Iron Complexes with Antarctic Krill–Derived Peptides Show Superior Effectiveness to Their Original Protein–Iron Complexes in Mice with Iron Deficiency Anemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Antarctic Krill Proteins and Preparation of Antarctic Krill Peptides
2.2. Preparation of Iron Complexes with Antarctic Krill Proteins or Peptides
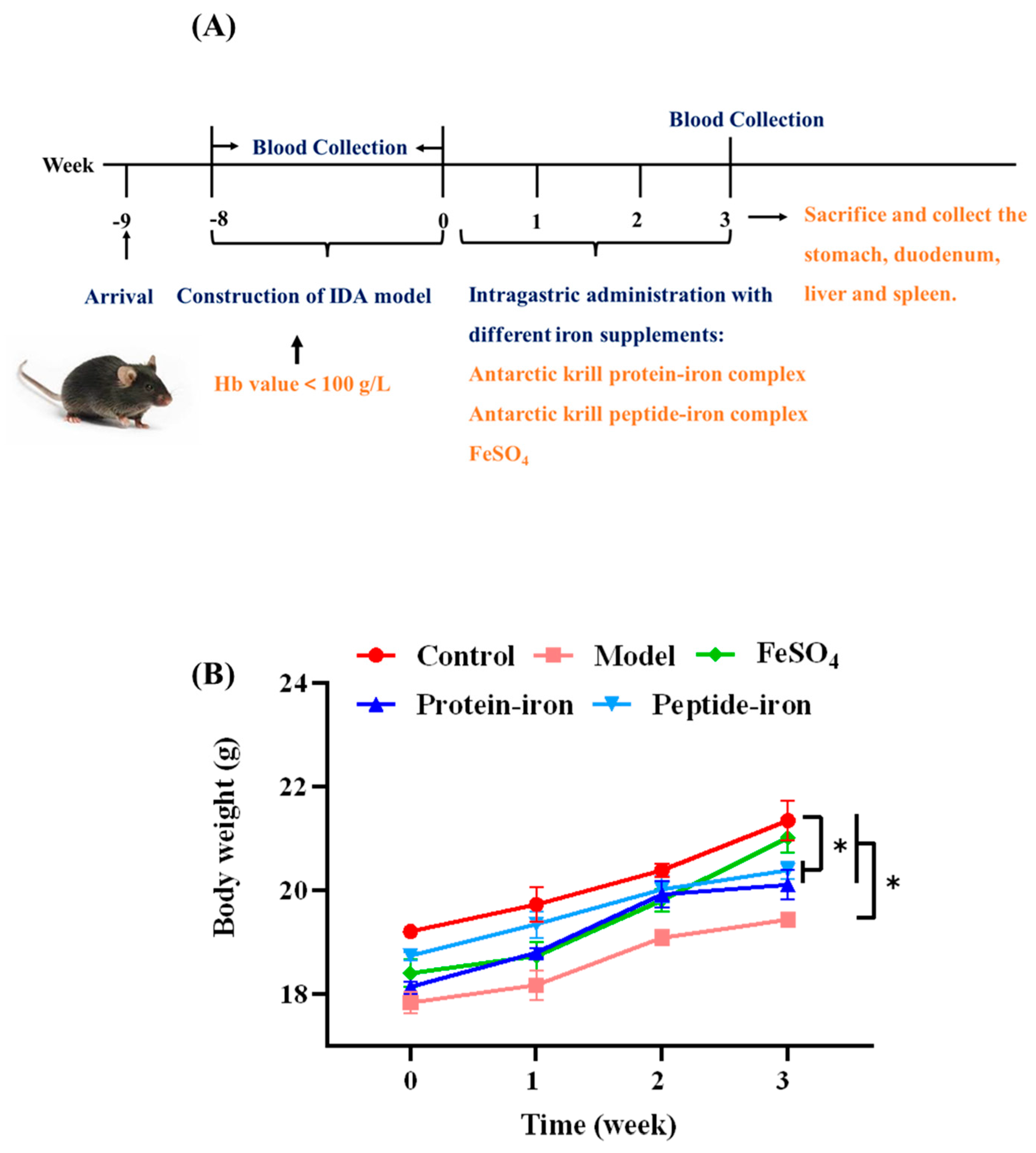
2.3. Animal Care and Treatment
2.4. Hematological Test
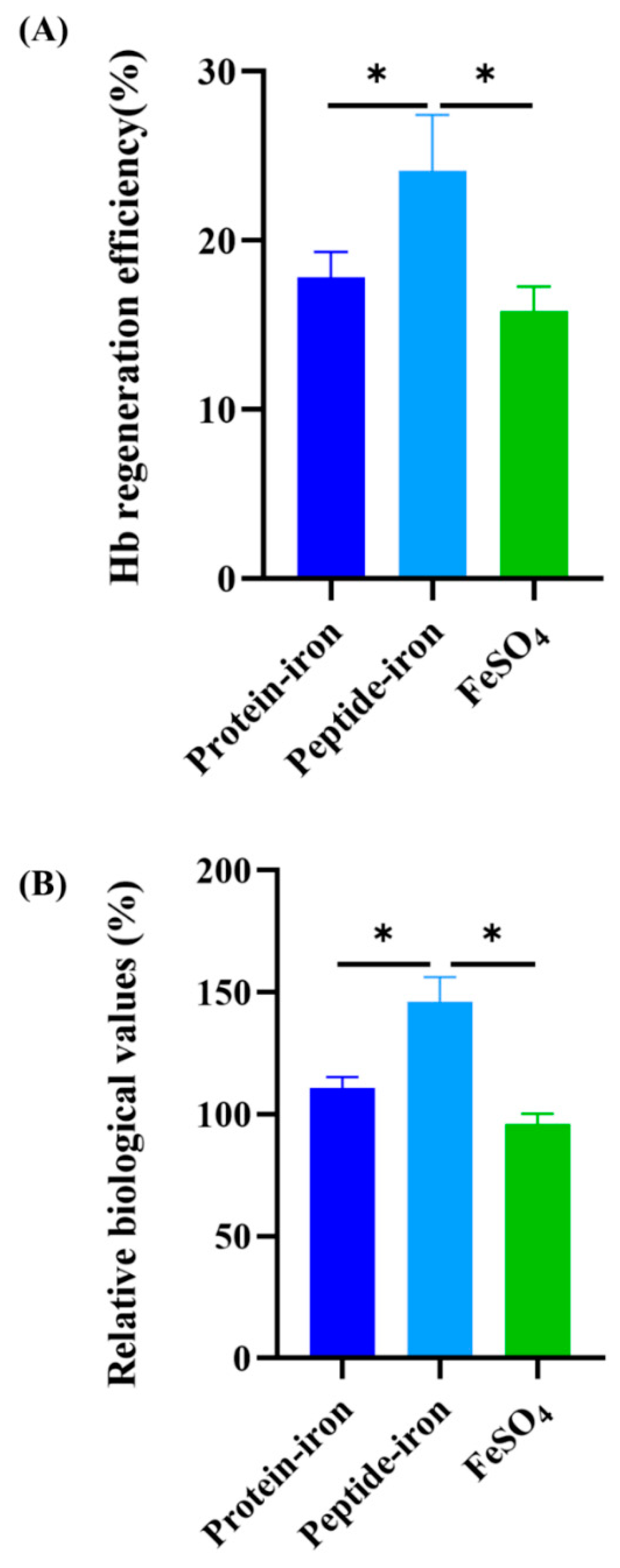
2.5. Relative Biological Value and Hb Regeneration Efficiency
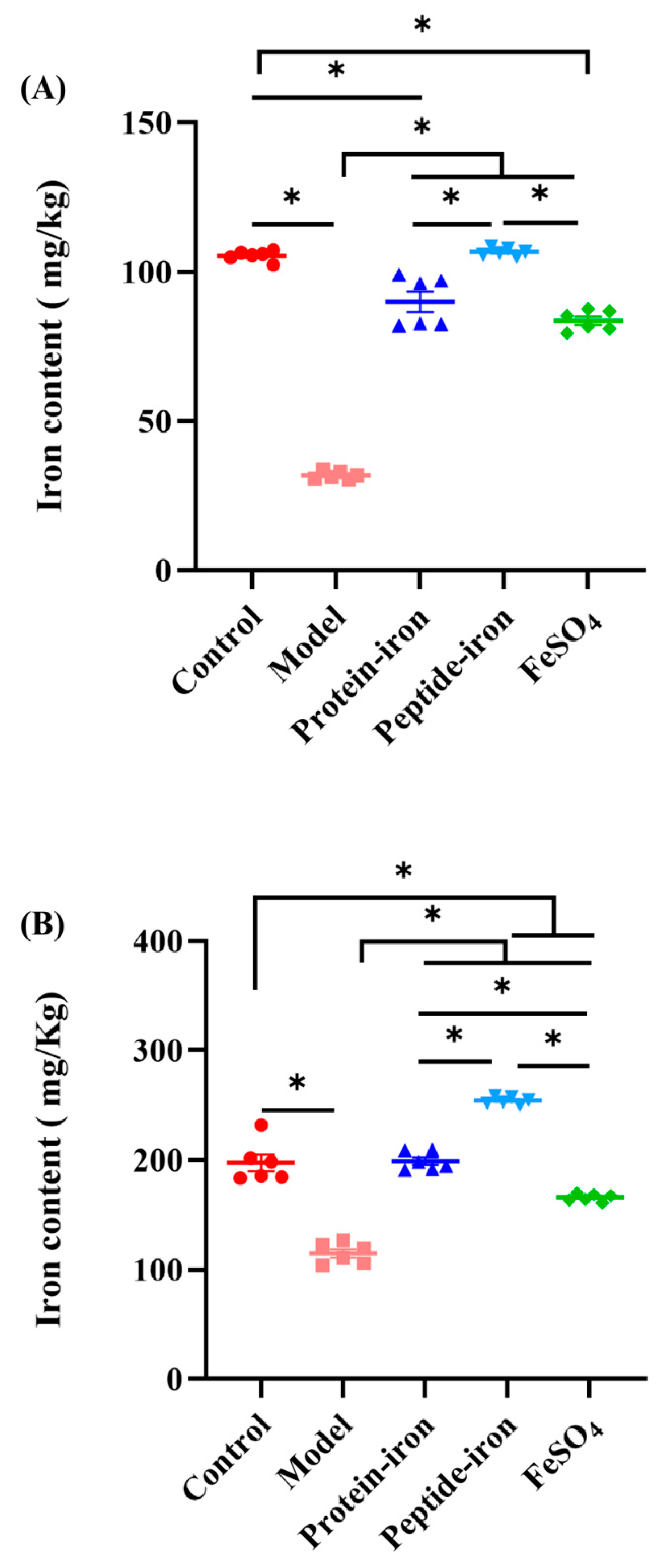
2.6. Analysis of Iron Content in Liver and Spleen
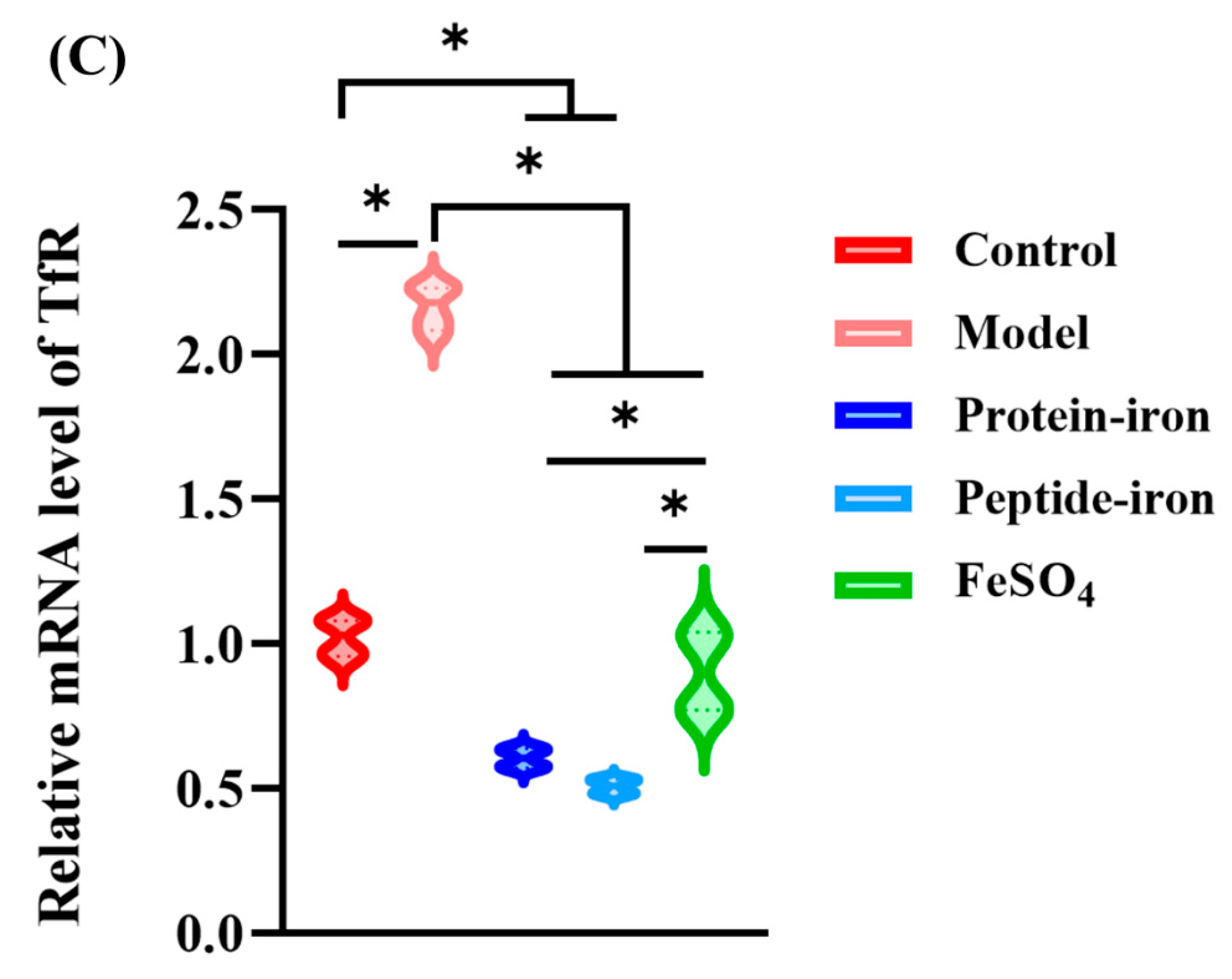
2.7. Real-Time (RT) PCR Analysis
2.8. The Detection of the SOD, GSH-PX, and MDA Levels in the Gastric Tissue
2.9. Histopathological Observation
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Growth of IDA Mice during Iron Supplementation
3.2. Hematological Parameter Analysis
3.3. Iron Content in the Liver and Spleen of Mice
3.4. Hemoglobin Regeneration Efficiency and Relative Biological Value
3.5. Expression of Iron-Regulated Genes
3.6. In Vivo Antioxidant Activities
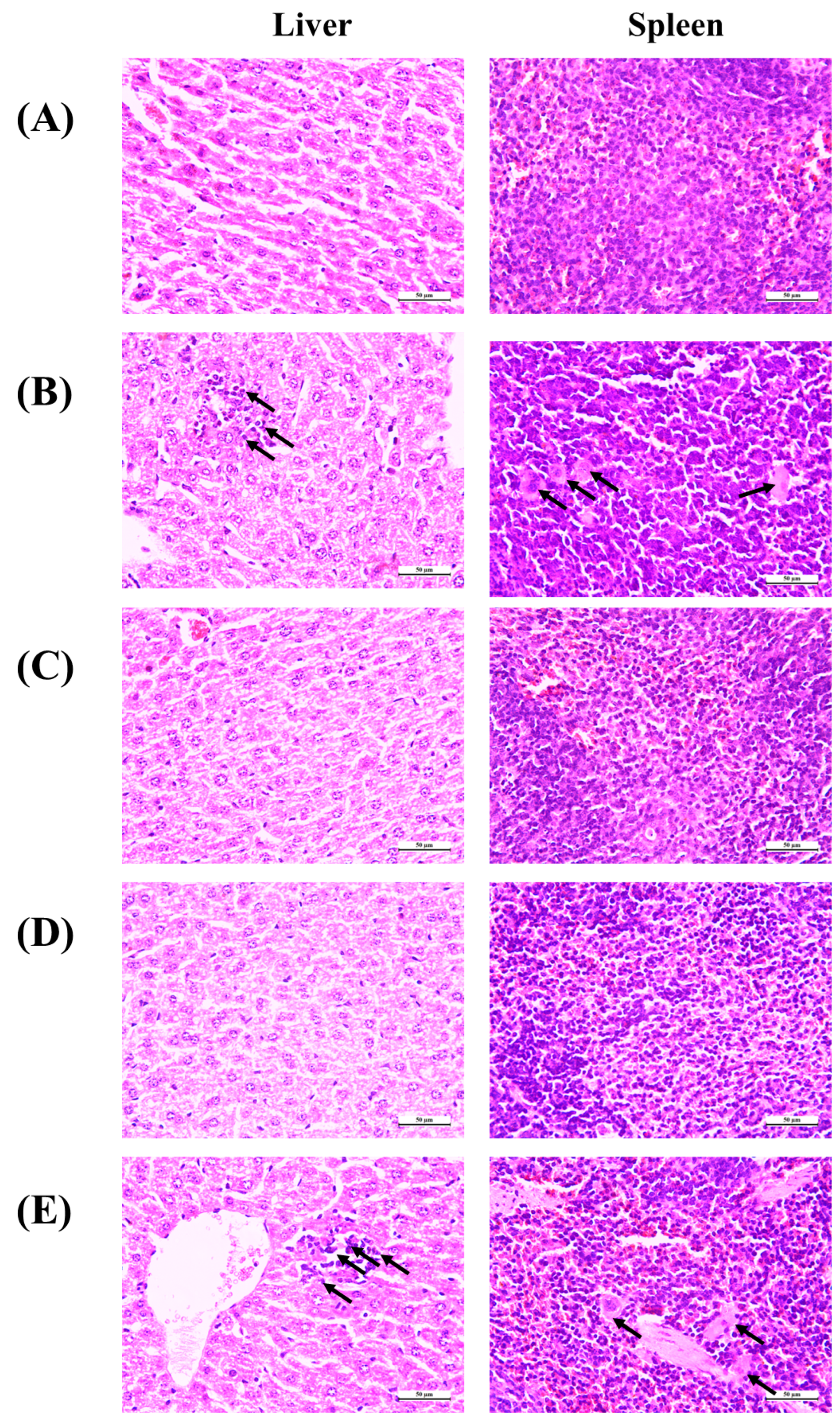
3.7. Histopathological Observation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.M. Evaluation and treatment of iron deficiency in adults. Nutr. Clin. Care 2002, 5, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Corrons, J.L.V.; Miguel-Garcia, A.; Pujades, M.A.; Miguel-Sosa, A.; Cambiazzo, S.; Linares, M.; Dibarrart, M.T.; Calvo, M.A. Increased susceptibility of microcytic red blood cells to in vitro oxidative stress. Eur. J. Haematol. 1995, 55, 327–331. [Google Scholar] [CrossRef]
- Sharp, P.; Srai, S.K. Molecular mechanisms involved in intestinal iron absorption. World J. Gastroenterol. WJG 2007, 13, 4716. [Google Scholar] [CrossRef]
