Abstract
This review aims to investigate and summarize adverse health events in children and adolescents associated with energy drink (ED) consumption, while also exploring the impact of simultaneous trigger factors and/or preexisting health conditions. We searched the database of PubMed, Cochrane library, and Web of Science for cases associated with ED consumption in minors up to 9 May 2023. The literature written in English met inclusion criteria if patients were <18 years of age and the ED consumption was confirmed. Records, relevant articles, and reports that met all inclusion criteria were fully read by two researchers. In total, 18 cases reporting adverse health events were included. Of those, 45% affected the cardiovascular system, 33% the neuropsychological system, and 22% other organ systems. In 33% of cases, additional triggers were reported. In 44% preexisting health conditions were present. This literature review suggests that ED intake may well be associated with adverse health events in minors. The cardiovascular and the neuropsychiatric systems seem to be predisposed. ED consumption in combination with potential trigger factors or in the presence of preexisting health conditions appears to be critical. To prevent adverse health events in the future, children and adolescents should be informed about risk factors and responsible consumption behaviors.
1. Introduction
Energy drinks (ED) are sweetened beverages containing stimulant compounds, such as caffeine or guarana [1] and are marketed as mental and physical enhancers [2]. Despite their well-known side effects on the cardiovascular system (e.g., arterial hypertension and cardiac arrhythmias) [3], ED consumption behavior remains very high, especially among teenagers [4]. Data from the NOMISMA-ARETÉ Consortium for the European Food Safety Authority (EFSA) demonstrated that the highest prevalence of ED consumption is reported in adolescents (68%), followed by adults (30%) and children (18%) [5]. Of those underaged ED consumers, the prevalence of high chronic consumption was estimated at 12% for adolescents and 16% for children, and the prevalence of high acute consumption at 12%, respectively [5].
In line with their increasing popularity among minors, the literature shows that emergency admissions associated with the consumption of ED have been increasing [6,7]. Especially the cardiovascular system seems to be affected by ED consumption: The excessive ED intake in combination with “party drugs” (i.e., illicit stimulants), alcohol, and/or in the presence of chronic medical conditions, may more frequently lead to adverse cardiovascular events such as cardiac arrhythmias or myocardial ischemia [8]. Interestingly, several clinical studies have demonstrated a significant increase in arterial blood pressure or QTc-alterations after acute ED consumption in adult subjects [9,10]. In addition, recent studies from our institution suggest that the cardiovascular system of minors may be more responsive to acute ED ingestion than that of adults [11,12]. Accordingly, the acute ED consumption led to a significantly higher blood pressure and arterial stiffness as well as a significantly lower left ventricular (LV) efficiency in juvenile study participants [12,13,14,15]. Further, we were able to demonstrate a higher disposition for cardiac arrhythmias visualized by a significantly higher prevalence of supraventricular extrasystoles after acute ED consumption [16].
Although most clinical trials have mainly focused on cardiovascular function, multiple case reports suggest that other organ systems, including the neuropsychological, hepatic, and renal system, are also affected by acute and chronic ED consumption.
Due to the fatal outcome after excessive ED intake in an adolescent patient of our institution, we reviewed published case reports and aimed to investigate as well as summarize adverse health events in children and adolescents associated with ED consumption. Moreover, the impact of simultaneous trigger factors and/or preexisting health conditions was explored.
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Inclusion Criteria
Inclusion criteria for case reports were as follows: (1) reported patients were <18 years of age, (2) the consumption of EDs was confirmed, (3) case reports were written in the English language. No publication date restriction was defined. Any research type including relevant case report data was included.
2.1.2. Exclusion Criteria
Exclusion criteria were the following: the inclusion criteria were not met; repeated publication of the same study population.
2.2. Information Sources
We searched the database of PubMed, Cochrane library and Web of Science up to 9 May 2023.
2.3. Search Strategy
We searched for the following text words (tw) terms, free-text terms and Medical Subject Heading (MeSH) terms individually or in combination: “Energy drink*”and “Child*” or “Adolescen*” or “Teen*” or “Youth*”and “case report*” or “Case Reports” or “Comment” or “Letter”. The search strategies for each database are available in Table 1.

