Exposure to a Vitamin D Best Practices Toolkit, Model, and E-Tools Increases Knowledge, Confidence, and the Translation of Research to Public Health and Practice †
Abstract
1. Introduction
1.1. Vitamin D Overview
1.2. Totality of Evidence
1.3. Healthcare Professionals’ Knowledge of Best Practices
1.4. Professional Organizations as a Platform for Disseminating Evidence-Based Practice
2. Materials
2.1. Toolkit Development
2.2. Model Development
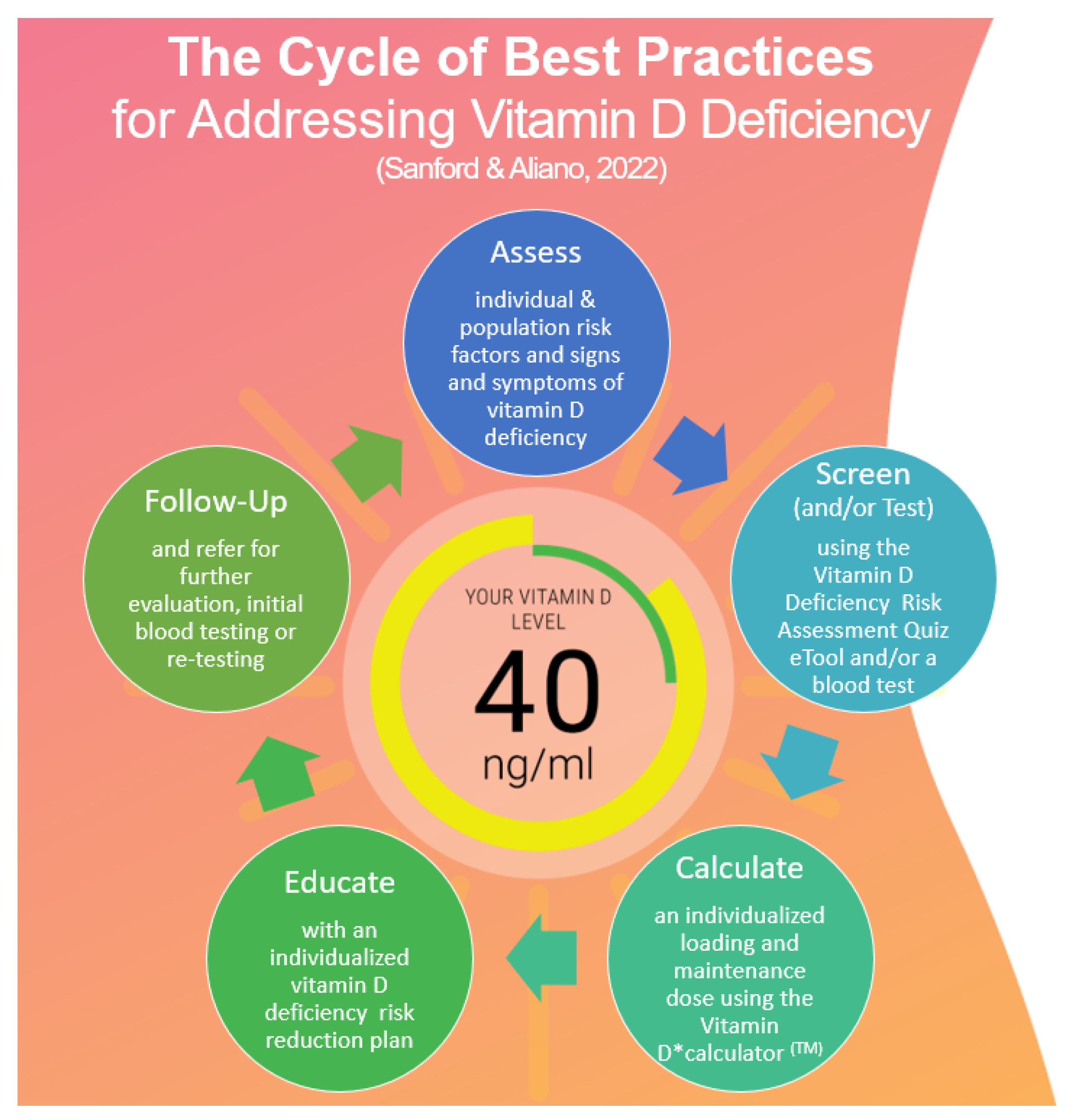
2.2.1. Model Components
Assess
Screen (or Test)
Calculate
Educate
- Addressing individual patient risk factors.
- Incorporating a safe sun or UVB exposure routine based on skin type, lifestyle, seasonality, and environmental determinants of health that affect the UV index, such as latitude, pollution, and inclement weather.
- Maintaining a healthy diet to maximize vitamin D absorption and the supplementation of necessary co-nutrients such as magnesium, K2, and essential fatty acids.
