Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of SCPs1
2.3. Determination of B16 Cell Viability
2.4. Determination of Melanin Content
2.5. Measurement of TYR Activity and ROS, GSH and cAMP Content
2.6. Western Blot
2.7. Real-Time Fluorescence Quantitative PCR (RT-PCR)
2.8. Data Analysis
3. Results and Discussion
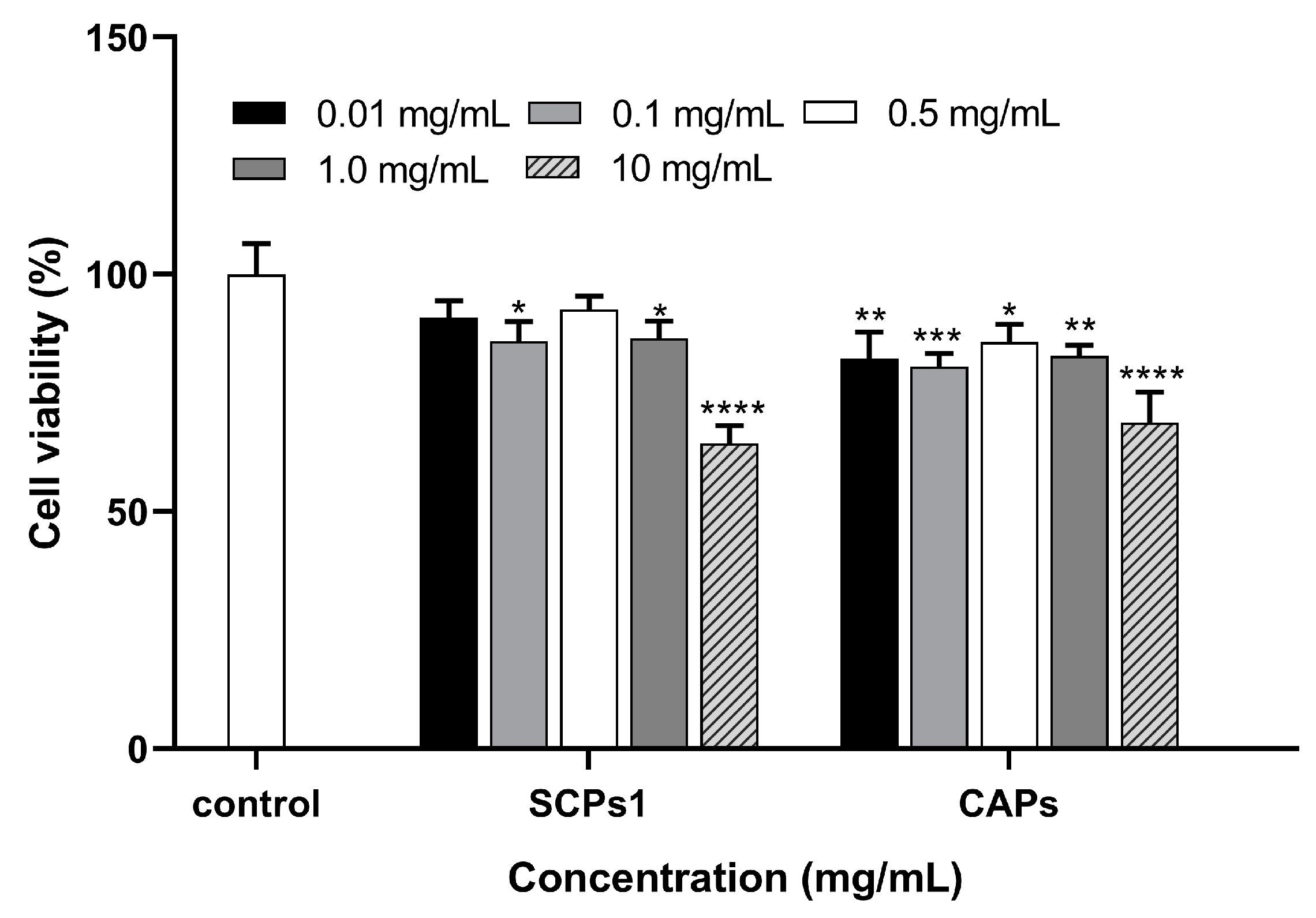
3.1. Effect of SCPs1 on the Viability of B16 Cells
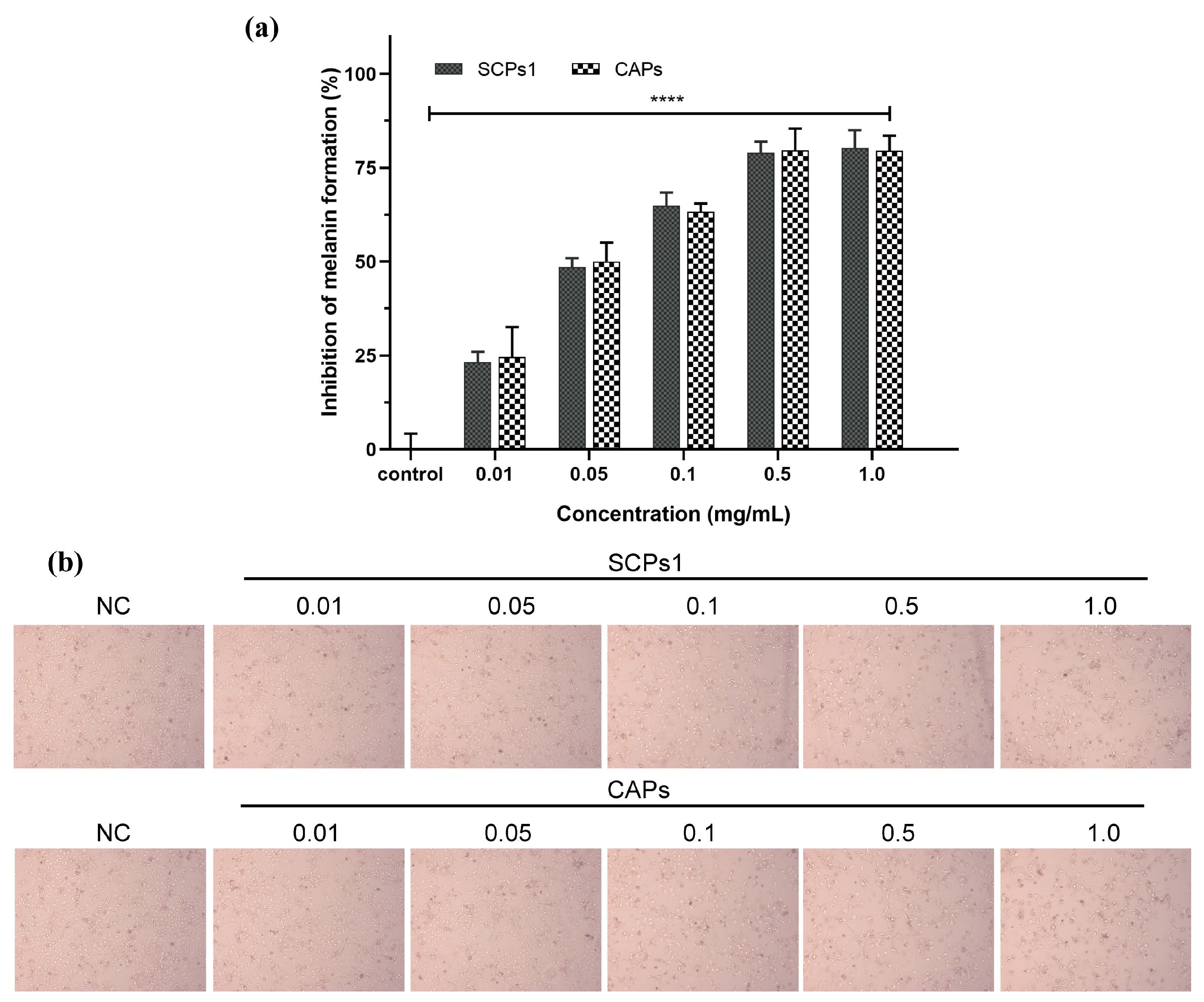
3.2. Inhibitory Effect of SCPs1 on Melanin
3.3. Effect of SCPs1 on TYR Activity and ROS, GSH and cAMP Content
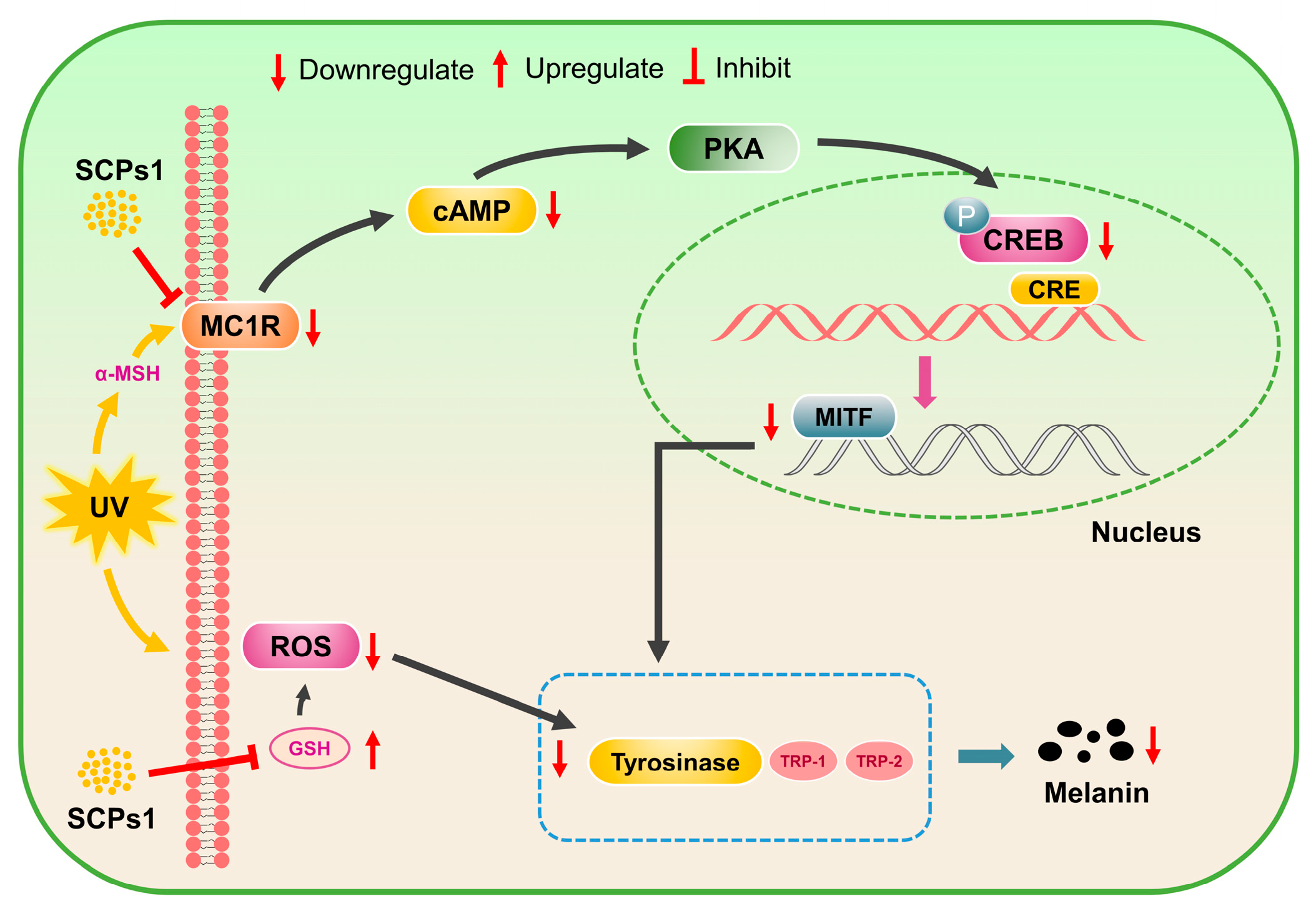
3.4. Downregulation of cAMP-CREB Signaling Pathway Protein Expression by SCPs1
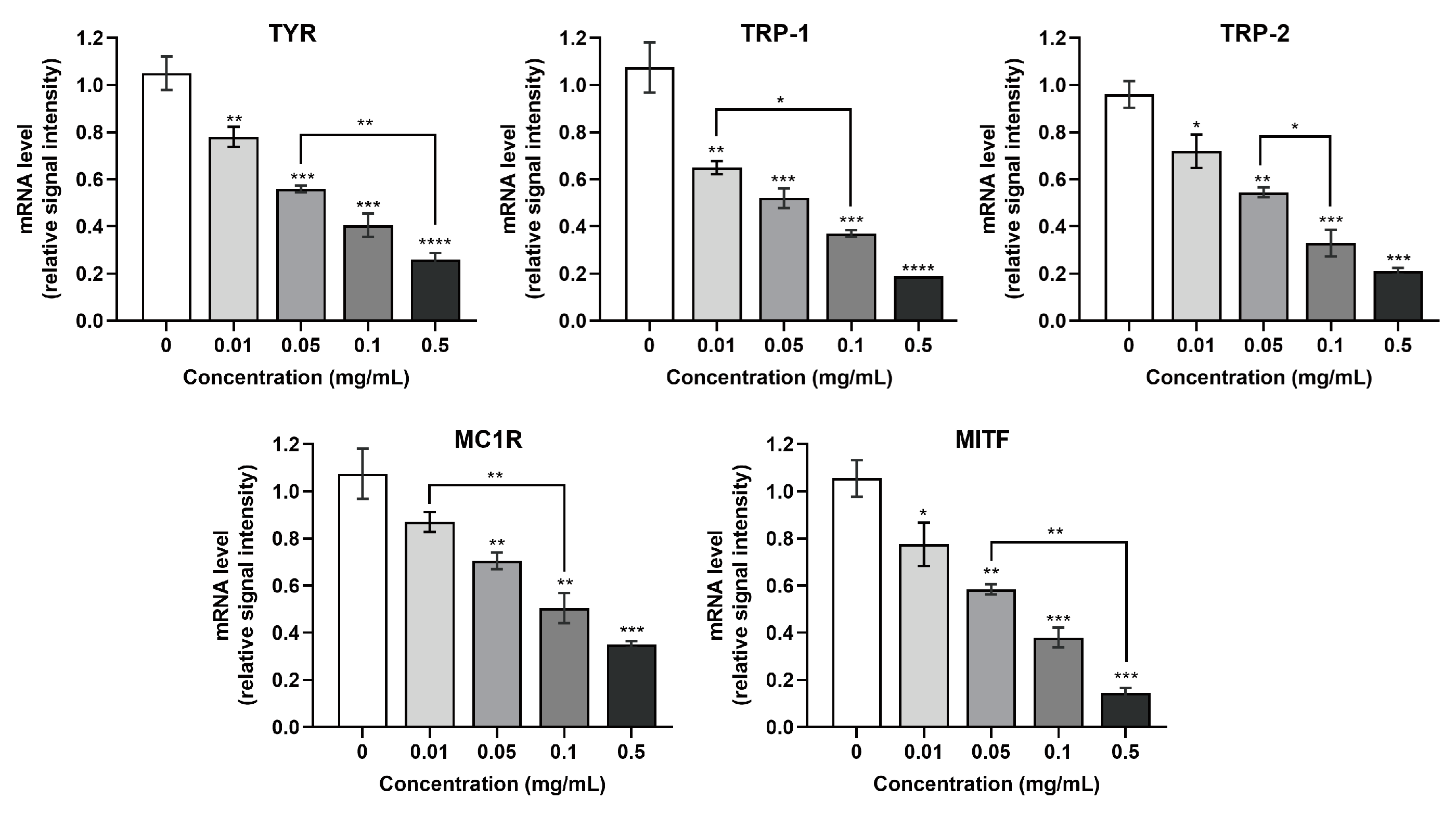
3.5. Effect of SCPs1 on the Transcription Levels of Genes in the cAMP-CREB Signaling Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzhussoeva, E.V.; Gorkun, A.A.; Zurina, I.M.; Kosheleva, N.V.; Kolokol’tsova, T.D.; Saburina, I.N. Influence of Fucoxanthin on Proliferative Activity of Human Melanocyte Culture. Bull. Exp. Biol. Med. 2020, 169, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Haung, X.-Y.; Chiu, C.-C.; Lin, M.-Y.; Lin, W.-H.; Chang, W.-T.; Tseng, C.-C.; Wang, H.-M.D. Inhibitions of melanogenesis via Phyllanthus emblica fruit extract powder in B16F10 cells. Food Biosci. 2019, 28, 177–182. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, G.; Lee, Y.; Kim, H.; Song, M.J.; Lee, D.H.; Chung, J.H. A natural compound harmine decreases melanin synthesis through regulation of the DYRK1A/NFATC3 pathway. J. Dermatol. Sci. 2021, 103, 16–24. