Inverse Association between Oxidative Balance Score and Incident Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Oxidative Balance Score
2.3. Assessment of T2DM
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
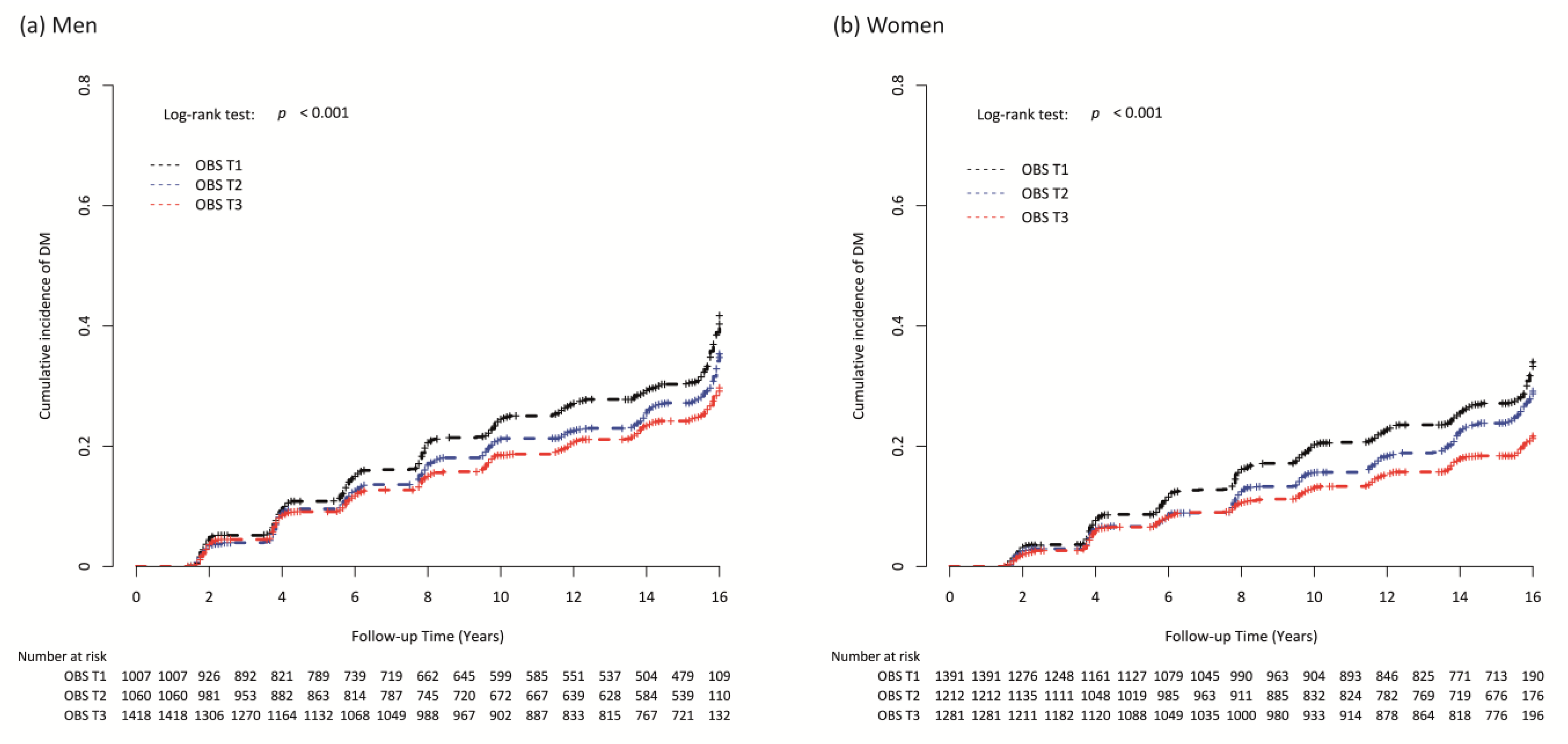
3.2. Longitudinal Association of OBS and Incident T2DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res. Clin. Prac. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.C. Trends of diabetes epidemic in korea. Diabetes Metab. J. 2018, 42, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Ku, H.; Park, K.S. Prevalence and socioeconomic burden of diabetes mellitus in south korean adults: A population-based study using administrative data. BMC Public Health 2021, 21, 548. [Google Scholar] [CrossRef]
- Cousin, E.; Schmidt, M.I.; Ong, K.L.; Lozano, R.; Afshin, A.; Abushouk, A.I.; Agarwal, G.; Agudelo-Botero, M.; Al-Aly, Z.; Alcalde-Rabanal, J.E.; et al. Burden of diabetes and hyperglycaemia in adults in the americas, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Diabetes Endocrinol. 2022, 10, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Khan, T.A.; Field, D.; Chen, V.; Ahmad, S.; Mejia, S.B.; Kahleová, H.; Rahelić, D.; Salas-Salvadó, J.; Leiter, L.A.; Uusitupa, M.; et al. Combination of multiple low-risk lifestyle behaviors and incident type 2 diabetes: A systematic review and dose-response meta-analysis of prospective cohort studies. Diabetes Care 2023, 46, 643–656. [Google Scholar] [CrossRef]
- Goodman, M.; Bostick, R.M.; Dash, C.; Flanders, W.D.; Mandel, J.S. Hypothesis: Oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol. 2007, 17, 394–399. [Google Scholar] [CrossRef]
- Hernández-Ruiz, Á.; García-Villanova, B.; Guerra-Hernández, E.; Amiano, P.; Ruiz-Canela, M.; Molina-Montes, E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients 2019, 11, 774. [Google Scholar] [CrossRef]
- Lakkur, S.; Goodman, M.; Bostick, R.M.; Citronberg, J.; McClellan, W.; Flanders, W.D.; Judd, S.; Stevens, V.L. Oxidative balance score and risk for incident prostate cancer in a prospective u.S. Cohort study. Ann. Epidemiol. 2014, 24, 475–478.e474. [Google Scholar] [CrossRef]
- Kong, S.Y.; Goodman, M.; Judd, S.; Bostick, R.M.; Flanders, W.D.; McClellan, W. Oxidative balance score as predictor of all-cause, cancer, and noncancer mortality in a biracial us cohort. Ann. Epidemiol. 2015, 25, 256–262.e251. [Google Scholar] [CrossRef]
- Cho, A.R.; Kwon, Y.J.; Lim, H.J.; Lee, H.S.; Kim, S.; Shim, J.Y.; Lee, H.R.; Lee, Y.J. Oxidative balance score and serum γ-glutamyltransferase level among korean adults: A nationwide population-based study. Eur. J. Nutr. 2018, 57, 1237–1244. [Google Scholar] [CrossRef]