- Providing education on the individualized vitamin D3 supplementation dosing routine, as outlined by the vitamin D*calculator™ recommendations.
- Providing high-quality patient education materials written by vitamin D researchers, such as the GrassrootsHealth Nutrient Research Institute’s IRB-approved “Know ’D‘ Number: Patient and Provider Guide to Understanding Vitamin D, Testing & Results” [100].
Follow Up
3. Methods
3.1. Setting and Participants
3.2. Toolkit Design and Use Process
3.3. Statistics
4. Results
4.1. Participant Demographics
4.2. Knowledge Assessment Results
4.3. Toolkit Feedback
4.4. Follow-Up Survey Results
- (1)
- Increased confidence in translating research to practice: participants (n = 86) reported increased confidence in translating research to practice. A paired t-test showed that the participants’ confidence scores increased significantly, from 2.0 to 3.3 on a scale of 1–5 (p < 0.0001) (see Table 6).
- (2)
- Follow-up survey results found that 100% of the follow-up participants (n = 72) reported translating research into practice within their sphere of influence or practice using at least one component of the model. The most commonly used model components were as follows: refer (54%), assess (50%), educate (46%), screen (25%), and calculate (18%), respectively (see Figure 2).
- (3)
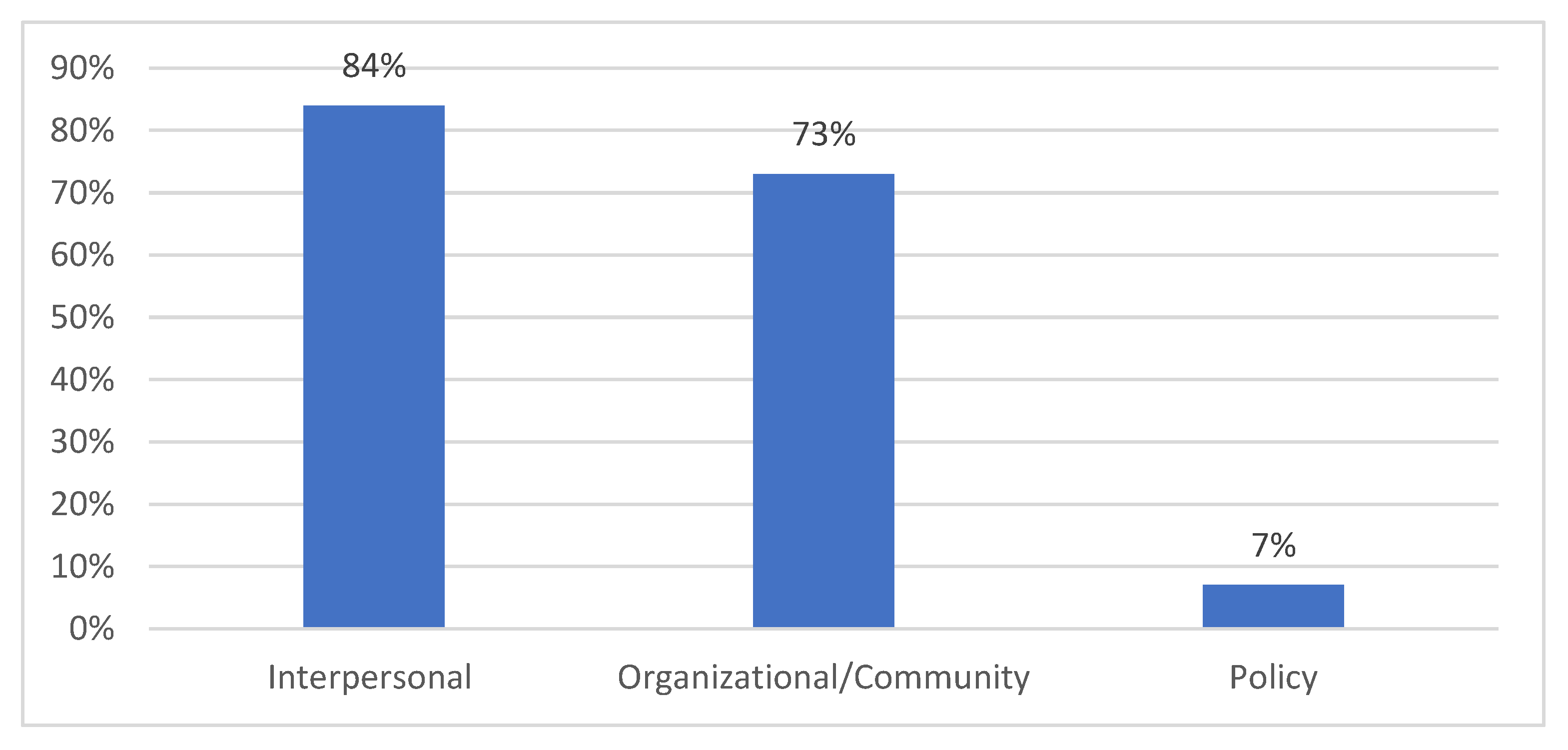
- The translation of research into practice or sphere of influence: of the participants, 94% (n = 85) shared knowledge within their practice or sphere of influence, with the most common socio-ecological model (SEM) levels being:
- Interpersonal (friends, family, and patients): 84%
- Organizational/community (coworkers and community members): 73%
- Policy (professional organization members, legislators, or health department staff): 7% (see Figure 3)
- (4)
- The most reported resource used to translate research into practice was the “Know “D” NUMBER: Patient and Provider Guide to Understanding Vitamin D, Testing and Results” [100].
