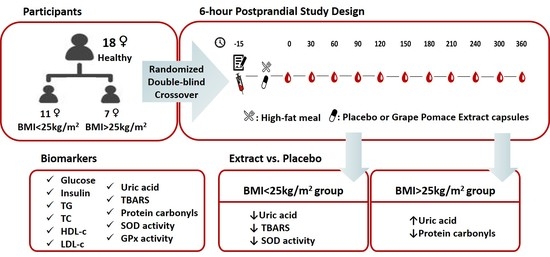
Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Grape Pomace Extract
2.4. Dietary and Physical Assessment
2.5. Anthropometry and Blood Sample Collection
2.6. Basic Biochemical Markers
2.7. Thiobarbituric Acid Reactive Substances (TBARS)
2.8. Protein Carbonyls
2.9. Superoxide Dismutase (SOD) Activity
2.10. Glutathione Peroxidase (GPx) Activity
2.11. Statistics
3. Results
3.1. Baseline Anthropometric, Biochemical, and Oxidative Stress Markers
3.2. Dietary Assessment and Study Compliance
3.3. Basic Biochemical Markers during Interventions
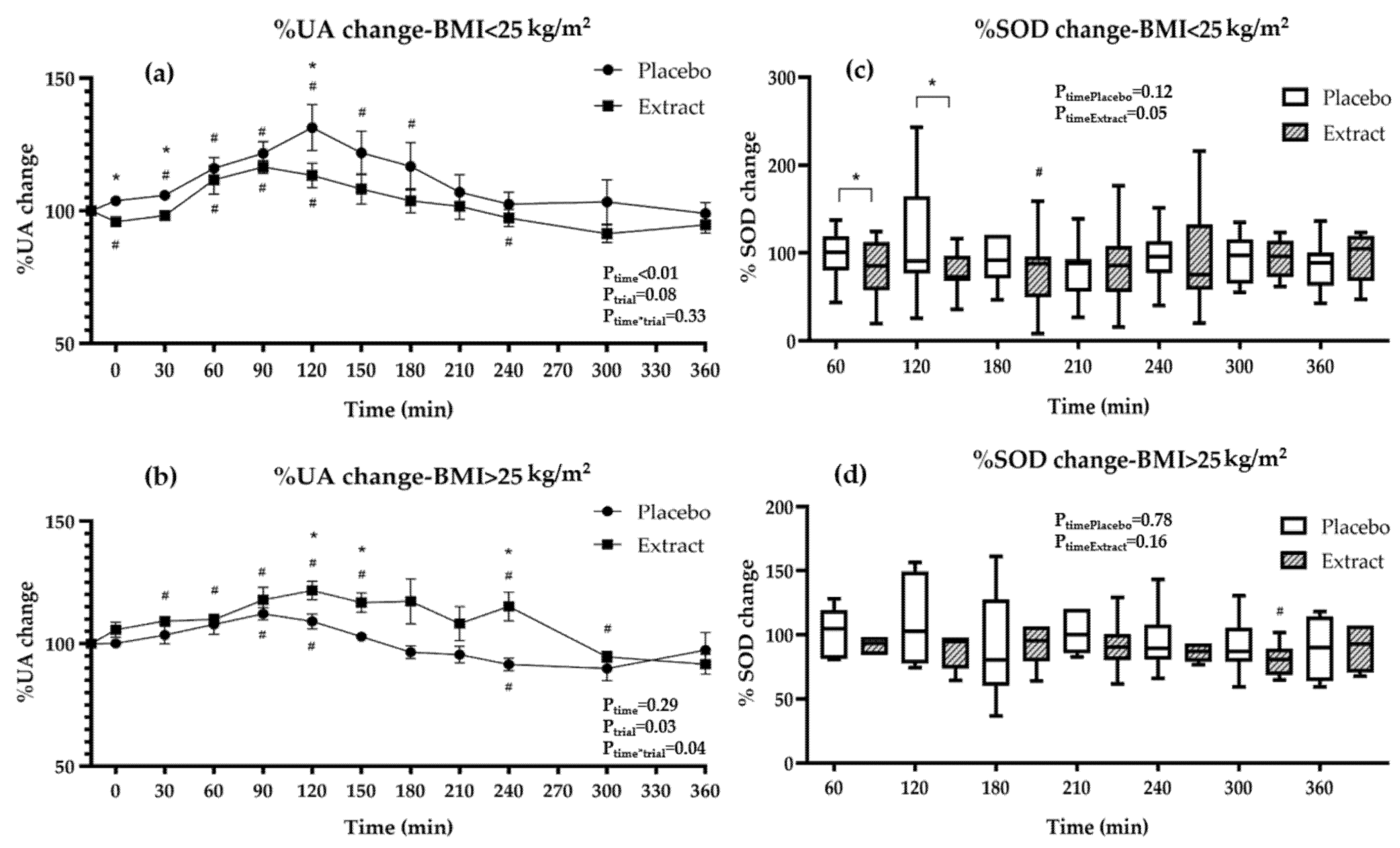
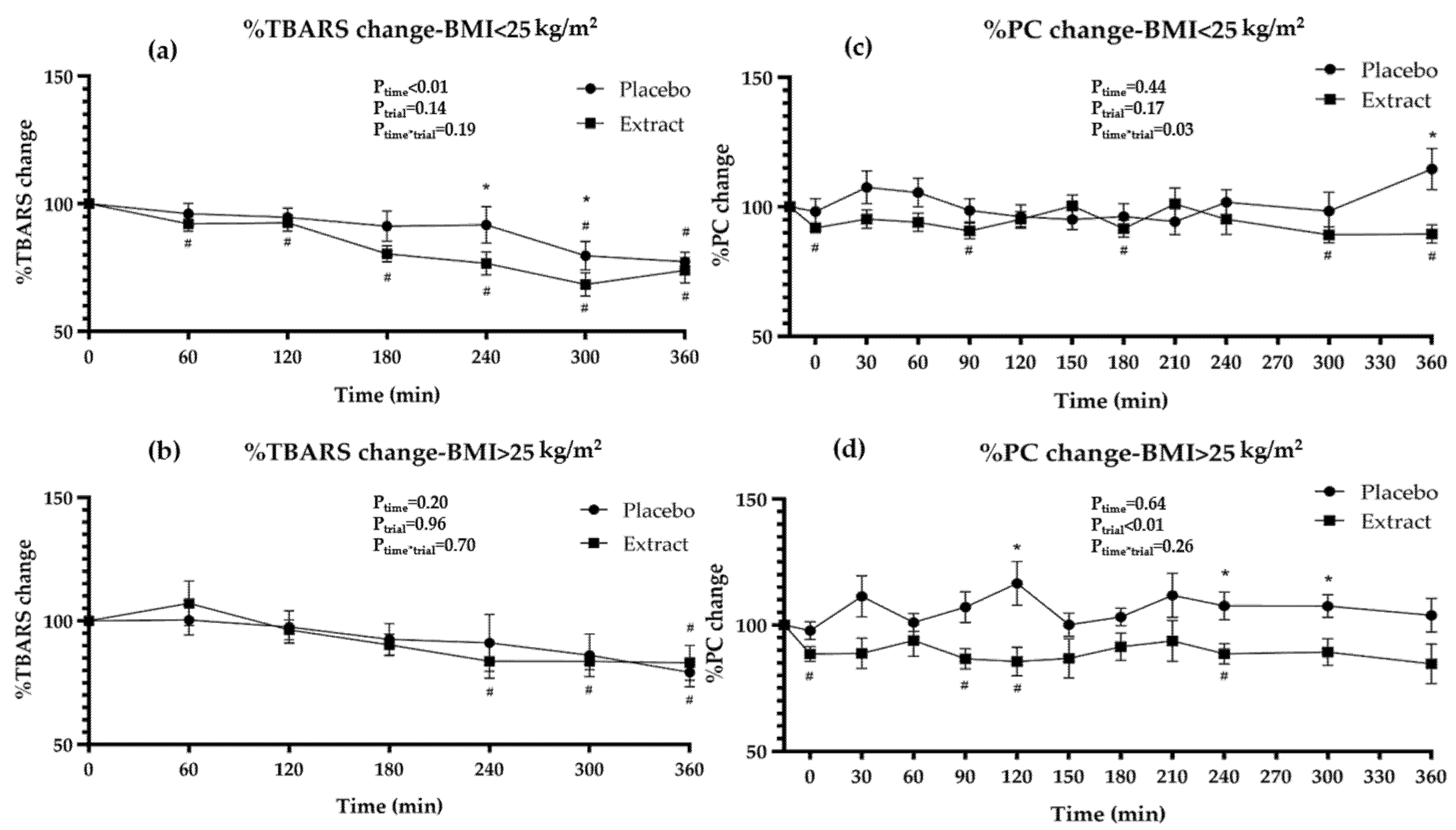
3.4. Oxidative Stress Markers during Interventions
3.5. Net iAUCs
3.6. Pilot Long-Term Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Liu, L.; Yang, S.; Liu, G.; Pan, L.; Gu, C.; Wang, Y.; Li, D.; Zhao, R.; Wu, M. Mechanisms of Atherosclerosis Induced by Postprandial Lipemia. Front. Cardiovasc. Med. 2021, 8, 636947. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.P.; Johnson, B.; Padilla, J.; Mather, K. Postprandial Lipaemia, Oxidative Stress and Endothelial Function: A Review. Int. J. Clin. Pract. 2010, 64, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Meessen, E.C.E.; Warmbrunn, M.V.; Nieuwdorp, M.; Soeters, M.R. Human Postprandial Nutrient Metabolism and Low-Grade Inflammation: A Narrative Review. Nutrients 2019, 11, 3000. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Fisher-Wellman, K.H. Systemic Oxidative Stress Is Increased to a Greater Degree in Young, Obese Women Following Consumption of a High Fat Meal. Oxid. Med. Cell. Longev. 2009, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Selby-Pham, S.; Colton, B.-L.; Bennett, L.; Williamson, G.; Dordevic, A.L. Does Timing of Phytonutrient Intake Influence the Suppression of Postprandial Oxidative Stress? A Systematic Literature Review. Redox Biol. 2021, 46, 102123. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–25. [Google Scholar] [CrossRef]
- St Leger, A.S.; Cochrane, A.L.; Moore, F. Factors Associated with Cardiac Mortality in Developed Countries with Particular Reference to the Consumption of Wine. Lancet 1979, 1, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and Its Metabolic Effects. A Comprehensive Review of Clinical Trials. Metabolism. 2018, 83, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; Gambert, P.; Fitó, M.; de la Torre, R. Wine and Oxidative Stress: Up-to-Date Evidence of the Effects of Moderate Wine Consumption on Oxidative Damage in Humans. Atherosclerosis 2010, 208, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Demopoulos, C.A.; Antonopoulou, S. Lipid Minor Constituents in Wines. A Biochemical Approach in the French Paradox. Int. J. Wine Res. 2009, 1, 131–143. [Google Scholar]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine Consumption Reduced Postprandial Platelet Sensitivity against Platelet Activating Factor in Healthy Men. Eur. J. Nutr. 2017, 56, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial Effects of Wine Consumption on Platelet Activating Factor Metabolic Enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Argyrou, C.; Detopoulou, M.; Tsitsou, S.; Seremeti, S.; Yannakoulia, M.; Antonopoulou, S.; Kolovou, G.; Kalogeropoulos, P. The Effect of Moderate Wine Consumption on Cytokine Secretion by Peripheral Blood Mononuclear Cells: A Randomized Clinical Study in Coronary Heart Disease Patients. Cytokine 2021, 146, 155629. [Google Scholar] [CrossRef] [PubMed]