- Garrick, M.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. Biometals 2003, 16, 41–54. [Google Scholar] [CrossRef]
- Conrad, M.E.; Umbreit, J.N.; Moore, E.G. Iron absorption and transport. Am. J. Med. Sci. 1999, 318, 213–229. [Google Scholar] [CrossRef]
- Scotland, P.B.; Heath, J.L.; Conway, A.E.; Porter, N.B.; Armstrong, M.B.; Walker, J.A.; Klebig, M.L.; Lavau, C.P.; Wechsler, D.S. The PICALM protein plays a key role in iron homeostasis and cell proliferation. PLoS ONE 2012, 7, e44252. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Jahan, T.A.; Vandenberg, A.; Glahn, R.P.; Tyler, R.T.; Reaney, M.J.; Tar’an, B. Iron Fortification and Bioavailability of Chickpea (Cicer arietinum L.) Seeds and Flour. Nutrients 2019, 11, 2240. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Chen, L.-Q.; Zhuang, H. Effects of heme iron enriched peptide on iron deficiency anemia in rats. Food Funct. 2014, 5, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-R.; Xie, Z.-G.; Ye, X.-Q.; Deng, S.-G.; Hu, Y.-Q.; Guo, X.; Chen, S.-G. Effectiveness of treatment of iron deficiency anemia in rats with squid ink melanin–Fe. Food Funct. 2014, 5, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [PubMed]
- Bries, A.; Wang, C.; Wels, B.; Agbemafle, I.; Meier, O.; Hurrell, R.; Reddy, M. Assessment of Gastrointestinal Symptoms and Non-transferrin Bound Iron after Oral Ferrous Sulfate and Iron-enriched Aspergillus Oryzae Supplementation in Women (P24-039-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1), nzz044.P24-039-19. [Google Scholar] [CrossRef]
- Huda, T.; Dibley, M.; El Arifeen, S.; Islam, S.; Ali, N.; Raihana, S.; Rahman, M.; Qadri, F.; Raqib, R.; Mordi, W.T.; et al. Assessing the Efficacy of Bovine Lactoferrin to Correct Iron Deficiency Anemia in Non-pregnant Non-lactating Women: A Randomized Controlled Trial (FS08-02-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1), nzz044.FS08-02-19. [Google Scholar] [CrossRef]