- Haggag Mel, S.; Elsanhoty, R.M.; Ramadan, M.F. Impact of dietary oils and fats on lipid peroxidation in liver and blood of albino rats. Asian Pac. J. Trop Biomed 2014, 4, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Pitaraki, E.E. The role of mediterranean diet and its components on the progress of osteoarthritis. J. Frailty Sarcopenia Falls 2017, 2, 45–52. [Google Scholar] [CrossRef]
- Romeu, M.; Aranda, N.; Giralt, M.; Ribot, B.; Nogues, M.R.; Arija, V. Diet, iron biomarkers and oxidative stress in a representative sample of mediterranean population. Nutr. J. 2013, 12, 102. [Google Scholar] [CrossRef]
- Valenzuela, R.; Rincón-Cervera, M.; Echeverría, F.; Barrera, C.; Espinosa, A.; Hernández-Rodas, M.C.; Ortiz, M.; Valenzuela, A.; Videla, L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2018, 45, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Annor, F.B.; Goodman, M.; Okosun, I.S.; Wilmot, D.W.; Il’yasova, D.; Ndirangu, M.; Lakkur, S. Oxidative stress, oxidative balance score, and hypertension among a racially diverse population. J. Am. Soc. Hypertens 2015, 9, 592–599. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort profile: The korean genome and epidemiology study (koges) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 korean society for the study of obesity guideline for the management of obesity in korea. J. Obes. Metab. Syndr. 2019, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and reliability of korean version of international physical activity questionnaire (ipaq) short form. J. Korean Acad. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, M.; Ayremlou, P.; Zarrin, R. Higher oxidative balance score is associated with better glycemic control among iranian adults with type-2 diabetes. Int. J. Vitam. Nutr. Res. 2021, 91, 31–39. [Google Scholar] [CrossRef]
- Son, D.H.; Lee, H.S.; Seol, S.Y.; Lee, Y.J.; Lee, J.H. Association between the oxidative balance score and incident chronic kidney disease in adults. Antioxidants 2023, 12, 335. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, D.H.; Kwon, Y.J. Association between oxidative balance score and new-onset hypertension in adults: A community-based prospective cohort study. Front. Nutr. 2022, 9, 1066159. [Google Scholar] [CrossRef]
- Noruzi, Z.; Jayedi, A.; Farazi, M.; Asgari, E.; Dehghani Firouzabadi, F.; Akbarzadeh, Z.; Djafarian, K.; Shab-Bidar, S. Association of oxidative balance score with the metabolic syndrome in a sample of iranian adults. Oxid. Med. Cell Longev. 2021, 2021, 5593919. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. Dash dietary pattern and cardiometabolic outcomes: An umbrella review of systematic reviews and meta-analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Thomas, M.S.; Calle, M.; Fernandez, M.L. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv. Nutr. 2023, 14, 44–54. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Yeh, H.C.; Duncan, B.B.; Schmidt, M.I.; Wang, N.Y.; Brancati, F.L. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: A cohort study. Ann. Intern. Med. 2010, 152, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the american diabetes association (ada) and the european association for the study of diabetes (easd). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [PubMed]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]

| OBS Components | Assignment Scheme * |
|---|---|
| 1. Saturated fatty acid [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 2. Omega-6 PUFA intake [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 3. Total iron intake [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 4. Smoking status [P] | 2 = never smoker, 1 = former smoker, 0 = current smoker |
| 5. Drinking status [P] | 2 = non-drinker, 1 = mild-to-moderate drinker (<30 g/day in men, <20 g/day in women), 0 = heavy drinker (≥30 g/day in men, ≥20 g/day in women) |
| 6. Overweight/obese [P] | 2 = normal, 1 = overweight, 0 = obese |
| 7. Abdominal obesity [P] | 1 = normal, 0 = abdominal obesity |
| 8. Omega-3 PUFA intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 9. Vitamin C intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 10. Vitamin E intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 11. Selenium intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 12. Total beta-carotene intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 13. Physical activity [A] | 0 = low (<7.5 METs-h/wk), 1 = moderate (7.5–30 METs-h/wk), 2 = high (>30 METs-h/wk) |
| Oxidative Balance Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||
| Variables | T1 (n = 1007) | T2 (n = 1060) | T3 (n = 1418) | p * | T1 (n = 1391) | T2 (n = 1212) | T3 (n = 1281) | p * |
| Age, years | 50.8 ± 8.5 | 51.8 ± 8.7 | 51.1 ± 8.7 | 0.501 | 53.9 ± 9.0 | 52.1 ± 8.9 | 50.0 ± 8.3 | <0.001 |
| MBP, mmHg | 98.2 ± 12.0 | 98.1 ± 12.3 | 96.5 ± 12.7 | 0.001 | 97.4 ± 13.8 | 95.2 ± 13.6 | 91.7 ± 13.0 | <0.001 |
| Glucose, mg/dL | 85.1 ± 9.4 | 84.9 ± 9.1 | 84.1 ± 8.7 | 0.008 | 81.9 ± 8.0 | 81.0 ± 7.6 | 80.5 ± 7.4 | <0.001 |
| Insulin, μU/mL | 6.8 [4.8; 9.5] | 6.5 [4.9; 8.9] | 6.1 [4.7; 8.3] | <0.001 | 7.7 [5.8; 10.2] | 7.3 [5.5; 9.9] | 7.1 [5.3; 9.3] | <0.001 |
| Total cholesterol, mg/dL | 193.8 ± 34.5 | 191.7 ± 35.3 | 188.8 ± 34.1 | <0.001 | 192.6 ± 34.0 | 189.7 ± 35.1 | 185.0 ± 32.0 | <0.001 |
| Triglyceride, mg/dL | 168.0 [124.0; 231.5] | 141.5 [109.0; 200.5] | 129.0 [95.0; 182.0] | <0.001 | 134.0 [100.0; 182.0] | 120.0 [91.5; 163.0] | 111.0 [87.0; 150.0] | <0.001 |
| HDL cholesterol, mg/dL | 42.7 ± 9.6 | 43.7 ± 9.5 | 44.6 ± 10.5 | <0.001 | 44.4 ± 9.7 | 46.4 ± 10.2 | 47.1 ± 9.9 | <0.001 |
| CRP, mg/dL | 0.16 [0.08; 0.27] | 0.15 [0.07; 0.26] | 0.13 [0.06; 0.22] | <0.001 | 0.14 [0.08; 0.24] | 0.14 [0.06; 0.23] | 0.11 [0.04; 0.20] | <0.001 |