- Choleva, M.; Argyrou, C.; Detopoulou, M.; Donta, M.-E.; Gerogianni, A.; Moustou, E.; Papaemmanouil, A.; Skitsa, C.; Kolovou, G.; Kalogeropoulos, P.; et al. Effect of Moderate Wine Consumption on Oxidative Stress Markers in Coronary Heart Disease Patients. Nutrients 2022, 14, 1377. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Muñiz, P. From Winery By-Product to Healthy Product: Bioavailability, Redox Signaling and Oxidative Stress Modulation by Wine Pomace Product. Crit. Rev. Food Sci. Nutr. 2022, 62, 7427–7448. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The Effect of Red Grape Seed Extract on Serum Paraoxonase Activity in Patients with Mild to Moderate Hyperlipidemia. Sao Paulo Med. J. Rev. Paul. Med. 2016, 134, 234–239. [Google Scholar] [CrossRef]
- Han, H.J.; Jung, U.J.; Kim, H.-J.; Cho, S.-J.; Kim, A.H.; Han, Y.; Choi, M.-S. Combined Supplementation with Grape Pomace and Omija Fruit Ethanol Extracts Dose-Dependently Improves Body Composition, Plasma Lipid Profiles, Inflammatory Status, and Antioxidant Capacity in Overweight and Obese Subjects. J. Med. Food 2016, 19, 170–180. [Google Scholar] [CrossRef]
- Yubero, N.; Sanz-Buenhombre, M.; Guadarrama, A.; Villanueva, S.; Carrión, J.M.; Larrarte, E.; Moro, C. LDL Cholesterol-Lowering Effects of Grape Extract Used as a Dietary Supplement on Healthy Volunteers. Int. J. Food Sci. Nutr. 2013, 64, 400–406. [Google Scholar] [CrossRef]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine Grape Pomace Flour Improves Blood Pressure, Fasting Glucose and Protein Damage in Humans: A Randomized Controlled Trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Turki, K.; Charradi, K.; Boukhalfa, H.; Belhaj, M.; Limam, F.; Aouani, E. Grape Seed Powder Improves Renal Failure of Chronic Kidney Disease Patients. EXCLI J. 2016, 15, 424–433. [Google Scholar] [CrossRef] [PubMed]
- De Groote, D.; Van Belleghem, K.; Devière, J.; Van Brussel, W.; Mukaneza, A.; Amininejad, L. Effect of the Intake of Resveratrol, Resveratrol Phosphate, and Catechin-Rich Grape Seed Extract on Markers of Oxidative Stress and Gene Expression in Adult Obese Subjects. Ann. Nutr. Metab. 2012, 61, 15–24. [Google Scholar] [CrossRef] [PubMed]
- van Mierlo, L.A.J.; Zock, P.L.; van der Knaap, H.C.M.; Draijer, R. Grape Polyphenols Do Not Affect Vascular Function in Healthy Men. J. Nutr. 2010, 140, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Sapwarobol, S.; Adisakwattana, S.; Changpeng, S.; Ratanawachirin, W.; Tanruttanawong, K.; Boonyarit, W. Postprandial Blood Glucose Response to Grape Seed Extract in Healthy Participants: A Pilot Study. Pharmacogn. Mag. 2012, 8, 192–196. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape Pomace Polyphenols Improve Insulin Response to a Standard Meal in Healthy Individuals: A Pilot Study. Clin. Nutr. 2019, 38, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Belelli, F.; Gentili, V.; Ursini, F.; Scaccini, C. Grape Seed Proanthocyanidins Prevent Plasma Postprandial Oxidative Stress in Humans. J. Agric. Food Chem. 2002, 50, 7720–7725. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Ferebee, D.E.; Fisher-Wellman, K.H.; Quindry, J.C.; Schilling, B.K. Postprandial Oxidative Stress: Influence of Sex and Exercise Training Status. Med. Sci. Sports Exerc. 2009, 41, 2111–2119. [Google Scholar] [CrossRef]