- Strbak, O.; Balejcikova, L.; Kmetova, M.; Gombos, J.; Trancikova, A.; Pokusa, M.; Kopcansky, P. Quantification of Iron Release from Native Ferritin and Magnetoferritin Induced by Vitamins B(2) and C. Int. J. Mol. Sci. 2020, 21, 6332. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef]
- Evcan, E.; Gulec, S. The development of lentil derived protein–iron complexes and their effects on iron deficiency anemia in vitro. Food Funct. 2020, 11, 4185–4192. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Wakasugi, E.; Yasui, R.; Kuwahata, M.; Kido, Y. Egg Yolk Protein Delays Recovery while Ovalbumin Is Useful in Recovery from Iron Deficiency Anemia. Nutrients 2015, 7, 4792–4803. [Google Scholar] [CrossRef]
- Li, B.; He, H.; Shi, W.; Hou, T. Effect of duck egg white peptide-ferrous chelate on iron bioavailability in vivo and structure characterization. J. Sci. Food Agric. 2019, 99, 1834–1841. [Google Scholar] [CrossRef]
- Eckert, E.; Lu, L.; Unsworth, L.D.; Chen, L.; Xie, J.; Xu, R. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins. J. Funct. Foods 2016, 25, 291–301. [Google Scholar] [CrossRef]
- Wang, T.; Lin, S.; Cui, P.; Bao, Z.; Liu, K.; Jiang, P.; Zhu, B.; Sun, N. Antarctic krill derived peptide as a nanocarrier of iron through the gastrointestinal tract. Food Biosci. 2020, 36, 100657. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Z.; Tan, L.; Zhu, Y.; Zhao, C.; Zeng, Q.-H.; Liu, G.; Wang, J.J.; Zhao, Y. Structural and functional properties of soluble Antarctic krill proteins covalently modified by rutin. Food Chem. 2022, 379, 132159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, M.; Zeng, H.; Zhao, H.; Zhang, M.; Yang, Z. Preparation, Characterization and Iron Absorption by Caco-2 Cells of the Casein Peptides-Iron Chelate. Int. J. Pept. Res. Ther. 2022, 28, 116. [Google Scholar] [CrossRef]