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Hu, X.; Chen, Q.; Shi, Y.; Zhuang, J.; Wang, Q. Cefotaxime sodium inhibited melanogenesis in B16F10 cells by cAMP/PKA/CREB pathways. Process Biochem. 2021, 110, 63–70. [Google Scholar] [CrossRef]
- Lee, H.R.; Jung, J.M.; Seo, J.Y.; Chang, S.E.; Song, Y. Anti-melanogenic property of ginsenoside Rf from Panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin. J. Ginseng Res. 2021, 45, 555–564. [Google Scholar] [CrossRef]
- An, X.; Lv, J.; Wang, F. Pterostilbene inhibits melanogenesis, melanocyte dendricity and melanosome transport through cAMP/PKA/CREB pathway. Eur. J. Pharmacol. 2022, 932, 175231. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Li, Y.; Liu, Z.; Lin, Y.; Huang, J.A. Anti-melanogenic effects of epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG) and gallocatechin-3-gallate (GCG) via down-regulation of cAMP/CREB/MITF signaling pathway in B16F10 melanoma cells. Fitoterapia 2020, 145, 104634. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Su, X.R.; Liu, S.S.; Yang, S.S.; Jiang, C.Y.; Zhang, Y.; Zhang, S. Zebrafish phosvitin-derived peptide Pt5 inhibits melanogenesis via cAMP pathway. Fish Physiol. Biochem. 2017, 43, 517–525. [Google Scholar] [CrossRef]