Table 1.
Search strategies.
2.4. Selection Process
Two researchers independently screened titles and abstracts identified from the search strategy. Records, relevant articles, and reports in the references that met all inclusion criteria were fully read by the same two researchers. Full texts that did not meet the inclusion criteria were excluded. Any disagreements between the two researchers were mediated through discussions with a third researcher to reach a final decision.
3. Results
3.1. Record Selection
The initial search consisted of 98 records. After excluding 15 duplicated records, two researchers reviewed the titles and abstracts independently and excluded 44 records. The remaining 39 records were fully reviewed by the same two researchers and the related references were selected according to the inclusion and exclusion criteria. Ultimately, 16 records containing 17 cases were considered relevant for this review (Figure 1).
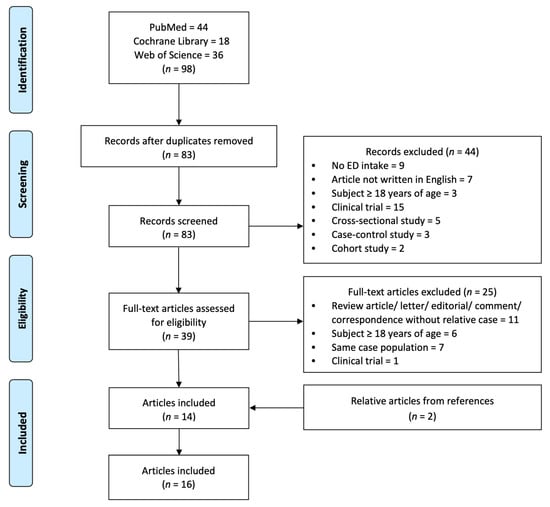
Figure 1.
Flow diagram of literature search and selection process.
3.2. Summary of Included Cases
A precise summary of all 16 records including 17 cases describing the age, gender, clinical presentation, and ED consumption behavior of the reported subjects is given in Table 2. In addition, our institutional case is added. Table 2 also shows information on abnormal results, diagnosis, potential triggers, preexisting health conditions, treatment, and outcome of each case. Figure 2 portrays affected organ systems, potential triggers, and preexisting health conditions associated with adverse health events after ED consumption.

Table 2.
Summary of included cases associated with adverse health events in children and adolescents after the consumption of energy drinks.
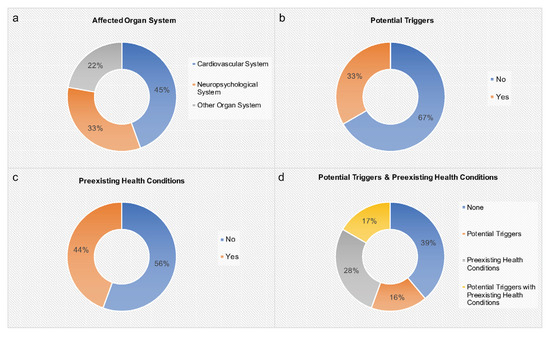
Figure 2.
Affected organ systems, potential triggers, and preexisting health conditions associated with adverse health events after ED consumption (n = 18). (a) Affected organ system, (b) potential triggers (coffee/cannabis/alcohol and baseline stimulant medication/alcohol and cardiovascular exercise), (c) preexisting health conditions (fatigue/fainting episodes/questionable myopericarditis/history of myocarditis (confirmed by biopsy)/generalized epilepsy/ADHD, asthma, allergies/seasonal allergies/liver transplantation and re-transplantation), (d) potential triggers and preexisting health conditions.
4. Discussion
In this literature review, we determined a connection between ED intake and adverse health events in minors (Table 2). In total, 18 cases were included. Most adverse health events concerned the cardiovascular or neuropsychiatric system (Figure 2). Most cases displayed complete remission. However, based on institutional experience, one fatal outcome after excessive ED consumption was demonstrated. Interestingly, in 61% of cases potential triggers and/or preexisting health conditions were present (Figure 2).