- (5)
- The participants reported that the most perceived barrier to translating vitamin D knowledge into practice were financial barriers, including the cost of testing and the lack of insurance coverage. Other identified barriers included resistance from interdisciplinary team members and individuals or the patients’ lack of interest in vitamin D information.
5. Discussion
5.1. Utilizing Evidence-Based Patient Care Technologies
5.1.1. Vitamin D Deficiency Risk Assessment Quiz
5.1.2. Vitamin D*Calculator™
6. Safe Sun Exposure Practices
7. Limitations
8. Recommendations for Continuing Education, Research and Quality Improvement
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin, D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grübler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; März, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]
- Peiris, A.N.; Bailey, B.A.; Manning, T. The relationship of vitamin D deficiency to health care costs in veterans. Mil. Med. 2008, 173, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.; Bailey, B.; El Abbassi, A.; Copeland, R.; Adebonojo, L.; Manning, T.; Peiris, A.N. Healthcare costs of Staphylococcus aureus and Clostridium difficile infections in Veterans: Role of vitamin D deficiency. Epidemiol. Infect. 2010, 138, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.; Bailey, B.; El-Abbassi, A.; Vannoy, M.; Manning, T.; Moorman, J.P.; Peiris, A.N. Healthcare costs of methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa infections in veterans: Role of vitamin D deficiency. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Fogleman, S.A.; Janney, C.; Cialdella-Kam, L.; Flint, J.H. Vitamin D Deficiency in the Military: It’s Time to Act! Mil. Med. 2022, 187, 144–148. [Google Scholar] [CrossRef]
- Niedermaier, T.; Gredner, T.; Kuznia, S.; Schöttker, B.; Mons, U.; Brenner, H. Vitamin D supplementation to the older adult population in Germany has the cost-saving potential of preventing almost 30 000 cancer deaths per year. Mol. Oncol. 2021, 15, 1986–1994. [Google Scholar] [CrossRef]
- Lacey, L.F.; Armstrong, D.J.; Royle, E.; Magee, P.; Pourshahidi, L.K.; Ray, S.; Strain, J.J.; McSorley, E. Cost-effectiveness of vitamin D3 supplementation in older adults with vitamin D deficiency in Ireland. BMJ Nutr. Prev. Health 2022, 5, 98–105. [Google Scholar] [CrossRef]
- Hannemann, A.; Wallaschofski, H.; Nauck, M.; Marschall, P.; Flessa, S.; Grabe, H.J.; Schmidt, C.O.; Baumeister, S.E. Vitamin D and health care costs: Results from two independent population-based cohort studies. Clin. Nutr. Edinb. Scotl. 2018, 37 Pt A, 2149–2155. [Google Scholar] [CrossRef]
- Buendía, J.A.; Patiño, D.G. Cost-utility of vitamin D supplementation to prevent acute respiratory infections in children. Cost. Eff. Resour. Alloc. 2023, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Scientists’ Call to D*action for Public Health. GrassrootsHealth. Available online: https://www.grassrootshealth.net/project/our-scientists/ (accessed on 15 October 2022).