- Faralizadeh, S.; Rahimabadi, E.Z.; Bahrami, S.H.; Hasannia, S. Extraction, characterization and biocompatibility evaluation of silver carp (Hypophthalmichthys molitrix) skin collagen. Sustain. Chem. Pharm. 2021, 22, 100454. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yang, F.; Wu, J.; Huang, D.; Huang, J.; Wang, S. Effects and mechanism of antifreeze peptides from silver carp scales on the freeze-thaw stability of frozen surimi. Food Chem. 2022, 396, 133717. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Li, X.; Wang, F.; Liu, Y.; Wu, J. Dual cryoprotective and antioxidant effects of silver carp (Hypophthalmichthys molitrix) protein hydrolysates on unwashed surimi stored at conventional and ultra-low frozen temperatures. LWT Food Sci. Technol. 2022, 153, 112563. [Google Scholar] [CrossRef]
- Liu, S.C.; Sheu, M.L.; Tsai, Y.C.; Lin, Y.C.; Chang, C.W.; Lai, D.W. Attenuation of in vitro and in vivo melanin synthesis using a Chinese herbal medicine through the inhibition of tyrosinase activity. Phytomedicine 2022, 95, 153876. [Google Scholar] [CrossRef]
- Zeng, H.J.; Li, Q.Y.; Ma, J.; Yang, R.; Qu, L.B. A comparative study on the effects of resveratrol and oxyresveratrol against tyrosinase activity and their inhibitory mechanism. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 251, 119405. [Google Scholar] [CrossRef]
- Ando, H.; Itoh, A.; Mishima, Y.; Ichihashi, M. Correlation between the number of melanosomes, tyrosinase mRNA levels, and tyrosinase activity in cultured murine melanoma cells in response to various melanogenesis regulatory agents. J. Cell. Physiol. 1995, 163, 608–614. [Google Scholar] [CrossRef]