- Gao, C.; Wang, F.; Yuan, L.; Liu, J.; Sun, D.; Li, X. Physicochemical property, antioxidant activity, and cytoprotective effect of the germinated soybean proteins. Food Sci. Nutr. 2019, 7, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wienk, K.J.H.; Marx, J.J.M.; Beynen, A.C. The concept of iron bioavailability and its assessment. Eur. J. Nutr. 1999, 38, 51–75. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, Q.; Liu, C.; Jia, S.; Wang, Y.; An, F.; Song, H. Effectiveness of AOS–iron on iron deficiency anemia in rats. RSC Adv. 2019, 9, 5053–5063. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zhang, Y.; You, R.-X.; Zeng, F.; Guo, D.; Wang, K.-P. Polysaccharide isolated from Angelica sinensis inhibits hepcidin expression in rats with iron deficiency anemia. J. Med. Food 2012, 15, 923–929. [Google Scholar] [CrossRef]
- Zielińska-Dawidziak, M.; Hertig, I.; Piasecka-Kwiatkowska, D.; Staniek, H.; Nowak, K.W.; Twardowski, T. Study on iron availability from prepared soybean sprouts using an iron-deficient rat model. Food Chem. 2012, 135, 2622–2627. [Google Scholar] [CrossRef]
- Linberg, R.; Conover, C.D.; Shum, K.L. Hemoglobin based oxygen carriers: How much methemoglobin is too much? Artif. Cells Blood Substit. Biotechnol. 1998, 26, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Ikeda, H.; Shigefuku, R.; Hattori, N.; Watanabe, T.; Matsunaga, K.; Hiraishi, T.; Tamura, T.; Noguchi, Y.; Fukuda, Y.; et al. Hemoglobin decrease with iron deficiency induced by daclatasvir plus asunaprevir combination therapy for chronic hepatitis C Virus genotype 1b. PLoS ONE 2016, 11, e0151238. [Google Scholar] [CrossRef] [PubMed]