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of Anti-Platelet Activity of Grape Pomace Extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef]
- Bountziouka, V.; Bathrellou, E.; Giotopoulou, A.; Katsagoni, C.; Bonou, M.; Vallianou, N.; Barbetseas, J.; Avgerinos, P.C.; Panagiotakos, D.B. Development, Repeatability and Validity Regarding Energy and Macronutrient Intake of a Semi-Quantitative Food Frequency Questionnaire: Methodological Considerations. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 659–667. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean Food Pattern Predicts the Prevalence of Hypertension, Hypercholesterolemia, Diabetes and Obesity, among Healthy Adults; the Accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A.; Maraki, M.I.; Kollia, M.; Gioxari, A.; Jansen, L.T.; Sidossis, L.S. Development, Reliability and Validity of a Physical Activity Questionnaire for Estimating Energy Expenditure in Greek Adults. Sci. Sports 2016, 31, e47–e53. [Google Scholar] [CrossRef]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Kotroyiannis, I.; Vassiliadou, C.; Panagiotakos, D.B.; Chrysohoou, C.; Pitsavos, C.; Stefanadis, C. Platelet Activating Factor (PAF) and Activity of Its Biosynthetic and Catabolic Enzymes in Blood and Leukocytes of Male Patients with Newly Diagnosed Heart Failure. Clin. Biochem. 2009, 42, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved Analysis of Malondialdehyde in Human Body Fluids. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- McCord, J.M. Analysis of Superoxide Dismutase Activity. Curr. Protoc. Toxicol. 2001, 7, 3–7. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Dimina, L.; Mariotti, F. The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances. Nutrients 2019, 11, 1963. [Google Scholar] [CrossRef]
- Fappi, A.; Mittendorfer, B. Different Physiological Mechanisms Underlie an Adverse Cardiovascular Disease Risk Profile in Men and Women. Proc. Nutr. Soc. 2020, 79, 210–218. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A Randomized Placebo-Controlled Cross-over Study on the Effects of Anthocyanins on Inflammatory and Metabolic Responses to a High-Fat Meal in Healthy Subjects. Redox Biol. 2022, 51, 102273. [Google Scholar] [CrossRef]
- Bantle, A.E.; Thomas, W.; Bantle, J.P. Metabolic Effects of Alcohol in the Form of Wine in Persons with Type 2 Diabetes Mellitus. Metabolism 2008, 57, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Rosiers, C.D.; Tardif, J.-C.; Nigam, A. The Role of Oxidative Stress in Postprandial Endothelial Dysfunction. Nutr. Res. Rev. 2012, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Title, L.M.; Cummings, P.M.; Giddens, K.; Nassar, B.A. Oral Glucose Loading Acutely Attenuates Endothelium-Dependent Vasodilation in Healthy Adults without Diabetes: An Effect Prevented by Vitamins C and E. J. Am. Coll. Cardiol. 2000, 36, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.A.; Sutherland, W.H.F.; McCormick, M.P.; de Jong, S.A.; Walker, R.J.; Wilkins, G.T. Impaired Endothelial Function Following a Meal Rich in Used Cooking Fat. J. Am. Coll. Cardiol. 1999, 33, 1050–1055. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R. Macronutrient Specific Postprandial Oxidative Stress: Relevance to the Development of Insulin Resistance. Curr. Diabetes Rev. 2009, 5, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Kabir, M.M.; Marshall, K.E.; Canale, R.E.; Farney, T.M. Postprandial Oxidative Stress in Response to Dextrose and Lipid Meals of Differing Size. Lipids Health Dis. 2010, 9, 79. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia Rapidly Suppresses Flow-Mediated Endothelium-Dependent Vasodilation of Brachial Artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Serin, O.; Konukoglu, D.; Firtina, S.; Mavis, O. Serum Oxidized Low Density Lipoprotein, Paraoxonase 1 and Lipid Peroxidation Levels during Oral Glucose Tolerance Test. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2007, 39, 207–211. [Google Scholar] [CrossRef]