- Ainiwaer, P.; Nueraihemaiti, M.; Li, Z.; Zang, D.; Jiang, L.; Li, Y.; Aisa, H.A. Chemical constituents of Ruta graveolens L. and their melanogenic effects and action mechanism. Fitoterapia 2022, 156, 105094. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, H.I.; Yang, I.; Nam, S.J.; Lim, K.M. Acremonidin E produced by Penicillium sp. SNF123, a fungal endophyte of Panax ginseng, has antimelanogenic activities. J. Ginseng Res. 2021, 45, 98–107. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Xu, K.; Wang, X.; Wen, Y.-T.; Wang, L.-J.; Huang, D.-Q.; Chen, X.-X.; Chai, W.-M. Punicalagin as a novel tyrosinase and melanin inhibitor: Inhibitory activity and mechanism. LWT Food Sci. Technol. 2022, 161, 113318. [Google Scholar] [CrossRef]
- Ju, X.; Cheng, S.; Li, H.; Xu, X.; Wang, Z.; Du, M. Tyrosinase inhibitory effects of the peptides from fish scale with the metal copper ions chelating ability. Food Chem. 2022, 390, 133146. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, T.; Hong, M.; Zhou, Y.; Huang, H.; Xiao, X.; Zheng, J.; Zhou, H.; Lu, Z. Quantitative proteomic analysis uncovers inhibition of melanin synthesis by silk fibroin via MITF/tyrosinase axis in B16 melanoma cells. Life Sci. 2021, 284, 119930. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, Y.-J. Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties. Process Biochem. 2019, 83, 176–182. [Google Scholar] [CrossRef]
- Li, C.; Fu, Y.; Dai, H.; Wang, Q.; Gao, R.; Zhang, Y. Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism. Food Sci. Hum. Well. 2022, 11, 218–229. [Google Scholar] [CrossRef]
