Pectin in Metabolic Liver Disease
Abstract
1. Introduction
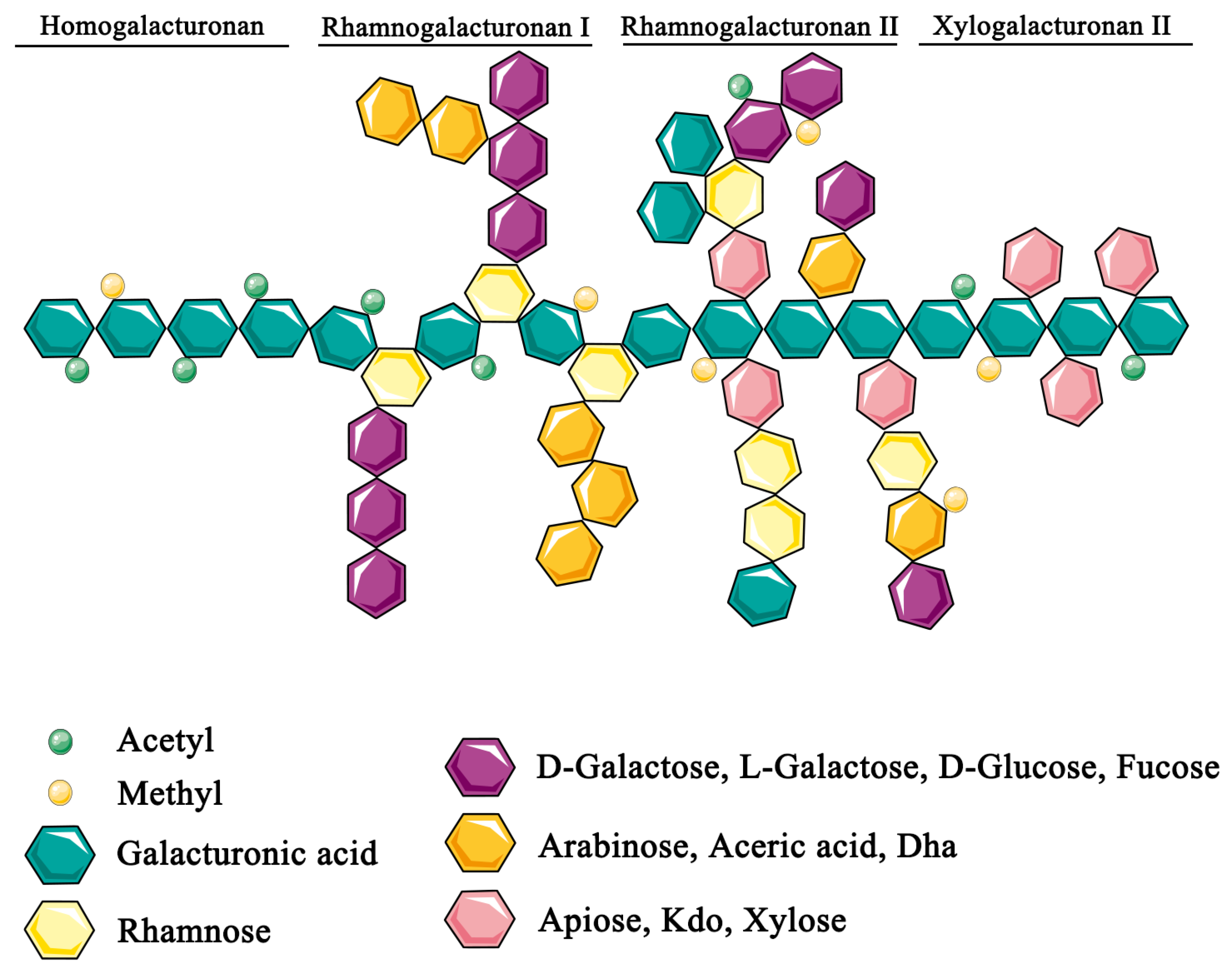
2. Biological Effects of Pectin
2.1. Physicochemical Properties of Pectin Modify Metabolite and Nutrient Availability
2.2. The Fermentation of Pectin by Gut Bacteria Produces Active Metabolites
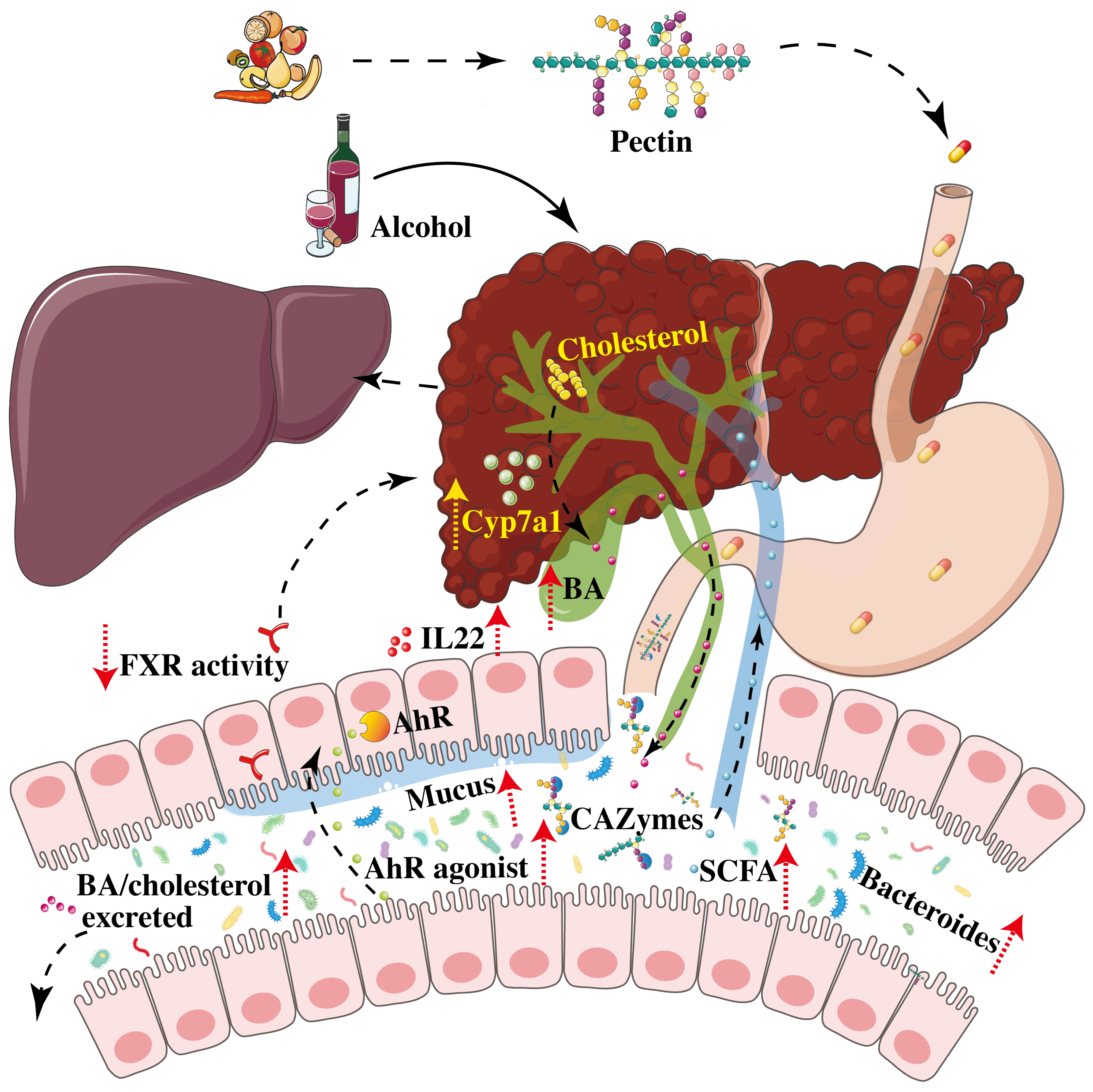
3. Pectin Alleviates NAFLD/MAFLD
4. Pectin Improves Alcoholic Liver Disease
5. Effect of Pectin on Hepatocellular Carcinoma (or Cancer)
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAT | Acyl CoA cholesterol acyltransferase |
| AhR | Aryl hydrocarbon receptor |
| ALD | Alcoholic liver disease |
| AMPK | AMP-activated protein kinase |
| ASBT | Apical sodium-dependent bile-salt transporter |
| BA | Bile acid |
| BAT | Brown adipose tissue |
| CAZymes | Carbohydrate active enzyme |
| CPT1 | Carnitine palmitoyl transferase 1 |
| C/EBP | CCAAT/enhancer binding protein |
| Cyp | Cytochrome P450 |
| DC | Dendritic cell |
| FAO | Fatty acid oxidation |
| FFAR | Free fatty acid receptor |
| FGF | Fibroblast growth factor |
| FXR | Farnesoid X receptor |
| GalA | Galacturonic acid |
| GLP-1 | Glucagon-like peptide 1 |
| GPCR | G-protein coupled receptor |
| GPR | G-protein coupled receptor |
| Glut | Glucose transporter |
| HAT | Histone acetyl transferase |
| HCAR | Hydroxycarboxylic acid receptor |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone deacetylase |
| HFD | High-fat diet |
| HMGCoA | Hydroxy-methylglutaryl coenzyme A |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| ILC | Innate lymphoid cell |
| IM | Intestinal microbiota |
| IRS-1 | Insulin receptor substrate-1 |
| LDL | Low-density lipoprotein |
| MAFLD | Metabolic-associated fatty liver disease |
| MO | Macrophage |
| MUFA | Mono-unsaturated fatty acid |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| PD1 | Programmed cell death protein 1 |
| PPAR | Peroxisome proliferator-activated receptor |
| PUFA | Poly-unsaturated fatty acid |
| PYY | Peptide YY |
| SCFA | Short-chain fatty acids |
| sAH | Severe alcoholic hepatitis |
| SFA | Saturated fatty acid |
| RG | Rhamnogalacturonan |
| TBA | Total bile acids |
| TG | Triglycerides |
| TGR5 | Takeda G-protein-coupled receptors |
| TLR | Toll-like receptor |
| Treg | Lymphocyte T regulator |
| TUDCA | Tauro-ursodeoxycholic acid |
| UCP1 | Uncoupling protein 1 |
| UDCA | Ursodeoxycholic acid |
| WAT | White adipose tissue |
| WT | Wild-type |
References
- Neuschwander-Tetri, B.A. Therapeutic Landscape for NAFLD in 2020. Gastroenterology 2020, 158, 1984–1998.e3. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. JAMA 2021, 326, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Eslam1, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.; Dufour, J.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Lang, S.; Schnabl, B. Microbiota and Fatty Liver Disease-the Known, the Unknown, and the Future. Cell Host Microbe 2020, 28, 233–244. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Amadieu, C.; Coste, V.; Neyrinck, A.M.; Thijssen, V.; Leyrolle, Q.; Bindels, L.B.; Piessevaux, H.; Stärkel, P.; Timary, P.D.; Delzenne, N.M. Restoring an adequate dietary fiber intake by inulin supplementation: A pilot study showing an impact on gut microbiota and sociability in alcohol use disorder patients. Gut Microbes 2022, 14, 2007042. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef]
- Papandreou, D.; Karabouta, Z.; Pantoleon, A.; Rousso, I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite 2012, 59, 939–944. [Google Scholar] [CrossRef]
- Alferink, L.J.M.; Erler, N.S.; de Knegt, R.J.; Janssen, H.L.A.; Metselaar, H.J.; Murad, S.D.; Jong, J.C.K. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur. J. Epidemiol. 2020, 35, 1069–1085. [Google Scholar] [CrossRef]
- Bhanja, A.; Sutar, P.P.; Mishra, M. Inulin-A polysaccharide: Review on its functional and prebiotic efficacy. J. Food Biochem. 2022, e14386. [Google Scholar] [CrossRef]