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and Vitamin D: Necessary for Public Health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Zgliczyński, W.S.; Rostkowska, O.M.; Sarecka-Hujar, B. Vitamin D Knowledge, Attitudes and Practices of Polish Medical Doctors. Nutrients 2021, 13, 2443. [Google Scholar] [CrossRef] [PubMed]
- Bodenheimer, T.; Sinsky, C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann. Fam. Med. 2014, 12, 573–576. [Google Scholar] [CrossRef]
- Dang, D.; Dearholt, S.; Bissett, K.; Ascenzi, J.; Whalen, M. Johns Hopkins Evidence-Based Practice for Nurses and Healthcare Professionals: Model and Guidelines, 4th ed.; Sigma Theta Tau International: Indianapolis, IN, USA, 2021. [Google Scholar]
- Reeder, A.I.; Jopson, J.A.; Gray, A.R. “Prescribing sunshine”: A national, cross-sectional survey of 1,089 New Zealand general practitioners regarding their sun exposure and vitamin D perceptions, and advice provided to patients. BMC Fam. Pract. 2012, 13, 85. [Google Scholar] [CrossRef]
- Sherman, E.M.; Svec, R.V. Barriers to vitamin D supplementation among military physicians. Mil. Med. 2009, 174, 302–307. [Google Scholar] [CrossRef]
- Bonevski, B.; Girgis, A.; Magin, P.; Horton, G.; Brozek, I.; Armstrong, B. Prescribing sunshine: A cross-sectional survey of 500 Australian general practitioners’ practices and attitudes about vitamin D. Int. J. Cancer 2012, 130, 2138–2145. [Google Scholar] [CrossRef]
- Rockwell, M.; Kraak, V.; Hulver, M.; Epling, J. Clinical Management of Low Vitamin D: A Scoping Review of Physicians’ Practices. Nutrients 2018, 10, 493. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Niño, J.F.M.; et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef]
- Mekonnen, W.; Feleke, Y.; Desalegn, Y.; Tarekegne, G.; Lambisso, B.; Haidar, J.; Zewede, T. Knowledge, attitude and practice of health care workers on measuring adult vitamin D level, diagnosis of deficiency, and management of consequent health conditions in three ecologies of Ethiopia: A cross-sectional study. BMC Nutr. 2020, 6, 77. [Google Scholar] [CrossRef]
- Al-Amri, F.; Gad, A.; Al-Habib, D.; Ibrahim, A.K. Knowledge, Attitude and Practice Regarding Vitamin D among Primary Health Care Physicians in Riyadh City, Saudi Arabia, 2015. World J. Food Sci. Technol. 2017, 1, 47. [Google Scholar] [CrossRef]
- Fallon, E.L.; Lanham-New, S.A.; Williams, P.; Ray, S. An investigation of the vitamin D Knowledge, Attitudes and Practice of UK practising doctors and nurses: The D-KAP study. Proc. Nutr. Soc. 2020, 79, E20. [Google Scholar] [CrossRef]
- Lhamo, Y.; Chugh, P.K.; Gautam, S.R.; Tripathi, C.D. Epidemic of Vitamin D Deficiency and Its Management: Awareness among Indian Medical Undergraduates. J. Environ. Public Health 2017, 2017, 2517207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mahajan, R.; Malhotra, P. Importance of Vitamin D, Awareness and Prevention of Its Deficiency among Female Medical Students. J. Cardiovasc. Dis. 2022, 13, 5. Available online: https://jcdronline.org/admin/Uploads/Files/62ef51aea85ac6.48677325.pdf (accessed on 5 November 2022).
- Walker, P.; Kifley, A.; Kurrle, S.; Cameron, I.D. Process outcomes of a multifaceted, interdisciplinary knowledge translation intervention in aged care: Results from the vitamin D implementation (ViDAus) study. BMC Geriatr. 2019, 19, 177. [Google Scholar] [CrossRef]
- Nowreen, N.; Hameed, R. Awareness regarding the importance of vitamin D and prevention of its deficiency among female undergraduate medical students. Int. J. Basic Clin. Pharmacol. 2019, 8, 865. [Google Scholar] [CrossRef]
- Manandhar, P.; Manandhar, N.; Joshi, S.K. Knowledge of Vitamin D among First-year Medical Undergraduate Students of a Medical College: A Descriptive Cross-sectional Study. J. Nepal. Med. Assoc. 2021, 59, 263. [Google Scholar] [CrossRef]
- Petrović, D.; Runjić, E.; Buljan, I.; Jeličić Kadić, A.; Markić, J. Knowledge and Practice of Pediatricians Regarding Hypovitaminosis D—A Survey across 33 European Countries. Children 2022, 9, 1831. [Google Scholar] [CrossRef]
- van Houwelingen, C.T.; Ettema, R.G.; Bleijenberg, N.; van Os-Medendorp, H.; Kort, H.S.; Cate, O.T. Educational intervention to increase nurses’ knowledge, self-efficacy and usage of telehealth: A multi-setting pretest-posttest study. Nurse Educ. Pract. 2021, 51, 102924. [Google Scholar] [CrossRef]
- Uko, C.; Utley, R. Implementing Evidence-Based Vitamin D Protocol in the Dialysis Clinic: An Educational Approach. Nephrol. Nurs. J. 2020, 47, 239–265. [Google Scholar] [CrossRef]
- Sanford, B.S.; Aliano, J.L.; Omary, C.S.; McDonnell, S.L.; Kimball, S.M.; Grant, W.B. Development of a Public Health Model for Translation of Best Practices in Addressing Vitamin D Deficiency; Rasmussen University: Fargo, ND, USA, 2022. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext; Feingold, K.R., Grunfeld, C., Anawalt, B., Boyce, A., Chrousos, G., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 18 October 2022).