- Hu, Z.-Z.; Ma, T.-X.; Sha, X.-M.; Zhang, L.; Tu, Z.-C. Improving tyrosinase inhibitory activity of grass carp fish scale gelatin hydrolysate by gastrointestinal digestion: Purification, identification and action mechanism. LWT Food Sci. Technol. 2022, 159, 113205. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Skin-care functions of peptides prepared from Chinese quince seed protein: Sequences analysis, tyrosinase inhibition and molecular docking study. Ind. Crop. Prod. 2020, 148, 112331. [Google Scholar] [CrossRef]
- Hou, H.U.; Zhao, X.U.E.; Li, B.; Zhang, Z.; Zhuang, Y. Inhibition of Melanogenic Activity by Gelatin and Polypeptides from Pacific Cod Skin in B16 Melanoma Cells. J. Food Biochem. 2011, 35, 1099–1116. [Google Scholar] [CrossRef]
- Castro-Jácome, T.P.; Alcántara-Quintana, L.E.; Montalvo-González, E.; Chacón-López, A.; Kalixto-Sánchez, M.A.; del Pilar Rivera, M.; López-García, U.M.; Tovar-Pérez, E.G. Skin-protective properties of peptide extracts produced from white sorghum grain kafirins. Ind. Crop. Prod. 2021, 167, 113551. [Google Scholar] [CrossRef]
- Gui, M.; Du, J.; Guo, J.; Xiao, B.; Yang, W.; Li, M. Aqueous Extract of Chrysanthemum morifolium (Ju Hua) Enhances the Antimelanogenic and Antioxidative Activities of the Mixture of Soy Peptide and Collagen Peptide. J. Tradit. Complement. Med. 2014, 4, 171–176. [Google Scholar] [CrossRef]
