Bone Disease in Chronic Kidney Disease and Kidney Transplant
Abstract
1. Introduction
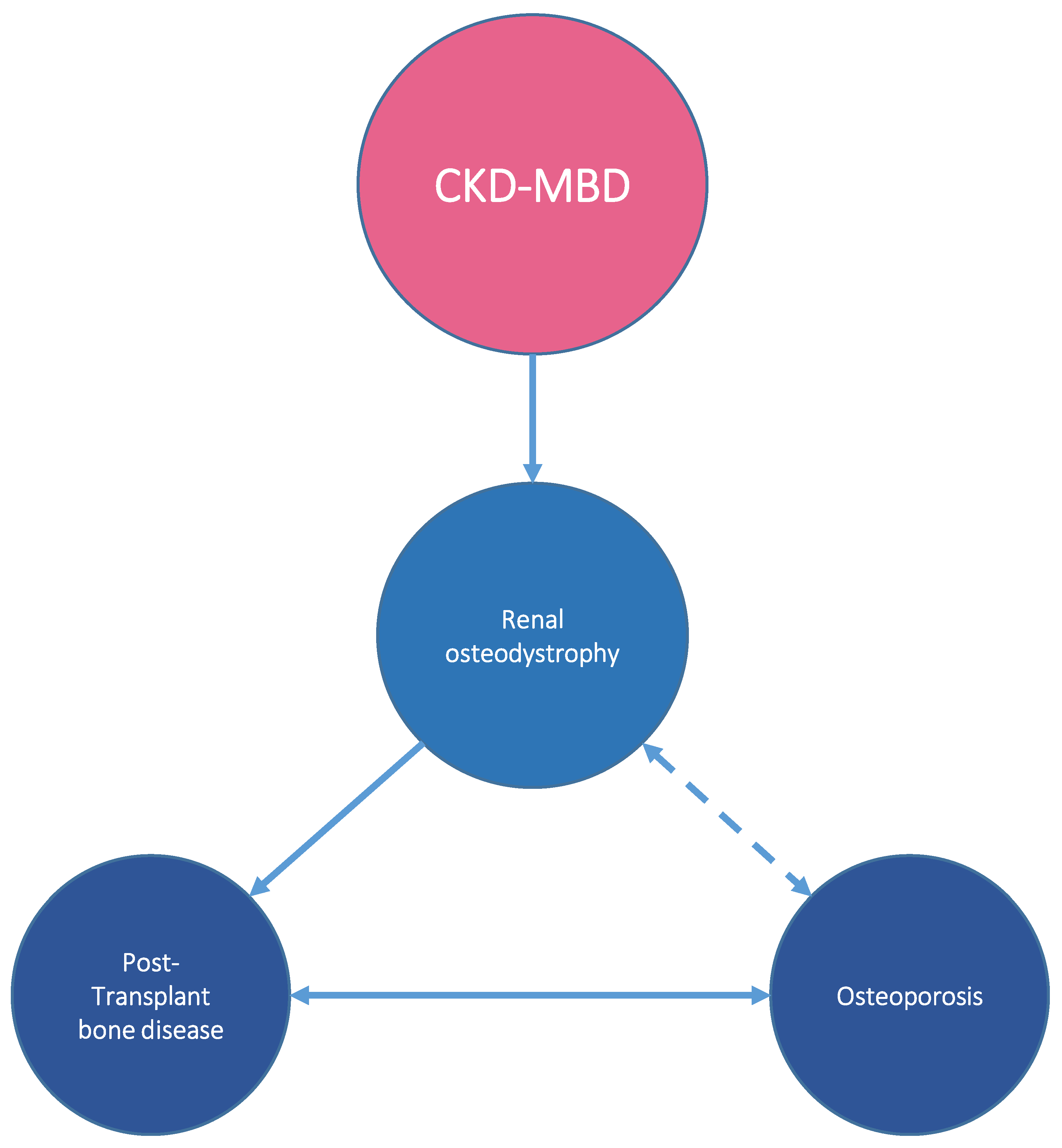
2. Bone Disease in CKD
3. Pathogenesis of Bone Disease in CKD-MBD
4. Bone Fracture in CKD
5. Bone Disease after Kidney Transplantation
6. Alterations of Bone Histology after Transplantation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, S1, 130. [Google Scholar]
- Moe, S.M.; Drueke, T.; Lameire, N.; Eknoyan, G. Chronic kidney disease-mineral-bone disorder: A new paradigm. Adv. Chronic Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef]
- Ball, A.M.; Gillen, D.L.; Sherrard, D.; Weiss, N.S.; Emerson, S.S.; Seliger, S.L.; Kestenbaum, B.R.; Stehman-Breen, C. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002, 288, 3014–3018. [Google Scholar] [CrossRef]
- Alem, A.M.; Sherrard, D.J.; Gillen, D.L.; Weiss, N.S.; Beresford, S.A.; Heckbert, S.R.; Wong, C.; Stehman-Breen, C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000, 58, 396–399. [Google Scholar] [CrossRef]
- Nickolas, T.L.; McMahon, D.J.; Shane, E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J. Am. Soc. Nephrol. 2006, 17, 3223–3232. [Google Scholar] [CrossRef]
- Coco, M.; Rush, H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am. J. Kidney Dis. 2000, 36, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.W.; Hwang, Y.T.; Huang, G.S.; Liang, C.C.; Lin, J. The influence of renal dialysis and hip fracture sites on the 10-year mortality of elderly hip fracture patients: A nationwide population-based observational study. Medicine 2017, 96, e7618. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Z.; Wang, J.J.; Chung, C.R.; Huang, P.C.; Su, B.A.; Cheng, K.C.; Chio, C.C.; Chien, C.C. Epidemiology and mortality of hip fracture among patients on dialysis: Taiwan National Cohort Study. Bone 2014, 64, 235–239. [Google Scholar] [PubMed]
- Desbiens, L.C.; Goupil, R.; Madore, F.; Mac-Way, F. Incidence of fractures in middle-aged individuals with early chronic kidney disease: A population-based analysis of CARTaGENE. Nephrol. Dial. Transplant. 2020, 35, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Urena-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in patients with CKD-diagnosis, treatment, and prevention: A review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef]
- Bellorin-Font, E.; Rojas, E.; Carlini, R.G.; Suniaga, O.; Weisinger, J.R. Bone remodeling after renal transplantation. Kidney Int. Suppl. 2003, 63, S125–S128. [Google Scholar] [CrossRef][Green Version]
- Weisinger, J.R.; Carlini, R.G.; Rojas, E.; Bellorin-Font, E. Bone disease after renal transplantation. Clin. J. Am. Soc. Nephrol. 2006, 1, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Cueto-Manzano, A.M.; Konel, S.; Hutchison, A.J.; Crowley, V.; France, M.W.; Freemont, A.J.; Adams, J.E.; Mawer, B.; Gokal, R. Bone loss in long-term renal transplantation: Histopathology and densitometry analysis. Kidney Int. 1999, 55, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin. Nephrol. 2013, 33, 191–203. [Google Scholar] [CrossRef]
- Monier-Faugere, M.C.; Malluche, H.H. Trends in renal osteodystrophy: A survey from 1983 to 1995 in a total of 2248 patients. Nephrol. Dial. Transplant. 1996, 11 (Suppl. 3), 111–120. [Google Scholar] [CrossRef][Green Version]
- Sprague, S.M. Renal bone disease. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Salusky, I.B.; Coburn, J.W.; Brill, J.; Foley, J.; Slatopolsky, E.; Fine, R.N.; Goodman, W.G. Bone disease in pediatric patients undergoing dialysis with CAPD or CCPD. Kidney Int. 1988, 33, 975–982. [Google Scholar] [CrossRef]
- Malluche, H.H.; Mawad, H.; Monier-Faugere, M.C. The importance of bone health in end-stage renal disease: Out of the frying pan, into the fire? Nephrol. Dial. Transplant. 2004, 19 (Suppl. 1), i9–i13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef]
- Cannata-Andia, J.B.; Rodriguez Garcia, M.; Gomez Alonso, C. Osteoporosis and adynamic bone in chronic kidney disease. J. Nephrol. 2013, 26, 73–80. [Google Scholar] [CrossRef]
- Bover, J.; Urena-Torres, P.; Torregrosa, J.V.; Rodriguez-Garcia, M.; Castro-Alonso, C.; Gorriz, J.L.; Laiz Alonso, A.M.; Cigarrán, S.; Benito, S.; López-Báez, V.; et al. Osteoporosis, bone mineral density and CKD-MBD complex (I): Diagnostic considerations. Nefrologia 2018, 38, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J. Posttransplantation bone disease. Transplantation 2005, 79, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.; Sprague, S.M.; Cannata-Andia, J.; Coco, M.; Cohen-Solal, M.; Fitzpatrick, L.; Goltzmann, D.; Lafage-Proust, M.-H.; Leonard, M.; Ott, S.; et al. Osteoporosis in chronic kidney disease. Am. J. Kidney Dis. 2004, 43, 566–571. [Google Scholar] [CrossRef]
- Barger-Lux, M.J.; Recker, R.R. Bone microstructure in osteoporosis: Transilial biopsy and histomorphometry. Top Magn. Reason. Imaging 2002, 13, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, C.E.M.; Dos Reis, L.M.; Quadros, K.R.D.S.; Roza, N.A.V.; Sano, R.; Carvalho, A.B.; Jorgetti, V.; De Oliveira, R.B. Renal osteodystrophy and clinical outcomes: Data from the Brazilian Registry of Bone Biopsies—REBRABO. J. Bras. Nefrol. 2020, 42, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Massry, S.G.; Coburn, J.W.; Chertow, G.M.; Hruska, K.; Langman, C.; Malluche, H.; Martin, K.; McCann, L.M.; McCarthy, J.T.; Moe, S.; et al. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42 (Suppl. 3), S1–S201. [Google Scholar]
- Sherrard, D.J.; Baylink, D.J.; Wergedal, J.E.; Maloney, N.A. Quantitative histological studies on the pathogenesis of uremic bone disease. J. Clin. Endocrinol. Metab. 1974, 39, 119–135. [Google Scholar] [CrossRef]
- Ellis, H.A.; Pierides, A.M.; Feest, T.G.; Ward, M.K.; Kerr, D.N. Histopathology of renal osteodystrophy with particular reference to the effects of 1alpha-hydroxyVitamin D3 in patients treated by long-term haemodialysis. Clin. Endocrinol. 1977, 7 (Suppl. 3), 1–8. [Google Scholar] [CrossRef]
- Feest, T.G.; Ward, M.K.; Ellis, H.A.; Conceicao, S.; Pierides, A.M.; Aird, E.; Simpson, W.; Cook, D.B.; Kerr, D.N. Renal bone disease--what is it and why does it happen? Clin. Endocrinol. 1977, 7, 19–23. [Google Scholar] [CrossRef]
- Spasovski, G.B.; Bervoets, A.R.; Behets, G.J.; Ivanovski, N.; Sikole, A.; Dams, G.; Couttenye, M.; De Broe, M.E.; D’Haese, P.C. Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrol. Dial. Transplant. 2003, 18, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Drueke, T.B.; Massy, Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016, 89, 289–302. [Google Scholar] [CrossRef]