- Martinez, T.M.; Meyer, R.K.; Duca, F.A. Therapeutic Potential of Various Plant-Based Fibers to Improve Energy Homeostasis via the Gut Microbiota. Nutrients 2021, 13, 3470. [Google Scholar] [CrossRef]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.H. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Chen, Q.; Xue, G.; Ni, Q.; Wang, Y.; Gao, Q.; Zhang, Y.; Xu, G. Physicochemical and rheological characterization of pectin-rich polysaccharides from Gardenia jasminoides J. Ellis flower. Food Sci. Nutr. 2020, 8, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimization of Pectin Enzymatic Extraction from Malus domestica ‘Falticeni’ Apple Pomace with Celluclast 1.5 L. Molecules 2019, 24, 2158. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Nie, S.; Wang, Q.; Xu, X. Chain conformations and steady-shear viscosity properties of pectic polysaccharides from apple and tomato. Food Chem. X 2022, 14, 100296. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; He, M.K.; Yao, Y.G.; Qin, Z.; Cai, X.S.; Wang, X.D. Pectic polysaccharides extracted from sesame seed hull: Physicochemical and functional properties. Int. J. Biol. Macromol. 2021, 192, 1075–1083. [Google Scholar] [CrossRef]
- Matharu, A.S.; Houghton, J.A.; Lucas-Torres, C.; Moreno, A. Acid-free microwave-assisted hydrothermal extraction of pectin and porous cellulose from mango peel waste—Towards a zero waste mango biorefinery. M Green Chem. 2016, 18, 5280. [Google Scholar] [CrossRef]
- Mendez, D.A.; Fabra, M.J.; Gomez-Mascaraque, L.; Lopez-Rubio, A.; Martinez-Abad, A. Modelling the Extraction of Pectin towards the Valorisation of Watermelon Rind Waste. Foods 2021, 10, 738. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Montserrat-de la Paz, S.; Martin, M.E. Pectins and Olive Pectins: From Biotechnology to Human Health. Biology 2021, 10, 860. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Microwave-assisted extraction of pectin from grape pomace. Sci. Rep. 2022, 12, 12722. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Influence of Extraction Conditions on the Yield and Physico-Chemical Parameters of Pectin from Grape Pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef]
- Valdivia-Rivera, S.; Herrera-Pool, I.E.; Ayora-Talavera, T.; Lizardi-Jimenez, M.A.; Garcia-Cruz, U.; Cuevas-Bernardino, J.C.; Cervantes-Uc, J.M.; Pacheco, N. Kinetic, Thermodynamic, Physicochemical, and Economical Characterization of Pectin from Mangifera indica L. cv. Haden Residues. Foods 2021, 10, 2093. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Wu, Z.; Qin, D.; Li, H.; Guo, D.; Cheng, H.; Sun, J.; Huang, M.; Ye, X.; Sun, B. Physicochemical and functional properties of Lycium ruthenicum pectin by different extraction methods. Front. Nutr. 2022, 9, 946606. [Google Scholar] [CrossRef]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. J. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. J. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Dongowski, G.; Lorenz, A.; Proll, J. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. J. Nutr. 2002, 132, 1935–1944. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Chung, W.S.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef] [PubMed]
- Shtriker, M.G.; Hahn, M.; Taieb, E.; Nyska, A.; Moallem, U.; Tirosh, O.; Madar, Z. Fenugreek galactomannan and citrus pectin improve several parameters associated with glucose metabolism and modulate gut microbiota in mice. Nutrition 2018, 46, 134–142.e3. [Google Scholar] [CrossRef] [PubMed]
- Shtriker, M.G.; Peri, I.; Taieb, E.; Nyska, A.; Tirosh, O.; Madar, Z. Galactomannan More than Pectin Exacerbates Liver Injury in Mice Fed with High-Fat, High-Cholesterol Diet. Mol. Nutr. Food Res. 2018, 62, e1800331. [Google Scholar] [CrossRef]
- Tian, F.; Chi, F.; Wang, G.; Liu, X.; Zhang, Q.; Chen, Y.; Zhang, H.; Chen, W. Lactobacillus rhamnosus CCFM1107 treatment ameliorates alcohol-induced liver injury in a mouse model of chronic alcohol feeding. J. Microbiol. 2015, 53, 856–863. [Google Scholar] [CrossRef]
- Kay, R.M. Dietary fiber. J. Lipid Res. 1982, 23, 221–242. [Google Scholar] [CrossRef]
- Terpstra, A.H.; Lapre, J.A.; de Vries, H.T.; Beynen, A.C. Dietary pectin with high viscosity lowers plasma and liver cholesterol concentration and plasma cholesteryl ester transfer protein activity in hamsters. J. Nutr. 1998, 128, 1944–1999. [Google Scholar] [CrossRef]
- Terpstra, A.H.; Lapre, J.A.; de Vries, H.T.; Beynen, A.C. The hypocholesterolemic effect of lemon peels, lemon pectin, and the waste stream material of lemon peels in hybrid F1B hamsters. Eur. J. Nutr. 2002, 41, 19–26. [Google Scholar] [CrossRef]
- Krzysik, M.; Grajeta, H.; Prescha, A.; Weber, R. Effect of cellulose, pectin and chromium(III) on lipid and carbohydrate metabolism in rats. J. Trace Elem. Med. Biol. 2011, 25, 97–102. [Google Scholar] [CrossRef]