- Montes-Nieto, R.; Insenser, M.; Murri, M.; Fernández-Durán, E.; Ojeda-Ojeda, M.; Martínez-García, M.Á.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Plasma Thiobarbituric Acid Reactive Substances (TBARS) in Young Adults: Obesity Increases Fasting Levels Only in Men Whereas Glucose Ingestion, and Not Protein or Lipid Intake, Increases Postprandial Concentrations Regardless of Sex and Obesity. Mol. Nutr. Food Res. 2017, 61, 1700425. [Google Scholar] [CrossRef]
- Bae, J.-H.; Schwemmer, M.; Lee, I.-K.; Lee, H.-J.; Park, K.-R.; Kim, K.-Y.; Bassenge, E. Postprandial Hypertriglyceridemia-Induced Endothelial Dysfunction in Healthy Subjects Is Independent of Lipid Oxidation. Int. J. Cardiol. 2003, 87, 259–267. [Google Scholar] [CrossRef]
- Tushuizen, M.E. Postprandial Dysmetabolism and Cardiovascular Disease in Type 2 Diabetes. Postgrad. Med. J. 2005, 81, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Evans, L.M.; Ellis, G.R.; Khan, N.; Morris, K.; Jackson, S.K.; Rees, A.; Lewis, M.J.; Frenneaux, M.P. Prolonged Deterioration of Endothelial Dysfunction in Response to Postprandial Lipaemia Is Attenuated by Vitamin C in Type 2 Diabetes. Diabet. Med. 2006, 23, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Evans, M.L.; Ellis, G.R.; Graham, J.; Morris, K.; Jackson, S.K.; Lewis, M.J.; Rees, A.; Frenneaux, M.P. The Relationships between Post-Prandial Lipaemia, Endothelial Function and Oxidative Stress in Healthy Individuals and Patients with Type 2 Diabetes. Atherosclerosis 2001, 154, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Calvagno, S.; Mauceri, B.; Misseri, M.; Tsami, A.; Vecchio, C.; Mastrosimone, G.; Di Pino, A.; Maiorca, D.; Judica, A.; et al. Effects of Antioxidants on Postprandial Oxidative Stress and Endothelial Dysfunction in Subjects with Impaired Glucose Tolerance and Type 2 Diabetes. Eur. J. Nutr. 2010, 49, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Signorelli, S.S.; Torrisi, B.; Pulvirenti, D.; Mauceri, B.; Abate, G.; Ignaccolo, L.; Bordonaro, F.; Cilio, D.; Calvagno, S.; et al. Effects of Antioxidant Supplementation on Postprandial Oxidative Stress and Endothelial Dysfunction: A Single-Blind, 15-Day Clinical Trial in Patients with Untreated Type 2 Diabetes, Subjects with Impaired Glucose Tolerance, and Healthy Controls. Clin. Ther. 2005, 27, 1764–1773. [Google Scholar] [CrossRef]
- Johnson, B.D.; Padilla, J.; Harris, R.A.; Wallace, J.P. Vascular Consequences of a High-Fat Meal in Physically Active and Inactive Adults. Appl. Physiol. Nutr. Metab. 2011, 36, 368–375. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater Oxidative Stress in Healthy Young Men Compared With Premenopausal Women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef]
- Spallarossa, P.; Garibaldi, S.; Barisione, C.; Ghigliotti, G.; Altieri, P.; Tracchi, I.; Fabbi, P.; Barsotti, A.; Brunelli, C. Postprandial Serum Induces Apoptosis in Endothelial Cells: Role of Polymorphonuclear-Derived Myeloperoxidase and Metalloproteinase-9 Activity. Atherosclerosis 2008, 198, 458–467. [Google Scholar] [CrossRef]