- Xiang, Z.; Xue, Q.; Gao, P.; Yu, H.; Wu, M.; Zhao, Z.; Li, Y.; Wang, S.; Zhang, J.; Dai, L. Antioxidant peptides from edible aquatic animals: Preparation method, mechanism of action, and structure-activity relationships. Food Chem. 2023, 404, 134701. [Google Scholar] [CrossRef]
- Zhang, C.; Du, B.; Song, Z.; Deng, G.; Shi, Y.; Li, T.; Huang, Y. Antioxidant activity analysis of collagen peptide-magnesium chelate. Polym. Test. 2023, 117, 107822. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Hernandez-Mendoza, A.; Gonzalez-Cordova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Guo, L.; Yin, Z.; Wen, L.; Xin, J.; Gao, X.; Zheng, X. Flower extracts from Paeonia decomposita and Paeonia ostii inhibit melanin synthesis via cAMP-CREB-associated melanogenesis signaling pathways in murine B16 melanoma cells. J. Food Biochem. 2019, 43, e12777. [Google Scholar] [CrossRef]
- Jeon, N.-J.; Kim, Y.-S.; Kim, E.-K.; Dong, X.; Lee, J.-W.; Park, J.-S.; Shin, W.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Inhibitory effect of carvacrol on melanin synthesis via suppression of tyrosinase expression. J. Funct. Foods 2018, 45, 199–205. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chien, Y.C.; Wu, C.H.; Kuo, Y.H.; Wu, W.C.; Pan, Y.Y.; Su, Y.H.; Wen, K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef]
- Hu, S.; Zheng, Z.; Zhang, X.; Chen, F.; Wang, M. Oxyresveratrol and trans-dihydromorin from the twigs of Cudrania tricuspidata as hypopigmenting agents against melanogenesis. J. Funct. Foods 2015, 13, 375–383. [Google Scholar] [CrossRef]