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Heaney, R.P.; Armas, L.A.G. Quantifying the vitamin D economy. Nutr. Rev. 2015, 73, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Moukayed, M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur. J. Clin. Nutr. 2020, 74, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, E1656. [Google Scholar] [CrossRef] [PubMed]
- Hamza, F.; Daher, S.; Fakhoury, H.; Grant, W.; Kvietys, P.; Alkattan, K. Immunomodulatory Properties of Vitamin D in the Intestinal and Respiratory Systems. Nutrients 2023, 15, 1696. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef]
- Bouillon, R.; Antonio, L. Nutritional rickets: Historic overview and plan for worldwide eradication. J. Steroid Biochem. Mol. Biol. 2020, 198, 105563. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef]
- Kadowaki, S.; Norman, A.W. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J. Clin. Invest. 1984, 73, 759–766. [Google Scholar] [CrossRef]
- Scragg, R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int. J. Epidemiol. 1981, 10, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Choukroun, J.; Volz, U.; Hueber, R.; Mourão, C.F.D.A.B.; Sader, R.; Kawase-Koga, Y.; Mazhari, R.; Amrein, K.; Meybohm, P.; et al. One hundred years after Vitamin D discovery: Is there clinical evidence for supplementation doses? Int. J. Growth Factors Stem Cells Dent. 2020, 3, 3. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Fakhoury, H.M.A.; Moukayed, M.; Pilz, S.; Al-Daghri, N.M. Evidence That Increasing Serum 25(OH)D Concentrations to 30 ng/mL in the Kingdom of Saudi Arabia and the United Arab Emirates Could Greatly Improve Health Outcomes. Biomedicines 2023, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Vimaleswaran, K.S.; Zhou, A. Genetic Determinants of 25-Hydroxyvitamin D Concentrations and Their Relevance to Public Health. Nutrients 2022, 14, 4408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef]
- Judd, S.E.; Morgan, C.J.; Panwar, B.; Howard, V.J.; Wadley, V.G.; Jenny, N.S.; Kissela, B.M.; Gutiérrez, O.M. Vitamin D deficiency and incident stroke risk in community-living black and white adults. Int. J. Stroke Off. J. Int. Stroke Soc. 2016, 11, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; A Oni, O.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.; Baggerly, L.; French, C.; Heaney, R.; Gorham, E.; Holick, M.; Scragg, R.; Garland, C. Incidence rate of type 2 diabetes is >50% lower in GrassrootsHealth cohort with median serum 25-hydroxyvitamin D of 41 ng/ml than in NHANES cohort with median of 22 ng/ml. J. Steroid Biochem. Mol. Biol. 2016, 155 Pt B, 239–244. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3097–3110. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Brunel, L.; Muscogiuri, G.; Kimball, S. Physiological serum 25-hydroxyvitamin D concentrations are associated with improved thyroid function-observations from a community-based program. Endocrine 2017, 58, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Kimball, S.M. The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements. Nutrients 2017, 9, E1244. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-Hydroxyvitamin D Concentrations ≥40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef]
- Ames, B.N.; Grant, W.B.; Willett, W.C. Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients 2021, 13, 499. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.; Uçar, N.; Soriano, J.M.; Llopis-Morales, A.; Sanford, B.S.; Grant, W.B. Vitamin D-Related Risk Factors for Maternal Morbidity and Mortality during Pregnancy: Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4124. [Google Scholar] [CrossRef]
- Suárez-Varela, M.M.; Uçar, N.; Peraita-Costa, I.; Huertas, M.F.; Soriano, J.M.; Llopis-Morales, A.; Grant, W.B. Vitamin D-Related Risk Factors for Maternal Morbidity during Pregnancy: A Systematic Review. Nutrients 2022, 14, 3166. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef] [PubMed]
- Kemény, L.V.; Robinson, K.C.; Hermann, A.L.; Walker, D.M.; Regan, S.; Yew, Y.W.; Lai, Y.C.; Theodosakis, N.; Rivera, P.D.; Ding, W.; et al. Vitamin D deficiency exacerbates UV/endorphin and opioid addiction. Sci. Adv. 2021, 7, eabe4577. [Google Scholar] [CrossRef] [PubMed]
- Akkus, M.; Davarci, P.Z.; Bas, S.; Odluyurt, H.; Aydogan, M. Evaluation of inflammatory parameters in patients who attempted suicide by taking drugs. Bratisl. Lek. Listy. 2022, 123, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.E.; Gibbons, J.B. The association between vitamin D serum levels, supplementation, and suicide attempts and intentional self-harm. PLoS ONE 2023, 18, e0279166. [Google Scholar] [CrossRef]
- Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077. [Google Scholar] [CrossRef]
- Kouba, B.R.; Torrá, A.C.N.C.; Camargo, A.; Rodrigues, A.L.S. The antidepressant-like effect elicited by vitamin D3 is associated with BDNF/TrkB-related synaptic protein synthesis. Metab. Brain Dis. 2023, 38, 601–611. [Google Scholar] [CrossRef]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Somoza-Moncada, M.M.; Turrubiates-Hernández, F.J.; Muñoz-Valle, J.F.; Gutiérrez-Brito, J.A.; Díaz-Pérez, S.A.; Aguayo-Arelis, A.; Hernández-Bello, J. Vitamin D in Depression: A Potential Bioactive Agent to Reduce Suicide and Suicide Attempt Risk. Nutrients 2023, 15, 1765. [Google Scholar] [CrossRef]
- Umhau, J.C.; George, D.T.; Heaney, R.P.; Lewis, M.D.; Ursano, R.J.; Heilig, M.; Hibbeln, J.R.; Schwandt, M.L. Low Vitamin D Status and Suicide: A Case-Control Study of Active Duty Military Service Members. PLoS ONE 2013, 8, e51543. [Google Scholar] [CrossRef]
- Yagci, I.; Avci, S. Biochemical predictors in presentations to the emergency department after a suicide attemp. Bratisl. Lek. Listy. 2021, 122, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zubizarreta, J.R.; Umhau, J.C.; Deuster, P.A.; Brenner, L.A.; King, A.J.; Petukhova, M.V.; Sampson, N.A.; Tizenberg, B.; Upadhyaya, S.K.; RachBeisel, J.A.; et al. Evaluating the heterogeneous effect of a modifiable risk factor on suicide: The case of vitamin D deficiency. Int. J. Methods Psychiatr. Res. 2022, 31, e1897. [Google Scholar] [CrossRef]
- Melough, M.M.; Li, M.; Hamra, G.; Palmore, M.; Sauder, K.A.; Dunlop, A.L.; LeWinn, K.Z.; Zhao, Q.; Kelly, R.S.; Switkowski, K.M.; et al. Greater Gestational Vitamin D Status is Associated with Reduced Childhood Behavioral Problems in the Environmental Influences on Child Health Outcomes Program. J. Nutr. 2023, 153, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Moukayed, M. A Narrative Review on the Potential Role of Vitamin D3 in the Prevention, Protection, and Disease Mitigation of Acute and Long COVID-19. Curr. Nutr. Rep. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.M.; Aguilar-Jimenez, W.; Trujillo-Gil, E.; Zapata, W.; Su, R.-C.; Ball, T.B.; Rugeles, M.T. Vitamin D treatment of peripheral blood mononuclear cells modulated immune activation and reduced susceptibility to HIV-1 infection of CD4+ T lymphocytes. PLoS ONE 2019, 14, e0222878. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs. 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Saeed, A.A.; Eid, M.; Ahmed, S.; Abboud, M.; Sami, B. Knowledge, attitude, and practice regarding Vitamin D deficiency among community pharmacists and prescribing doctors in Khartoum city, Sudan, 2020. Matrix Sci. Pharma. 2020, 4, 41. [Google Scholar] [CrossRef]
- Farooq, M.; Mohamedally, S.; Doshani, A.; Mousa, H. PMM.61 Are doctors well informed on Vitamin D and its’ prescribing during pregnancy? Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99 (Suppl. 1), A143. [Google Scholar] [CrossRef]
- Simon, A.E.; Ahrens, K.A. Adherence to Vitamin D Intake Guidelines in the United States. Pediatrics 2020, 145, e20193574. [Google Scholar] [CrossRef]
- Ross, E.J.; Fitzpatrick, J.J.; Click, E.R.; Krouse, H.J.; Clavelle, J.T. Transformational leadership practices of nurse leaders in professional nursing associations. J. Nurs. Adm. 2014, 44, 201–206. [Google Scholar] [CrossRef]
- Titler, M.G. The Evidence for Evidence-Based Practice Implementation. In Patient Safety and Quality: An. Evidence-Based Handbook for Nurses; Hughes, R.G., Ed.; Advances in Patient Safety; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. Available online: http://www.ncbi.nlm.nih.gov/books/NBK2659/ (accessed on 15 October 2022).
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice Hall: Upper Saddle River, NJ, USA, 1986. [Google Scholar]
- GrassrootsHealth. Moving Vitamin D Research into Practice: Addressing Vitamin D Deficiency to Improve Patient Outcomes, Population Health & Reduce Costs; GrassrootsHealth: Encinitas, CA, USA, 2023; Available online: https://www.grassrootshealth.net/blog/announcing-new-vitamin-deducation-course-great-everyone-ceu-approved/ (accessed on 12 February 2023).
- Golden, T.L.; Wendel, M.L. Public Health’s Next Step in Advancing Equity: Re-evaluating Epistemological Assumptions to Move Social Determinants From Theory to Practice. Front. Public Health 2020, 8, 131. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00131 (accessed on 15 October 2022). [CrossRef]
- MUSC Health. Vitamin D Testing and Treatment Protocol. 2017. Available online: https://www.grassrootshealth.net/wp-content/uploads/2017/10/newman-protocol-letter.pdf?_ga=2.20709048.1817993166.1668455725-1671503586.1657558075 (accessed on 3 January 2023).