4.1. Effects of Enery Drinks on the Pediatric Cardiovascular System
Cardiovascular adverse health events were reported in 45% of cases, including cardiac arrhythmia, arterial hypertension, acute coronary artery vasospasm, and spontaneous coronary artery dissection (Table 2, Figure 2). In five subjects, potential triggers and/or preexisting health conditions were described. In the two subjects without potential triggers and/or preexisting health conditions, very large ED amounts were consumed over multiple days. One healthy male adolescent displayed spontaneous coronary artery dissection after the consumption of a reasonable ED amount (80 mg of caffeine) [17]. In this case no potential triggers were described [17]. Hence, this literature review suggests that adverse cardiovascular events associated with the consumption of EDs particularly occur if preexisting health conditions and/or additional trigger factors (e.g., alcohol, drugs, physical exercise) are present and/or very large ED amounts are consumed.
The adverse cardiovascular health effects associated with the consumption of EDs can partially be attributed to their high caffeine content [33]. Caffeine was demonstrated to act vasoconstrictive and to increase LV inotropy leading to an augmentation of blood pressure [33]. In addition, a proarrhythmic potential of caffeinated EDs is assumed [34]. Recent studies of our department support these findings by investigating the acute effects of EDs on blood pressure, arterial stiffness, LV function, as well as heart rhythm and electrocardiographic time intervals in up to 27 healthy children and adolescents [12,13,14,15,16]. Subjects received a bodyweight-adjusted ED amount (3 mg caffeine per kg of body weight) or a placebo beverage (similar sugar content, no conventional ED ingredients) on two consecutive days [12,13,14,15,16]. Interestingly, the acute ED consumption led to significantly higher systolic and diastolic blood pressure [12,13]. Moreover, arterial stiffness of the common carotid arteries was found to be significantly higher after acute ED consumption in the examined pediatric cohort [14]. The ED consumption led to a significantly lower LV efficiency in juvenile study participants [15]. Further, a disposition for cardiac arrhythmias visualized by a significantly higher prevalence of supraventricular extrasystoles after the acute ED consumption was demonstrated [16]. In contrast to adult studies [9,35,36,37], a significant increase in the QTc interval was not observed [16]. However, it must be pointed out that in these recently published pediatric studies, the acute maximal caffeine consumption considered as safe for healthy children and teenagers by the EFSA, was not surpassed [12,13,14,15,16,38]. It remains rather unknown how the pediatric cardiovascular system reacts to higher ED amounts, particularly if additional triggers and/or preexisting health conditions are present. Moreover, due to their high sugar content the chronic intake of EDs can lead to sugar metabolism disorders and excess weight ultimately increasing long-term cardiovascular morbidity.
In addition to caffeine, EDs may contain taurine. In the literature, the amino acid taurine is considered to decrease blood pressure and to be anti-arrhythmic [9,39,40]. The cardiovascular effects of other stimulants often added to EDs, such as B vitamins, L-carnitine or glucuronolactone, are understudied and require further research [9,33].
4.2. Effects of Energy Drinks on the Pediatric Neuropsychiatric System
The relationship between the compounds of an ED and neuropsychiatric diseases is still rather uncharted territory. The main compound caffeine may reduce the epileptological threshold if given in low dosage and chronic use may have a protective effect [37,41]. Another example presents caffeinated analgetic medication for diverse types of headaches [42]. In contrast, it has been suggested that caffeine as a stimulant may trigger psychotic episodes and panic disorders [43]. Caffeine consumption may lead to a state of hyperexcitability in the brain potentially causing adverse reactions. A common disturbance from daily intake of caffeinated EDs may be the presence of chronic headaches due to overexcitability of the cortex [44]. A rising concern regarding EDs is their link to seizures. A study reviewing 4 adult cases of new-onset seizures found that they all consumed EDs that contained caffeine and taurine. The seizures resolved when subjects abstained from ED consumption [45].