- Cardona, F.; Túnez, I.; Tasset, I.; Garrido-Sánchez, L.; Collantes, E.; Tinahones, F.J. Circulating Antioxidant Defences Are Decreased in Healthy People after a High-Fat Meal. Br. J. Nutr. 2008, 100, 312–316. [Google Scholar] [CrossRef]
- Urquiaga, I.; Ávila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxid. Med. Cell. Longev. 2017, 2017, 8361493. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Roseland, J.M.; Haytowitz, D.B.; Pehrsson, P.R.; Ershow, A.G. Availability and Quality of Published Data on the Purine Content of Foods, Alcoholic Beverages, and Dietary Supplements. J. Food Compos. Anal. 2019, 84, 103281. [Google Scholar] [CrossRef]
- Miglio, C.; Peluso, I.; Raguzzini, A.; Villaño, D.V.; Cesqui, E.; Catasta, G.; Toti, E.; Serafini, M. Fruit Juice Drinks Prevent Endogenous Antioxidant Response to High-Fat Meal Ingestion. Br. J. Nutr. 2014, 111, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Randolph, J.; Cheema, M.; Tadapaneni, R.; Park, E.; Burton-Freeman, B.; Kappagoda, T. Effect of Grape Seed Extract on Postprandial Oxidative Status and Metabolic Responses in Men and Women with the Metabolic Syndrome—Randomized, Cross-over, Placebo-Controlled Study. Funct. Foods Health Dis. 2013, 2, 508. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.X.; Kong, L.D.; Yang, C.; Cheng, C.H.K.; Zhang, X. Administration of Procyanidins from Grape Seeds Reduces Serum Uric Acid Levels and Decreases Hepatic Xanthine Dehydrogenase/Oxidase Activities in Oxonate-Treated Mice. Basic Clin. Pharmacol. Toxicol. 2004, 94, 232–237. [Google Scholar] [CrossRef]
- Lu, M.-C.; Yang, M.-D.; Li, P.-C.; Fang, H.-Y.; Huang, H.-Y.; Chan, Y.-C.; Bau, D.-T. Effect of Oligomeric Proanthocyanidin on the Antioxidant Status and Lung Function of Patients with Chronic Obstructive Pulmonary Disease. In Vivo 2018, 32, 753–758. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Strigunova, E.N.; Kostyuk, T.V.; Afanas’ev, I.B. Experimental Evidence that Flavonoid Metal Complexes May Act as Mimics of Superoxide Dismutase. Arch. Biochem. Biophys. 2004, 428, 204–208. [Google Scholar] [CrossRef]
| Placebo BMI < 25 kg/m2 (n = 11) | Extract BMI < 25 kg/m2 (n = 11) | Placebo BMI > 25 kg/m2 (n = 7) | Extract BMI > 25 kg/m2 (n = 7) | |
|---|---|---|---|---|
| Age (years) | 26.5 ± 3.0 | 26.5 ± 3.0 | 29.0 ± 2.9 | 29.0 ± 2.9 |
| Weight (kg) | 57.2 (56.2–62.8) $ | 57 (55.4–61.5) $ | 81 (66.1–82.4) | 81.5 (64.9–83.9) |
| Height (m) | 1.63 ± 0.03 | 1.63 ± 0.03 | 1.64 ± 0.07 | 1.64 ± 0.07 |
| BMI (kg/m2) | 22.4 ± 1.1 $ | 22.2 ± 1.1 $ | 29.5 ± 4.3 | 29.5 ± 4.6 |
| Waist circumference (cm) | 69.6 ± 3.3 $ | 69.2 ± 4.2 $ | 82.6 ± 9.7 | 83.6 ± 8.8 |
| Hip circumference (cm) | 96.8 ± 5.2 $ | 96.6 ± 5.0 $ | 114.0 ± 9.0 | 114.4 ± 9.5 |
| Waist-to-hip ratio | 0.72 ± 0.04 | 0.72 ± 0.04 | 0.72 ± 0.03 | 0.73 ± 0.03 |
| SBP (mmHg) | 108.8 ± 10.0 | 108.5 ± 11.5 | 113.6 ± 7.8 | 117.3 ± 8.2 |
| DBP (mmHg) | 63 (61–78) | 67 (62–82) | 72 (71–80) | 72 (70–84) |
| Heart rate (pulses) | 75 (72–92) | 80 (66–84) | 77 (62–80) | 77 (60–95) |
| Body fat (%) | 23.4 (19.8–9.9) $ | 23.9 (22.7–4.8) $ | 32.6 (32–42.4) | 35.7 (31.7–43.0) |
| Body fat (kg) | 13.3 (11.3–8.8) $ | 13.9 (12.5–4.5) $ | 26.5 (21.1–34.9) | 27.7 (20.8–36.2) |
| Body fat-free mass (kg) | 44.5 ± 1.7 $ | 43.7 ± 1.4 $ | 50.2 ± 4.2 | 49.9 ± 4.9 |
| Glucose (mg/dL) | 82.3 ± 5.4 | 83.6 ± 6.4 | 85.1 ± 6.4 | 87.2 ± 4.5 |