- Zhu, R.G.; Sun, Y.D.; Hou, Y.T.; Fan, J.G.; Chen, G.; Li, T.P. Pectin penta-oligogalacturonide reduces cholesterol accumulation by promoting bile acid biosynthesis and excretion in high-cholesterol-fed mice. Chem. Biol. Interact. 2017, 272, 153–159. [Google Scholar] [CrossRef]
- Song, M.; Lopez-Pena, C.L.; McClements, D.J.; Decker, E.A.; Xiao, H. Safety evaluation and lipid-lowering effects of food-grade biopolymer complexes (epsilon-polylysine-pectin) in mice fed a high-fat diet. Food Funct. 2017, 8, 1822–1829. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Liu, F.; Zhang, P.; Muhammad, Z.; Pan, S. Role of the Gut Microbiota and Their Metabolites in Modulating the Cholesterol-Lowering Effects of Citrus Pectin Oligosaccharides in C57BL/6 Mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef]
- Bagabaldo, P.A.A.; Atienza, L.M.; Castillo-Israel, K.A.T.; Estacio, M.A.C.; Gaban, P.J.V.; Maniwang, J.R.C.; Gapasin, R.P.; Estribillo, A.G.M.; Cena-Navarro, R.B. ‘Saba’ banana (Musa acuminata x balbisiana BBB Group) peel pectin supplementation improves biomarkers of obesity and associated blood lipid disorders in obese hypercholesterolemic mice. Curr. Res. Food Sci. 2022, 5, 251–260. [Google Scholar] [CrossRef]
- Dongowski, G.; Lorenz, A. Intestinal steroids in rats are influenced by the structural parameters of pectin. J. Nutr. Biochem. 2004, 15, 196–205. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Nemeth-Balogh, M.; Paulovicsova, B.; Bergstrom, A.; Wilcks, A.; Licht, T.R.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Ahn, J.; Nathani, S.; Reeves, R.D. Dietary soluble fiber and cholesterol affect serum cholesterol concentration, hepatic portal venous short-chain fatty acid concentrations and fecal sterol excretion in rats. J. Nutr. 1992, 122, 246–253. [Google Scholar] [CrossRef]
- Zhu, R.; Hou, Y.; Sun, Y.; Li, T.; Fan, J.; Chen, G.; Wei, J. Pectin Penta-Oligogalacturonide Suppresses Intestinal Bile Acids Absorption and Downregulates the FXR-FGF15 Axis in High-Cholesterol Fed Mice. Lipids 2017, 52, 489–498. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, L.; Meng, Q.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs. J. Anim. Sci. 2018, 96, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diez, F.; Garcia-Mediavilla, V.; Bayon, J.E.; Gonzalez-Gallego, J. Pectin feeding influences fecal bile acid excretion, hepatic bile acid and cholesterol synthesis and serum cholesterol in rats. J. Nutr. 1996, 126, 1766–1771. [Google Scholar] [PubMed]
- Trautwein, E.A.; Kunath-Rau, A.; Erbersdobler, H.F. Effect of different varieties of pectin and guar gum on plasma, hepatic and biliary lipids and cholesterol gallstone formation in hamsters fed on high-cholesterol diets. Br. J. Nutr. 1998, 79, 463–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, P.J. Dietary agents that target gastrointestinal and hepatic handling of bile acids and cholesterol. J. Clin. Lipidol. 2008, 2, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Holter, M.M.; Chirikjian, M.K.; Govani, V.N.; Cummings, B.P. TGR5 Signaling in Hepatic Metabolic Health. Nutrients 2020, 12, 2598. [Google Scholar] [CrossRef]
- Lin, X.; Liddle, D.M.; Neizer, H.R.; Robinson, L.E.; Wright, A.J. Acute whole apple consumption did not influence postprandial lipaemia: A randomised crossover trial. Br. J. Nutr. 2020, 123, 807–817. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef]
- Schwartz, S.E.; Levine, G.D. Effects of dietary fiber on intestinal glucose absorption and glucose tolerance in rats. Gastroenterology 1980, 79, 833–836. [Google Scholar] [CrossRef]
- Haber, G.B.; Heaton, K.W.; Murphy, D.; Burroughs, L.F. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet 1977, 2, 679–682. [Google Scholar] [CrossRef]
- Flourie, B.; Vidon, N.; Florent, C.H.; Bernier, J.J. Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut 1984, 25, 936–941. [Google Scholar] [CrossRef]
- Palou, M.; Sanchez, J.; Garcia-Carrizo, F.; Palou, A.; Pico, C. Pectin supplementation in rats mitigates age-related impairment in insulin and leptin sensitivity independently of reducing food intake. Mol. Nutr. Food Res. 2015, 59, 2022–2033. [Google Scholar] [CrossRef]
