, 32, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.B.; Han, J.W.; Song, J.; Lee, K.; Kim, T.H.; Kwak, K.P.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Moon, S.W. Hypohomocysteinemia may increases the risk of dementia and Alzheimer’s disease: A nationwide population-based prospective cohort study. Clin. Nutr. 2021, 40, 4579–4584. [Google Scholar] [CrossRef]
- Cullen, C.E.; Carter, G.T.; Weiss, M.D.; Grant, P.A.; Saperstein, D.S. Hypohomocysteinemia: A potentially treatable cause of peripheral neuropathology? Phys. Med. Rehabil. Clin. 2012, 23, 59–65. [Google Scholar] [CrossRef]
- Mietus-Snyder, M.L.; Shigenaga, M.K.; Suh, J.H.; Shenvi, S.V.; Lal, A.; McHugh, T.; Olson, D.; Lilienstein, J.; Krauss, R.M.; Gildengoren, G. A nutrient-dense, high-fiber, fruit-based supplement bar increases HDL cholesterol, particularly large HDL, lowers homocysteine, and raises glutathione in a 2-wk trial. FASEB J. 2012, 26, 3515. [Google Scholar] [CrossRef]
- Zulli, A.; Lau, E.; Wijaya, B.P.; Jin, X.; Sutarga, K.; Schwartz, G.D.; Learmont, J.; Wookey, P.J.; Zinellu, A.; Carru, C.; et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: Association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension 2009, 53, 1017–1022. [Google Scholar] [CrossRef]
- Ahn, C.S. Effect of taurine supplementation on plasma homocysteine levels of the middle-aged Korean women. In Taurine 7; Springer: New York, NY, USA, 2009; pp. 415–422. [Google Scholar]
- Deminice, R.; Rosa, F.T.; da Silva, L.E.C.M.; Jordao, A.A. Taurine supplementation does not decrease homocysteine levels and liver injury induced by a choline-deficient diet. Life Sci. 2014, 105, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhao, J.; Xu, J.; Jiang, W.; Tang, C.S.; Qi, Y.F. Effects of taurine and homocysteine on calcium homeostasis and hydrogen peroxide and superoxide anions in rat myocardial mitochondria. Clin. Exp. Pharmacol. Physiol. 2004, 31, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Tsujino, T.; Watari, Y.; Emoto, N.; Yokoyama, M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: Amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation 2001, 104, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, N.L.; Zeng, Y.; Liu, B.; Kumthip, K.; Wang, T.T.; Huo, D.; Ingels, J.F.; Lu, L.; Shang, J. The molecular chaperone GRP78 contributes to toll-like receptor 3-mediated innate immune response to hepatitis C virus in hepatocytes. J. Biol. Chem. 2016, 291, 12294–12309. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Hao, Y.J.; Jiao, Y.; Wang, Y.H.; Xu, L.B.; Mao, C.Y.; Yang, X.L.; Yang, A.N.; Tian, J.; Zhang, M.H. Homocysteine-induced oxidative stress through TLR4/NF-κB/DNMT1-mediated LOX-1 DNA methylation in endothelial cells. Mol. Med. Rep. 2017, 16, 9181–9188. [Google Scholar] [CrossRef]
- Gao, S.; Wang, L.; Liu, W.; Wu, Y.; Yuan, Z. The synergistic effect of homocysteine and lipopolysaccharide on the differentiation and conversion of raw264. 7 macrophages. J. Inflamm. 2014, 11, 13. [Google Scholar] [CrossRef]
- Zaric, B.L.; Radovanovic, J.N.; Gluvic, Z.; Stewart, A.J.; Essack, M.; Motwalli, O.; Gojobori, T.; Isenovic, E.R. Atherosclerosis Linked to Aberrant Amino Acid Metabolism and Immunosuppressive Amino Acid Catabolizing Enzymes. Front. Immunol. 2020, 11, 551758. [Google Scholar] [CrossRef]
- Santos, M.G.; Pegoraro, M.; Sandrini, F.; Macuco, E.C. Risk factors for the development of atherosclerosis in childhood and adolescence. Arq. Bras. Cardiol. 2008, 90, 276–283. [Google Scholar] [CrossRef]
- Camare, C.; Pucelle, M.; Negre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Murakami, S. Taurine and atherosclerosis. Amino Acids 2014, 46, 73–80. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2020, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Kowara, M.; Cudnoch-Jedrzejewska, A. Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. Int. J. Mol. Sci. 2021, 22, 3513. [Google Scholar] [CrossRef]