- Spasovski, G.B. Bone biopsy in the diagnosis of renal osteodystrophy. Prilozi 2004, 25, 83–93. [Google Scholar] [PubMed]
- Malluche, H.H.; Mawad, H.W.; Monier-Faugere, M.C. Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J. Bone Miner. Res. 2010, 26, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.M.; Bellorin-Font, E.; Jorgetti, V.; Carvalho, A.B.; Malluche, H.H.; Ferreira, A.; D’Haese, P.C.; Drüeke, T.B.; Du, H.; Manley, T.; et al. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients with CKD Treated by Dialysis. Am. J. Kidney Dis. 2016, 67, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Couttenye, M.M.; D’Haese, P.C.; Van Hoof, V.O.; Lemoniatou, E.; Goodman, W.; Verpooten, G.A.; De Broe, M.E. Low serum levels of alkaline phosphatase of bone origin: A good marker of adynamic bone disease in haemodialysis patients. Nephrol. Dial. Transplant. 1996, 11, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, J.C.; Plaza, C.; Torres, A.; Vega, N.; Anabitarte, A.; Fernandez, A.; Lorenzo, A.; Hortal, L.; Palop, L. Low turnover bone disease is the more common form of bone disease in CAPD patients. Adv. Perit. Dial. 1992, 8, 376–380. [Google Scholar]
- Sherrard, D.J.; Hercz, G.; Pei, Y.; Maloney, N.A.; Greenwood, C.; Manuel, A.; Saiphoo, C.; Fenton, S.S.; Segre, G.V. The spectrum of bone disease in end-stage renal failure--an evolving disorder. Kidney Int. 1993, 43, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Lorenzo, V.; Hernandez, D.; Rodriguez, J.C.; Concepcion, M.T.; Rodriguez, A.P.; Hernández, A.; de Bonis, E.; Darias, E.; González-Posada, J.M.; et al. Bone disease in predialysis, hemodialysis, and CAPD patients: Evidence of a better bone response to PTH. Kidney Int. 1995, 47, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A.; Kanis, J.A.; Beneton, M.N.; Brown, C.B.; Juttmann, J.R.; Jordans, J.G.; Josse, S.; Meyrier, A.; Lins, R.L.; Fairey, I.T. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 1995, 310, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Ballanti, P.; Bonucci, E.; Calabria, S.; Costantini, S.; Ferrannini, M.; Giustini, M.; Giordano, R.; Nicolai, G.; Manni, M.; et al. Renal Osteodystrophy in Predialysis and Hemodialysis Patients: Comparison of Histologic Patterns and Diagnostic Predictivity of Intact PTH. Nephron 2002, 91, 103–111. [Google Scholar] [CrossRef]
- Coen, G.; Ballanti, P.; Bonucci, E.; Calabria, S.; Centorrino, M.; Fassino, V.; Manni, M.; Mantella, D.; Mazzaferro, S.; Napoletano, I.; et al. Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol. Dial. Transpl. 1998, 13, 2294–2302. [Google Scholar] [CrossRef]
- Ferreira, A.; Frazao, J.M.; Monier-Faugere, M.C.; Gil, C.; Galvao, J.; Oliveira, C.; Baldaia, J.; Rodrigues, I.; Santos, C.; Ribeiro, S. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J. Am. Soc. Nephrol. 2008, 19, 405–412. [Google Scholar] [CrossRef]
- Salam, S.; Gallagher, O.; Gossiel, F.; Paggiosi, M.; Khwaja, A.; Eastell, R. Diagnostic Accuracy of Biomarkers and Imaging for Bone Turnover in Renal Osteodystrophy. J. Am. Soc. Nephrol. 2018, 29, 1557–1565. [Google Scholar] [CrossRef]
- Malluche, H.; Ritz, E.; Lange, H. Bone histology in incipient and advanced renal failure. Kidney Int. 1976, 9, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, C.; Carvalho, A.B.; Higa, A.; Jorgetti, V.; Draibe, S.A.; Canziani, M.E. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J. Bone Miner. Res. 2010, 25, 499–504. [Google Scholar] [CrossRef]
- Graciolli, F.G.; Neves, K.R.; Barreto, F.; Barreto, D.V.; Dos Reis, L.; Canziani, M.E.; Sabbagh, Y.; Carvalho, A.B.; Jorgetti, V.; Elias, R.M.; et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017, 91, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- El-Husseini, A.; Abdalbary, M.; Lima, F.; Issa, M.; Ahmed, M.-T.; Winkler, M.; Srour, H.; Davenport, D.; Wang, G.; Faugere, M.-C.; et al. Low Turnover Renal Osteodystrophy with Abnormal Bone Quality and Vascular Calcification in Patients With Mild-to-Moderate CKD. Kidney Int. Rep. 2022, 7, 1016–1026. [Google Scholar] [CrossRef]
- Coen, G.; Mazzaferro, S.; Bonucci, E.; Ballanti, P.; Massimetti, C.; Donato, G.; Landi, A.; Smacchi, A.; Della Rocca, C.; Cinotti, G.A.; et al. Treatment of secondary hyperparathyroidism of predialysis chronic renal failure with low doses of 1,25(OH)2D3: Humoral and histomorphometric results. Miner. Electrolyte Metab. 1986, 12, 375–382. [Google Scholar] [PubMed]
- Sperschneider, H.; Humbsch, K.; Abendroth, K. Oral calcitriol pulse therapy in hemodialysis patients. Effects on histomorphometry of bone in renal hyperparathyroidism. Med. Klin. 1997, 92, 597–603. [Google Scholar] [CrossRef]
- Behets, G.J.; Spasovski, G.; Sterling, L.R.; Goodman, W.G.; Spiegel, D.M.; De Broe, M.E.; D’Haese, P.C. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015, 87, 846–856. [Google Scholar] [CrossRef]
- Malluche, H.; Monier-Faugere, M.-C.; Wang, G.; O, J.M.F.; Charytan, C.; Coburn, J.; Coyne, D.; Kaplan, M.; Baker, N.; McCary, L.; et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin. Nephrol. 2008, 69, 269–278. [Google Scholar] [CrossRef]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Slatopolsky, E.; Finch, J.; Clay, P.; Martin, D.; Sicard, G.; Singer, G.; Gao, P.; Cantor, T.; Dusso, A. A novel mechanism for skeletal resistance in uremia. Kidney Int. 2000, 58, 753–761. [Google Scholar] [CrossRef]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 819–823. [Google Scholar] [CrossRef]
- Evenepoel, P.; D’Haese, P.; Brandenburg, V. Sclerostin and DKK1: New players in renal bone and vascular disease. Kidney Int. 2015, 88, 235–240. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; dos Reis, L.M.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, A.R.; Spasovski, G.B.; Behets, G.J.; Dams, G.; Polenakovic, M.H.; Zafirovska, K.; Van Hoof, V.O.; De Broe, M.E.; D’Haese, P.C. Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am. J. Kidney Dis. 2003, 41, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Canziani, M.E.; Tomiyama, C.; Higa, A.; Mozar, A.; Glorieux, G.; Vanholder, R.; A Massy, Z.; De Carvalho, A.B. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J. Bras. Nefrol. 2014, 36, 289–296. [Google Scholar] [CrossRef]