- Adam, C.L.; Williams, P.A.; Garden, K.E.; Thomson, L.M.; Ross, A.W. Dose-dependent effects of a soluble dietary fibre (pectin) on food intake, adiposity, gut hypertrophy and gut satiety hormone secretion in rats. PLoS ONE 2015, 10, e0115438. [Google Scholar] [CrossRef]
- Adam, C.L.; Williams, P.A.; Dalby, M.J.; Garden, K.; Thomson, L.M.; Richardson, A.J.; Gratz, S.W.; Ross, A.W. Different types of soluble fermentable dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr. Metab. 2014, 11, 36. [Google Scholar] [CrossRef]
- Seyrig, J.A.; Naveau, S.; Gonzales, R.; Petit, R. Pectines. Gastroenterol. Clin. Biol. 1983, 7, 1031–1037. [Google Scholar]
- Wrzosek, L.; Ciocan, D.; Hugot, C.; Spatz, M.; Dupeux, M.; Houron, C.; Lievin-Le Moal, V.; Puchois, V.; Ferrere, G.; Trainel, N.; et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 2021, 70, 1299–1308. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Munoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Hino, S.; Sonoyama, K.; Bito, H.; Kawagishi, H.; Aoe, S.; Morita, T. Low-methoxyl pectin stimulates small intestinal mucin secretion irrespective of goblet cell proliferation and is characterized by jejunum Muc2 upregulation in rats. J. Nutr. 2013, 143, 34–40. [Google Scholar] [CrossRef]
- Ferrere, G.; Wrzosek, L.; Cailleux, F.; Turpin, W.; Puchois, V.; Spatz, M.; Ciocan, D.; Rainteau, D.; Humbert, L.; Hugot, C.; et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 2017, 66, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Llopis, M.; Cassard, A.M.; Wrzosek, L.; Boschat, L.; Bruneau, A.; Ferrere, G.; Puchois, V.; Martin, J.C.; Lepage, P.; Le Roy, T.; et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016, 65, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Wicksteed, B.; Schiltz, G.E.; Gilchrist, A.; Layden, B.T. SCFA Receptors in Pancreatic beta Cells: Novel Diabetes Targets? Trends Endocrinol. Metab. 2016, 27, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Hand, T.W. The Role of the Microbiota in Shaping Infectious Immunity. Trends Immunol. 2016, 37, 647–658. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015, 21, 698–708. [Google Scholar] [CrossRef]
- Sepahi, A.; Liu, Q.; Friesen, L.; Kim, C.H. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal. Immunol. 2021, 14, 317–330. [Google Scholar] [CrossRef]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef]
- Wu, J.; Chen, M.; Shi, S.; Wang, H.; Li, N.; Su, J.; Liu, R.; Huang, Z.; Jin, H.; Ji, X.; et al. Hypoglycemic effect and mechanism of a pectic polysaccharide with hexenuronic acid from the fruits of Ficus pumila L. in C57BL/KsJ db/db mice. Carbohydr. Polym. 2017, 178, 209–220. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Ruiz, L.R.; Conde, A.K.; Sun, D.M.; Erickson, S.K.; McNamara, D.J. Psyllium reduces plasma LDL in guinea pigs by altering hepatic cholesterol homeostasis. J. Lipid Res. 1995, 36, 1128–1138. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Vergara-Jimenez, M.; Romero, A.L.; Erickson, S.K.; McNamara, D.J. Gender differences in response to dietary soluble fiber in guinea pigs: Effects of pectin, guar gum, and psyllium. J. Lipid Res. 1995, 36, 2191–2202. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Dong, Y.; Zhu, R.; Liu, Y. Antioxidant activity of penta-oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014, 145, 335–341. [Google Scholar] [CrossRef]
- Li, T.P.; Zhu, R.G.; Dong, Y.P.; Liu, Y.H.; Li, S.H.; Chen, G. Effects of pectin pentaoligosaccharide from Hawthorn (Crataegus pinnatifida Bunge. var. Major) on the activity and mRNA levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. J. Agric. Food Chem. 2013, 61, 7599–7605. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrne, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef]
- Adam, C.L.; Thomson, L.M.; Williams, P.A.; Ross, A.W. Soluble Fermentable Dietary Fibre (Pectin) Decreases Caloric Intake, Adiposity and Lipidaemia in High-Fat Diet-Induced Obese Rats. PLoS ONE 2015, 10, e0140392. [Google Scholar] [CrossRef]
- Fak, F.; Jakobsdottir, G.; Kulcinskaja, E.; Marungruang, N.; Matziouridou, C.; Nilsson, U.; Stalbrand, H.; Nyman, M. The physico-chemical properties of dietary fibre determine metabolic responses, short-chain Fatty Acid profiles and gut microbiota composition in rats fed low- and high-fat diets. PLoS ONE 2015, 10, e0127252. [Google Scholar] [CrossRef]