- Yu, D.; Liao, J.K. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc. Res. 2022, 118, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Woo, M.; Kim, M.; Noh, J.S.; Song, Y.O. Antioxidative and Cholesterol-Lowering Effects of Lemon Essential Oil in Hypercholesterolemia-Induced Rabbits. Prev. Nutr. Food Sci. 2018, 23, 8–14. [Google Scholar] [CrossRef]
- Matsushima, Y.; Sekine, T.; Kondo, Y.; Sakurai, T.; Kameo, K.; Tachibana, M.; Murakami, S. Effects of taurine on serum cholesterol levels and development of atherosclerosis in spontaneously hyperlipidaemic mice. Clin. Exp. Pharmacol. Physiol. 2003, 30, 295–299. [Google Scholar] [CrossRef]
- Murakami, S.; Kondo-Ohta, Y.; Tomisawa, K. Improvement in cholesterol metabolism in mice given chronic treatment of taurine and fed a high-fat diet. Life Sci. 1999, 64, 83–91. [Google Scholar] [CrossRef]
- Huseini, H.F.; Anvari, M.S.; Khoob, Y.T.; Rabbani, S.; Sharifi, F.; Arzaghi, S.M.; Fakhrzadeh, H. Anti-hyperlipidemic and anti-atherosclerotic effects of Pinus eldarica Medw. nut in hypercholesterolemic rabbits. Daru 2015, 23, 32. [Google Scholar] [CrossRef]
- Mahamuni, S.P.; Khose, R.D.; Menaa, F.; Badole, S.L. Therapeutic approaches to drug targets in hyperlipidemia. BioMedicine 2012, 2, 137–146. [Google Scholar] [CrossRef]
- Kondo, Y.; Toda, Y.; Kitajima, H.; Oda, H.; Nagate, T.; Kameo, K.; Murakami, S. Taurine inhibits development of atherosclerotic lesions in apolipoprotein E-deficient mice. Clin. Exp. Pharmacol. Physiol. 2001, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Zhang, X.; Du, Y.; Chen, G.; Xiang, P.; Ling, W.; Wang, D. Terpene Lactucopicrin Limits Macrophage Foam Cell Formation by a Reduction of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 in Lipid Rafts. Mol. Nutr. Food Res. 2022, 66, e2100905. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Shah, P.K.; Galis, Z.S. Matrix metalloproteinase hypothesis of plaque rupture: Players keep piling up but questions remain. Circulation 2001, 104, 1878–1880. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.A.; Bustanji, Y.K.; Abdalla, S.S. Hypocholesterolemic effect of beta-caryophyllene in rats fed cholesterol and fat enriched diet. J. Clin. Biochem. Nutr. 2018, 62, 230–237. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Doleski, P.H.; Boligon, A.A.; Stefani, L.M.; Monteiro, S.G. Hypolipidemic effect of beta-caryophyllene to treat hyperlipidemic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 215–223. [Google Scholar] [CrossRef]
- Hao, M.X.; Jiang, L.S.; Fang, N.Y.; Pu, J.; Hu, L.H.; Shen, L.H.; Song, W.; He, B. The cannabinoid WIN55,212-2 protects against oxidized LDL-induced inflammatory response in murine macrophages. J. Lipid Res. 2010, 51, 2181–2190. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Z.; Liu, Y.; Xue, J.; Tian, Y.; Liu, W.; Zhang, W.; Shen, Y.; Xu, W.; Liang, X.; et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J. Cardiovasc. Pharmacol. 2010, 55, 292–298. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Zhang, W.; Xue, J.; Wu, Y.Z.; Xu, W.; Liang, X.; Chen, T.; Kishimoto, C.; Yuan, Z. WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur. J. Pharmacol. 2010, 649, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013, 715, 46–55. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef] [PubMed]
- Staels, B. PPAR agonists and the metabolic syndrome. Therapie 2007, 62, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Abu-Soud, H.M.; Hazen, S.L. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 2000, 28, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Marsche, G.; Zimmermann, R.; Horiuchi, S.; Tandon, N.N.; Sattler, W.; Malle, E. Class B scavenger receptors CD36 and SR-BI are receptors for hypochlorite-modified low density lipoprotein. J. Biol. Chem. 2003, 278, 47562–47570. [Google Scholar] [CrossRef]
- Park, E.; Schuller-Levis, G.; Quinn, M.R. Taurine chloramine inhibits production of nitric oxide and TNF-alpha in activated RAW 264.7 cells by mechanisms that involve transcriptional and translational events. J. Immunol. 1995, 154, 4778–4784. [Google Scholar] [CrossRef]