- Lima, F.; El-Husseini, A.; Monier-Faugere, M.C.; David, V.; Mawad, H.; Quarles, D.; Malluche, H.H. FGF-23 serum levels and bone histomorphometric results in adult patients with chronic kidney disease on dialysis. Clin. Nephrol. 2014, 82, 287. [Google Scholar] [CrossRef]
- Cianciolo, G.; La Manna, G.; Della Bella, E.; Cappuccilli, M.L.; Angelini, M.L.; Dormi, A.; Capelli, I.; Laterza, C.; Costa, R.; Alviano, F.; et al. Effect of Vitamin D receptor activator therapy on Vitamin D receptor and osteocalcin expression in circulating endothelial progenitor cells of hemodialysis patients. Blood Purif. 2013, 35, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Slatopolsky, E.; Gonzalez, E.; Martin, K. Pathogenesis and treatment of renal osteodystrophy. Blood Purif. 2003, 21, 318–326. [Google Scholar] [CrossRef]
- Slatopolsky, E. The role of calcium, phosphorus and Vitamin D metabolism in the development of secondary hyperparathyroidism. Nephrol. Dial Transplant. 1998, 13, 3–8. [Google Scholar] [CrossRef][Green Version]
- Hruska, K.A.; Sugatani, T.; Agapova, O.; Fang, Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 2017, 100, 80–86. [Google Scholar] [CrossRef]
- Cannata-Andia, J.B.; Martin-Carro, B.; Martin-Virgala, J.; Rodriguez-Carrio, J.; Bande-Fernandez, J.J.; Alonso-Montes, C.; Carrillo-López, N. Chronic Kidney Disease-Mineral and Bone Disorders: Pathogenesis and Management. Calcif. Tissue Int. 2021, 108, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ortiz, M.E.; Rodriguez, M. Recent advances in understanding and managing secondary hyperparathyroidism in chronic kidney disease. F1000Res 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; on behalf of the Chronic Renal Insufficiency Cohort (CRIC) Study Group; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Gogusev, J.; Duchambon, P.; Hory, B.; Giovannini, M.; Goureau, Y.; Sarfati, E.; Drüeke, T.B. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997, 51, 328–336. [Google Scholar] [CrossRef]
- Rodriguez, M.; Nemeth, E.; Martin, D. The calcium-sensing receptor: A key factor in the pathogenesis of secondary hyperparathyroidism. Am. J. Physiol. Renal. Physiol. 2005, 288, F253–F264. [Google Scholar] [CrossRef]
- Centeno, P.P.; Herberger, A.; Mun, H.-C.; Tu, C.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Slatopolsky, E.; Brown, A.; Dusso, A. Pathogenesis of secondary hyperparathyroidism. Kidney Int. Suppl. 1999, 73, S14–S19. [Google Scholar] [CrossRef]
- Slatopolsky, E.; Brown, A.; Dusso, A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am. J. Kidney Dis. 2001, 37, S54–S57. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; Floege, J.; Ketteler, M. Bone and Mineral Disorders in Chronic Kidney Disease. In Comprehensive Clinical Nephrology, 6th ed.; Feehally, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Cianciolo, G.; La Manna, G.; Donati, G.; Dormi, A.; Cappuccilli, M.L.; Cuna, V.; Legnani, C.; Palareti, G.; Colì, L.; Stefoni, S. Effects of unfractioned heparin and low-molecular-weight heparin on osteoprotegerin and RANKL plasma levels in haemodialysis patients. Nephrol. Dial. Transpl. 2011, 26, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Asci, G.; Ok, E.; Savas, R.; Ozkahya, M.; Duman, S.; Toz, H.; Kayikcioglu, M.; Branscum, A.J.; Monier-Faugere, M.-C.; Herberth, J.; et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol. Dial Transpl. 2011, 26, 1010–1015. [Google Scholar] [CrossRef]
- Fang, Y.; Ginsberg, C.; Seifert, M.; Agapova, O.; Sugatani, T.; Register, T.C.; Freedman, B.I.; Monier-Faugere, M.-C.; Malluche, H.; Hruska, K.A. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 2014, 25, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 44-1986. An 80-year-old woman with Paget’s disease and severe hypercalcemia after a recent fracture. N. Engl. J. Med. 1986, 315, 1209–1219. [Google Scholar] [CrossRef]
- Elias, R.M.; Dalboni, M.A.; Coelho, A.C.E.; Moyses, R.M.A. CKD-MBD: From the Pathogenesis to the Identification and Development of Potential Novel Therapeutic Targets. Curr. Osteoporos. Rep. 2018, 16, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Juppner, H.; Azucena-Serrano, C.E.; Yadin, O.; Salusky, I.B.; Wesseling-Perry, K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 2009, 45, 1161–1168. [Google Scholar] [CrossRef]
- Wesseling-Perry, K.; Pereira, R.C.; Wang, H.; Elashoff, R.M.; Sahney, S.; Gales, B.; Juppner, H.; Salusky, I.B. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J. Clin. Endocrinol. Metab. 2009, 94, 511–517. [Google Scholar] [CrossRef]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Ferrari, G.O.; Neves, K.R.; Cavallari, R.T.; Dominguez, W.V.; Dos Reis, L.M.; Graciolli, F.G.; Oliveira, E.C.; Liu, S.; Sabbagh, Y.; et al. Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease—Role of sclerostin? PLoS ONE 2013, 8, e79721. [Google Scholar] [CrossRef]
- Roforth, M.M.; Fujita, K.; McGregor, U.I.; Kirmani, S.; McCready, L.K.; Peterson, J.M.; Drake, M.T.; Monroe, D.G.; Khosla, S. Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone 2014, 59, 1–6. [Google Scholar] [CrossRef]
- Keller, H.; Kneissel, M. SOST is a target gene for PTH in bone. Bone 2005, 37, 148–158. [Google Scholar] [CrossRef]
- Kulkarni, N.H.; Halladay, D.L.; Miles, R.R.; Gilbert, L.M.; Frolik, C.A.; Galvin, R.J.; Gillespie, M.; Onyia, J. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell Biochem. 2005, 95, 1178–1190. [Google Scholar] [CrossRef]
- Santos, M.F.P.; Hernandez, M.J.; de Oliveira, I.B.; Siqueira, F.R.; Dominguez, W.V.; dos Reis, L.M.; Carvalho, A.B.; Moysés, R.M.A.; Jorgetti, V. Comparison of clinical, biochemical and histomorphometric analysis of bone biopsies in dialysis patients with and without fractures. J. Bone Miner. Metab. 2019, 37, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Haarhaus, M.; Evenepoel, P.; European Renal Osteodystrophy workgroup; Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD) working group of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA). Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int. 2021, 100, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin, D.; PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.J.; Haymann, J.P.; Ben M’Rad, M.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.; Ott, U.; Kaemmerer, D.; Schuetze, J.; Wolf, G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3–5. Clin. Nephrol. 2008, 70, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Beaubrun, A.C.; Kilpatrick, R.D.; Freburger, J.K.; Bradbury, B.D.; Wang, L.; Brookhart, M.A. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 1461–1469. [Google Scholar] [CrossRef]