| Insulin (μΙU/mL) | 5.5 (4.1–6.3) | 6.0 (4.5–6.8) | 6.6 (5.5–7.9) | 6.6 (5.6–7.7) |
| HOMA IR | 1.1 (0.8–1.3) | 1.2 (0.9–1.5) | 1.5 (1.2–1.6) | 1.5 (1.2–1.6) |
| Triglycerides (mg/dL) | 62.8 ± 17.0 | 57.9 ± 16.5 | 63.7 ± 10.1 | 67.6 ± 8.8 |
| Total cholesterol (mg/dL) | 148.4 ± 36.2 | 153.7 ± 29.0 | 158.0 ± 34.8 | 160.2 ± 26.6 |
| HDL-c (mg/dL) | 52.2 ± 22.3 | 50.3 ± 19.2 | 50.7 ± 10.0 | 48.0 ± 11.7 |
| LDL-c (mg/dL) | 83.7 ± 20.9 | 91.8 ± 20.3 | 94.6 ± 34.0 | 98.7 ± 16.3 |
| Placebo BMI < 25 kg/m2 (n = 11) | Extract BMI < 25 kg/m2 (n = 11) | Placebo BMI > 25 kg/m2 (n = 7) | Extract BMI > 25 kg/m2 (n = 7) | |
|---|---|---|---|---|
| Uric acid (mg/dL) | 3.6 ± 1.0 | 4.1 ± 0.8 | 3.6 ± 1.1 | 3.7 ± 0.8 |
| TBARS (μM) | 2.2 ± 0.8 | 2.8 ± 2.0 | 2.4 ± 1.2 | 2.8 ± 1.9 |
| PC (μmol/μg pr) | 0.61 (0.60–0.63) | 0.55 (0.50–0.78) | 0.56 (0.53–0.62) | 0.65 (0.49–0.74) |
| SOD (U/mg pr) | 9.7 ± 5.1 | 9.7 ± 3.8 | 11.1 ± 4.5 | 10.9 ± 4.1 |
| GPx (U/L) | 35.6 (31.1–38.0) | 32.1 (29.7–36.5) | 34.5 (28.9–36.2) | 34.1 (29.5–35.7) |
| Placebo BMI < 25 kg/m2 (n = 11) | Extract BMI < 25 kg/m2 (n = 11) | Placebo BMI > 25 kg/m2 (n = 7) | Extract BMI > 25 kg/m2 (n = 7) | |
|---|---|---|---|---|
| Energy intake (kcal) | 1539.4 (1389.1–2565.1) | 1546.0 $ (1344.2–1893.7) | 1973.1 (1571.2–2362.5) | 1997.8 (1813.2–2520.3) |
| Carbohydrates (% of total energy) | 39.3 ± 9.6 | 34.2 ± 5.3 | 34.9 ± 7.8 | 38.3 ± 7.6 |
| Proteins (% of total energy) | 17.0 ± 4.1 | 19.7 ± 5.3 | 18.0 ± 5.1 | 15.9 ± 1.4 |
| Lipids (% of total energy) | 39.4 ± 7.5 | 44.3 ± 5.7 | 45.4 ± 5.6 | 44.2 ± 6.2 |
| Protein intake/body weight (g/kg) | 1.3 ± 0.4 | 1.4 ± 0.4 $ | 1.1 ± 0.4 | 1.0 ± 0.2 |
| SFA (% of total energy) | 12.9 ± 3.0 | 13.2 ± 2.6 | 14.6 ± 2.1 | 15.3 ± 2.2 |
| MUFA (% of total energy) | 16.0 ± 5.1 $ | 19.1 ± 5.9 | 20.7 ± 3.4 | 18.6 ± 3.8 |
| PUFA (% of total energy) | 5.7 ± 2.6 | 7.1 ± 2.3 | 7.3 ± 2.1 | 6.7 ± 1.6 |
| Fiber (g) | 17.7 (12.0–21.3) | 11.7 (11.1–17.1) | 13.0 (12.0–13.9) | 13.1 (11.5–26.0) |
| Vitamin A (IU) | 1479.9 (1109.9–5090.7) | 4068 (1782.9–14012.2) | 5352.1 (3673.4–8264.2) | 3127.2 (2634.0–6482.8) |
| Vitamin C (mg) | 28.5 (13.3–37.8) $ | 49.7 (19.8–78.0) $ | 76.5 (32.3–96.1) | 108.9 (82.7–229.2) |
| Dairy (servings/day) | 1.0 (0.4–2.6) | 0.7 (0.6–2.6) | 0.2 (0.0–2.1) | 0.2 (0.0–1.3) |
| Fruits and vegetables (servings/d) | 1.6 ± 1.2 | 2.3 ± 1.4 | 2.7 ± 1.8 | 3.6 ± 3.1 |
| Legumes (servings/day) | 0.0 (0.0–0.4) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.3) |
| Fish (servings/day) | 0.0 (0.0–0.7) | 0.6 (0.0–0.7) | 0.1 (0.0–0.3) | 0.0 (0.0–0.3) |
| Red and processed meat (servings/day) | 1.1 ± 0.9 $ | 1.4 ± 1.2 | 2.5 ± 1.2 | 2.1 ± 0.7 |
| Chicken/turkey (servings/day) | 1.2 ± 1.0 | 0.8 ± 0.5 | 0.7 ± 0.6 | 0.5 ± 0.8 |
| Whole grains (servings/day) | 0.8 (0.4–1.3) | 0.2 (0.0–1.7) | 0.5 (0.3–2.5) | 1.4 (0.4–2.2) |
| Refined grains (servings/day) | 3.5 ± 2.1 | 2.5 ± 1.5 | 3.6 ± 1.7 | 3.4 ± 1.5 |
| Nuts (servings/day) | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.2 ± 0.2 | 0.4 ± 0.4 |
| Sweets (servings/day) | 0.5 (0.2–0.9) | 0.6 (0.0–1.1) | 0.4 (0.0–1.8) | 0.9 (0.8–1.5) |
| Physical activity levels | 1.4 ± 0.2 $ | 1.4 ± 0.2 $ | 1.7 ± 0.2 | 1.8 ± 0.2 |
| −15 | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 300 | 360 | ^ ptime ^ ptime*trial ^ ptrial | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %Glucose-Placebo | 100 | 104.3 ± 11.4 | 112.5 ± 23.4 # | 92.6 ± 14.5 # | 98.4 ± 14.6 | 106.0 ± 17.3 | 103.5 ± 11.1 | 101.7 ± 13.3 | 101.1 ± 9.7 | 100.5 ± 9.8 | 100.7 ± 9.2 | 99.0 ± 9.4 | 0.06 |
