In-Season Consumption of Locally Produced Tomatoes Decreases Cardiovascular Risk Indices
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Determination of Lycopene Content
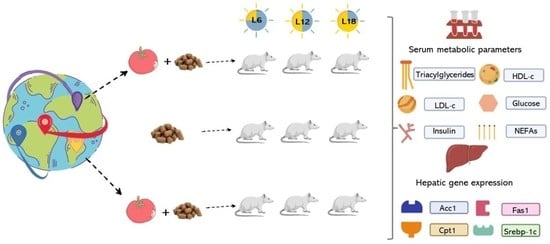
2.3. Animals
2.4. Serum Analysis
2.5. Cardiovascular Risk Indices and HOMA Index
2.6. Hepatic Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Lycopene Content
3.2. Biochemical Parameters
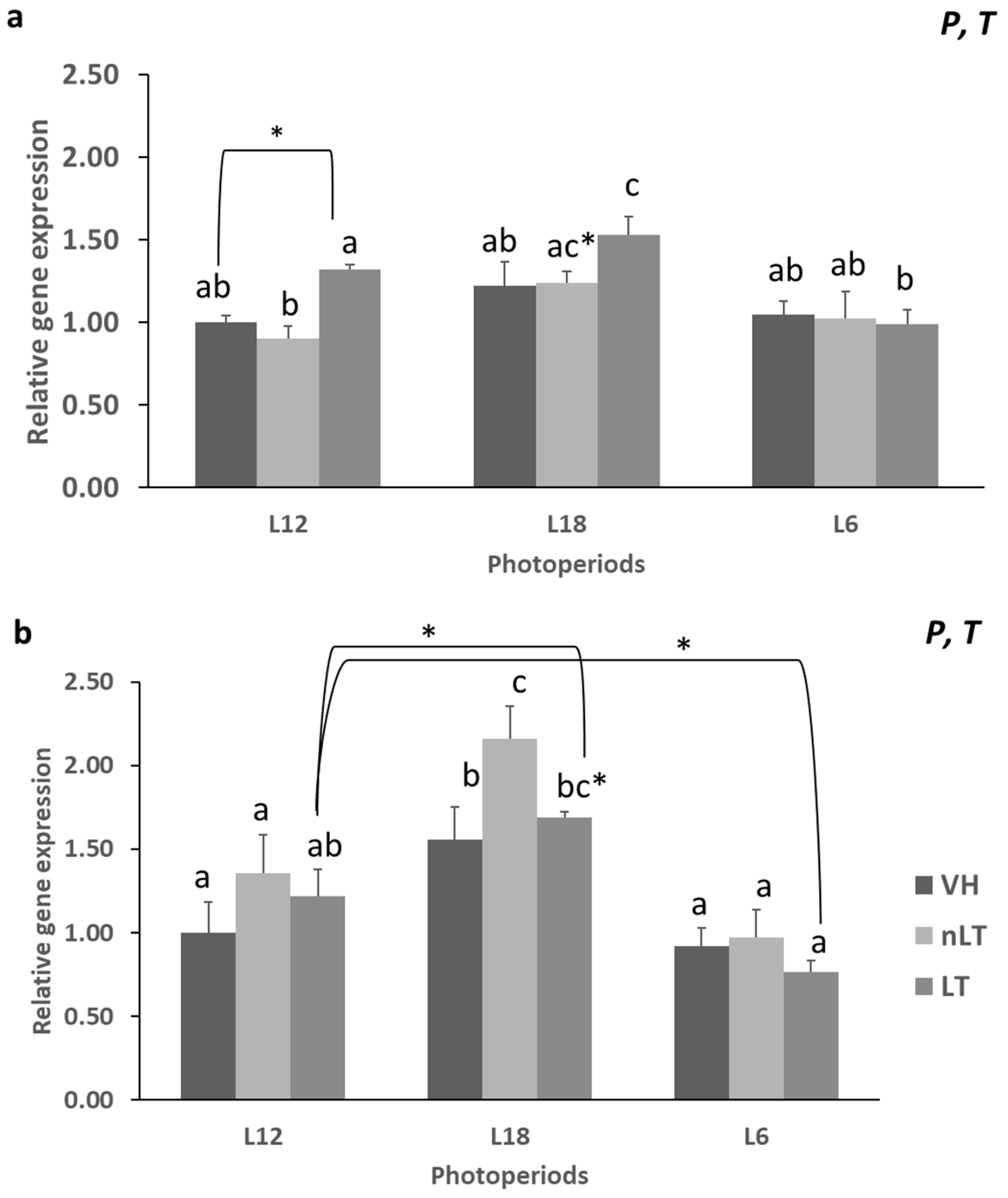
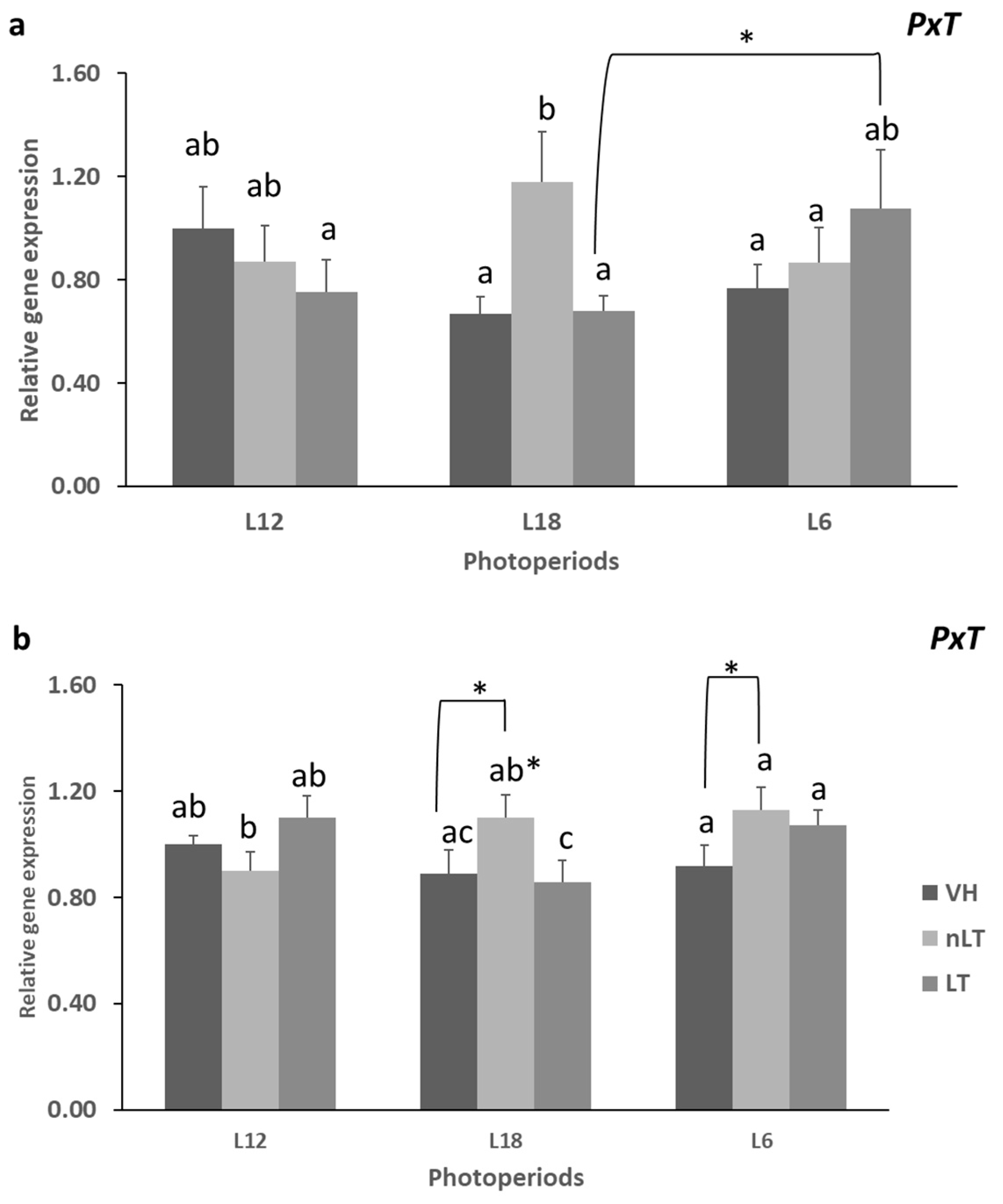