- Moe, S.M.; Nickolas, T.L. Fractures in Patients with CKD: Time for Action. Clin. J. Am. Soc. Nephrol. 2016, 11, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Urena-Torres, P.; Cozzolino, M.; Rodriguez-Garcia, M.; Gomez-Alonso, C. The Non-invasive Diagnosis of Bone Disorders in CKD. Calcif. Tissue Int. 2021, 108, 512–527. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Bellorin-Font, E.; Benghanem Gharbi, M.; Braam, B.; Feehally, J.; Harris, D.C.; Jha, V.; Jindal, K.; et al. Global overview of health systems oversight and financing for kidney care. Kidney Int. Suppl. 2018, 8, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014, 85, 166–173. [Google Scholar] [CrossRef]
- Naylor, K.L.; McArthur, E.; Leslie, W.D.; Fraser, L.A.; Jamal, S.A.; Cadarette, S.M.; Pouget, J.G.; Lok, C.E.; Hodsman, A.B.; Adachi, J.D.; et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014, 86, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Klawansky, S.; Komaroff, E.; Cavanaugh, P.F.; Mitchell, D.Y., Jr.; Gordon, M.J.; Connelly, J.E.; Ross, S.D. Relationship between age, renal function and bone mineral density in the US population. Osteoporos. Int. 2003, 14, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, M.; Albert, J.M.; Akiba, T.; Akizawa, T.; Arab, L.; Bragg-Gresham, J.L.; Mason, N.; Prutz, K.G.; Young, E.W.; Pisoni, R.L. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006, 70, 1358–1366. [Google Scholar] [CrossRef]
- Danese, M.D.; Kim, J.; Doan, Q.V.; Dylan, M.; Griffiths, R.; Chertow, G.M. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am. J. Kidney Dis. 2006, 47, 149–156. [Google Scholar] [CrossRef]
- Dukas, L.; Schacht, E.; Stahelin, H.B. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005, 16, 1683–1690. [Google Scholar]
- Naylor, K.L.; Leslie, W.D.; Hodsman, A.B.; Rush, D.N.; Garg, A.X. FRAX predicts fracture risk in kidney transplant recipients. Transplantation 2014, 97, 940–945. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kido, R.; Onishi, Y.; Fukuma, S.; Akizawa, T.; Fukagawa, M.; Kazama, J.J.; Narita, I.; Fukuhara, S. Use of renin-angiotensin system inhibitors is associated with reduction of fracture risk in hemodialysis patients. PLoS ONE 2015, 10, e0122691. [Google Scholar] [CrossRef]
- Malluche, H.H.; Porter, D.S.; Monier-Faugere, M.C.; Mawad, H.; Pienkowski, D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J. Am. Soc. Nephrol. 2012, 23, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, C.; Ix, J.H. Diagnosis and Management of Osteoporosis in Advanced Kidney Disease: A Review. Am. J. Kidney Dis. 2022, 79, 427–436. [Google Scholar] [CrossRef]
- Tentori, F.; Albert, J.M.; Young, E.W.; Blayney, M.J.; Robinson, B.M.; Pisoni, R.L.; Akiba, T.; Greenwood, R.N.; Kimata, N.; Levin, N.W.; et al. The survival advantage for haemodialysis patients taking Vitamin D is questioned: Findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transpl. 2009, 24, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Urena, P.; Bernard-Poenaru, O.; Ostertag, A.; Baudoin, C.; Cohen-Solal, M.; Cantor, T.; De Vernejoul, M.C. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol. Dial. Transpl. 2003, 18, 2325–2331. [Google Scholar] [CrossRef]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.C. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes CKDMBDUWG. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Iimori, S.; Mori, Y.; Akita, W.; Kuyama, T.; Takada, S.; Asai, T.; Kuwahara, M.; Sasaki, S.; Tsukamoto, Y. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—A single-center cohort study. Nephrol. Dial. Transpl. 2012, 27, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yenchek, R.H.; Ix, J.H.; Shlipak, M.G.; Bauer, D.C.; Rianon, N.J.; Kritchevsky, S.; Harris, T.B.; Netwman, A.B.; A Cauley, J.; Fried, L.F. Bone Mineral Density and Fracture Risk in Older Individuals with CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1130–1136. [Google Scholar] [CrossRef]
- Naylor, K.L.; Garg, A.X.; Zou, G.; Langsetmo, L.; Leslie, W.D.; Fraser, L.A.; Adachi, J.D.; Morin, S.; Goltzman, D.; Lentle, B.; et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin. J. Am. Soc. Nephrol. 2015, 10, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Poiana, C.; Dusceac, R.; Niculescu, D.A. Utility of Trabecular Bone Score (TBS) in Bone Quality and Fracture Risk Assessment in Patients on Maintenance Dialysis. Front. Med. 2021, 8, 782837. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Abdalbary, M.; Sobh, M.; Elnagar, S.; Elhadedy, M.A.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; Sabry, A.; Halawa, A.; El-Husseini, A. Management of osteoporosis in patients with chronic kidney disease. Osteoporos. Int. 2022, 33, 2259–2274. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Marques, I.D.B.; Hans, D.; Dempster, D.; Zhou, H.; Patel, P.; Pereira, R.M.R.; Jorgetti, V.; Moyses, R.M.A.; Nickolas, T.L. The trabecular bone score: Relationships with trabecular and cortical microarchitecture measured by HR-pQCT and histomorphometry in patients with chronic kidney disease. Bone 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Nickolas, T.L.; Stein, E.M.; Dworakowski, E.; Nishiyama, K.K.; Komandah-Kosseh, M.; Zhang, C.A.; McMahon, D.J.; Liu, X.S.; Boutroy, S.; Cremers, S.; et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Miner. Res. 2013, 28, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.L.; Del Valle, E.E.; Zanchetta, M.B.; Nobaru, M.; Silveira, F.; Puddu, M.; Barone, R.; Bogado, C.E.; Zanchetta, J.R. Evaluation of bone microarchitecture by high-resolution peripheral quantitative computed tomography (HR-pQCT) in hemodialysis patients. Osteoporos. Int. 2012, 23, 2543–2550. [Google Scholar] [CrossRef]
- Drechsler, C.; Verduijn, M.; Pilz, S.; Krediet, R.T.; Dekker, F.W.; Wanner, C.; Ketteler, M.; Boeschoten, E.W.; Brandenburg, V.; NECOSAD Study Group. Bone alkaline phosphatase and mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1752–1759. [Google Scholar] [CrossRef]
- Kobayashi, I.; Shidara, K.; Okuno, S.; Yamada, S.; Imanishi, Y.; Mori, K.; Ishimura, E.; Shoji, S.; Yamakawa, T.; Inaba, M. Higher serum bone alkaline phosphatase as a predictor of mortality in male hemodialysis patients. Life Sci. 2012, 90, 212–218. [Google Scholar] [CrossRef]
