TNF-α Mediates the Association between Dietary Inflammatory Index and Depressive Symptoms in Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Sample Size
2.3. Data Collection and Measurement
2.3.1. Assessment of Physical Activity Level
2.3.2. Assessment of DepS and Anxiety Symptoms
2.3.3. Assessment of Dietary Intake and Calculation of E-DII
2.4. Assessment of Inflammatory Markers
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Nutrients Intake
3.3. Plasma Inflammatory Markers
3.4. Association between E-DII and DepS
3.5. Associations between E-DII and Inflammatory Markers
3.6. Associations between Inflammatory Markers and DepS
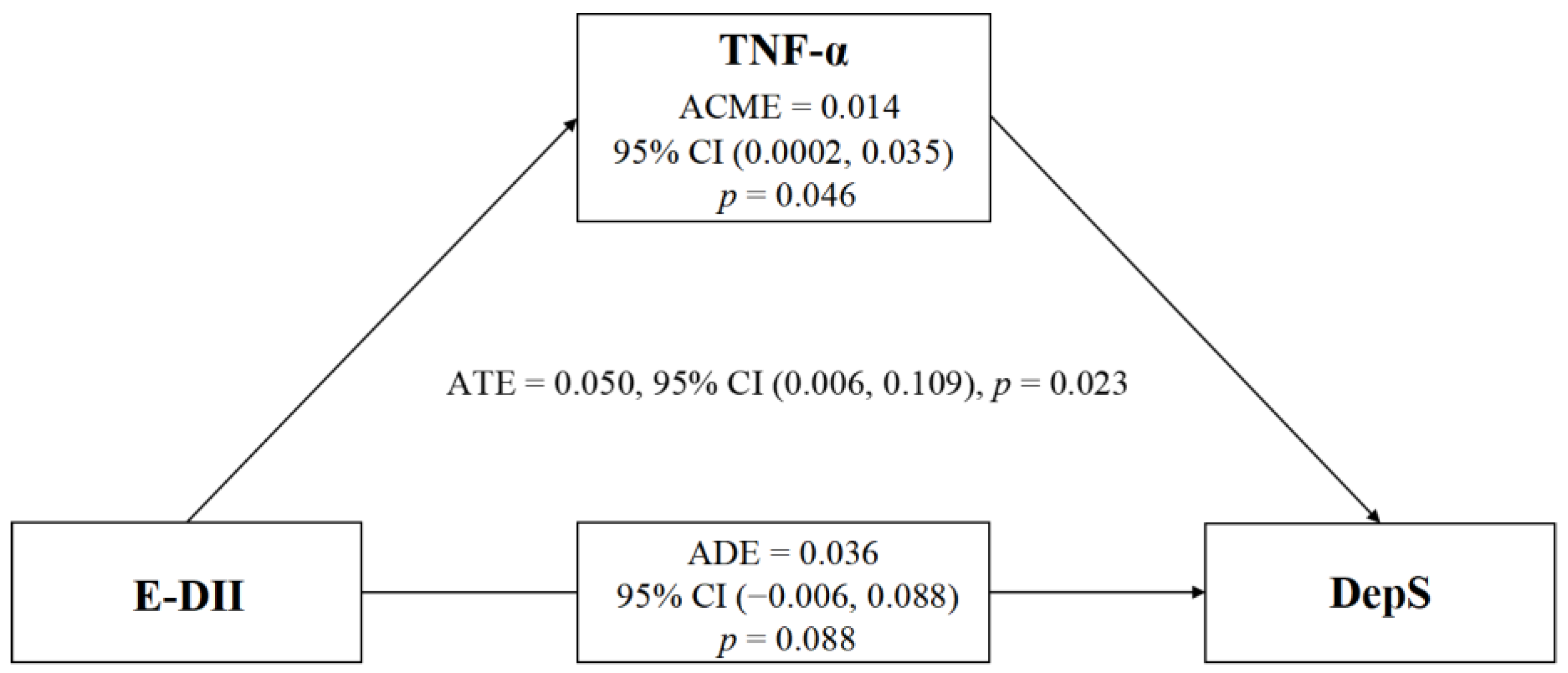
3.7. Association between E-DII and DepS with TNF-α as a Mediator
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Würtzen, H.; Dalton, S.O.; Elsass, P.; Sumbundu, A.D.; Steding-Jensen, M.; Karlsen, R.V.; Andersen, K.K.; Flyger, H.L.; Pedersen, A.E.; Johansen, C. Mindfulness significantly reduces self-reported levels of anxiety and depression: Results of a randomised controlled trial among 336 Danish women treated for stage I–III breast cancer. Eur. J. Cancer 2013, 49, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Linden, W.; Vodermaier, A.; Mackenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Maass, S.W.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.; de Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef]
- Lan, B.; Jiang, S.; Li, T.; Sun, X.; Ma, F. Depression, anxiety, and their associated factors among Chinese early breast cancer in women under 35 years of age: A cross sectional study. Curr. Probl. Cancer 2020, 44, 100558. [Google Scholar] [CrossRef]
- Walker, J.; Hansen, C.H.; Martin, P.; Symeonides, S.; Ramessur, R.; Murray, G.; Sharpe, M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014, 1, 343–350. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cncer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef]
- Mauskopf, J.A.; Simon, G.E.; Kalsekar, A.; Nimsch, C.; Dunayevich, E.; Cameron, A. Nonresponse, partial response, and failure to achieve remission: Humanistic and cost burden in major depressive disorder. Depress. Anxiety 2009, 26, 83–97. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Paul Amminger, G.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. International Society for Nutritional Psychiatry Research consensus position statement: Nutritional medicine in modern psychiatry. World Psychiatry 2015, 14, 370–371. [Google Scholar] [CrossRef]
- Quirk, S.E.; Williams, L.J.; O’Neil, A.; Pasco, J.A.; Jacka, F.N.; Housden, S.; Berk, M.; Brennan, S.L. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry 2013, 13, 175. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Vuksanovic, D.; Sanmugarajah, J.; Lunn, D.; Sawhney, R.; Eu, K.; Liang, R. Unmet needs in breast cancer survivors are common, and multidisciplinary care is underutilised: The Survivorship Needs Assessment Project. Breast Cancer 2021, 28, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yeter, K.; Rock, C.L.; Pakiz, B.; Bardwell, W.A.; Nichols, J.F.; Wilfley, D.E. Depressive symptoms, eating psychopathology, and physical activity in obese breast cancer survivors. Psychooncology 2006, 15, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Ortuño Sánchez-Pedreño, F.; Van der Does, W.; Angel Martínez-González, M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Oddy, W.H.; Allen, K.L.; Trapp, G.S.A.; Ambrosini, G.L.; Black, L.J.; Huang, R.C.; Rzehak, P.; Runions, K.C.; Pan, F.; Beilin, L.J.; et al. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav. Immun. 2018, 69, 428–439. [Google Scholar] [CrossRef]
- Lai, J.S.; Oldmeadow, C.; Hure, A.J.; McEvoy, M.; Hiles, S.A.; Boyle, M.; Attia, J. Inflammation mediates the association between fatty acid intake and depression in older men and women. Nutr. Res. 2016, 36, 234–245. [Google Scholar] [CrossRef]
- Bouchard, L.C.; Antoni, M.H.; Blomberg, B.B.; Stagl, J.M.; Gudenkauf, L.M.; Jutagir, D.R.; Diaz, A.; Lechner, S.; Glück, S.; Derhagopian, R.P.; et al. Postsurgical Depressive Symptoms and Proinflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosom. Med. 2016, 78, 26–37. [Google Scholar] [CrossRef]
- Wu, T.; Hsu, F.C.; Pierce, J.P. Acid-Producing Diet and Depressive Symptoms among Breast Cancer Survivors: A Longitudinal Study. Cancers 2020, 12, 3183. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Shakya, P.R.; Melaku, Y.A.; Shivappa, N.; Hébert, J.R.; Adams, R.J.; Page, A.J.; Gill, T.K. Dietary inflammatory index (DII®) and the risk of depression symptoms in adults. Clin. Nutr. 2021, 40, 3631–3642. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. Dietary Inflammatory Potential and the Risk of Incident Depression in Adults: A Systematic Review. Adv. Nutr. 2019, 10, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, Y.; He, J.; Yi, J.; Wang, Y.; Zhang, J.; Zhu, X. Emotional suppression and depressive symptoms in women newly diagnosed with early breast cancer. BMC Womens Health 2015, 15, 91. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psych. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Hann, D.; Winter, K.; Jacobsen, P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J. Psychosom. Res. 1999, 46, 437–443. [Google Scholar] [CrossRef]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Imai, C.; Takimoto, H.; Fudono, A.; Tarui, I.; Aoyama, T.; Yago, S.; Okamitsu, M.; Sasaki, S.; Mizutani, S.; Miyasaka, N.; et al. Application of the Nutrient-Rich Food Index 9.3 and the Dietary Inflammatory Index for Assessing Maternal Dietary Quality in Japan: A Single-Center Birth Cohort Study. Nutrients 2021, 13, 2854. [Google Scholar] [CrossRef]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Popa, T.A.; Ladea, M. Nutrition and depression at the forefront of progress. J. Med. Life 2012, 5, 414–419. [Google Scholar] [PubMed]