| Education level, n (%) | 0.595 | <0.001 | ||||||
| Elementary/middle school | 427 (42.4%) | 451 (42.7%) | 569 (40.3%) | 1052 (76.3%) | 797 (66.3%) | 724 (56.7%) | ||
| High school | 366 (36.4%) | 378 (35.8%) | 514 (36.4%) | 274 (19.9%) | 317 (26.4%) | 436 (34.2%) | ||
| College/university | 213 (21.2%) | 226 (21.4%) | 330 (23.4%) | 53 (3.8%) | 88 (7.3%) | 116 (9.1%) | ||
| Household income, n (%) | 0.005 | <0.001 | ||||||
| <100 million South Korean Won | 259 (25.9%) | 272 (25.8%) | 380 (26.9%) | 658 (48.1%) | 474 (39.6%) | 414 (32.9%) | ||
| 100–200 million South Korean Won | 338 (33.8%) | 286 (27.1%) | 447 (31.7%) | 375 (27.4%) | 361 (30.2%) | 345 (27.4%) | ||
| >200 million South Korean Won | 402 (40.2%) | 496 (47.1%) | 584 (41.4%) | 336 (24.5%) | 362 (30.2%) | 498 (39.6%) | ||
| Energy intake, kcal/day | 1798.5 ± 487.5 | 1979.5 ± 650.1 | 2250.6 ± 748.2 | <0.001 | 1607.3 ± 513.4 | 1876.1 ± 656.9 | 2211.7 ± 858.5 | <0.001 |
| Oxidative Balance Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||
| Variables | T1 (n = 1007) | T2 (n = 1060) | T3 (n = 1418) | p * | T1 (n = 1391) | T2 (n = 1212) | T3 (n = 1281) | p * |
| Saturated fatty acid, g/day | 8.7 ± 4.0 | 10.5 ± 6.2 | 12.9 ± 7.7 | <0.001 | 7.9 ± 4.5 | 10.5 ± 6.3 | 13.9 ± 9.6 | <0.001 |
| Omega-6 PUFA intake, g/day | 7.6 ± 4.0 | 8.6 ± 5.5 | 9.2 ± 5.4 | <0.001 | 7.5 ± 4.1 | 8.6 ± 5.6 | 9.8 ± 6.4 | <0.001 |
| Total iron intake, mg/day | 16.2 ± 6.1 | 19.3 ± 8.8 | 23.5 ± 10.9 | <0.001 | 14.6 ± 6.2 | 18.8 ± 8.5 | 24.5 ± 12.9 | <0.001 |
| Smoking status, n (%) | <0.001 | <0.001 | ||||||
| Current smoker | 647 (64.3%) | 519 (49.0%) | 471 (33.2%) | 86 (6.2%) | 30 (2.5%) | 7 (0.5%) | ||
| Former smoker | 273 (27.1%) | 352 (33.2%) | 442 (31.2%) | 27 (1.9%) | 12 (1.0%) | 4 (0.3%) | ||
| Never smoker | 87 (8.6%) | 189 (17.8%) | 505 (35.6%) | 1278 (91.9%) | 1170 (96.5%) | 1270 (99.1%) | ||
| Drinking status, n (%) | <0.001 | <0.001 | ||||||
| Heavy drinker | 295 (29.3%) | 200 (18.9%) | 167 (11.8%) | 37 (2.7%) | 12 (1.0%) | 8 (0.6%) | ||
| Mild-to-moderate drinker | 565 (56.1%) | 588 (55.5%) | 680 (48.0%) | 433 (31.1%) | 315 (26.0%) | 236 (18.4%) | ||
| Non-drinker | 147 (14.6%) | 272 (25.7%) | 571 (40.3%) | 921 (66.2%) | 885 (73.0%) | 1037 (81.0%) | ||
| Obesity status, n (%) | <0.001 | <0.001 | ||||||
| Obese | 596 (59.2%) | 426 (40.2%) | 322 (22.7%) | 892 (64.1%) | 523 (43.2%) | 267 (20.8%) | ||
| Overweight | 228 (22.6%) | 301 (28.4%) | 411 (29.0%) | 345 (24.8%) | 298 (24.6%) | 384 (30.0%) | ||
| Normal weight | 183 (18.2%) | 333 (31.4%) | 685 (48.3%) | 154 (11.1%) | 391 (32.3%) | 630 (49.2%) | ||
| Abdominal obesity, n (%) | 350 (34.8%) | 226 (21.3%) | 126 (8.9%) | <0.001 | 755 (54.3%) | 374 (30.9%) | 199 (15.5%) | <0.001 |