- Evenepoel, P.; Cavalier, E.; D’Haese, P.C. Biomarkers Predicting Bone Turnover in the Setting of CKD. Curr. Osteoporos. Rep. 2017, 15, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.; Jones, R.G.; Rayner, H.C.; Harnden, P.; Hordon, L.D.; Aaron, J.E.; Oldroyd, B.; Brownjohn, A.M.; Turney, J.H.; Smith, M.A. Assessment of renal osteodystrophy in dialysis patients: Use of bone alkaline phosphatase, bone mineral density and parathyroid ultrasound in comparison with bone histology. Nephron 1997, 75, 412–419. [Google Scholar] [CrossRef]
- Coen, G.; Ballanti, P.; Balducci, A.; Calabria, S.; Fischer, M.S.; Jankovic, L.; Manni, M.; Morosetti, M.; Moscaritolo, E.; Sardella, D.; et al. Serum osteoprotegerin and renal osteodystrophy. Nephrol. Dial. Transpl. 2002, 17, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Moyses, R.M.; Neves, K.R.; Canziani, M.E.; Draibe, S.A.; Jorgetti, V.; Carvalho, A.B. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008, 73, 771–777. [Google Scholar] [CrossRef]
- Lehmann, G.; Stein, G.; Huller, M.; Schemer, R.; Ramakrishnan, K.; Goodman, W.G. Specific measurement of PTH (1-84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int. 2005, 68, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Haarhaus, M.; Monier-Faugere, M.C.; Magnusson, P.; Malluche, H.H. Bone alkaline phosphatase isoforms in hemodialysis patients with low versus non-low bone turnover: A diagnostic test study. Am. J. Kidney Dis. 2015, 66, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.D.; Araujo, M.J.; Graciolli, F.G.; Reis, L.M.; Pereira, R.M.; Custodio, M.R.; Jorgetti, V.; Elias, R.M.; David-Neto, E.; Moysés, R.M.A. Biopsy vs. peripheral computed tomography to assess bone disease in CKD patients on dialysis: Differences and similarities. Osteoporos. Int. 2017, 28, 1675–1683. [Google Scholar] [CrossRef]
- Grotz, W.H.; Mundinger, F.A.; Gugel, B.; Exner, V.; Kirste, G.; Schollmeyer, P.J. Bone fracture and osteodensitometry with dual energy X-ray absorptiometry in kidney transplant recipients. Transplantation 1994, 58, 912–915. [Google Scholar] [CrossRef]
- Nikkel, L.E.; Hollenbeak, C.S.; Fox, E.J.; Uemura, T.; Ghahramani, N. Risk of fractures after renal transplantation in the United States. Transplantation 2009, 87, 1846–1851. [Google Scholar] [CrossRef]
- O’Shaughnessy, E.A.; Dahl, D.C.; Smith, C.L.; Kasiske, B.L. Risk factors for fractures in kidney transplantation. Transplantation 2002, 74, 362–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khairallah, P.; Nickolas, T.L. Bone and Mineral Disease in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2022, 17, 121–130. [Google Scholar] [CrossRef]
- Patel, S.; Kwan, J.T.; McCloskey, E.; McGee, G.; Thomas, G.; Johnson, D.; Wills, R.; Ogunremi, L.; Barron, J. Prevalence and causes of low bone density and fractures in kidney transplant patients. J. Bone Miner. Res. 2001, 16, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Grotz, W.H.; Mundinger, F.A.; Gugel, B.; Exner, V.M.; Kirste, G.; Schollmeyer, P.J. Bone mineral density after kidney transplantation. A cross-sectional study in 190 graft recipients up to 20 years after transplantation. Transplantation 1995, 59, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Durieux, S.; Mercadal, L.; Orcel, P.; Dao, H.; Rioux, C.; Bernard, M.; Rozenberg, S.; Barrou, B.; Bourgeois, P.; Deray, G.; et al. Bone mineral density and fracture prevalence in long-term kidney graft recipients. Transplantation 2002, 74, 496–500. [Google Scholar] [CrossRef]
- Julian, B.A.; Laskow, D.A.; Dubovsky, J.; Dubovsky, E.V.; Curtis, J.J.; Quarles, L.D. Rapid loss of vertebral mineral density after renal transplantation. N. Engl. J. Med. 1991, 325, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.K.; de Jonge, H.; Naesens, M.; Bammens, B.; Claes, K.; Kuypers, D.; Vanrenterghem, Y. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin. J. Am. Soc. Nephrol. 2008, 3, 1829–1836. [Google Scholar] [CrossRef]
- Perrin, P.; Caillard, S.; Javier, R.M.; Braun, L.; Heibel, F.; Borni-Duval, C.; Muller, C.; Olagne, J.; Moulin, B. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am. J. Transplant. 2013, 13, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Lou, I.; Foley, D.; Odorico, S.K.; Leverson, G.; Schneider, D.F.; Sippel, R.; Chen, H. How Well Does Renal Transplantation Cure Hyperparathyroidism? Ann. Surg. 2015, 262, 653–659. [Google Scholar] [CrossRef]
- Cianciolo, G.; Cozzolino, M. FGF23 in kidney transplant: The strange case of Doctor Jekyll and Mister Hyde. Clin. Kidney J. 2016, 9, 665–668. [Google Scholar] [CrossRef]
- Lobo, P.I.; Cortez, M.S.; Stevenson, W.; Pruett, T.L. Normocalcemic hyperparathyroidism associated with relatively low 1:25 Vitamin D levels post-renal transplant can be successfully treated with oral calcitriol. Clin. Transplant. 1995, 9, 277–281. [Google Scholar]
- Iyer, S.P.; Nikkel, L.E.; Nishiyama, K.K.; Dworakowski, E.; Cremers, S.; Zhang, C.; McMahon, D.J.; Boutroy, S.; Liu, X.S.; Ratner, L.E.; et al. Kidney transplantation with early corticosteroid withdrawal: Paradoxical effects at the central and peripheral skeleton. J. Am. Soc. Nephrol. 2014, 25, 1331–1341. [Google Scholar] [CrossRef]
- Batteux, B.; Gras-Champel, V.; Lando, M.; Brazier, F.; Mentaverri, R.; Desailly-Henry, I.; Rey, A.; Bennis, Y.; Masmoudi, K.; Choukroun, G.; et al. Early steroid withdrawal has a positive effect on bone in kidney transplant recipients: A propensity score study with inverse probability-of-treatment weighting. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20953357. [Google Scholar] [CrossRef]