- Aly, J.; Engmann, O. The Way to a Human’s Brain Goes through Their Stomach: Dietary Factors in Major Depressive Disorder. Front. Neurosci. 2020, 14, 582853. [Google Scholar] [CrossRef] [PubMed]
- Elstgeest, L.E.M.; Visser, M.; Penninx, B.; Colpo, M.; Bandinelli, S.; Brouwer, I.A. Bidirectional associations between food groups and depressive symptoms: Longitudinal findings from the Invecchiare in Chianti (InCHIANTI) study. Br. J. Nutr. 2019, 121, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef]
- Perez-Cornago, A.; de la Iglesia, R.; Lopez-Legarrea, P.; Abete, I.; Navas-Carretero, S.; Lacunza, C.I.; Lahortiga, F.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Zulet, M.A. A decline in inflammation is associated with less depressive symptoms after a dietary intervention in metabolic syndrome patients: A longitudinal study. Nutr. J. 2014, 13, 36. [Google Scholar] [CrossRef]
- Firth, J.; Veronese, N.; Cotter, J.; Shivappa, N.; Hebert, J.R.; Ee, C.; Smith, L.; Stubbs, B.; Jackson, S.E.; Sarris, J. What Is the Role of Dietary Inflammation in Severe Mental Illness? A Review of Observational and Experimental Findings. Front. Psychiatry 2019, 10, 350. [Google Scholar] [CrossRef]
- Lucas, M.; Chocano-Bedoya, P.; Schulze, M.B.; Shulze, M.B.; Mirzaei, F.; O’Reilly, É.J.; Okereke, O.I.; Hu, F.B.; Willett, W.C.; Ascherio, A. Inflammatory dietary pattern and risk of depression among women. Brain Behav. Immun. 2014, 36, 46–53. [Google Scholar] [CrossRef]
- Manigault, A.W.; Kuhlman, K.R.; Irwin, M.R.; Cole, S.W.; Ganz, P.A.; Crespi, C.M.; Bower, J.E. Vulnerability to inflammation-related depressive symptoms: Moderation by stress in women with breast cancer. Brain Behav. Immun. 2021, 94, 71–78. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiat. 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Raad, T.; Griffin, A.; George, E.S.; Larkin, L.; Fraser, A.; Kennedy, N.; Tierney, A.C. Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients 2021, 13, 3506. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Pace, T.W.; Liu, T.; Felger, J.C.; Mister, D.; Doho, G.H.; Kohn, J.N.; Barsevick, A.M.; Long, Q.; Miller, A.H. Predictors of depression in breast cancer patients treated with radiation: Role of prior chemotherapy and nuclear factor kappa B. Cancer 2013, 119, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Doherty, M.; Atkinson, T.M.; O’Hanlon, R.; Breitbart, W.; Nelson, C.J.; Miller, A.H. Cancer-related inflammation and depressive symptoms: Systematic review and meta-analysis. Cancer 2022, 128, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Huang, S.C.; Wei, J.C.; Wu, D.J.; Huang, Y.C. Vitamin B(6) supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur. J. Clin. Nutr. 2010, 64, 1007–1013. [Google Scholar] [CrossRef]
- Mozaffari, H.; Askari, M.; Bellissimo, N.; Azadbakht, L. Associations between dietary intake of B vitamins and cardiovascular risk factors in elderly men: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14691. [Google Scholar] [CrossRef]