- Al Samarraie, A.; Pichette, M.; Rousseau, G. Role of the Gut Microbiome in the Development of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 5420. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease. Front. Cardiovasc. Med. 2022, 9, 900381. [Google Scholar] [CrossRef] [PubMed]
- Muradi, A.; Jasirwan, C.O.M.; Simanjuntak, C.D.; Pratama, D.; Suhartono, R.; Darwis, P.; Kekalih, A. The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients. Life 2022, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.E.; Lai, K.S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Z.; Shen, S.; Shan, W. Effects of taurine on gut microbiota and metabolism in mice. Amino Acids 2016, 48, 1601–1617. [Google Scholar] [CrossRef]
- Valerii, M.C.; Turroni, S.; Ferreri, C.; Zaro, M.; Sansone, A.; Dalpiaz, A.; Botti, G.; Ferraro, L.; Spigarelli, R.; Bellocchio, I.; et al. Effect of a Fiber D-Limonene-Enriched Food Supplement on Intestinal Microbiota and Metabolic Parameters of Mice on a High-Fat Diet. Pharmaceutics 2021, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.F.d.A.; Lacerda, R.d.S.; Correia, J.P.d.A.; de Melo, T.R.; Ferreira, S.B. Potential Antibacterial Action of α-Pinene. Med. Sci. 2022, 12, 11. [Google Scholar] [CrossRef]
- Santos, E.S.; de Sousa Machado, S.T.; Rodrigues, F.B.; da Silva, Y.A.; Matias, L.C.X.; Lopes, M.J.P.; Gomes, A.D.S.; Ribeiro, T.F.; Farcia, F.A.B.; Coultinho, H.D.M.; et al. Potential anti-inflammatory, hypoglycemic, and hypolipidemic activities of alpha-pinene in diabetic rats. Process Biochem. 2023, 126, 80–86. [Google Scholar] [CrossRef]
- Santos, E.S.; Abrantes Coelho, G.L.; Saraiva Fontes Loula, Y.K.; Saraiva Landim, B.L.; Fernandes Lima, C.N.; Tavares de Sousa Machado, S.; Pereira Lopes, M.J.; Soares Gomes, A.D.; Martins da Costa, J.G.; Alencar de Menezes, I.R.; et al. Hypoglycemic, Hypolipidemic, and Anti-Inflammatory Effects of Beta-Pinene in Diabetic Rats. Evid. Based Complement. Alternat. Med. 2022, 2022, 8173307. [Google Scholar] [CrossRef]
- Park, T.; Lee, K. Dietary taurine supplementation reduces plasma and liver cholesterol and triglyceride levels in rats fed a high-cholesterol or a cholesterol-free diet. Adv. Exp. Med. Biol. 1998, 442, 319–325. [Google Scholar] [CrossRef]
- Murakami, S.; Kondo, Y.; Sakurai, T.; Kitajima, H.; Nagate, T. Taurine suppresses development of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis 2002, 163, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gokce, G.; Ozsarlak-Sozer, G.; Oran, I.; Oktay, G.; Ozkal, S.; Kerry, Z. Taurine suppresses oxidative stress-potentiated expression of lectin-like oxidized low-density lipoprotein receptor and restenosis in balloon-injured rabbit iliac artery. Clin. Exp. Pharmacol. Physiol. 2011, 38, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Faravelli Inocorporated PhytoCann BP: Next Generation Black Pepper Extract. Available online: https://www.bevnet.com/sponsored/phytocann-bp-next-generation-black-pepper-extract/ (accessed on 2 May 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiderski, J.; Sakkal, S.; Apostolopoulos, V.; Zulli, A.; Gadanec, L.K. Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients 2023, 15, 2562. https://doi.org/10.3390/nu15112562
Swiderski J, Sakkal S, Apostolopoulos V, Zulli A, Gadanec LK. Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients. 2023; 15(11):2562. https://doi.org/10.3390/nu15112562
Chicago/Turabian StyleSwiderski, Jordan, Samy Sakkal, Vasso Apostolopoulos, Anthony Zulli, and Laura Kate Gadanec. 2023. "Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases" Nutrients 15, no. 11: 2562. https://doi.org/10.3390/nu15112562
APA StyleSwiderski, J., Sakkal, S., Apostolopoulos, V., Zulli, A., & Gadanec, L. K. (2023). Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients, 15(11), 2562. https://doi.org/10.3390/nu15112562