- Nikkel, L.E.; Mohan, S.; Zhang, A.; McMahon, D.J.; Boutroy, S.; Dube, G.; Ratner, L.; Hollenbeak, C.S.; Leonard, M.B.; Shane, E.; et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am. J. Transplant. 2012, 12, 649–659. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.R.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients. Kidney Int. 2019, 95, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Julian, B.A.; Bartolucci, A.; Saag, K.G. Bone mineral density changes within six months of renal transplantation. Transplantation 2003, 75, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Almond, M.K.; Kwan, J.T.; Evans, K.; Cunningham, J. Loss of regional bone mineral density in the first 12 months following renal transplantation. Nephron 1994, 66, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Carlini, R.G.; Rojas, E.; Weisinger, J.R.; Lopez, M.; Martinis, R.; Arminio, A.; Bellorin-Font, E. Bone disease in patients with long-term renal transplantation and normal renal function. Am. J. Kidney Dis. 2000, 36, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Ketteler, M.; Heussen, N.; Politt, D.; Frank, R.D.; Westenfeld, R.; Ittel, T.H.; Floege, J. Lumbar bone mineral density in very long-term renal transplant recipients: Impact of circulating sex hormones. Osteoporos. Int. 2005, 16, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Carrero, J.J.; Evans, M.; Fellander-Tsai, L.; Berg, H.E.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Fractures after kidney transplantation: Incidence, predictors, and association with mortality. Bone 2020, 140, 115554. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Behets, G.; Bammens, B.; Claes, K.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D.R.J.; Cavalier, E.; D’Haese, P.; et al. Natural History of Bone Disease following Kidney Transplantation. J. Am. Soc. Nephrol. 2022, 33, 638–652. [Google Scholar] [CrossRef]
- Monier-Faugere, M.C.; Mawad, H.; Qi, Q.; Friedler, R.M.; Malluche, H.H. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J. Am. Soc. Nephrol. 2000, 11, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Carlini, R.G.; Rojas, E.; Arminio, A.; Weisinger, J.R.; Bellorin-Font, E. What are the bone lesions in patients with more than four years of a functioning renal transplant? Nephrol. Dial. Transpl. 1998, 13, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Behets, G.J.; Viaene, L.; D’Haese, P.C. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int. 2017, 91, 469–476. [Google Scholar] [CrossRef]
- Neves, C.L.; dos Reis, L.M.; Batista, D.G.; Custodio, M.R.; Graciolli, F.G.; Martin Rde, C.; Neves, K.R.; Dominguez, W.V.; Moyses, R.M.; Jorgetti, V. Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation 2013, 96, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.A.; Lugon, J.R.; Jorgetti, V.; Draibe, S.A.; Carvalho, A.B. Histologic evolution of bone disease 6 months after successful kidney transplantation. Am. J. Kidney Dis. 2004, 44, 747–756. [Google Scholar] [CrossRef]
- Keronen, S.; Martola, L.; Finne, P.; Burton, I.S.; Kroger, H.; Honkanen, E. Changes in Bone Histomorphometry after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 894–903. [Google Scholar] [CrossRef]
- Rojas, E.; Carlini, R.G.; Clesca, P.; Arminio, A.; Suniaga, O.; De Elguezabal, K.; Weisinger, J.R.; Hruska, K.A.; Bellorin-Font, E. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003, 63, 1915–1923. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Ferreira, A.C.; D’Haese, P.; Haarhaus, M.; Vervloet, M.; Lafage-Proust, M.H.; Ferreira, A.; Evenepoel, P.; European Renal Osteodystrophy (EUROD) workgroup; Chronic Kidney Disease—Mineral and Bone Disorder Working Group (CKD-MBD WG). Bone histomorphometry for the diagnosis of renal osteodystrophy: A call for harmonization of reference ranges. Kidney Int. 2022, 102, 431–434. [Google Scholar] [CrossRef]

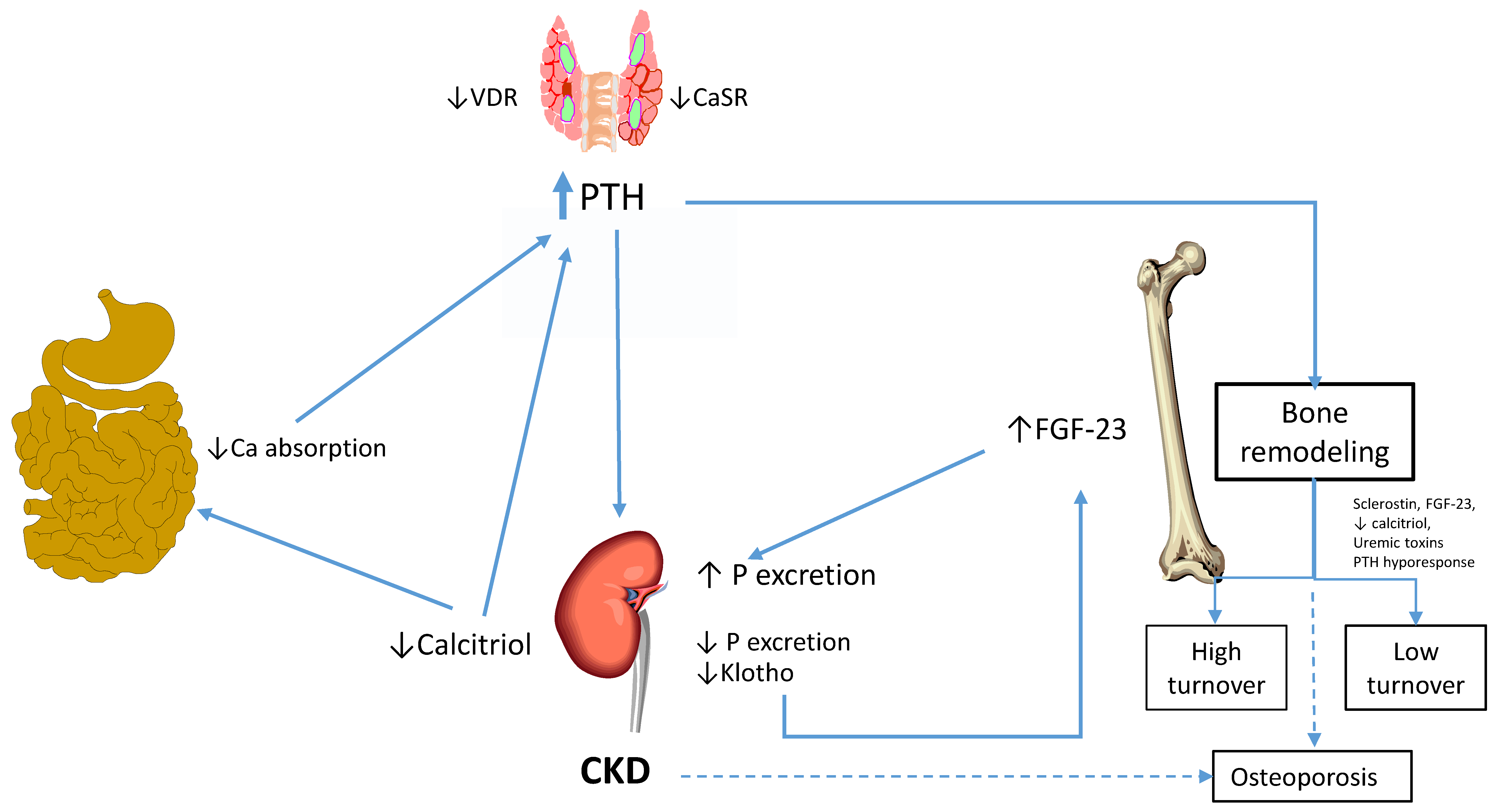
| Reference | Country | Patients Number | Age Years | Kidney Function | Low BTO % Patients | High BTO % Patients | Normal BTO % Patients | Comments |
|---|---|---|---|---|---|---|---|---|
| Couttenye [36] 1996 | Belgium, Greece, Egypt, Argentina, Slovakia, Luxemburg | 103 Female 53 | 59.7 ± 1.3 | ESKD | 46.7 | 40.8 | 12.6 | P P-binder: 89 pts (CaCO3, 33, Al (OH)3 31, both 31). Vit D: 42 pts. Al overload: 12 pts PTX: 8 pts. Previous kidney transplant: 8 pts |
| Salusky [17] 1988 | USA | 44 Fem 22 | 11.8 ± 5.8 | ESKD CAPD | 20 | 64 | 16 | P-binder: 45% of pts on Al (OH)3. CaCO3 54.5% of pts. All pts receiving PO calcitriol Bone surface AL in 10 out 20 pts receiving Al (OH)3. |
| Rodriguez-Perez [37] 1992 | Spain | 26 | NA | ESKD | 57 | 43 | NA | NA |
| Sherard [38] 1993 | USA 239 Canada 20 pts | 259 Female 63 | PD: 60 ± 12 Female 63, HD: 52 ± 1.4 Female 39 | ESKD | 66 38 | 44 62 | NR | P-binder: Al (OH)3 predominantly early in the study. CaCO3 predominantly later. Vitamin D: 12.7% of the pts). Surface bone Al 25% in 31% of the pts with low BTO. Serum Al higher in HD. Low BTO more frequent in PD. Diabetes: 30% of pts on PD and 19% of pts on HD PTX: 4% of pts on PD, 6% of pts on HD. Dialysis fluid Ca concentration 3.25 meq/L |
| Torres [39] 1995 | Spain (Canary Islands) | 119 Female 80 | 47 ± 16 | Predialysis 38 HD: 49 CAPD: 32 | 60 44 59 | 40 56 61 | NR | P-binder: CaCaO3 was primary. Al (OH)3 added to 40% of predialysis pts and 56% of dialysis pts.). Vitamin D (PO alfa-calcifediol): HD: 6, CAPD 12, predialysis none |
| Hamdy [40] 1995 | Belgium, France, Netherlands, United Kingdom. | 176 * | 18–81 | CrCl 15–50 mL/min | 4 7 | 91 93 | NA | * Placebo 87 Alfacalcidol 89 |
| Monier-Faugere [15] 1996 | USA | 2248 Female 1144 | Female 54 ± 0.4 Male: 50 ± 0.5 | ESKD HD: 81.4% CAPD 18.6% | 22.6 | 77.4 | NA | P-binder: Al containing more used between 1983 and 1987. Ca-based binders use increased progressively. Vitamin D: Calcitriol: 20% of pts; use of IV progressively increased in time. Al staining at 30% of trabecular surface in 85% of pts. Trend decreased progressively. BTO type varied within the study period. Proportion of use of DFO decreased along the survey. |
| Coen [41] 2002 | Italy | Predialysis 79 HD 107 | 51 ± 14 51.1 ± 12 | S. creatinine 5.2 ± 2.5 ESKD | Predialysis 21.5 HD 14 | Predialysis 65.8 HD 86 | Predialysis 12.6 NA | P-binder or Vitamin D: Predialysis patients: No. HD pts: CaCO3 Ca None of the pts were on Vitamin D. Bone Al higher in HD pts. |
| Coen [42] | Italy | 41 | 51.9 ± 12.4 | ESKD HD | 22 | 78 | P-binder: Ca-based in most patients. Al agents in 39% of pts for short periods. Calcitriol in a minority of pts. | |
| Spazovsky [31] 2003 | Macedonia | 84 Female 40 | 54.2 ± 12 | CCl < 5 Non-dialysis | 35 | 27 | 38 | P-binder: CaCO3 in 70% of pts). Vitamin D analog: None of the pts. |
| Ferreira 2008 [43] | Portugal | 68 Female | 55. ± 15.4 * 53.9 ± 13.7 ** | ESKD | * 67 > 56 ** 60 > 54 | 33 > 46 40 > 46 | NR | P-binders: * Sevelamer N = 33. ** Ca-based N = 35. Pts underwent bone biopsy prior to and at the end of treatment with either P-binder. BTO is shown at both periods. |