- Azarmanesh, D.; Bertone-Johnson, E.R.; Pearlman, J.; Liu, Z.; Carbone, E.T. The dietary inflammatory index is inversely associated with depression, which is minimally mediated by C-reactive protein. Nutr. Res. 2022, 97, 11–21. [Google Scholar] [CrossRef]
- Boomsma, D.I.; Willemsen, G.; Sullivan, P.F.; Heutink, P.; Meijer, P.; Sondervan, D.; Kluft, C.; Smit, G.; Nolen, W.A.; Zitman, F.G.; et al. Genome-wide association of major depression: Description of samples for the GAIN Major Depressive Disorder Study: NTR and NESDA biobank projects. Eur. J. Hum. Genet. 2008, 16, 335–342. [Google Scholar] [CrossRef]
- Kaster, M.P.; Gadotti, V.M.; Calixto, J.B.; Santos, A.R.S.; Rodrigues, A.L.S. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology 2012, 62, 419–426. [Google Scholar] [CrossRef]
- Tyring, S.; Gottlieb, A.; Papp, K.; Gordon, K.; Leonardi, C.; Wang, A.; Lalla, D.; Woolley, M.; Jahreis, A.; Zitnik, R.; et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised phase III trial. Lancet 2006, 367, 29–35. [Google Scholar] [CrossRef]
- Guloksuz, S.; Wichers, M.; Kenis, G.; Russel, M.G.; Wauters, A.; Verkerk, R.; Arts, B.; van Os, J. Depressive symptoms in Crohn’s disease: Relationship with immune activation and tryptophan availability. PLoS ONE 2013, 8, e60435. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Yu, J. Inflammation: A mechanism of depression? Neurosci. Bull. 2014, 30, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Egziabher, F.; Rabasa-Lhoret, R.; Bonnet, F.; Bastard, J.P.; Desage, M.; Skilton, M.R.; Vidal, H.; Laville, M. Nutritional intervention to reduce the n-6/n-3 fatty acid ratio increases adiponectin concentration and fatty acid oxidation in healthy subjects. Eur. J. Clin. Nutr. 2008, 62, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The efficacy of an energy-restricted anti-inflammatory diet for the management of obesity in younger adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Muzsik, A.; Krzyżanowska-Jankowska, P.; Walkowiak, J.; Bajerska, J. The effect of habitual fat intake, IL6 polymorphism, and different diet strategies on inflammation in postmenopausal women with central obesity. Nutrients 2019, 11, 1557. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.; Cardoos, A.; Walker, R.; Mischoulon, D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Su, K.P.; Lai, H.C.; Yang, H.T.; Su, W.P.; Peng, C.Y.; Chang, J.P.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatry 2014, 76, 559–566. [Google Scholar] [CrossRef]
- Shelton, R.C.; Pencina, M.J.; Barrentine, L.W.; Ruiz, J.A.; Fava, M.; Zajecka, J.M.; Papakostas, G.I. Association of obesity and inflammatory marker levels on treatment outcome: Results from a double-blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs. J. Clin. Psychiatry 2015, 76, 1635–1641. [Google Scholar] [CrossRef]
- Corley, J.; Shivappa, N.; Hébert, J.R.; Starr, J.M.; Deary, I.J. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers among Older Adults in the Lothian Birth Cohort 1936 Study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef] [PubMed]