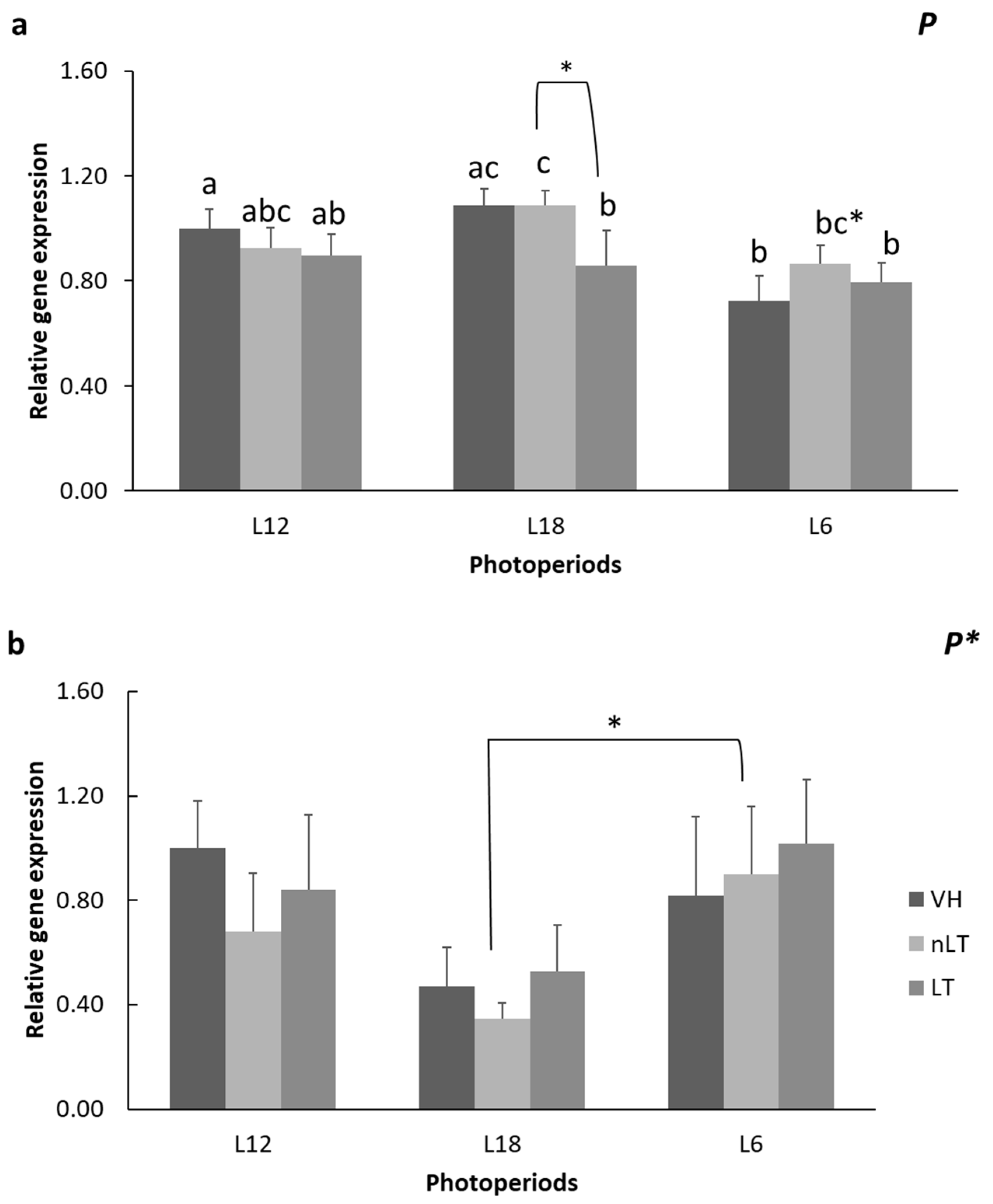
3.3. Gene Expression of Enzymes Related to Lipid Metabolism
4. Discussion
- The photoperiod affects the lipid and carbohydrate metabolism of all treatments.
- Tomato consumption has differential effects on lipid metabolism depending on its geographical origin.
- The consumption of TL in season, L12, decreases CR1 and At.Coef.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the Chemical Cues of Other Species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; I Serrazanetti, D.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-R.; Choi, E.-H.; Kim, G.-T.; Park, T.-S.; Shim, S.-M. Bioefficacy of Graviola leaf extracts in scavenging free radicals and upregulating antioxidant genes. Food Funct. 2016, 7, 861–871. [Google Scholar] [CrossRef]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R.; Matthews, D.C.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 2018, 90, E1789–E1798. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 5 March 2022).