| Malluche [34] 2011 | USA Europe | 630 Black 87 White 543 | 55 ± 1 Black 50.7 ± 1.4 White 56.3 ± 0.6 | ESKD HD: 600 PD: 30 | 58 | 24 | 18 | Black patients were younger, had less time on dialysis, more treatment with vit D analog and non-Ca/non-Al containing P-binders. Diabetes prevalence: 25.3% black vs. 20.4% white pts, respectively. P-binder: CaCO3 429 pts. Active Vitamin D analogs: 109 pts. Dialysate Ca: 2.5 meq/l in 371 pts, Ca 3.3 meq/l in 259 pts. |
| Sprague [35] 2016 | Brazil Portugal, Turkey Venezuela | 492 Female 218 | 49.5± | ESKD | 52 | 48 | MR |
P-binders: Ca-based 379; Al salts 52, Sevelamer 42 pts, respectively. Vit D 143 pts. Corticosteroids 24, immunosuppressives 20 pts. Previous kidney transplant 46. Parathyroidectomy 5. |
| Salam [44] 2018 | UK | 43 | 59 ± 12 | CKD 4–5 65% predialysis | 26 | 40 | 34 | Diabetes: 28% of pts vs. 0 in controls. Previous fragility fracture 22% of pts vs. 7% in controls. By HR-pQCT, CKD pts had lower BMD, trabecular thickness, and trabecular bone volume at distal radius and distal tibia compared with controls. |
| Carbonara [25] 2020 | Brazil | 260 | 51 ± 12 | 21.6 | 76.6 | 1.8 | Osteoporosis in 35% of pts irrespective of ROD type. Al staining in 38% of the biopsies. |
| Reference | Country | Patients Number | Age Years | Kidney Function CKD Stage or GFR ml/min/1.73 m2 | Low BTO % Patients | High BTO % Patients | Normal BTO % Patients | Comments |
|---|---|---|---|---|---|---|---|---|
| Malluche 1976 [45] | Germany | 50 Female 31 | 43.2. ± 11 | 6–80 | NA | Most patients | Not reported | BTO increased with decreasing GFR. None of the pts were receiving vit D analogs, PO phosphate binders or Ca supplements. |
| Bervoets [58] 2003 | 84 | Predialysis ESKD | 35 | 27 | 38 | Active Vitamin D in 30.8% of the pts across CKD stages, mostly CKD stages 4 to 5D. Calcium containing P-binder in 21.2% of pts, mostly in CKD stage 4 to 5D | ||
| Tomiyama [46] 201 | Brazil | 50 Female 66 | GFR 15–90 | CKD 2: 100 CKD 3: 88 CKD 4: 78 | 0 0 2 | 0 12 20 | No treatment with P-binders or vit D analogs. High prevalence of hypertension, diabetes, overweight/obesity dyslipidemia. CAC detected in 66% of pts correlated with bone turnover. | |
| Barreto [59] 2014; Drueke [32] * 2016 | Brazil | 49 Female 32 | 52 | CKD stage 2–5 GFR 36 ± 17 | CKD 2–3: Predominant low BTO | CKD 4–5: Predominant high BTO | Not reported | No P-binder or vit D treatment Association of indoxyl sulfate with osteoblast surface and bone fibrosis * Reanalysis of the same data |
| Lima [60] 2019 | USA | 104 Female 75 | 59 ± 15 | CKD stage 2: 22 pts CKD stage. 3: 29 pts CKD stage 4–5: 19 pts ESKD HD: 34 pts | 55 | 33 | 13 | Treatment with active Vitamin D in 30.8% of the patients across the different stages of CKD, mostly in CKD st 4 to 5D Calcium containing P-binder in 21.2% of patients, mostly in CKD stage 4 to 5D |
| Graciolli [47] 2017 | Brazil | 148 Female 51 | 50–54 | CKD stage 2–5 CKD stage 2–3 CKD stage 4 CKD stage 5 | 83 94 83 81 | 17 6 17 19 | Not reported | P-binder or Vitamin D: Predialysis patients: No. HD pts: CaCO3 Ca None of the pts were on Vitamin D. Bone Al higher in HD pts. Predictive value of iPTH is higher in HD in HD. |
| El-Husseini [48] 2022 | USA | 32 | 61 ± 11 | 44 ± 16 | 84 | 16 | Not reported | Calcium supplement 2 pts, Vitamin D none. Diuretics 15 pts. In white pts, eGFR correlated negatively with BTO. Most pts had VC >80%. VC correlated positively with serum P, FGF-23, and activin. TBS correlated negatively with coronary calcification |
| Reference | Cohort Number of pts (N) | CKD Stage/CCl ml/min or eGFR ml/min/1.73 m2 | Fracture Type and Number | Fracture Incidence 1000 Person-Years | Comments |
|---|---|---|---|---|---|
| Alem [5] 2000 | USRDS data base N: 326,464 person-year | ESKD | Hip 6542 | Men 7.45 Women 13.63 | Relative risk highest in younger people. Added incidence of fracture increased with age and was greater for women than for men. |
| Coco [6] 2000 | N: 4039 person-year, N: 1272 pts treated | ESKD HD | Hip 56 | Men 11.7 Women 24.1 | The one-year mortality rate from hip fracture was ~2.5 times higher in dialysis pts compared with general population. |
| Jadoul [99] 2006 | DOPPS: HD pts. 12,782 | ESKD HD | Hip 174 Any 498 | 8.9 for hip 25.6 for any new fracture | Older age, female sex, prior kidney transplant and low serum albumin were predictive of new fracture. PTH > 900 was associated with risk of new fracture |
| Danese [100] 2006 | DMMS data base N: 9007 pts | NA | Hip and vertebral | 580/1000 vs. 217/1000 in the general dialysis population | Age and sex-adjusted mortality rate after fracture 2.7 times greater than the dialysis population. Pts with lower PTH were more likely to sustain a hip fracture than those with higher PTH. |
| Dukas [101] 2005 | Cross sectional N: Women 5313 N: Men 3238 | CrCl ml/min 60.9% < 65 39.1% ≥ 65 | Not reported | Not reported | CCl < 65 increased risk of experiencing falls and risk for hip fracture (OR 1.57, 95%CI 1.18–2.09, p = 0.002), and for vertebral fracture |
| Lin [8] 2014 | Taiwan NHIRD N: 51,473 incident dialysis patients | ESKD Dialysis | Hip 1903 | 8.92 Men 7.54 Female 10.12 | HD pts had a 31% higher incidence of hip fracture than PD patients (HR 1.31, 95% CI: 1.01–1.70). Patients ≥65 years old had more than 13 times the risk of a hip fracture than those 18–44 years old (HR: 13.65; 95% CI: 10.12–18.40) |
| Tentori [90] 2014 | International DOPPS N: 34,579 | ESKD/HD | NA | Japan 1 Belgium 45 | Fracture pts had 3.7-fold higher rates of death compared to DOPPS population. In most countries, mortality rates exceeded 500 per 1000 patient-year |
| Naylor [97] 2014 | Data base from Ontario, Canada N: 679,114 |
eGFR ml/min/1.73 m2: ≥60; 45–59; 30–44; 15–29; <15 | Hip Forearm Pelvis Humerus | Not available in women ≥ 60 | Fracture rate in women ≥ 65 years old at different eGFR (ml/min/1.73 m2): >60: 4.3% 45–59: 43%: 5.8% 30–44: 47.9%: 6.5% 15–29: 54.4%: 7.8% <15.54.2%: 9.6 |
| Naylor [102] 2015 | 2107 320 individuals with eGFR < 60 mL/min/1.73m2 1787 individuals with eGFR ≥ 60 mL/min/m2 |
eGFR ml/min/1.73 m2: ≥60; 45–59; 30–44 15–29; <15 | 64 (3%) over 4.8 years | Not available | The 5-year observed major osteoporotic fracture risk was 5.3% in individuals with eGFR < 60 mL/min/1.73m2 was 5.3%, comparable to the FRAX predicted fracture risk. No difference in the AUC values for FRAX in individuals with eGFR < 60 mL/min/1.73 m2 vs. those with eGFR ≥ 60 mL/min/m2 |
| Yamamoto [103] 2015 | 3276 | * 1.48 ** 2.33 | Mortality was lower in pts * using ACEI/ARB than those ** not using ACEI/ARB 13.6% vs. 16.8% | ||