| Omega-3 PUFA intake, g/day | 1.1 ± 0.6 | 1.3 ± 0.9 | 1.5 ± 0.9 | <0.001 | 1.0 ± 0.6 | 1.3 ± 0.9 | 1.6 ± 1.1 | <0.001 |
| Vitamin C intake, mg/day | 73.8 ± 45.1 | 109.4 ± 85.9 | 160.0 ± 116.9 | <0.001 | 81.5 ± 64.8 | 135.1 ± 116.3 | 205.6 ± 149.7 | <0.001 |
| Vitamin E intake, mg/day | 10.5 ± 4.0 | 13.6 ± 6.6 | 17.8 ± 8.5 | <0.001 | 9.2 ± 4.4 | 13.6 ± 7.1 | 19.2 ± 10.5 | <0.001 |
| Selenium intake, μg/day | 36.9 ± 18.5 | 49.5 ± 31.0 | 66.2 ± 38.4 | <0.001 | 29.6 ± 18.6 | 46.0 ± 28.6 | 67.1 ± 47.8 | <0.001 |
| Beta-carotene intake, μg/day | 2212.2 ± 1530.4 | 3409.7 ± 2930.3 | 4938.1 ± 3940.7 | <0.001 | 1980.8 ± 1415.3 | 3283.4 ± 2557.0 | 5325.7 ± 4545.6 | <0.001 |
| Physical activity, n (%) | <0.001 | <0.001 | ||||||
| Low (<7.5 METs-h/day) | 103 (10.2%) | 51 (4.8%) | 47 (3.3%) | 197 (14.2%) | 97 (8.0%) | 63 (4.9%) | ||
| Moderate (7.5–30 METs-h/day) | 682 (67.7%) | 629 (59.3%) | 774 (54.6%) | 847 (60.9%) | 787 (64.9%) | 770 (60.1%) | ||
| High (>30 METs-h/day) | 222 (22.0%) | 380 (35.8%) | 597 (42.1%) | 347 (24.9%) | 328 (27.1%) | 448 (35.0%) | ||
| Oxidative Balance Score Tertiles | Numbers, n | New-Onset Cases, n | Follow-Up Period, Person-Year | Incidence Rate Per 1000 Person-Years | Unadjusted | Adjusted |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||
| Men | ||||||
| Continuous (per 1 increment) | 0.94 (0.91–0.96) | 0.96 (0.94–0.99) | ||||
| T1 | 1007 | 306 | 11,130.3 | 27.49 | 1 (reference) | 1 (reference) |
| T2 | 1060 | 284 | 12,238.5 | 23.21 | 0.85 (0.72–0.99) | 0.86 (0.73–1.02) |
| T3 | 1418 | 318 | 16,218.5 | 19.61 | 0.72 (0.62–0.85) | 0.83 (0.70–0.99) |
| p for trend | <0.001 | 0.035 | ||||
| Women | ||||||
| Continuous (per 1 increment) | 0.91 (0.89–0.94) | 0.95 (0.92–0.98) | ||||
| T1 | 1391 | 367 | 16,206.6 | 22.65 | 1 (reference) | 1 (reference) |
| T2 | 1212 | 281 | 14,717.9 | 19.09 | 0.84 (0.72–0.98) | 0.94 (0.80–1.11) |
| T3 | 1281 | 232 | 16,025.8 | 14.48 | 0.64 (0.54–0.75) | 0.78 (0.65–0.94) |
| p for trend | <0.001 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-J.; Park, H.-M.; Lee, J.-H. Inverse Association between Oxidative Balance Score and Incident Type 2 Diabetes Mellitus. Nutrients 2023, 15, 2497. https://doi.org/10.3390/nu15112497
Kwon Y-J, Park H-M, Lee J-H. Inverse Association between Oxidative Balance Score and Incident Type 2 Diabetes Mellitus. Nutrients. 2023; 15(11):2497. https://doi.org/10.3390/nu15112497
Chicago/Turabian StyleKwon, Yu-Jin, Hye-Min Park, and Jun-Hyuk Lee. 2023. "Inverse Association between Oxidative Balance Score and Incident Type 2 Diabetes Mellitus" Nutrients 15, no. 11: 2497. https://doi.org/10.3390/nu15112497
APA StyleKwon, Y.-J., Park, H.-M., & Lee, J.-H. (2023). Inverse Association between Oxidative Balance Score and Incident Type 2 Diabetes Mellitus. Nutrients, 15(11), 2497. https://doi.org/10.3390/nu15112497