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Li, M.; Sui, Y.; Zhou, Y.; Sun, Y. Effects of lycopene on metabolism of glycolipid and inflammation in non-alcoholic fatty liver disease rats. Wei Sheng Yan Jiu 2020, 49, 254–271. [Google Scholar] [CrossRef]
- Periago, M.J.; García-Alonso, J.; Jacob, K.; Olivares, A.B.; Bernal, M.J.; Iniesta, M.D.; Martínez, C.; Ros, G. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int. J. Food Sci. Nutr. 2009, 60, 694–708. [Google Scholar] [CrossRef]
- Periago, M.J.; Martínez-Valverde, I.; Chesson, A.; Provan, G. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Johnston, J.D.; Morgan, P.J.; Johnstone, A.M. The Big Breakfast Study: Chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 2018, 43, 174–183. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and polyphenols: Roles and diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Carrión, Á.; de Azua, M.J.R.; Mulero, M.; Arola-Arnal, A.; Suárez, M. Oxidative stress in rats is modulated by seasonal consumption of sweet cherries from different geographical origins: Local vs. non-local. Nutrients 2020, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Carrión, Á.J.C.; de Azua, M.J.R.; Bravo, F.I.I.; Aragonès, G.; Muguerza, B.; Suarez, M.; Arola-Arnal, A. Tomatoes consumed in-season prevents oxidative stress in Fischer 344 rats: Impact of geographical origin. Food Funct. 2021, 12, 8340–8350. [Google Scholar] [CrossRef] [PubMed]
- Gibert-Ramos, A.; Palacios-Jordan, H.; Salvadó, M.J.; Crescenti, A. Consumption of out-of-season orange modulates fat accumulation, morphology and gene expression in the adipose tissue of Fischer 344 rats. Eur. J. Nutr. 2020, 59, 621–631. [Google Scholar] [CrossRef]
- Cruz-Carrión, Á.; Calani, L.; de Azua, M.J.R.; Mena, P.; Del Rio, D.; Suárez, M.; Arola-Arnal, A. (Poly)phenolic composition of tomatoes from different growing locations and their absorption in rats: A comparative study. Food Chem. 2022, 388, 132984. [Google Scholar] [CrossRef]
- Motilva, M.J.; Macià, A.; Romero, M.P.; Labrador, A.; Domínguez, A.; Peiró, L. Optimisation and validation of analytical methods for the simultaneous extraction of antioxidants: Application to the analysis of tomato sauces. Food Chem. 2014, 163, 234–243. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Quantification strategies in real-time PCR. AZ Quant. PCR 2004, 1, 89–113. [Google Scholar]
- Leonardi, C.; Ambrosino, P.; Esposito, F.; Fogliano, V. Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes. J. Agric. Food Chem. 2000, 48, 4723–4727. [Google Scholar] [CrossRef]
- Domínguez, I.; Lafuente, M.T.; Hernández-Muñoz, P.; Gavara, R. Influence of modified atmosphere and ethylene levels on quality attributes of fresh tomatoes (Lycopersicon esculentum Mill.). Food Chem. 2016, 209, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Capítulo 1. Cosecha. Available online: https://www.fao.org/3/y4893s/y4893s04.htm (accessed on 13 December 2022).