Currently there are four caffeine-induced disorders recognized in the Diagnostic and Statistical Manual of Mental Disorders 4th edition for psychiatric health [46]. These include caffeine-induced anxiety, caffeine-related disorder, caffeine-induced sleep disorder, and caffeine intoxication [47]. A randomized placebo-controlled study, involving 90 professional athletes, indicated that insomnia, activity, and nervousness increased after ED consumption [48]. Another study examined over 1727 5th–12th grade students and concluded that children who consumed EDs had a 66% increased risk of hyperactivity and inattention compared to students who did not consume caffeine [49].
The amount of caffeine contained in EDs varies considerably. The pharmacokinetics of caffeine are mainly due to its antagonism of A1 and A2 adenosine receptors, preventing the neuron from blocking the release of glutamate and dopamine potentially leading to a lower seizure threshold [8]. The resulting cardiovascular effects are well studied, and it is suggested that EDs may lead to a reduction in cerebral blood flow [50], potentially triggering headaches. Finally, the literature supports the notion that ED consumption, particularly in adolescents, has adverse effects on the developing brain, possibly leading to insomnia, hyperactivity, and attention deficit disorder [51].
To the best of our knowledge, there are no controlled studies investigating the acute effects of EDs on the neuropsychological system in healthy children and teenagers so far.
4.3. Effects of Energy Drinks on Other Pediatric Organ Systems
4.3.1. Hepatic System
Two cases affecting the hepatic system were identified (Table 2). EDs are often highly enriched in niacin. The pediatric EFSA reference value for niacin ranges between 12 mg and 19 mg per day [52]. Five hundred mL of ED can contain around 40 mg of niacin, which is about 200% of the reference value. It is assumed that the excessive ingestion of niacin can lead to drug-induced liver injury as described in the case by Apestegui et al. (2011) [29]. However, it should be noted that in this particular case the subject received multiple liver transplantations and was therefore predisposed to liver toxins [29]. Another case described the onset of non-alcoholic steatohepatitis (NASH) after chronic ED consumption. EDs contain large amounts of sugar. In a common 16 oz ED, the sugar content can range between 37 g and 62 g, making EDs a main source of empty calories [53]. In addition, there are data implicating that caffeine intake may decrease insulin sensitivity [44]. The chronic consumption of EDs can lead to the development of sugar metabolism disorders and excess weight, which are both considered as risk factors for NASH [54]. The case of Robin et al. (2017) underlines these considerations [30]. The long-term ED intake was made responsible for the NASH onset in the obese 17-year-old subject. The initiated lifestyle modifications, including the restraint from ED consumption, led to partial remission [30].
4.3.2. Renal System
A case reported by Schöffl et al. (2011) linked excessive ED and alcohol consumption with acute renal failure in a 17-year-old boy [31]. EDs contain large amounts of taurine. It is suggested that 95% of taurine is metabolized in the kidneys. Taurine is considered to modify renal blood flow and control osmolarity in the renal medulla [55]. The authors assume the excessive taurine intake to be potentially involved in the pathophysiology of acute renal failure by inducing tubular necrosis [31]. However, the negative effects of excessive alcohol consumption on the renal system (e.g., rhabdomyolysis) should also be considered in this case [56]. The chronic ED consumption can lead to arterial hypertension, sugar metabolism disorders and excess weight, all of which are known risk factors for chronic kidney disease [57].
4.3.3. Skin
One case by Yazdi et al. (2008) described the presence of a multilocular lichen aureus in an 11-year-old boy [32]. Interestingly, the lesions resolved after the regular ED consumption was ceased by the subject. While the pathophysiology of this disease is rather unknown, the authors speculate ED ingredients to be a potential trigger of its onset [32].