- Samout, N.; Bouzenna, H.; Dhibi, S.; Ncib, S.; ElFeki, A.; Hfaiedh, N. Therapeutic effect of apple pectin in obese rats. Biomed. Pharmacother. 2016, 83, 1233–1238. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced non-alcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Drew, J.E.; Reichardt, N.; Williams, L.M.; Mayer, C.D.; Walker, A.W.; Farquharson, A.J.; Kastora, S.; Farquharson, F.; Milligan, G.; Morrison, D.J.; et al. Dietary fibers inhibit obesity in mice, but host responses in the cecum and liver appear unrelated to fiber-specific changes in cecal bacterial taxonomic composition. Sci. Rep. 2018, 8, 15566. [Google Scholar] [CrossRef]
- Bray, J.K.; Chiu, G.S.; McNeil, L.K.; Moon, M.L.; Wall, R.; Towers, A.E.; Freund, G.G. Switching from a high-fat cellulose diet to a high-fat pectin diet reverses certain obesity-related morbidities. Nutr. Metab. 2018, 15, 55. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, X.; Sun, X.; Li, W.; Liu, T.; Zhang, X.; Li, Y.; Li, T.; Li, S. Pectic Oligogalacturonide Facilitates the Synthesis and Activation of Adiponectin to Improve Hepatic Lipid Oxidation. Mol. Nutr. Food Res. 2021, 65, e2100167. [Google Scholar] [CrossRef] [PubMed]
- Houron, C.; Ciocan, D.; Trainel, N.; Mercier-Nome, F.; Hugot, C.; Spatz, M.; Perlemuter, G.; Cassard, A.M. Gut Microbiota Reshaped by Pectin Treatment Improves Liver Steatosis in Obese Mice. Nutrients 2021, 13, 3725. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.C.; Warren, D.C.; Lateef, S.N.; Benedito, V.A.; Tou, J.C. Apple Pomace Consumption Favorably Alters Hepatic Lipid Metabolism in Young Female Sprague-Dawley Rats Fed a Western Diet. Nutrients 2018, 10, 1882. [Google Scholar] [CrossRef] [PubMed]
- Jakobsdottir, G.; Jadert, C.; Holm, L.; Nyman, M.E. Propionic and butyric acids, formed in the caecum of rats fed highly fermentable dietary fibre, are reflected in portal and aortic serum. Br. J. Nutr. 2013, 110, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2017, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1927633. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- Ciocan, D.; Rebours, V.; Voican, C.S.; Wrzosek, L.; Puchois, V.; Cassard, A.M.; Perlemuter, G. Characterization of intestinal microbiota in alcoholic patients with and without alcoholic hepatitis or chronic alcoholic pancreatitis. Sci. Rep. 2018, 8, 4822. [Google Scholar] [CrossRef]
- Ciocan, D.; Voican, C.S.; Wrzosek, L.; Hugot, C.; Rainteau, D.; Humbert, L.; Cassard, A.M.; Perlemuter, G. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol. Ther. 2018, 48, 961–974. [Google Scholar] [CrossRef]
- Ciocan, D.; Spatz, M.; Trainel, N.; Hardonniere, K.; Domenichini, S.; Mercier-Nome, F.; Desmons, A.; Humbert, L.; Durand, S.; Kroemer, G.; et al. Modulation of the Bile Acid Enterohepatic Cycle by Intestinal Microbiota Alleviates Alcohol Liver Disease. Cells 2022, 11, 968. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Lv, L.; Jiang, H.; Yan, R.; Wang, S.; Lu, Y.; Wu, Z.; Shen, J.; Jiang, S.; et al. Identification of a protective Bacteroides strain of alcoholic liver disease and its synergistic effect with pectin. Appl. Microbiol. Biotechnol. 2022, 106, 3735–3749. [Google Scholar] [CrossRef]
- Maino Vieytes, C.A.; Taha, H.M.; Burton-Obanla, A.A.; Douglas, K.G.; Arthur, A.E. Carbohydrate Nutrition and the Risk of Cancer. Curr. Nutr. Rep. 2019, 8, 230–239. [Google Scholar] [CrossRef]
- Daillere, R.; Vetizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Zhang, S.L.; Mao, Y.Q.; Zhang, Z.Y.; Li, Z.M.; Kong, C.Y.; Chen, H.L.; Cai, P.R.; Han, B.; Ye, T.; Wang, L.S. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021, 11, 4155–4170. [Google Scholar] [CrossRef]
- Liu, H.Y.; Huang, Z.L.; Yang, G.H.; Lu, W.Q.; Yu, N.R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef]
- Park, S.N.; Noh, K.T.; Jeong, Y.I.; Jung, I.D.; Kang, H.K.; Cha, G.S.; Lee, S.J.; Seo, J.K.; Kang, D.H.; Hwang, T.H.; et al. Rhamnogalacturonan II is a Toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells. Exp. Mol. Med. 2013, 45, e8. [Google Scholar] [CrossRef][Green Version]
- Goldsworthy, T.L.; Hamm, T.E., Jr.; Rickert, D.E.; Popp, J.A. The effect of diet on 2,6-dinitrotoluene hepatocarcinogenesis. Carcinogenesis 1986, 7, 1909–1915. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e22. [Google Scholar] [CrossRef]