- Thole, V.; Vain, P.; Yang, R.; Almeida, J.; Enfissi, E.M.A.; Nogueira, M.; Price, E.J.; Alseekh, S.; Fernie, A.R.; Fraser, P.D.; et al. Analysis of Tomato Post-Harvest Properties: Fruit Color, Shelf Life, and Fungal Susceptibility. Curr. Protoc. Plant Biol. 2020, 5, e20108. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, M.J.; Cruz-Carrión, Á.; Muguerza, B.; Arola-Arnal, A.; Suarez, M. Seasonal consumption of cherries from different origins affects metabolic markers and gene expression of lipogenic enzymes in rat liver: A preliminary study. Nutrients 2021, 13, 3643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional Regulation of Hepatic Lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678. [Google Scholar] [CrossRef]
- Maxson & Mitchell Energy Metabolism in the Liver. Physiol. Behav. 2016, 176, 139–148. [CrossRef]
- Abu-Elheiga, L.; Brinkley, W.R.; Zhong, L.; Chirala, S.S.; Woldegiorgis, G.; Wakil, S.J. The subcellular localization of acetyl-CoA carboxylase 2. Proc. Natl. Acad. Sci. USA 2000, 97, 1444–1449. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Klein, S.; Carraro, F.; Weber, J.M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. J. Physiol. Endocrinol. Metab. 1990, 258, E382–E389. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of altered photoperiod on circadian clock and lipid metabolism in rats. Chronobiol. Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef]
- AL Mulla, N.; Simonsen, L.; Bülow, J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: The effects of exercise intensity. J. Physiol. 2000, 524 Pt 3, 919–928. [Google Scholar] [CrossRef]
- Pisonero-Vaquero, S.; Martínez-Ferreras, Á.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Fernández, A.; Benet, M.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; Sánchez-Campos, S. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2015, 59, 879–893. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Navarro-González, I.; Rodrigo-García, J.; Seva, J.; García-Alonso, J.; Periago-Castón, M.J. Consumption of Spinach and Tomato Modifies Lipid Metabolism, Reducing Hepatic Steatosis in Rats. Antioxidants 2020, 9, 1041. [Google Scholar] [CrossRef]
- Firani, N.K.; Adi, P.; Assalami, R.; Anriz, R. Tomato fruit (Solanum lycopersicum) peel extract improves fasting blood glucose and insulin resistance in type 2 diabetes wistar rats. Med. Plants 2018, 10, 120–124. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C. Digestive enzyme inhibition activity of the phenolic substances in selected fruits, vegetables and tea as compared to black legumes. J. Funct. Foods 2017, 38, 644–655. [Google Scholar] [CrossRef]
- Moussa, M.; Gouranton, E.; Gleize, B.; Yazidi, C.E.; Niot, I.; Besnard, P.; Borel, P.; Landrier, J.F. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol. Nutr. Food Res. 2011, 55, 578–584. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Navarro-González, I.; González-Barrio, R.; Martín-Pozuelo, G.; Doménech, G.; Seva, J.; García-Alonso, J.; Periago-Castón, M.J. Tomato juice supplementation influences the gene expression related to steatosis in rats. Nutrients 2018, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Dobiášová, M. Atherogenic impact of lecithin-cholesterol acyltransferase and its relation to cholesterol esterification rate in HDL (FERHDL) and AIP [log(TG/HDL-C)] biomarkers: The butterfly effect? Physiol. Res. 2017, 66, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Pharm, I.J.; Sci, B.; Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.K.; Tyagi, S. Atherogenic Index of Plasma, Castelli Risk Index and Atherogenic Coefficient-New Parameters in Assessing Cardiovascular Risk. Int. J. Pharm. Biol. Sci. 2013, 3, 359–364. [Google Scholar]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12485966/ (accessed on 15 July 2021).