4.4. Prevention of Adverse Health Events Associated with Energy Drink Consumption
While children and teenagers represent a large percentage of the ED consumer market, studies examining the effects of their consumption; acute or chronic, on pediatric health are lacking. Medical associations, such as the American Academy of Pediatrics apprehend a potential health risk in association with these beverages in minors and advise against ED consumption [58]. Furthermore, several countries, including Lithuania and Latvia, banned the sale of EDs to minors [59,60]. To reduce future adverse reactions connected to ED intake, children and adolescents should be informed about the associated risk factors and responsible ED consumption behaviors. Minors with preexisting health conditions should be discouraged from drinking EDs. In addition, ED consumption in combination with potential trigger factors (e.g., physical activity, long-term medication, drug/alcohol abuse) should be avoided.
4.5. Limitations
A potential limitation of this review is publication bias. With 18 cases included, this literature review can be considered relatively small. In addition, in some cases, the exact ED ingredients, their concentrations, or the specific ED amount were not described. In addition, in some cases, information about specific trigger factors (e.g., physical activity, illicit drugs, etc.,) was not reported. It is possible that some subjects withheld information about concomitant drug use. These limitations must be taken into consideration, as they could have altered the demonstrated results.
5. Conclusions
This literature review suggests that ED consumption may well be associated with adverse health events in minors. The cardiovascular and the neuropsychiatric systems seem to be predisposed. Particularly, ED consumption in combination with potential trigger factors or in the presence of preexisting health conditions appears to be critical. To prevent adverse health effects in the future, children and adolescents should be informed about ED-associated risk factors and responsible ED consumption behaviors. Minors with preexisting health conditions should be discouraged from drinking EDs. In addition, ED consumption in combination with potential trigger factors (e.g., physical activity, drug intake) should be avoided. To better understand and determine the possible side-effects of ED consumption in minors with or without preexisting health conditions and to investigate possible trigger factors, we highlight the necessity of future controlled studies and clinical trials. The validated data may not only aid the acute clinical manifestations but also support the primary prevention.
Author Contributions
N.A.H. conceptualized and designed the study and critically reviewed and revised the manuscript. P.L. and G.M. designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript. A.J. and R.D.-P. critically reviewed and revised the manuscript. F.S.O. conceptualized and designed the study, coordinated, and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This study (project title: EDUCATE-Study: Energy-Drinks—Unexplored Cardiovascular Alterations in TEens and TwEens) was supported by the German Heart Foundation/German Foundation of Heart Research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hampton, T. Energy Drinks Pose Worrisome Risks to Adolescents’ Cardiovascular Health. Circulation 2016, 134, 1052–1053. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Syed, W.; Medici, V.; Sferra, T.J. Impact of Energy Drinks on Health and Well-being. Curr. Nutr. Rep. 2018, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hellín, J.; Varillas-Delgado, D. Energy Drinks and Sports Performance, Cardiovascular Risk, and Genetic Associations; Future Prospects. Nutrients 2021, 13, 715. [Google Scholar] [CrossRef]