- Coban, E.; Ozdogan, M.; Timuragaoglu, A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004, 112, 126–128. [Google Scholar] [CrossRef]
- Yamanishi, H.; Iyama, S.; Yamaguchi, Y.; Kanakura, Y.; Iwatani, Y. Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity. Clin. Chem. 2003, 49, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Fang, S.; Hu, Q.; Huang, D.; Feng, J. Digital gene expression profiling analysis of duodenum transcriptomes in SD rats administered ferrous sulfate or ferrous glycine chelate by gavage. Sci. Rep. 2016, 6, 37923. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Martínez-Maqueda, D.; Cruz-Huerta, E.; Miralles, B.; Recio, I. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides. Food Res. Int. 2014, 63, 170–181. [Google Scholar] [CrossRef]
- Hu, S.; Lin, S.; Liu, Y.; He, X.; Zhang, S.; Sun, N. Exploration of iron-binding mode, digestion Kinetics, and iron absorption behavior of Antarctic Krill-derived heptapeptide-iron complex. Food Res. Int. 2022, 154, 110996. [Google Scholar] [CrossRef]
- Storcksdieck, S.; Bonsmann, G.; Hurrell, R. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J. Food Sci. 2007, 72, S019–S029. [Google Scholar] [CrossRef]
- Giorgi, G.; Roque, M.E. Immunohistochemical studies on duodenum, spleen and liver in mice: Distribution of ferroportin and prohepcidin in an inflammation model. Int. J. Morphol. 2011, 29, 747–753. [Google Scholar]
- Huh, M.; Shin, M.; Lee, Y.; Sohn, H. Effect of soybean hull iron on growth, iron bioavailability, and behavioral function in anemic rats induced by iron deficiency during gestation or lactation. Nutr. Res. 1999, 19, 1749–1761. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Wei, G.-X.; Wang, W.-N.; Ma, G.-D.; Tang, P.; Chen, X.-Q. Effects of Fe-YM1504 on iron deficiency anemia in rats. Food Funct. 