| Variables | Depressed (n = 60) | Non-Depressed (n = 160) | p-Value |
|---|---|---|---|
| Age (years) 1 | 52.5 (45.0, 64.8) | 53.0 (46.0, 59.0) | 0.648 |
| BMI (kg/m2) 1 | 23.2 (21.0, 25.7) | 23.4 (21.5, 25.3) | 0.654 |
| E-DII score 1 | 0.65 (−0.30, 1.97) | −0.61 (−1.41, 0.82) | <0.001 |
| SAS score 1 | 41.9 (37.8, 45.0) | 32.5 (30.0, 36.3) | <0.001 |
| Menopausal status, n (%) 2 | |||
| Pre-menopausal | 25 (41.7) | 64 (40.0) | 0.823 |
| Post-menopausal | 35 (58.3) | 96 (60.0) | |
| Marital status, n (%) 2 | |||
| Married | 53 (88.3) | 144 (90.0) | 0.719 |
| Widowed/divorced/single | 7 (11.7) | 16 (10.0) | |
| Education level, n (%) 2 | |||
| Primary school or lower | 17 (28.3) | 20 (12.5) | 0.036 |
| Middle school | 23 (38.3) | 70 (43.8) | |
| High school/ secondary school | 10 (16.7) | 28 (17.5) | |
| Junior college or higher | 10 (16.7) | 42 (26.3) | |
| Employment, n (%) 2 | |||
| Unemployed | 21 (35.0) | 48 (30.0) | 0.599 |
| Employed | 19 (31.7) | 47 (29.4) | |
| Retired | 20 (33.3) | 65 (40.6) | |
| Residence, n (%) 2 | |||
| Rural areas | 24 (40.0) | 41 (25.6) | 0.040 |
| Towns | 10 (16.7) | 50 (31.3) | |
| Urban areas | 26 (43.3) | 69 (43.1) | |
| Family monthly income (RMB), n (%) 2 | |||
| <2000 | 11 (18.3) | 14 (8.8) | 0.031 |
| 2000–5000 | 32 (53.3) | 74 (46.3) | |
| >5000 | 17 (28.3) | 72 (45.0) | |
| Cancer stage, n (%) 2 | |||
| I | 13 (21.7) | 37 (23.1) | 0.511 |
| II | 29 (48.3) | 87 (54.4) | |
| III | 18 (30.0) | 36 (22.5) | |
| Surgery type, n (%) 2 | |||
| Lumpectomy | 17 (28.3) | 50 (31.3) | 0.675 |
| Mastectomy | 43 (71.7) | 110 (68.8) | |
| Presence of comorbidities, n (%) 2 | |||
| No | 39 (65.0) | 119 (74.4) | 0.169 |
| Yes | 21 (35.0) | 41 (25.6) | |
| Physical activity level, n (%) 3 | |||
| Low | 28 (46.7) | 42 (26.3) | 0.012 |
| Moderate | 31 (51.7) | 110 (68.8) | |
| High | 1 (1.7) | 8 (5.0) | |
| Smoking status, n (%) 3 | |||
| Never | 58 (96.7) | 160 (100) | 0.073 |
| Former/current | 2 (3.3) | 0 (0.0) | |
| Drinking status, n (%) 3 | |||
| Never | 55 (91.7) | 158 (98.8) | 0.017 |
| Former/current | 5 (8.3) | 2 (1.3) |
| Variables | Depressed (n = 60) | Non-Depressed (n = 160) | p-Value |
|---|---|---|---|
| Carbohydrate (g/d) | 186.5 (152.9, 247.3) | 169.5 (133.9, 219.3) | 0.018 |
| Dietary fiber (g/d) | 11.8 (8.3, 17.8) | 12.6 (8.8, 17.1) | 0.642 |
| Vitamin A (μgRAE/d) | 379.0 (274.3, 642.0) | 542.0 (363.0, 729.0) | 0.007 |
| Vitamin B2 (mg/d) | 1.0 (0.7, 1.4) | 1.1 (0.8, 1.6) | 0.019 |
| Vitamin B6 (mg/d) | 0.1 (0.1, 0.2) | 0.2 (0.1, 0.3) | 0.042 |
| Vitamin C (mg/d) | 136.1 (73.8, 237.3) | 166.6 (95.7, 229.6) | 0.152 |
| Vitamin D (μg/d) | 0.9 (0.1, 3.0) | 1.3 (0.0, 5.2) | 0.272 |
| Vitamin E (mg/d) | 24.9 (16.1, 31.3) | 25.8 (20.4, 33.4) | 0.286 |
| Folic acid (μg/d) | 124.1 (62.8, 174.7) | 144.2 (89.2, 196.2) | 0.033 |
| Niacin (mg/d) | 12.2 (9.8, 18.3) | 14.8 (11.4, 19.6) | 0.055 |
| β-Carotene (mg/d) | 1.2 (0.4, 2.0) | 1.7 (1.0, 2.9) | 0.005 |