- Hendrikx, T.; Duan, Y.; Wang, Y.; Oh, J.H.; Alexander, L.M.; Huang, W.; Starkel, P.; Ho, S.B.; Gao, B.; Fiehn, O.; et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2018, 68, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhanel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]

| Pectin Sources | Yield (%) | DM (%) | Mw (kg/mol) | Reference |
|---|---|---|---|---|
| Apple pomace | 4.2–25.3 | 41.7–96.02 | 142–899 | [20,22,23,24] |
| Banana peel | 2–9 | 40–80 | 87–248 | [22,28] |
| Beet pulp | 20.0–24.87 | 52–58.92 | 116–311 | [20,32] |
| Carrot pomace | 5–15.2 | 45.2–77 | 114–1460 | [20] |
| Chicory | 12.2 | 44.7 | 260 | [20] |
| Citron peels | 13.4–37.52 | 37.5 -82.2 | 342.7 -918 | [20,32] |
| Cocoa pod husks | 4.2 | 8.1 | - | [20] |
| Cubiu fruit | 14.2 | 62 | 628 | [20] |
| Eggplant peel waste | 26.1 | 60.2 | - | [20] |
| Fresh watermelon rinds | 19.3 | 63.0 | 34.51 | [20] |
| Gardenia jasminoides J. Ellis flower | 18.04 ± 1.81 | 32.76 ± 1.58 | 141.50 ± 52.09 | [21] |
| Grape pomace | 3.96–11.23 | 62.14–83.11 | 41.5–53 | [30,31] |
| Grapefruit peel | 25–30 | 67.59, 69.03 | 132.01, 385.5 | [20,28] |
| Green tea leaf | 5.3–9.2 | 21.1–26.5 | 276–396 | [20] |
| Jackfruit rinds | 14.59 | - | - | [20] |
| Lime peel | 13–26 | 78.49 | 794.7 | [20,28] |
| Lycium ruthenicum | 3.1–7.31 | 2.96–31.03 | 38.24–5291 | [34] |
| Lyophilized watermelon rinds | 14.2 | 61.5 | 40.39 | [20] |
| Mango peel residues | 1.36–20.9 | 70–88.38 | 14.13–2858 | [22,26,29,32,33] |
| Medlar fruit | - | 62.9 | 198 | [20] |
| Orange peel | 24 | 37 | - | [28] |
| Papaya peel | 16 | 53.4 | - | [20] |
| Passion fruit | 10–14.8 | 9.57–60 | 802 | [20,28] |
| Pomegranate peel | 8.5 | 75 | 549 | [20] |
| Pomelo peels | 6–37 | 57.87 | 353 | [20,28] |
| Ponkan peel | 25.6 | 85.7 | 86.0 | [20] |
| Potato pulp | 14.34 | 37.45 | 320 | [20] |
| Pumpkin waste | 7.4 | 3–18 | 139–289 | [22,28] |
| Sesame seed hull | 0.03–8.07 | 33.11–41.53 | 22.7–44.6 | [25] |
| Stems of E. arvense | 5.9 | 16 | 360 | [20] |
| Sugar beet pulp | 7.1, 24 | 28–52 | 651, - | [20,28] |
| Sweet prickly pear | - | 26.83 | 204.08 | [20] |
| Tomato | 7.55–32.6 | 45.7–88.98 | 19 | [24] |
| Unripe banana | 11.63 | - | - | [20] |
| Watermelon peel | 2.1–28 | 41.2–87.28 | 34.9–119 | [20,27,28] |
| Animal Species | Animal Model | Type of Pectin | Pectin Amount % or g/day/kg | Duration in Days and (weeks) | Weight Gain Fat Mass Adipose Tissue | Liver Steatosis Liver Lipid Metabolism | Plasma Lipids Plasma Metabolites | ALT Liver Inflammation Liver Metabolism | Bile Acid and Cholesterol Metabolism or Metabolites SCFA | Glucose Homeostatis | IM Composition and Gut Homeostasis | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chow Diet | C57BL/Ksj db/db mice, male | Standard chow diet UNK | Ficus pumila Linn Pectin HM | 100 or 200 mg/kg/day gavage | curative 28 days (4 w) in 12 w | no effect on BW, Food & water intake | ↓ steatosis mRNA & protein ↓ G6Pase, PEPCK, pIRS-1, pGS ↑ GK, pAkt, pGSK3 & pAMPK | ↓ fasting blood glucose ↓ serum insulin ↓ HOMA-IR ↑ liver glycogen | [89] | ||||
| Wistar rats, male | Standard chow diet UNK 3.3 kcal/g 8% kcal from fat 4% cellulose | Apple pectin HE | 10% | preventive 30 days (1 m) | ↓ BW gain and cumulative food intake ↓ fat content ↑ lean mass WAT: ↓ Prkaa2, IRS1, AKT, pAKT, Ppar, Acaca, Fasn, Gpam, Scd1, Lpl, Slc2a4, Pnpla2 ↑ STAT3, pSTAT3, AMPK | ↓ Irs1, pIrs1, Prkaa2, Lepr, AMPK & pAMPK, Srebf1, Mlxipl, Gpam, Fasn, Scd1, ACC ↑ STAT3 & pSTAT3, Pnpla2, CPT1, | ↓ plasma leptin ↑ adiponectin = ↓ L/A ratio | ↓ fasted blood glucose and insulin ↓ HOMA-IR index | [71] | ||||
| High Chol Diet | Hartley guinea pigs, male and female | Protein 23% Fat 15.1% Carbohydrate 52.1% Simple sugar UNK Fiber 12.5% Chol 0.04% | Lime peels pectin vs. cellulose as control vs. gum guar | 12.5% | preventive 28 days (4 w) | ↓ free & total & esterified Chol | ↓ TC, VLDL, LDL | ↑ HMG-CoA reductase, chol 7a-hydroxylase, apoB/E receptor ↓ hepatic ACAT ↑ reductase activity | [91] | ||||
| Hartley guinea pigs, male | ↓ free & esterified Chol | ↓ Chol ↓ ApoB | ↑ chol 7a-hydroxylase | ↓ Intestinal Chol absorption ↑ LDL FCR | [90] | ||||||||
| Kunming mice, males | Protein UNK Fat UNK (Lard 5%) Carbohydrate UNK Simple sugar UNK Fiber UNK Chol 2% vs. 0.4% | pectin HPPS | 300 mg/kg BW oral infusion | preventive 8 days (4 w) | ↓ BW gain, serum & hepatic TC level | ↓ hepatic TC level | ↓ BA in liver, ileal, small intestine levels, total BA pool size ↑ BA content in gallbladder, feces mRNA or protein: ↓ ileal FXR, FGF15, SHP1, ↑ ASBT ↓ liver FGFR4, Cyp7a1 ↑ liver Cyp7a1 | [51] | |||||