| %Glucose-Extract | 100 | 104.3 ± 9.7 | 112.8 ± 15.0 # | 97.8 ± 10.7 | 103.6 ± 15.6 | 104.7 ± 14.7 | 102.5 ± 7.3 | 102.2 ± 9.8 | 102.5 ± 7.7 | 100.8 ± 10.8 | 98.1 ± 8.8 | 94.7 ± 8.9 # | 0.69 |
| † pP-E | - | 0.98 | 0.95 | 0.13 | 0.27 | 0.65 | 0.69 | 0.87 | 0.52 | 0.92 | 0.27 | 0.08 | 0.86 |
| %Insulin-Placebo | 100 | - | 706.1 ± 472.1 # | 449.7 ± 171.6 # | 420.1 ± 196.1 # | 474.2 ± 210.2 # | 394.1 ± 173.9 # | - | - | - | - | - | <0.01 |
| %Insulin-Extract | 100 | - | 689.9 ± 281.7 # | 584.9 ± 328.2 # | 457.6 ± 194.5 # | 459.3 ± 186.9 # | 369.2 ± 110.1 # | - | - | - | - | - | 0.44 |
| † pP-E | - | - | 0.85 | 0.04 | 0.40 | 0.69 | 0.58 | - | - | - | - | - | 0.67 |
| %TG-Placebo | 100 | 97.1 ± 10.0 | 125.2 ± 23.7 # | 154.2 ± 37.5 # | 171.7 ± 48.5 # | 195.3 ± 63.4 # | 195.5 ± 79.9 # | 188.7 ± 65.1 # | 178.8 ± 65.8 # | 159.4 ± 52.1 # | 168.1 ± 71.7 # | 163.8 ± 58.2 # | <0.01 |
| %TG-Extract | 100 | 97.5 ± 10.5 | 120.0 ± 20.9 # | 152.5 ± 31.4 # | 173.1 ± 32.7 # | 180.0 ± 37.4 # | 176.3 ± 38.5 # | 175.2 ± 59.6 # | 165.4 ± 43.4 # | 167.1 ± 41.8 # | 136.7 ± 22.5 # | 140.6 ± 34.3 # | 0.29 |
| † pP-E | - | 0.87 | 0.34 | 0.82 | 0.90 | 0.35 | 0.31 | 0.50 | 0.44 | 0.60 | 0.04 | 0.11 | 0.41 |
| %Total cholesterol-Placebo | 100 | 98.9 ± 7.0 | 101.9 ± 7.5 | 100.5 ± 8.9 | 106.2 ± 7.3 | 102.3 ± 9.1 | 105.5 ± 10.2 | 100.0 ± 12.1 | 102.1 ± 8.2 | 98.0 ± 6.8 | 100.7 ± 11.1 | 102.7 ± 8.3 | 0.13 |
| %Total cholesterol-Extract | 100 | 99.4 ± 4.8 | 100.9 ± 4.9 | 99.6 ± 5.6 | 103.4 ± 7.4 | 98.5 ± 6.6 | 102.2 ± 8.1 | 97.4 ± 7.4 | 96.6 ± 6.7 | 97.4 ± 6.8 | 96.8 ± 5.4 | 101.3 ± 5.5 | 0.41 |
| † pP-E | - | 0.82 | 0.69 | 0.68 | 0.21 | 0.15 | 0.20 | 0.31 | 0.01 | 0.74 | 0.12 | 0.54 | 0.25 |
| %HDL-c-Placebo | 100 | 103.2 ± 8.0 | 99.6 ± 7.3 | 102.0 ± 9.7 | 97.4 ± 10.4 | 97.1 ± 10.9 | 95.1 ± 12.0 | 96.3 ± 11.3 | 95.5 ± 10.2 | 97.6 ± 9.0 | 99.7 ± 10.7 | 99.8 ± 10.3 | <0.01 |
| %HDL-c-Extract | 100 | 100.2 ± 4.8 | 101.2 ± 8.2 | 98.6 ± 7.7 | 95.9 ± 7.5 | 94.7 ± 8.6 # | 94.7 ± 7.9 # | 92.0 ± 10.1 # | 92.1 ± 8.2 # | 94.4 ± 10.1 # | 96.1 ± 7.5 # | 101.1 ± 10.0 | 0.27 |
| † pP-E | - | 0.17 | 0.53 | 0.27 | 0.59 | 0.45 | 0.90 | 0.26 | 0.31 | 0.36 | 0.27 | 0.74 | 0.45 |
| %LDL-c-Placebo | 100 | 97.5 ± 11.0 | 99.6 ± 10.2 | 91.7 ± 13.0 # | 102.0 ± 11.8 | 91.4 ± 13.1 # | 98.1 ± 9.4 | 90.2 ± 17.3 # | 94.9 ± 12.3 | 90.1 ± 13.5 # | 91.9 ± 17.7 | 96.8 ± 14.6 | 0.03 |
| %LDL-c-Extract | 100 | 99.3 ± 7.0 | 97.0 ± 7.8 | 93.0 ± 7.8 # | 97.7 ± 9.9 | 89.0 ± 9.8 # | 95.6 ± 8.9 # | 88.7 ± 8.8 # | 88.8 ± 6.5 # | 88.4 ± 8.7 # | 91.8 ± 8.0 # | 96.4 ± 7.9 | 0.68 |
| † pP-E | - | 0.62 | 0.46 | 0.73 | 0.30 | 0.47 | 0.42 | 0.70 | 0.08 | 0.64 | 0.99 | 0.92 | 0.51 |
| −15 | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 300 | 360 | ^ ptime ^ ptime*trial ^ ptrial | §† ptime | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %UA-Placebo | 100 | 102.4 ± 6.1 | 105.0 ± 7.5 # | 112.9 ± 12.9 # | 118.0 ± 12.8 # | 122.7 ± 25.2 # | 114.5 ± 23.0 # | 108.9 ± 25.3 | 102.5 ± 18.7 | 98.2 ± 13.5 | 98.1 ± 23.6 | 98.4 ± 15.5 | <0.01 | - |
| %UA-Extract | 100 | 99.7 ± 7.7 | 102.6 ± 6.9 | 111.0 ± 14.1 # | 117.0 ± 10.3 # | 111.6 ± 16.3 # | 113.5 ± 18.8 # | 109.0 ± 19.8 | 104.2 ± 16.9 | 104.3 ± 15.3 | 92.6 ± 9.3 # | 93.5 ± 10.4 # | 0.48 | - |
| †pP-E | - | 0.33 | 0.36 | 0.63 | 0.76 | 0.29 | 0.58 | 0.99 | 0.71 | 0.17 | 0.21 | 0.14 | 0.62 | - |