- Grasser, E.K.; Miles-Chan, J.L.; Charrière, N.; Loonam, C.R.; Dulloo, A.G.; Montani, J.-P. Energy Drinks and Their Impact on the Cardiovascular System: Potential Mechanisms. Adv. Nutr. 2016, 7, 950–960. [Google Scholar] [CrossRef]
- Zucconi, S.; Volpato, C.; Adinolfi, F.; Gandini, E.; Gentile, E.; Loi, A.; Fioriti, L. Gathering consumption data on specific consumer groups of energy drinks. EFSA Support. Publ. 2013, 10, 394E. [Google Scholar] [CrossRef]
- Mattson, M.E. Update on Emergency Department Visits Involving Energy Drinks: A Continuing Public Health Concern. In The CBHSQ Report; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2013; pp. 1–7. [Google Scholar]
- Seifert, S.M.; Seifert, S.A.; Schaechter, J.L.; Bronstein, A.C.; Benson, B.E.; Hershorin, E.R.; Arheart, K.L.; Franco, V.I.; Lipshultz, S.E. An analysis of energy-drink toxicity in the National Poison Data System. Clin. Toxicol. 2013, 51, 566–574. [Google Scholar] [CrossRef]
- Ehlers, A.; Marakis, G.; Lampen, A.; Hirsch-Ernst, K.I. Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem. Toxicol. 2019, 130, 109–121. [Google Scholar] [CrossRef]
- Shah, S.A.; Szeto, A.; Farewell, R.; Shek, A.; Fan, D.; Quach, K.N.; Bhattacharyya, M.; Elmiari, J.; Chan, W.; O’Dell, K.; et al. Impact of High Volume Energy Drink Consumption on Electrocardiographic and Blood Pressure Parameters: A Randomized Trial. J. Am. Heart Assoc. 2019, 8, e011318. [Google Scholar] [CrossRef]
- Basrai, M.; Schweinlin, A.; Menzel, J.; Mielke, H.; Weikert, C.; Dusemund, B.; Putze, K.; Watzl, B.; Lampen, A.; Bischoff, S.C. Energy Drinks Induce Acute Cardiovascular and Metabolic Changes Pointing to Potential Risks for Young Adults: A Randomized Controlled Trial. J. Nutr. 2019, 149, 441–450. [Google Scholar] [CrossRef]
- Shah, S.A.; Chu, B.W.; Lacey, C.S.; Riddock, I.C.; Lee, M.; Dargush, A.E. Impact of Acute Energy Drink Consumption on Blood Pressure Parameters: A Meta-analysis. Ann. Pharmacother. 2016, 50, 808–815. [Google Scholar] [CrossRef]
- Oberhoffer, F.S.; Li, P.; Jakob, A.; Dalla-Pozza, R.; Haas, N.A.; Mandilaras, G. Energy Drinks: Effects on Blood Pressure and Heart Rate in Children and Teenagers. A Randomized Trial. Front. Cardiovasc. Med. 2022, 9, 862041. [Google Scholar] [CrossRef] [PubMed]
- Oberhoffer, F.S.; Dalla-Pozza, R.; Jakob, A.; Haas, N.A.; Mandilaras, G.; Li, P. Energy drinks: Effects on pediatric 24-h ambulatory blood pressure monitoring. A randomized trial. Pediatr Res. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mandilaras, G.; Jakob, A.; Dalla-Pozza, R.; Haas, N.A.; Oberhoffer, F.S. Energy Drinks and Their Acute Effects on Arterial Stiffness in Healthy Children and Teenagers: A Randomized Trial. J. Clin. Med. 2022, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Oberhoffer, F.S.; Li, P.; Jakob, A.; Dalla-Pozza, R.; Haas, N.A.; Mandilaras, G. Energy Drinks Decrease Left Ventricular Efficiency in Healthy Children and Teenagers: A Randomized Trial. Sensors 2022, 22, 7209. [Google Scholar] [CrossRef]
- Mandilaras, G.; Li, P.; Dalla-Pozza, R.; Haas, N.A.; Oberhoffer, F.S. Energy Drinks and Their Acute Effects on Heart Rhythm and Electrocardiographic Time Intervals in Healthy Children and Teenagers: A Randomized Trial. Cells 2022, 11, 498. [Google Scholar] [CrossRef]
- Polat, N.; Ardıç, I.; Akkoyun, M.; Vuruşkan, E. Spontaneous coronary artery dissection in a healthy adolescent following consumption of caffeinated “energy drinks”. Turk. Kardiyol. Dern Ars. 2013, 41, 738–742. [Google Scholar] [CrossRef]
- Dufendach, K.A.; Horner, J.M.; Cannon, B.C.; Ackerman, M.J. Congenital type 1 long QT syndrome unmasked by a highly caffeinated energy drink. Heart Rhythm 2012, 9, 285–288. [Google Scholar] [CrossRef]