| Primers Names | Gene Accession Numbers | Primers Sequences (5’-3’) | |
|---|---|---|---|
| M-actin | NM_007393.5 | sense | CTGAGAGGGAAATCGTGCGT |
| antisense | CCACAGGATTCCATACCCAAGA | ||
| M-tyr | NM_011661 | sense | CAAATTGTACAGAGAAGCGAGTCTT |
| antisense | GGATGACATAGACTGAGCTGATAGT | ||
| M-trp-1 | NM_031202 | sense | CTCACAGTCAGGAGAAATCTTCTAG |
| antisense | CAGTATGTCTTCTAACCTCCTTGTG | ||
| M-trp-2 | NM_010024 | sense | CACAGGAAACTTTGCTGGTTATAAT |
| antisense | GATCACGTAGTCTGGATGGATACTC | ||
| M-mtif | NM_001113198 | sense | CAAGTACCACATACAGCAAGCTC |
| antisense | CTTATAAAATGCCTCTTTTTCACAG | ||
| M-mc1r | NM_008559 | sense | GCTGGAGACTACTATCATCCTGCT |
| antisense | GAGATGTAGCGGTCTATAGCAATG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-L.; Li, M.-J.; Xiong, G.-Q.; Cai, J.; Liao, T.; Zu, X.-Y. Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway. Nutrients 2023, 15, 2449. https://doi.org/10.3390/nu15112449
Li H-L, Li M-J, Xiong G-Q, Cai J, Liao T, Zu X-Y. Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway. Nutrients. 2023; 15(11):2449. https://doi.org/10.3390/nu15112449
Chicago/Turabian StyleLi, Hai-Lan, Mei-Jin Li, Guang-Quan Xiong, Jun Cai, Tao Liao, and Xiao-Yan Zu. 2023. "Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway" Nutrients 15, no. 11: 2449. https://doi.org/10.3390/nu15112449
APA StyleLi, H.-L., Li, M.-J., Xiong, G.-Q., Cai, J., Liao, T., & Zu, X.-Y. (2023). Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway. Nutrients, 15(11), 2449. https://doi.org/10.3390/nu15112449