| Magnesium (mg/d) | 286.5 (216.5, 354.0) | 313.0 (224.0, 408.0) | 0.059 |
| Iron (mg/d) | 16.8 (12.2, 20.6) | 19.1 (14.6, 23.4) | 0.029 |
| Zinc (mg/d) | 8.5 (6.2, 11.6) | 9.9 (7.8, 13.5) | 0.012 |
| Selenium (μg/d) | 42.2 (26.5, 78.9) | 58.5 (33.5, 90.9) | 0.053 |
| SFA (g/d) | 15.3 (11.1, 19.3) | 14.6 (11.1, 18.8) | 0.359 |
| PUFA (g/d) | 14.2 (9.8, 18.7) | 14.6 (10.0, 18.0) | 0.454 |
| Omega-3 fatty acids (g/d) | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.3) | 0.707 |
| Omega-6 fatty acids (g/d) | 1.3 (0.8, 1.6) | 1.2 (0.8, 1.6) | 0.454 |
| Variables | Depressed (n = 34) | Non-Depressed (n = 89) | p-Value |
|---|---|---|---|
| CRP (mg/L) | 1.89 (1.78, 2.04) | 1.82 (1.69, 1.92) | 0.006 |
| TNF-α (pg/mL) | 12.89 (12.30, 14.09) | 12.25 (10.60, 13.08) | 0.001 |
| IL-6 (pg/mL) | 7.23 (6.62, 7.63) | 6.20 (5.42, 6.81) | <0.001 |
| IL-1β (pg/mL) | 11.25 (10.62, 11.84) | 10.57 (9.52, 11.59) | 0.010 |
| IL-4 (pg/mL) | 6.67 (6.15, 7.05) | 6.69 (6.10, 7.01) | 0.655 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| E-DII (Continuous) | 1.53 | 1.19, 1.97 | 0.001 |
| E-DII (Categorical) | |||
| Lowest tertile (−4.72~−0.92) | 1.00 | Reference | 0.002 * |
| Middle tertile (−0.91~0.63) | 1.75 | 0.59, 5.21 | |
| Highest tertile (0.64~3.72) | 5.13 | 1.76, 14.96 | |
| Nutrients | |||
| Carbohydrate | 1.00 | 0.99, 1.01 | 0.863 |
| Vitamin A | 1.00 | 0.99, 1.00 | 0.341 |
| Vitamin B2 | 0.39 | 0.17, 0.91 | 0.029 |
| Vitamin B6 | 0.07 | 0.01, 1.03 | 0.053 |
| Folic acid | 1.00 | 0.99, 1.00 | 0.224 |
| β-Carotene | 0.84 | 0.66, 1.08 | 0.170 |
| Iron | 0.92 | 0.86, 0.98 | 0.005 |
| Zinc | 0.82 | 0.72, 0.94 | 0.003 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| CRP | 11.40 | 0.74, 176.52 | 0.082 |
| TNF-α | 1.72 | 1.18, 2.50 | 0.005 |
| IL-6 | 4.80 | 2.02, 11.37 | <0.001 |
| IL-1β | 1.46 | 0.96, 2.22 | 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Maitiniyazi, G.; Li, Z.; Li, T.; Liu, Y.; Zhang, R.; Cao, X.; Gu, D.; Xia, S. TNF-α Mediates the Association between Dietary Inflammatory Index and Depressive Symptoms in Breast Cancer. Nutrients 2023, 15, 84. https://doi.org/10.3390/nu15010084
Chen Y, Maitiniyazi G, Li Z, Li T, Liu Y, Zhang R, Cao X, Gu D, Xia S. TNF-α Mediates the Association between Dietary Inflammatory Index and Depressive Symptoms in Breast Cancer. Nutrients. 2023; 15(1):84. https://doi.org/10.3390/nu15010084
Chicago/Turabian StyleChen, Yue, Gusonghan Maitiniyazi, Ziyuan Li, Tong Li, Yuan Liu, Rong Zhang, Xiaoyun Cao, Danfeng Gu, and Shufang Xia. 2023. "TNF-α Mediates the Association between Dietary Inflammatory Index and Depressive Symptoms in Breast Cancer" Nutrients 15, no. 1: 84. https://doi.org/10.3390/nu15010084
APA StyleChen, Y., Maitiniyazi, G., Li, Z., Li, T., Liu, Y., Zhang, R., Cao, X., Gu, D., & Xia, S. (2023). TNF-α Mediates the Association between Dietary Inflammatory Index and Depressive Symptoms in Breast Cancer. Nutrients, 15(1), 84. https://doi.org/10.3390/nu15010084