| Hung [7] 2017 | Taiwan’s NHIRD Total of 61,346 first fragility hip fracture nationwide. 997 dialysis hip fracture patients were matched to 4985 non-dialysis hip fracture subjects | ESKD Dialysis | Hip 997 | Not available |
Higher proportion of femoral neck fractures in the dialysis group compared to the non-dialysis group (51% and 42%, respectively;
p < 0.001) The mortality rate was significantly higher for patients in the dialysis group, with a mortality rate of 91% compared to 71% for those in the non-dialysis group ( p < 0.001). |
| Desbiens [9] 2020 | CARTaGENE data base (CAG) N: 679,114 19,391pts with CKD included | Non-CKD: 9521 CKD: 2: 9114 CKD 3: 756 | 829 Various type | Non-CKD: 6.9 CKD 2: 7.6 CKD 3: 11.3 | Compared with the median eGFR of 90 mL/min/1.73 m2, eGFRs of 60 mL/min/1.73 m2 were associated with increased fracture incidence [adjusted hazard ratio (HR) ¼ 1.25 (95% confidence interval 1.05–1.49) for 60 mL/min/1.73 m2; 1.65 (1.14–2.37) for 45 mL/min/1.73 m2]. The effect of decreased eGFR on fracture incidence was higher in younger individuals [HR 2.45 (1.28–4.67) at 45 years; 1.11 (0.73–1.67) at 65 years and in men. |
| Reference | Country | Number of Patients | Age Years | Kidney Function | Comments |
|---|---|---|---|---|---|
| Coutteneye [36] 1996 | Belgium, Greece, Egypt, Argentina, Slovakia, Luxemburg | 103 Female 53 | 59.7 ± 1.3 | ESKD | Cut-off: BALP ≤ 27 U/l, osteocalcin 1≤; PTH: ≤ 150 pg/mL had the best specificity and sensitivity for detection of adynamic bone disease. |
| Salusky [17] 1988 | USA | 44 Female 22 | 11.8 ± 5.8 | ESKD on CAPD | Bone formation rate correlated with serum PTH. |
| Sherard [38] 1993 | USA and Canada | 259 Female 63 | PD: 60 ± 12 HD: 52 ± 1.4 | ESKD | iPTH correlated directly with bone formation: higher in high bone turnover lesions, intermediate in mild lesions with normal bone formation |
| Torres [39] 1995 | Spain (Canary Islands) | 119 Female 80 | 47 ± 16 | Predialysis 38 ESKD HD: 49 pts ESKD CAPD: 32 pts | iPTH level 120–250 pg/mL needed to avoid low bone turnover and HPT bone disease. |
| Monier-Faugere [15] 1996 | USA | 2248 Female 1144 | Female 54 ± 0.4 Male: 50 ± 0.5 | ESKD HD 81.4% CAPD18.6% | Serum ALP significantly greater in patients with HPT, and in low BTO. iPTH greater in pts with HPT than in those with MUO, and low BTO,, respectively. |
| Fletcher [123] 1997 | USA | 73 | Dialysis | PTH > 100 pg/mL sensitivity of 81%, specificity of 66% for high BTO | |
| Coen 2002 [124] | Italy | 186 | Predialysis: 79 pts HD: 107 pts | In dialysis pts, iPTH level of 150 pg/mL had a negative predictive value for low BTO of 96.4%, and a Youden index of 0.69. In predialysis pts. The Youden index was 0.57. AP had a Youden Index of 0.64. | |
| Bervoets [58] 2003 | 84 | For ABD, osteocalcin 41mcg/L, sensitivity of 83% and specificity of 67%. PPV 47%. Combination with BALP 23 U/L or less increased sensitivity, specificity, and PPV to 72%, 89% and 77%, respectively | |||
| Barreto [125] 2008 | Brazil | 97 | ESRD on dialysis | For low turnover: PTH < 150 pg/mL, sensitivity of 50%, specificity of 85%, PPV 83% For high bone turnover: PTH >300 pg/mL, sensitivity 0.69, specificity 0.75, PPV 0.62 | |
| Lehman [126] 2005 | USA | 132 | CKD 3–5 | Patients with CKD stage 3–4 and low BTO had BI-PTH (biointact PTH) and iPTH (intact PTH) levels (in pg/mL ± SD) of 35 ± 34 and 59 ± 63. For high BTO, BI-PTH and iPTH 141 ± 60 and 221 ± 106, respectively. In CKD stage 5 and low BTO BI-PTH 51 ± 38 and iPTH 90 ± 60 pg/mL. For high BTO BI-PTH and iPTH levels of 237 ± 214 and 461 ± 437 pg/mL, respectively. Areas under the ROC curves for distinguishing low BTO from high BTO were 0.94 for BI-PTH and 0.91 for iPTH, respectively in stages 3–4. For CKD stage 5, values were 0.86 and 0.85, respectively. | |
| Malluche [34] 2011 | USA Europe | 630 Female 301 | 55 ± 1 | ESKD HD: 600 PD: 30 | PTH not a significant predictor of bone turnover in black dialysis patients. PTH was a predictor of low bone turnover in white patients on dialysis |
| Haarhaus [127] 2015 | PTH, BALP, Bix have similar diagnostic accuracy in distinguishing low from non-low BTO. BALP (AUC, 0.89) and PTH (AUC, 0.85) are useful for the diagnosis of non-low BTO. B1x can be used for the diagnosis of low BTO (area under the curve (0.83) | ||||
| Sprague [35] 2016 | Brazil 156 Portugal 89 Turkey 133 Venezuela 114 | 492 Female 218 | 49.5± | ESKD |
iPTH and BALP allowed discrimination of low from non-low and high from non-high BTO. Optimal iPTH to discriminate low from non-low BTO: 104/pg/mL, and for high vs. non-high bone turnover: 323 pg/mL. Optimal BALP: 33.1 U/L, better for diagnosing low BTO. Combination of iPTH and BALP did not improve discrimination between low and high BTO |
| Marques [128] 2017 | Brazil | 31 Female 19 | 41 ± 11 | ESKD HD 27, PD 3 No dialysis 1 |
Patients with low BTO: low PTH, BAP, and TRAP5b. BAP was the best predictor of BTO status. Best cut-off 66.6 U/L. Sensitivity 85, specificity 82. |
| Graciolli [47] 2017 | Brazil | 148 Female 51 | 50–54 | CKD stage 2 -5 |
ROC PTH and FGF-23 curves were able to predict high BTO and low BTO. None of the markers was able to predict bone mineralization |
| Salam [44] 2018 | UK | 43 | 59 ± 12 | CKD 4–5 including HD | BALP, intact PINP, and TRAP 5b better than iPTH for discriminating low from non-low BTO. iPTH can discriminate high BTO from non-high BTO |
| Lima [60] 2019 | USA | 104 Female 75 | 59 ± 15 | CKD stage 2: 22 CKD stage 3: 29 CKD stage 4–519 ESKD on HD: 34 | Serum activin increased with declining eGFR Activin showed similar AUC results, specificity, and sensitivity in predicting high turnover as iPTH, BSAP, and FGF-23 for discrimination of high vs. non-high bone turnover. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellorin-Font, E.; Rojas, E.; Martin, K.J. Bone Disease in Chronic Kidney Disease and Kidney Transplant. Nutrients 2023, 15, 167. https://doi.org/10.3390/nu15010167
Bellorin-Font E, Rojas E, Martin KJ. Bone Disease in Chronic Kidney Disease and Kidney Transplant. Nutrients. 2023; 15(1):167. https://doi.org/10.3390/nu15010167
Chicago/Turabian StyleBellorin-Font, Ezequiel, Eudocia Rojas, and Kevin J. Martin. 2023. "Bone Disease in Chronic Kidney Disease and Kidney Transplant" Nutrients 15, no. 1: 167. https://doi.org/10.3390/nu15010167
APA StyleBellorin-Font, E., Rojas, E., & Martin, K. J. (2023). Bone Disease in Chronic Kidney Disease and Kidney Transplant. Nutrients, 15(1), 167. https://doi.org/10.3390/nu15010167