2016, 7, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, C.; Song, W.; Che, S.; Wang, C.; Feng, X.; Li, B.; Dai, Y. Evaluating the efficacy of a ferrous-ion-chelating peptide from Alaska pollock frame for the improvement of iron nutritional status in rats. Food Funct. 2019, 10, 4888–4896. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Nagashima, Y.; Tabata, T.; Oda, H. Immunochemical analysis of iron transporters and M2 macrophages in ovarian endometrioma and clear cell adenocarcinoma. Mol. Clin. Oncol. 2021, 15, 159. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, G.; Zheng, J.; Chen, M.; Maimaitiming, Z.; Chen, M.; Liu, S.; Jiang, R.; Fuqua, B.K.; Dunaief, J.L.; et al. Hephaestin and ceruloplasmin facilitate iron metabolism in the mouse kidney. Sci. Rep. 2016, 6, 39470. [Google Scholar] [CrossRef] [PubMed]
- Aigner, E.; Weiss, G.; Datz, C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver. World. J. Hepatol. 2015, 7, 177–188. [Google Scholar] [CrossRef]
- Kleven, M.D.; Jue, S.; Enns, C.A. Transferrin Receptors TfR1 and TfR2 Bind Transferrin through Differing Mechanisms. Biochemistry 2018, 57, 1552–1559. [Google Scholar] [CrossRef]
- Sanyear, C.; Butthep, P.; Eamsaard, W.; Fucharoen, S.; Svasti, S.; Masaratana, P. Iron homeostasis in a mouse model of thalassemia intermedia is altered between adolescence and adulthood. PeerJ 2020, 8, e8802. [Google Scholar] [CrossRef]
- Mukherjee, S.; Banerjee, S.K.; Maulik, M.; Dinda, A.K.; Talwar, K.K.; Maulik, S.K. Protection against acute adriamycin-induced cardiotoxicity by garlic: Role of endogenous antioxidants and inhibition of TNF-α expression. BMC Pharmacol. 2003, 3, 16. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.-T.; Chen, X.-N.; Zhu, A.-X.; Wu, B.