- Screening, Brief Intervention, and Referral to Treatment (SBIRT). Available online: https://www.samhsa.gov/sbirt (accessed on 15 October 2022).
- Sanford, B.; Aliano, J. Cycle of Best Practices for Addressing Vitamin D Deficiency. 2022. Available online: https://www.researchgate.net/publication/366189944_Cycle_of_Best_Practices_for_Addressing_Vitamin_D_Deficiency?channel=doi&linkId=6396913d095a6a7774229362&showFulltext=true (accessed on 12 December 2022).
- Wagner, C.L.; Baggerly, C.; McDonnell, S.; Baggerly, K.A.; French, C.B.; Baggerly, L.; Hamilton, S.A.; Hollis, B.W. Post-hoc analysis of vitamin D status and reduced risk of preterm birth in two vitamin D pregnancy cohorts compared with South Carolina March of Dimes 2009-2011 rates. J. Steroid Biochem. Mol. Biol. 2016, 155 Pt B, 245–251. [Google Scholar] [CrossRef]
- Why the Same Dose Does Not Work for Everyone; GrassrootsHealth: Encinitas, CA, USA, 2021; Available online: https://www.grassrootshealth.net/blog/dose-not-work-everyone/ (accessed on 16 January 2023).
- Kimball, S.M.; Holick, M.F. Official recommendations for vitamin D through the life stages in developed countries. Eur. J. Clin. Nutr. 2020, 74, 1514–1518. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC–Oxford study. Public. Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Everyone Responds Differently to Vitamin D Infographic; GrassrootsHealth: Encinitas, CA, USA, 2022; Available online: https://www.grassrootshealth.net/document/everyone-responds-differently-vitamin-d-infographic/ (accessed on 1 November 2022).
- Vitamin D Deficiency Risk Assessment Quiz; GrassrootsHealth: Encinitas, CA, USA, 2022; Available online: https://www.grassrootshealth.net/project/vitamin-d-deficiency-risk-assessment-quiz/ (accessed on 16 January 2023).
- GrassrootsHealth. Vitamin D*calculatorTM; GrassrootsHealth: Encinitas, CA, USA, 2020; Available online: https://www.grassrootshealth.net/project/dcalculator/ (accessed on 27 October 2022).
- KNOW “D” NUMBER Patient and Provider Guide to Understanding Vitamin D, Testing & Results Booklet; GrassrootsHealth: Encinitas, CA, USA, 2022; Available online: https://www.grassrootshealth.net/document/know-d-number-patient-provider-guide-understanding-vitamin-d-testing-results-booklet/ (accessed on 27 October 2022).
- Create Courses Online|#1 E-learning Software Platform. Create an online course easily|Easygenerator. Available online: https://www.easygenerator.com/en/ (accessed on 15 October 2022).
- Khammarnia, M.; Haj Mohammadi, M.; Amani, Z.; Rezaeian, S.; Setoodehzadeh, F. Barriers to implementation of evidence based practice in zahedan teaching hospitals, iran, 2014. Nurs. Res. Pract. 2015, 2015, 357140. [Google Scholar] [CrossRef] [PubMed]
- Achieve and Manage Your Optimal Vitamin D Levels; GrassrootsHealth: Encinitas, CA, USA, 2022; Available online: https://grassrootshealth.org/project/achieve-manage-optimal-vitamin-d-levels/ (accessed on 15 October 2022).
- NEW Loading Dose Vitamin D*Calculator! GrassrootsHealth: Encinitas, CA, USA, 2020; Available online: https://www.grassrootshealth.net/blog/new-loading-dose-vitamin-dcalculator/ (accessed on 26 December 2022).