| High Chol Diet | Kunming mice, male | Protein UNK Fat 10% (Lard 10%) Carbohydrate UNK Simple UNK Fiber UNK Chol 2% | pectin HPPS GA 98% | 50, 150 or 300 mg/kg BW oral infusion | preventive 28 or 70 days (4 w or 10 w) | ↓ BW gain in mice fed a HFD ↓ fat accumulation | ↑ FA oxidation-e activities ↑ CPT-I & 3KCT (4, 10 w), DCR & ACO (10 w), ↑ activities of peroxisomal 3KCT, ACO DCR, mitochondrial CPT-I ↑ PPAR | ↓ FFA ↓ TG | ↑ fecal total lipids | [93] | |||
| preventive 70 days (10 w) | ↓ BW gain ↓ eWAT ↓ perirenal fat pads in HFD fed mice | ↓ liver TG, GPAT & PAP activity ↓ lipid steatosis | ↑ antioxidant enzyme activities: SOD, GSH-Px, CAT, GSH & TAC ↓ MDA | [92] | |||||||||
| High Chol Diet | Wistar rats, male | Protein 20% Fat 28% (Lard 23%) Carbohydrate 44% Simple sugar 10% Fiber 34% Chol 2% | Apple pectin HE 70–75% +/− guar gum | 8% | preventive 14, 28, 42 days (2 w, 4 w or 6 w) | ↓ BW gain ↓ fat content | ↓ liver weight ↓ TG ↓ Chol | ↓ Chol 6 weeks: ↓ serum MCP-1 | 2 weeks: ↑ serum & cecal acetate ↑ serum propioniate ↑ serum & cecal total SCFA | ↑ Bacteroides (guar gum), ↑ Akkermansia (fibre-free), great individual variance (pectin) 2 weeks: ↑ weight of cecal content | [94] | ||
| Protein 12% Fat 10% Carbohydrate 62.1% Simple sugar 10% Fiber 52.1 % | Citrus pectin vs. guar gum vs. FOS | 10% | preventive 12 days (1.7 w) | ↑ acetate in cecum and portal serum: correlation between cecum-formed and absorbed SCFA | ↓ caecum tissue weight | [104] | |||||||
| Protein 20% Fat 28% (Lard 23%) Carbohydrate 35% Simple sugar 10% Fiber 25% Chol 2% | Citrus peel pectin LM (24%) or HM (70%) | 8% | preventive 21 days (3 w) | ↓ BW gain, epididymal fat pad, liver & spleen weight, ↓ liver fat | ↓ TG ↓ chol (HMp), | ↓ TG (LMp), no change of chol | ↑ serum & cecal SCFA ↑ acetate (HMp)—no changes for propioniate butyrate | ↓ blood glucose | ↑ Akkermansiano effect in Lactobacillus, Bacteroides et Bifidobacterium | [96] | |||
| UNK except Fat 10% sheep fat | Apple pectin | 0,5 mg/kg/day (gastric gavage) | preventive 49 days (7 w) | ↓ BW gain ↓ eWAT ↓ perirenal fat pads in HFD fed mice | ↓ serum TC, LDL-C, TG levels ↑ HDL-C ↓ TBARS level ↑ SOD, CAT and GSH-Px activities | restore normal AST, ALT ↑ SOD, CAT and GSH-Px, activities (liver, kidney) ↓ TBARS level (liver, kidney) | [97] | ||||||
| Sprague Dawley rats, male | AIN-93 modified Protein 26.7% Fat 23.7% (Lard 19.4%) Carbohydrate 32.8% Simple sugar 10.5% Fiber 22.8% | Apple pectin HM and HE > 50% | 10% | curative 28 days (4 w) in 11 w | ↓ Final BW & BW gain ↓ fat mass & body fat percentage ↓ total lean mass ↑ total body lean | ↓ liver total fat ↓ TG levels | ↓ total chol & TG ↑ Plasma PYY ↓ Plasma leptin | ↓ serum insulin | ↓ Cumulative caloric intake ↑ small intestine and caecum weights and lengths | [95] | |||
| Sprague-Dawley rats female | Protein 19.5% Fat 23% (Lard 21%) Carbohydrate 51% Simple sugar 34% Fiber 11% | Apple pectin (Apple pomace) | 10% | preventive 56 days (8 w) | ↓ fat vacuoles & histology scores ↑ palmitic acid (16:0) ↓ palmitoleic acid & oleic acid content ↓ liver DGAT2 mRNA | ↓ total BA concentration | [103] | ||||||
| C57BL/6J mice, male | Protein UNK Fat 30% (Lard 30%) Carbohydrate UNK Simple sugar UNK Fiber UNK Chol UNK | Citrus peel pectin GA > 74% | 4% & 8% | preventive 84 days (12 w) | ↓ BW gain ↓ BMI ↓ eWAT weight ↓ fat index ↓ adipocyte size | ↓ TG, TC, NEFA ↓ FAS, ACC & ChREBP levels ↓ SFA, MUPA, palmitic acid levels ↑ PUFA ↓ hepatic fat accumulation | ↓ TG, TC, LDL-C ↑ HDL-C | ↓ ALT, AST ↓ liver NF-B, TNF, PPAR & MDA, p-ERK, p-JNK, p-p38, Nrf2 levels, ratios of pERK/ERK and pJNK/JNK ↑ GSH-Px, SOD activities | ↓ cecal isobutyric acid, isovaleric acid & valeric acid levels ↑ cecal total SCFA, acetate & propioniate levels | ↑ Firmicutes, Bacteroides, Parabacteroides, Allobaculum, Bifidobacterium, Olsenella, Barnesiella, Anaerobacterium, Clostridium IV ↓ Lachnospiraceae, Lactobacillaceae, Lactobacillus, Helicobacter, Alistipes, Clostridium XIVa | [98] | ||
| Protein 30% Fat 40% (Lard UNK) Carbohydrate 30% Simple sugar UNK Fiber UNK | Pectin UNK | 10% | curative 35 days (5w) in 17w | ↓ BW (LFP diet) ↓ BW gain (HFP diet) | ↓ liver adiposity | ↓ fasted blood glucose | [100] | ||||||