- Di Rocco, J.R.; During, A.; Morelli, P.J.; Heyden, M.; Biancaniello, T.A. Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: Two case reports. J. Med. Case Rep. 2011, 5, 18. [Google Scholar] [CrossRef]
- Usman, A.; Jawaid, A. Hypertension in a young boy: An energy drink effect. BMC Res. Notes 2012, 5, 591. [Google Scholar] [CrossRef]
- Terlizzi, R.; Rocchi, C.; Serra, M.; Solieri, L.; Cortelli, P. Reversible postural tachycardia syndrome due to inadvertent overuse of Red Bull. Clin. Auton. Res. 2008, 18, 221–223. [Google Scholar] [CrossRef]
- Wilson, R.E.; Kado, H.S.; Samson, R.; Miller, A.B. A case of caffeine-induced coronary artery vasospasm of a 17-year-old male. Cardiovasc. Toxicol. 2012, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Butragueño Laiseca, L.; Toledo del Castillo, B.; Miranda Herrero, M.C. Energy drinks as a trigger factor for seizures in paediatric patients: A case report. Neurología 2019, 34, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Staikoglou, N.; Polanagnostaki, A.; Lamprou, V.; Chartampilas, E.; Pavlou, E.; Tegos, T.; Finitsis, S. Posterior cerebral artery dissection after excessive caffeine consumption in a teenager. Radiol. Case Rep. 2022, 17, 2081–2084. [Google Scholar] [CrossRef]
- Babu, K.M.; Zuckerman, M.D.; Cherkes, J.K.; Hack, J.B. First-Onset Seizure After Use of an Energy Drink. Pediatr. Emerg. Care 2011, 27, 539–540. [Google Scholar] [CrossRef]
- Yamada-Takeda, M.; Patel, A.; Fenton, G. Energy Drink-Induced Breakthrough Seizure in a Patient on Valproic Acid-Considering Herbal Safety in Epilepsy. J. Pharm. Pract. 2019, 32, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D. Reversible cerebral vasoconstriction syndrome: Another health hazard of energy drinks? Clin. Pediatr. 2015, 54, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Quadri, S.; Harding, L.; Lillig, M. An Energy Drink-Induced Manic Episode in an Adolescent. Prim. Care Companion CNS Disord. 2018, 20, 18102318. [Google Scholar] [CrossRef]
- Apestegui, C.A.; Julliard, O.; Ciccarelli, O.; Duc, D.K.; Lerut, J. Energy drinks: Another red flag for the liver allograft. Liver Transpl. 2011, 17, 1117–1118. [Google Scholar] [CrossRef]
- Robin, S.; Buchanan, R.; Poole, R. Energy drinks and adolescents—A hepatic health hazard? J. Hepatol. 2018, 68, 856–857. [Google Scholar] [CrossRef]
- Schöffl, I.; Kothmann, J.F.; Schöffl, V.; Rupprecht, H.D.; Rupprecht, T. “Vodka energy”: Too much for the adolescent nephron? Pediatrics 2011, 128, e227–e231. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Mayser, P.; Sander, C.A. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J. Cutan. Pathol. 2008, 35, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Frankel, D.S. Arrhythmogenic effects of energy drinks. J. Cardiovasc. Electrophysiol. 2017, 28, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.A.; Lacey, C.S.; Aaron, M.; Kolasa, M.; Occiano, A.; Shah, S.A. Randomized Controlled Trial of High-Volume Energy Drink Versus Caffeine Consumption on ECG and Hemodynamic Parameters. J. Am. Heart Assoc. 2017, 6, e004448. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Occiano, A.; Nguyen, T.A.; Chan, A.; Sky, J.C.; Bhattacharyya, M.; O’Dell, K.M.; Shek, A.; Nguyen, N.N. Electrocardiographic and blood pressure effects of energy drinks and Panax ginseng in healthy volunteers: A randomized clinical trial. Int. J. Cardiol. 2016, 218, 318–323. [Google Scholar] [CrossRef]
- Kozik, T.M.; Shah, S.; Bhattacharyya, M.; Franklin, T.T.; Connolly, T.F.; Chien, W.; Charos, G.S.; Pelter, M.M. Cardiovascular responses to energy drinks in a healthy population: The C-energy study. Am. J. Emerg. Med. 2016, 34, 1205–1209. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar]