| %TBARS-Placebo | 100 | - | - | 97.8 ± 14.2 | - | 95.8 ± 13.7 | - | 91.7 ± 18.0 | - | 91.5 ± 25.7 | 82.1 ± 19.8 # | 78.0 ± 13.3 # | <0.01 | - |
| %TBARS-Extract | 100 | - | - | 97.9 ± 17.6 | - | 94.0 ± 10.7 # | - | 84.2 ± 11.6 # | - | 79.4 ± 16.2 # | 74.3 ± 14.9 # | 77.5 ± 17.4 # | 0.14 | - |
| † pP-E | - | - | - | 0.97 | - | 0.60 | - | 0.08 | - | 0.08 | 0.08 | 0.91 | 0.27 | - |
| %PC-Placebo | 100 | 98.0 ± 13.7 | 109.0 ± 20.8 | 103.7 ± 15.4 | 101.9 ± 15.6 | 104.1 ± 20.6 | 97.0 ± 12.6 | 99.0 ± 14.1 | 101.1 ± 20.9 | 104.0 ± 15.3 | 101.9 ± 20.6 | 110.4 ± 23.4 | 0.74 | - |
| %PC-Extract | 100 | 90.6 ± 6.8 # | 92.8 ± 13.5 # | 93.9 ± 13.3 | 89.1 ± 10.2 # | 91.4 ± 12.6 # | 95.1 ± 17.7 | 91.5 ± 12.2 # | 98.2 ± 20.6 | 92.6 ± 16.5 | 89.3 ± 11.5 # | 87.6 ± 15.5 # | 0.03 | - |
| † pP-E | - | 0.06 | 0.02 | 0.08 | 0.02 | 0.06 | 0.68 | 0.08 | 0.69 | 0.06 | 0.04 | <0.01 | <0.01 | - |
| %SOD-Placebo | 100 | - | - | 102.7 (81.1–119.2) | - | 99.9 (77.2–151.1) | - | 88.6 (68.2–122.2) | 90.4 (82.2–103.9) | 92.6 (79.6–109.3) | 92.0 (71.2–108.0) | 89.4 (63.4–103.9) | - | 0.25 |
| %SOD-Extract | 100 | - | - | 89.1 (67.9–109.1) | - | 77.5 (70.9–97.4) # | - | 89.5 (65.5–102.6) # | 87.7 (70.5–102.4) | 82.6 (70.4–128.1) | 82.0 (71.6–104.5) | 101.6 (68.4–117.2) | - | 0.04 |
| † pP-E | - | - | - | 0.03 | - | 0.01 | - | 0.81 | 0.55 | 0.50 | 0.44 | 0.21 | ||
| %GPx-Placebo | 100 | 103.7 ± 4.0 # | 99.1 ± 4.8 | 99.6 ± 5.2 | 100.2 ± 7.8 | 97.8 ± 6.7 | 101.0 ± 9.1 | 99.1 ± 5.7 | 100.2 ± 9.9 | 99.3 ± 8.4 | 101.1 ± 7.0 | 102.5 ± 5.8 | 0.49 | - |
| %GPx-Extract | 100 | 104.6 ± 5.5 # | 101.7 ± 4.8 | 99.9 ± 6.2 | 99.7 ± 8.3 | 99.1 ± 7.4 | 102.6 ± 8.6 | 101.0 ± 8.9 | 98.8 ± 8.4 | 100.9 ± 7.4 | 101.7 ± 6.6 | 103.7 ± 9.3 | 0.91 | - |
| † pP-E | - | 0.62 | 0.12 | 0.82 | 0.85 | 0.62 | 0.60 | 0.41 | 0.67 | 0.47 | 0.79 | 0.68 | 0.58 | - |
| Placebo (n = 4) | Extract (n = 4) | |
|---|---|---|
| %Glucose | 100.2 (96.7–106.2) | 102.4 (97.0–104.6) |
| %Insulin | 94.6 (81.8–105.4) | 105.8 (61.7–121.0) |
| %Triglycerides | 122.0 (82.3–133.3) | 85.8 (76.8–109.8) |
| %Total Cholesterol | 93.6 (89.2–111.4) | 89.8 (85.0–94.1) |
| %HDL-c | 100.7 (96.3–124.0) | 99.1 (82.8–126.0) |
| %LDL-c | 81.0 (70.6–114.4) | 81.7 (65.4–102.7) |
| %Uric acid | 104.8 (74.5–141.2) | 101.8 (87.4–131.2) |
| %TBARS | 116.1 (91.2–165.2) | 102.4 (79.8–137.6) |
| %Protein Carbonyls | 97.3 (74.9–144.3) | 87.6 (78.5–98.0) |
| %SOD | 86.1 (70.5–127.1) | 100.1 (79.1–146.9) |
| %GPx | 102.4 (95.3–106.5) | 100.1 (95.3–103.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choleva, M.; Matalliotaki, E.; Antoniou, S.; Asimomyti, E.; Drouka, A.; Stefani, M.; Yannakoulia, M.; Fragopoulou, E. Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2023, 15, 156. https://doi.org/10.3390/nu15010156
Choleva M, Matalliotaki E, Antoniou S, Asimomyti E, Drouka A, Stefani M, Yannakoulia M, Fragopoulou E. Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients. 2023; 15(1):156. https://doi.org/10.3390/nu15010156
Chicago/Turabian StyleCholeva, Maria, Eleni Matalliotaki, Sokratis Antoniou, Eirini Asimomyti, Archontoula Drouka, Maria Stefani, Mary Yannakoulia, and Elizabeth Fragopoulou. 2023. "Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study" Nutrients 15, no. 1: 156. https://doi.org/10.3390/nu15010156
APA StyleCholeva, M., Matalliotaki, E., Antoniou, S., Asimomyti, E., Drouka, A., Stefani, M., Yannakoulia, M., & Fragopoulou, E. (2023). Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients, 15(1), 156. https://doi.org/10.3390/nu15010156