| Protein 23% Fat 23.5% (Lard 21%) Carbohydrate 46.5% Simple sugar 20% Fiber 14.3% | Apple pectin | 10% | preventive 56 days (8w) | ↓ BW gain ↓ fat mass | ↓ liver lipid | ↓ plasma leptin, resistin | ↓ insulin fed (or fasted, unclear) | ↑ Bacteroidetes, Proteobacteria, Deltaproteobacteria ↓ cecal Claudin5, Trefoil Factor3 gene expressions | [99] | ||||
| Protein 26% Fat 35% (Lard 31%) Carbohydrate 32% Simple sugar 9.5% Fiber 6.5% | Apple pectin | 2% (0.06 g pectin/30 g of mouse = 2 g/kg) | Curative 56 days (8 w) in (16 w) | eWAT ↓ semi-quantified adipocyte diameter | ↓ TG, liver/body ratio ↓ lipid droplet size in BAT | ↓ Firmicutes, Ruminococcus, Desulfovibrionaceae, proteobacteria ↑ Bacteroidetes, S24_7, Prevotellaceae, Turicibacteraceae | [102] | ||||||
| Kunming mice, male | UNK HFK Bioscience Chow and High fat diets | Hawthorn pectin oligosaccha ride (POS) | 0.25, 0.75, 1.5 g/kg diet (0.025%, 0.075%, 0.15%) | preventive 70 days (10 w) | WAT mRNA and protein ↑ cAMP, AC, C/EBP, PPAR, RXR, PKA, Pap1, pSRC, pERK, pCREB | mRNA or protein:↑ ADPN, LKB1, ACO, CPT-1, adipoR1 (1.5 g/kg), PPAR, PGC-1, NRF-1 (0.75 & 1.5 g/kg). For all diets ↑ AMPK, p-AMPK, adipoR1 ↓ ACC | ↓ TG, TC, total lipids, ADPN level | [101] |
| Animal Species | Type of Pectin | Pectin Amount % or g/day/kg | Duration in Days and (weeks) | Liver Steatosis Lipid Metabolism | Plasma Lipids Plasma Metabolites | ALT Liver Inflammation Liver Metabolism | Bile Acid and Cholesterol Metabolism or Metabolites SCFA | IM Composition and Gut Homeostasis | Ref |
|---|---|---|---|---|---|---|---|---|---|
| C57BL/6J mice, female | Apple pectinHM (73%) | 6.5% | Prevention 21 days (3 w) | ↓ steatosis ↓ TG | ↓ ALT ↓ liver weight ↓ liver TNF, IL1, IL6, CCL2, TGF | ↓ fecal BA, TUDCA & UDCA, | ↑ Bacteroides, proportion of Enterobacteriaceae ↓ reduced IM diversity ↑ goblet cells, Reg3 (colon & Ileum), reg3 (Ileum) | [81] | |
| C57BL/6J mice, female | Apple pectin | 0.4%, 1%, 2% and 6.5% | Curative 7 days (1 w) in 28 days (4 w) | ↓ steatosis ↓ TG | not modify alcohol absorption | ↓ ALT, CCL2, CCL3, TNF, IL1 ↑ bacterial genes involved in carbohydrate, lipid, and amino-acid metabolism | ↓ Tryptophan, Indole ↑ total AhR agonists | ↑ Bacteroides, Bacteroidetes, Lactobacillus ↓ Firmicutes ↑ Proteobacteria and Enterobacteriaceae (6.5% pectin) ↑ Reg3, reg3(colon & Ileum) (2% & 6.5% pectin)↑ Cyp1a1, AhRr, il17, il22(colon) (2% & 6.5% pectin) | [75] |
| C57BL/6J mice, female | Apple pectin | 6.5% | Prevention 21 days (3 w) | ↓ TG ↓ BAT UCP1 | ↓ ALT ↓ liver TNF, IL1, CCR2, CCL2, CCL3 | ↓ plasma total BA ↓ plasma CA, MCA, MCA, DCA, TCA, TMCA ↓ liver MCA, TDCA ↑ liver TCDCA ↑ caecum CA, CDCA, UDCA, TCA, TMCA, TUDCA ↓ caecum MCA, DCA, LCA, TCDCA, TDCA ↓ ileum MRP2, SGLT1, Glut2, CD36, Fabp1 mRNA ↑ ileum MRP3 mRNA ↑ colon ASBT, OST mRNA | ↑ Bacteroides, Enterobacteriacae ↓ Lactobacillus and Enterococcus | [111] | |
| C57BL/6J mice, female | Apple pectin (PE) vs. PE + B. fragilis ATCC25285 (BFPE) | 2% | Prevention 10 days (1.4 w) | ↓ steatosis and neutrophil infiltration ↓ TG ↓ IL-1, IL-1, and TNF-, CD36, PPAR mRNA, ↓ Liver TLR4 mRNA | ↓ plasma LPS, LBP, IL-2, IL-12 | ↓ ALT (BFPE) | ↑ acetate (BFPE), propioniate, butyrate in cecal contents ↑ IPA & IAA & Tryptophan (BFPE), ILA in colon contents | ↑ shannon index ↑ Bacteroides, B. fragilis, Bacteroidetes, Bacteroidales, Proteobacteria, Enterobacterales, Escherichia-Shigella, Lachnospirales ↓ Firmicutes, Erysipelotrichales, Monoglobales, Peptostreptococcales-Tissierellales, Dubosiella, Monoglobus, Allobaculum, Faecalibaculum, Romboutsia ↑ colon goblet cell counts, MUC2 mRNA ↑ colon ZO-1(BFPE), IL-22, Reg3, and Reg3 | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Cassard, A.-M.; Ciocan, D. Pectin in Metabolic Liver Disease. Nutrients 2023, 15, 157. https://doi.org/10.3390/nu15010157
Hu W, Cassard A-M, Ciocan D. Pectin in Metabolic Liver Disease. Nutrients. 2023; 15(1):157. https://doi.org/10.3390/nu15010157
Chicago/Turabian StyleHu, Wanchao, Anne-Marie Cassard, and Dragos Ciocan. 2023. "Pectin in Metabolic Liver Disease" Nutrients 15, no. 1: 157. https://doi.org/10.3390/nu15010157
APA StyleHu, W., Cassard, A.-M., & Ciocan, D. (2023). Pectin in Metabolic Liver Disease. Nutrients, 15(1), 157. https://doi.org/10.3390/nu15010157