- Multivalent Feedback Regulation of HMG CoA Reductase, a Control Mechanism Coordinating Isoprenoid Synthesis and Cell Growth—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/6995544/?dopt=Abstract (accessed on 22 May 2020).
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects of bioactive compounds of Tomato juice binding to hepatic HMGCR: In vivostudy and molecular modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef]

| L6 | L12 | L18 | 2wA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VH | nLT | LT | VH | nLT | LT | VH | nLT | LT | ||
| Serum Parameters | ||||||||||
| TAG (mmol/L) | 1.14 ± 0.11 c | 1.40 ± 0.07 a | 0.88 ± 0.09 b | 1.17 ± 0.09 c | 1.15 ± 0.10 a*c | 0.99 ± 0.08 bc | 0.95 ± 0.08 bc | 1.10 ± 0.10 b*c | 0.95 ± 0.08 bc | T |
| TC (mmol/L) | 1.80 ± 0.16 ab | 2.03 ± 0.26 a | 2.15 ± 0.25 ab* | 1.97 ± 0.20 ab* | 2.02 ± 0.23 ab* | 2.15 ± 0.25 a | 2.11 ± 0.24 a | 1.96 ± 0.21 ab* | 1.39 ± 0.13 b | |
| HDL-c | 0.75 ± 0.12 | 0.80 ± 0.21 | 0.64 ± 0.22 | 0.50 ± 0.12 | 0.53 ± 0.01 | 0.66 ± 0.14 | 0.38 ± 0.16 | 0.85 ± 0.11 | 0.67 ± 0.12 | |
| LDL-c | 0.16 ± 0.06 | 0.14 ± 0.06 | 0.19 ± 0.08 | 0.09 ± 0.03 | 0.15 ± 0.04 | 0.14 ± 0.03 | 0.13 ± 0.02 | 0.08 ± 0.03 | 0.09 ± 0.02 | |
| NEFAs | 0.79 ± 0.10 ab | 1.04 ± 0.27 a | 0.87 ± 0.09 ab | 0.78 ± 0.06 b | 0.76 ± 0.10 a*b | 0.77 ± 0.11 b | 0.76 ± 0.08 ab | 0.70 ± 0.04 b | 0.62 ± 0.06 b | P * |
| Glucose | 8.87 ± 0.53 ac* | 8.79 ± 0.55 ac* | 7.20 ± 0.27 b | 10.18 ± 0.55 c | 9.52 ± 0.64 ac | 9.22 ± 0.42 ac | 8.50 ± 0.19 a | 8.71 ± 0.45 a | 8.27 ± 0.56 ab | T, P |
| Insulin (ng/mL) | 5.38 ± 0.68 b | 6.27 ± 0.86 ab | 6.68 ± 1.24 ab | 9.93 ± 1.53 a | 7.82 ± 1.10 ab | 8.05 ± 1.63 ab | 8.80 ± 0.54 ab* | 6.78 ± 1.30 ab | 8.61 ± 1.60 ab* | P |
| Ratios | ||||||||||
| HOMA | 0.09 ± 0.01 a | 0.11 ± 0.02 a | 0.09 ± 0.02 a | 0.19 ± 0.04 b | 0.14 ± 0.02 ab | 0.14 ± 0.03 ab | 0.13 ± 0.01 ab* | 0.11 ± 0.02 a | 0.13 ± 0.03 a | P |
| Atherogenic Index | 0.23 ± 0.12 | 0.21 ± 0.10 | 0.21 ± 0.10 | 0.45 ± 0.12 | 0.29 ± 0.05 | 0.24 ± 0.13 | 0.48 ± 0.24 | 0.14 ± 0.04 | 0.19 ± 0.09 | |
| CR1 | 1.97 ± 0.34 b | 2.91 ± 0.71 abc* | 4.67 ± 1.15 c | 3.97 ± 0.51 a | 3.34 ±0.53 ab | 2.17 ± 0.52 a*b # | 4,71 ± 1.59 a | 2.09 ± 0.11 b | 2.71 ± 0.43 a*b | PxT |
| CR2 | 0.14 ± 0.07 a*b | 0.24 ± 0.12 ab | 0.26 ± 0.07 ab | 0.10 ± 0.03 b | 0.40 ± 0.12 a | 0.34 ± 0.13 ab* | 0.33 ±0.13 ab | 0.13 ± 0.05 ab | 0.26 ± 0.09 ab | |
| At.Coef | 0.97 ± 0.34 b | 2.24 ± 0.73 abc* | 3.67 ± 1.15 c | 2.97 ± 0.51 a | 2.34 ± 0.53 ab | 1.17 ± 0.52 a*b # | 3.71 ± 1.59 a | 1.09 ± 0.11 b | 1.71 ± 0.43 a*b | PxT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz de Azua, M.J.; Cruz-Carrión, Á.; Muguerza, B.; Aragonès, G.; Arola-Arnal, A.; Romero, M.P.; Bravo, F.I.; Suarez, M. In-Season Consumption of Locally Produced Tomatoes Decreases Cardiovascular Risk Indices. Nutrients 2023, 15, 43. https://doi.org/10.3390/nu15010043
Ruiz de Azua MJ, Cruz-Carrión Á, Muguerza B, Aragonès G, Arola-Arnal A, Romero MP, Bravo FI, Suarez M. In-Season Consumption of Locally Produced Tomatoes Decreases Cardiovascular Risk Indices. Nutrients. 2023; 15(1):43. https://doi.org/10.3390/nu15010043
Chicago/Turabian StyleRuiz de Azua, Ma. Josefina, Álvaro Cruz-Carrión, Begoña Muguerza, Gerard Aragonès, Anna Arola-Arnal, María Paz Romero, Francisca Isabel Bravo, and Manuel Suarez. 2023. "In-Season Consumption of Locally Produced Tomatoes Decreases Cardiovascular Risk Indices" Nutrients 15, no. 1: 43. https://doi.org/10.3390/nu15010043
APA StyleRuiz de Azua, M. J., Cruz-Carrión, Á., Muguerza, B., Aragonès, G., Arola-Arnal, A., Romero, M. P., Bravo, F. I., & Suarez, M. (2023). In-Season Consumption of Locally Produced Tomatoes Decreases Cardiovascular Risk Indices. Nutrients, 15(1), 43. https://doi.org/10.3390/nu15010043