- van Groningen, L.; Opdenoordt, S.; van Sorge, A.; Telting, D.; Giesen, A.; de Boer, H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur. J. Endocrinol. 2010, 162, 805–811. [Google Scholar] [CrossRef]
- Engelsen, O. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef]
- Engelsen, O.; Brustad, M.; Aksnes, L.; Lund, E. Daily Duration of Vitamin D Synthesis in Human Skin with Relation to Latitude, Total Ozone, Altitude, Ground Cover, Aerosols and Cloud Thickness. Photochem. Photobiol. 2005, 81, 1287. [Google Scholar] [CrossRef]
- Estupiñán, J.G.; Raman, S.; Crescenti, G.H.; Streicher, J.J.; Barnard, W.F. Effects of Clouds and Haze on UV-B Radiation. J. Geophys. Res. 1996, 101, 16807–16816. Available online: https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1029/96JD01170 (accessed on 7 February 2023). [CrossRef]
- Leal, A.C.G.B.; Corrêa, M.P.; Holick, M.F.; Melo, E.V.; Lazaretti-Castro, M. Sun-induced production of vitamin D3 throughout 1 year in tropical and subtropical regions: Relationship with latitude, cloudiness, UV-B exposure and solar zenith angle. Photochem. Photobiol. Sci. 2021, 20, 265–274. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]

| Body System/Condition | Optimal 25(OH)D Concentration | Findings | Reference |
|---|---|---|---|
| All-cause mortality | ≥30–36 ng/mL | HR 1.9 (95% confidence interval = 1.6, 2.2; p < 0.001) | Garland and Grant, 2014 [51] |
| Stroke | ≥30 ng/mL | aHR 1.85 (95% CI, 1.17–2.93) <20 ng/mL vs. >30 ng/mL | Judd et al. [52] |
| Hypertension | ≥40 ng/mL | Lowered BP and reduced the prevalence of hypertension among hypertensive patients. | Mirhosseini et al. [57] |
| Myocardial infarction | ≥30 ng/mL | Acharya et al. [53] | |
| Type 2 diabetes from prediabetes | ≥40 ng/mL | Pittas et al. [80] | |
| Cancer, all-cause | ≥40 ng/mL | Women with concentrations ≥40 ng/mL had a 67% lower risk of cancer than women with concentrations <20 ng/mL (HR = 0.33, 95% CI = 0.12–0.90). | McDonnell et al. [62] |
| Breast Cancer | ≥60 ng/mL | Women with concentrations ≥60 ng/mL had an 80% lower risk of breast cancer than women with concentrations <20 ng/mL (HR = 0.20, p = 0.03). | McDonnell et al. [81] |
| Preterm birth | ≥40 ng/mL | McDonnell et al. [21] | |
| Thyroid function | ≥50 ng/mL | Mirhosseini et al. [56] | |
| Alzheimer’s disease/dementia/brain health | ≥30 ng/mL | Grant et al. [48] | |
| COVID-19 | 50 ng/mL | Gibbons et al. [59] Kaufman et al. [60] | |
| Autoimmune disease | ≥30 ng/mL | Concentrations of 40–60 ng/mL may be needed for optimal risk reduction. | Sîrbe et al. [61] |
| Healthcare Discipline | N | Percent |
|---|---|---|
| Nurses (RN/LPN) | 102 | 86% |
| Dietitians (LDN, LRN) | 16 | 13% |
| Did not disclose | 1 | 1% |
| Educational Degree | N | Percent |
|---|---|---|
| Licensed Practical Nurse | 17 | 14% |
| Associate Degree | 14 | 12% |
| Bachelor’s Degree | 54 | 45% |
| Master’s Degree | 27 | 23% |
| Doctoral Degree | 6 | 5% |
| Prefer not to state | 1 | 1% |
| Healthcare Discipline | N | Percent |
|---|---|---|
| Nurses (RN/LPN) | 72 | 83% |
| Dietitians (LDN, LRN) | 14 | 16% |
| Did not disclose | 1 | 1% |
| Knowledge Scores | Pre-Test Score | Post-Test Score |
|---|---|---|
| 31% | 65% |
| Confidence Scores | Pre-Score | Post-Score |
|---|---|---|
| 2.0 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanford, B.S.; Aliano, J.L.; Omary, C.S.; McDonnell, S.L.; Kimball, S.M.; Grant, W.B. Exposure to a Vitamin D Best Practices Toolkit, Model, and E-Tools Increases Knowledge, Confidence, and the Translation of Research to Public Health and Practice. Nutrients 2023, 15, 2446. https://doi.org/10.3390/nu15112446
Sanford BS, Aliano JL, Omary CS, McDonnell SL, Kimball SM, Grant WB. Exposure to a Vitamin D Best Practices Toolkit, Model, and E-Tools Increases Knowledge, Confidence, and the Translation of Research to Public Health and Practice. Nutrients. 2023; 15(11):2446. https://doi.org/10.3390/nu15112446
Chicago/Turabian StyleSanford, Beth S., Jennifer L. Aliano, Courtney S. Omary, Sharon L. McDonnell, Samantha M. Kimball, and William B. Grant. 2023. "Exposure to a Vitamin D Best Practices Toolkit, Model, and E-Tools Increases Knowledge, Confidence, and the Translation of Research to Public Health and Practice" Nutrients 15, no. 11: 2446. https://doi.org/10.3390/nu15112446
APA StyleSanford, B. S., Aliano, J. L., Omary, C. S., McDonnell, S. L., Kimball, S. M., & Grant, W. B. (2023). Exposure to a Vitamin D Best Practices Toolkit, Model, and E-Tools Increases Knowledge, Confidence, and the Translation of Research to Public Health and Practice. Nutrients, 15(11), 2446. https://doi.org/10.3390/nu15112446