- Guan, L.; Miao, P. The effects of taurine supplementation on obesity, blood pressure and lipid profile: A meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2020, 885, 173533. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef]
- van Koert, R.R.; Bauer, P.R.; Schuitema, I.; Sander, J.W.; Visser, G.H. Caffeine and seizures: A systematic review and quantitative analysis. Epilepsy Behav. 2018, 80, 37–47. [Google Scholar] [CrossRef]
- Lipton, R.B.; Diener, H.-C.; Robbins, M.S.; Garas, S.Y.; Patel, K. Caffeine in the management of patients with headache. J. Headache Pain 2017, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.A.; Hoirisch-Clapauch, S.; Nardi, A.E.; Freire, R.C. Panic Disorder and Chronic Caffeine Use: A Case-control Study. Clin. Pract. Epidemiol. Ment. Health 2019, 15, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Alsunni, A.A. Energy Drink Consumption: Beneficial and Adverse Health Effects. Int. J. Health Sci. 2015, 9, 468–474. [Google Scholar] [CrossRef]
- Babu, K.M.; Church, R.J.; Lewander, W. Energy Drinks: The New Eye-Opener for Adolescents. Clin. Pediatr. Emerg. Med. 2008, 9, 35–42. [Google Scholar] [CrossRef]
- Guze, S.B. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). Am. J. Psychiatry 1995, 152, 1228. [Google Scholar]
- Moussa, M.; Hansz, K.; Rasmussen, M.; Gillman, C.; Pollard, C.; Kwak, E.; Izsak, E. Cardiovascular Effects of Energy Drinks in the Pediatric Population. Pediatr. Emerg. Care 2021, 37, 578–582. [Google Scholar] [CrossRef]
- Nordt, S.P.; Vilke, G.M.; Clark, R.F.; Cantrell, F.L.; Chan, T.C.; Galinato, M.; Nguyen, V.; Castillo, E.M. Energy Drink Use and Adverse Effects Among Emergency Department Patients. J. Community Health 2012, 37, 976–981. [Google Scholar] [CrossRef]
- Schwartz, D.L.; Gilstad-Hayden, K.; Carroll-Scott, A.; Grilo, S.A.; McCaslin, C.; Schwartz, M.; Ickovics, J.R. Energy drinks and youth self-reported hyperactivity/inattention symptoms. Acad. Pediatr. 2015, 15, 297–304. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Van Batenburg-Eddes, T.; Lee, N.C.; Weeda, W.D.; Krabbendam, L.; Huizinga, M. The potential adverse effect of energy drinks on executive functions in early adolescence. Front. Psychol. 2014, 5, 457. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014, 12, 3759. [Google Scholar] [CrossRef]
- Schneider, M.B.; Benjamin, H.J.; Committee on Nutrition and the Council on Sports Medicine and Fitness. Sports Drinks and Energy Drinks for Children and Adolescents: Are They Appropriate? Pediatrics 2011, 127, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Greene, E.; Oman, K.; Lefler, M. Energy drink-induced acute kidney injury. Ann. Pharmacother. 2014, 48, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Deighan, C.J.; Wong, K.M.; McLaughlin, K.J.; Harden, P. Rhabdomyolysis and acute renal failure resulting from alcohol and drug abuse. QJM 2000, 93, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Emerging risk factors and markers of chronic kidney disease progression. Nat. Rev. Nephrol. 2009, 5, 677–689. [Google Scholar] [CrossRef]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef]
- Lithuania Bans Sale of Energy Drinks to Minors. 2015. Available online: https://www.lrt.lt/en/news-in-english/19/43504/lithuania-bans-sale-of-energy-drinks-to-minors (accessed on 2 March 2023).
- Broadcasting, L.P. Minors-People Less than 18 Years Old-Will No Longer Be Able to Buy Energy Drinks as the Saeima on Thursday Adopted Amendments to Energy Drink Circulation Law, Reported LSM’s Latvian-Language Service. 2016. Available online: https://eng.lsm.lv/article/society/society/latvia-bans-energy-drink-sales-to-minors.a165212/ (accessed on 2 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).