-H. Remote ischemic postconditioning protects against gastric mucosal lesions in rats. World J. Gastroenterol. 2014, 20, 9519–9527. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-H.; Maeng, H.-Y.; Sun, Y.-K.; Kim, Y.-A.; Park, D.-W.; Park, T.S.; Lee, S.T.; Choi, J.-R. Oxidative status in iron-deficiency anemia. J. Clin. Lab. Anal. 2009, 23, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Wang, Q.Y.; Luan, H.Y.; Kang, Z.C.; Wang, C.B. Effects of L-carnitine against oxidative stress in human hepatocytes: Involvement of peroxisome proliferator-activated receptor alpha. J. Biomed. Sci. 2012, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Ahmad, S.I. Correction of iron deficiency anemia in pregnancy and its effects on Superoxide dismutase. Pak. J. Pharm. Sci. 2012, 25, 423–427. [Google Scholar]
- Ding, Y.; Ko, S.-C.; Moon, S.-H.; Lee, S.-H. Protective effects of novel antioxidant peptide purified from alcalase hydrolysate of velvet antler against oxidative stress in chang liver cells in vitro and in a zebrafish model in vivo. Int. J. Mol. Sci. 2019, 20, 5187. [Google Scholar] [CrossRef]
- Rahimpour, A.; Heidarzadehpilehrood, R.; Aghel, M.; Jamalpoor, Z.; Heydarian, P.; Ghasemi, S.A.; Pirhoushiaran, M. Bioinformatics Analysis of MicroRNA Profiles Unveils Novel Biological Markers of Alzheimer’s Disease. Neurochem. J. 2022, 16, 334–342. [Google Scholar] [CrossRef]
- Gordon, M. The mechanism of antioxidant action in vitro. In Food Antioxidants; Springer: Dordrecht, The Netherlands, 1990; pp. 1–18. [Google Scholar]
- Ghoneum, M.; Abdulmalek, S.; Pan, D. Reversal of age-associated oxidative stress in mice by PFT, a novel kefir product. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420950149. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Cao, G.; Angerosa, M. Ferrous sulfate, but not iron polymaltose complex, aggravates local and systemic inflammation and oxidative stress in dextran sodium sulfate-induced colitis in rats. Drug Des. Dev. Ther. 2015, 9, 2585. [Google Scholar] [CrossRef] [PubMed]





| Gens | Primers | Sequences | Products (bp) |
|---|---|---|---|
| Tf | Forward | GCAGTGTCAGAGCACGAGAATAC | 156 |
| Reverse | GGTCATAGCATCGGCTTCACTT | ||
| TfR | Forward | CGTGGAGACTACTTCCGTGCTAC | 139 |
| Reverse | GAGATACATAGGGCGACAGGAAG | ||
| DMT1 | Forward | CTGCCTACAGCAACTCATCCCT | 136 |
| Reverse | GTGAACGCCCAGAGTTTACGA | ||
| GAPDH | Forward | CCTCGTCCCGTAGACAAAATG | 133 |
| Reverse | TGAGGTCAATGAAGGGGTCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Lin, S.; Feng, Q.; He, X.; Xu, H.; Chen, L.; Sun, N. Iron Complexes with Antarctic Krill–Derived Peptides Show Superior Effectiveness to Their Original Protein–Iron Complexes in Mice with Iron Deficiency Anemia. Nutrients 2023, 15, 2510. https://doi.org/10.3390/nu15112510
Hu S, Lin S, Feng Q, He X, Xu H, Chen L, Sun N. Iron Complexes with Antarctic Krill–Derived Peptides Show Superior Effectiveness to Their Original Protein–Iron Complexes in Mice with Iron Deficiency Anemia. Nutrients. 2023; 15(11):2510. https://doi.org/10.3390/nu15112510
Chicago/Turabian StyleHu, Shengjie, Songyi Lin, Qi Feng, Xueqing He, Haowei Xu, Lei Chen, and Na Sun. 2023. "Iron Complexes with Antarctic Krill–Derived Peptides Show Superior Effectiveness to Their Original Protein–Iron Complexes in Mice with Iron Deficiency Anemia" Nutrients 15, no. 11: 2510. https://doi.org/10.3390/nu15112510
APA StyleHu, S., Lin, S., Feng, Q., He, X., Xu, H., Chen, L., & Sun, N. (2023). Iron Complexes with Antarctic Krill–Derived Peptides Show Superior Effectiveness to Their Original Protein–Iron Complexes in Mice with Iron Deficiency Anemia. Nutrients, 15(11), 2510. https://doi.org/10.3390/nu15112510








