Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TβRI-p38-MAPK-Depending RUNX2 Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Synthesis and Verification of the Soy Peptide (CBP)
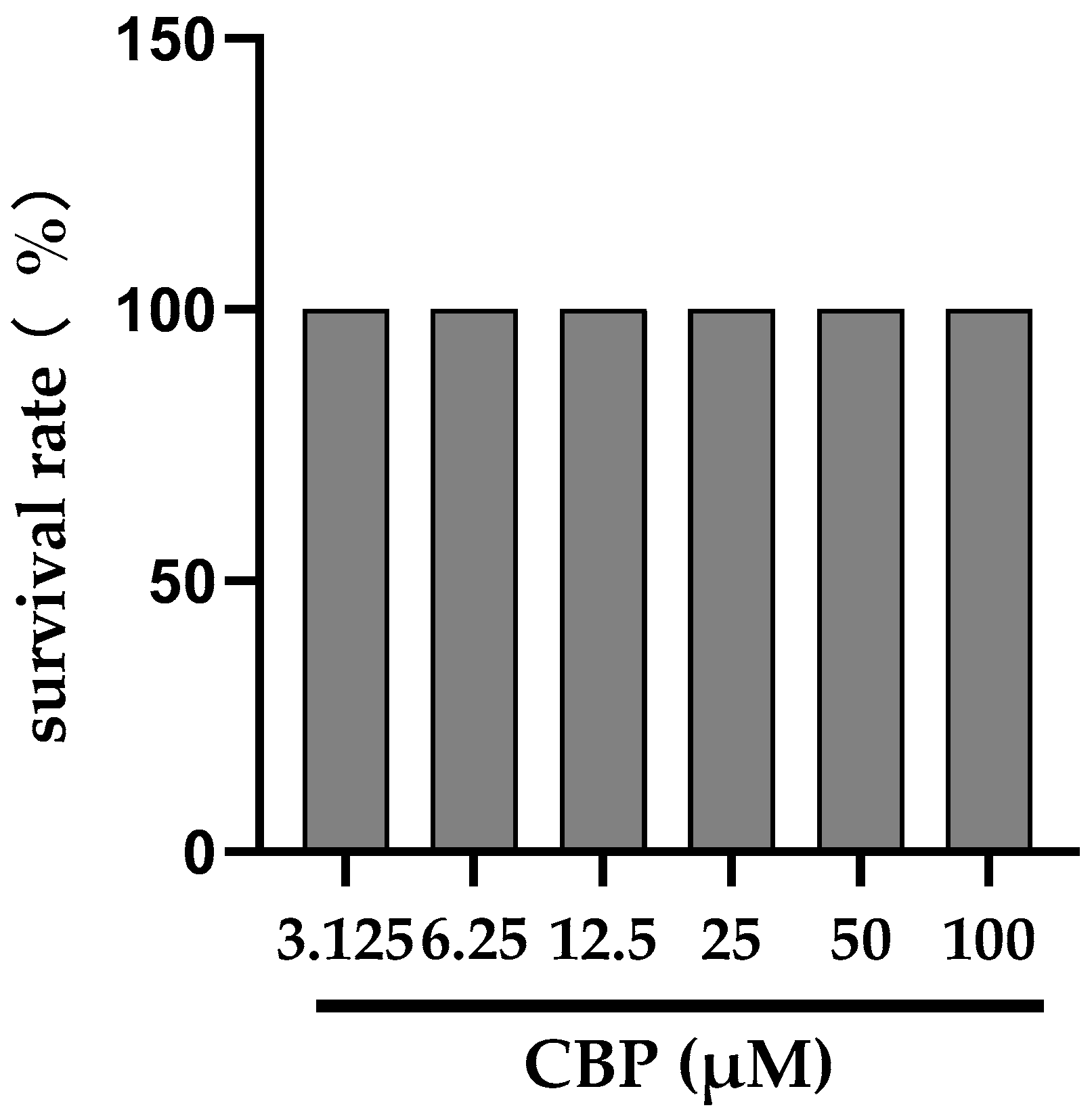
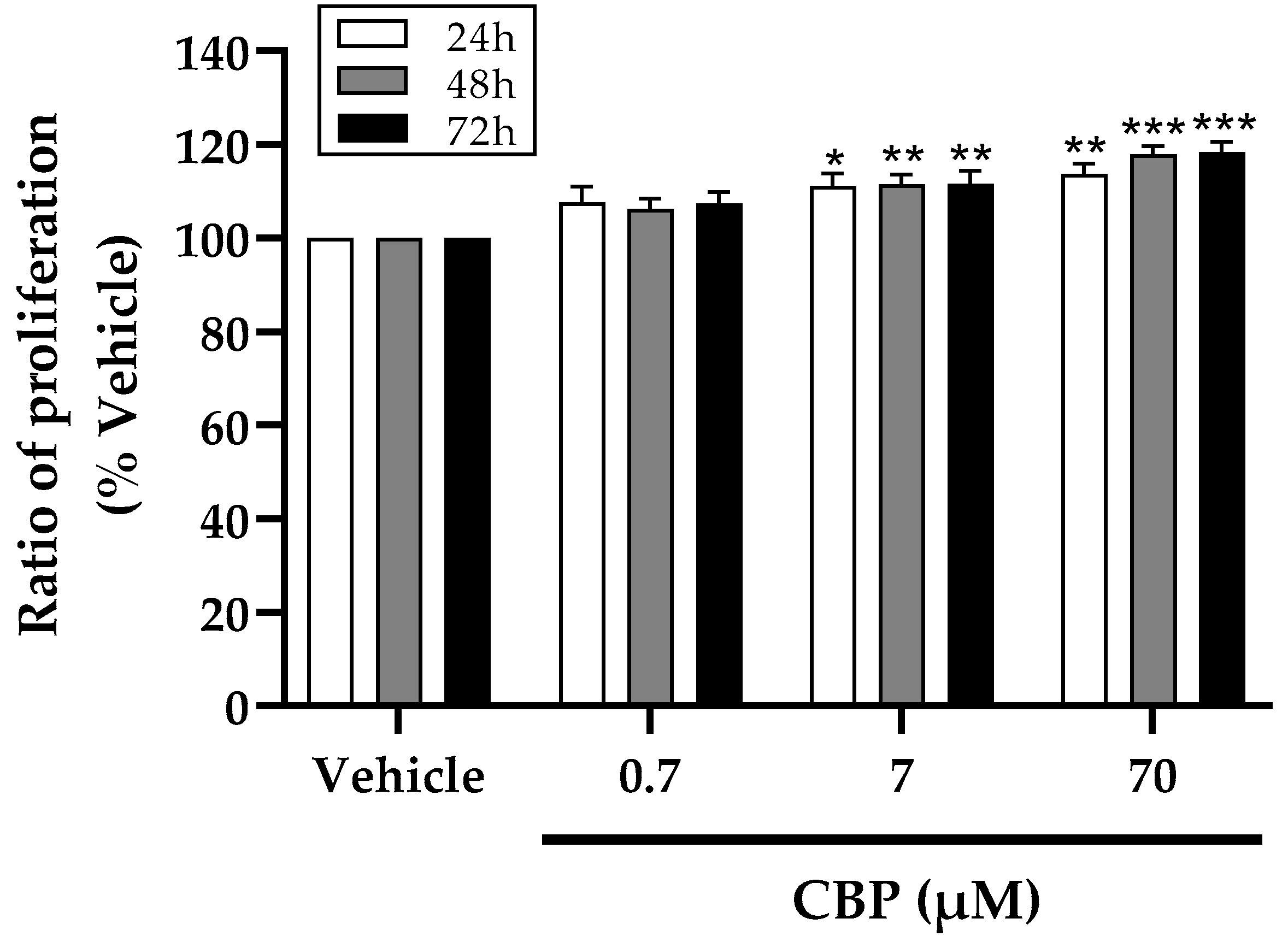
2.4. Proliferation Assay of MC3T3-E1 Cell
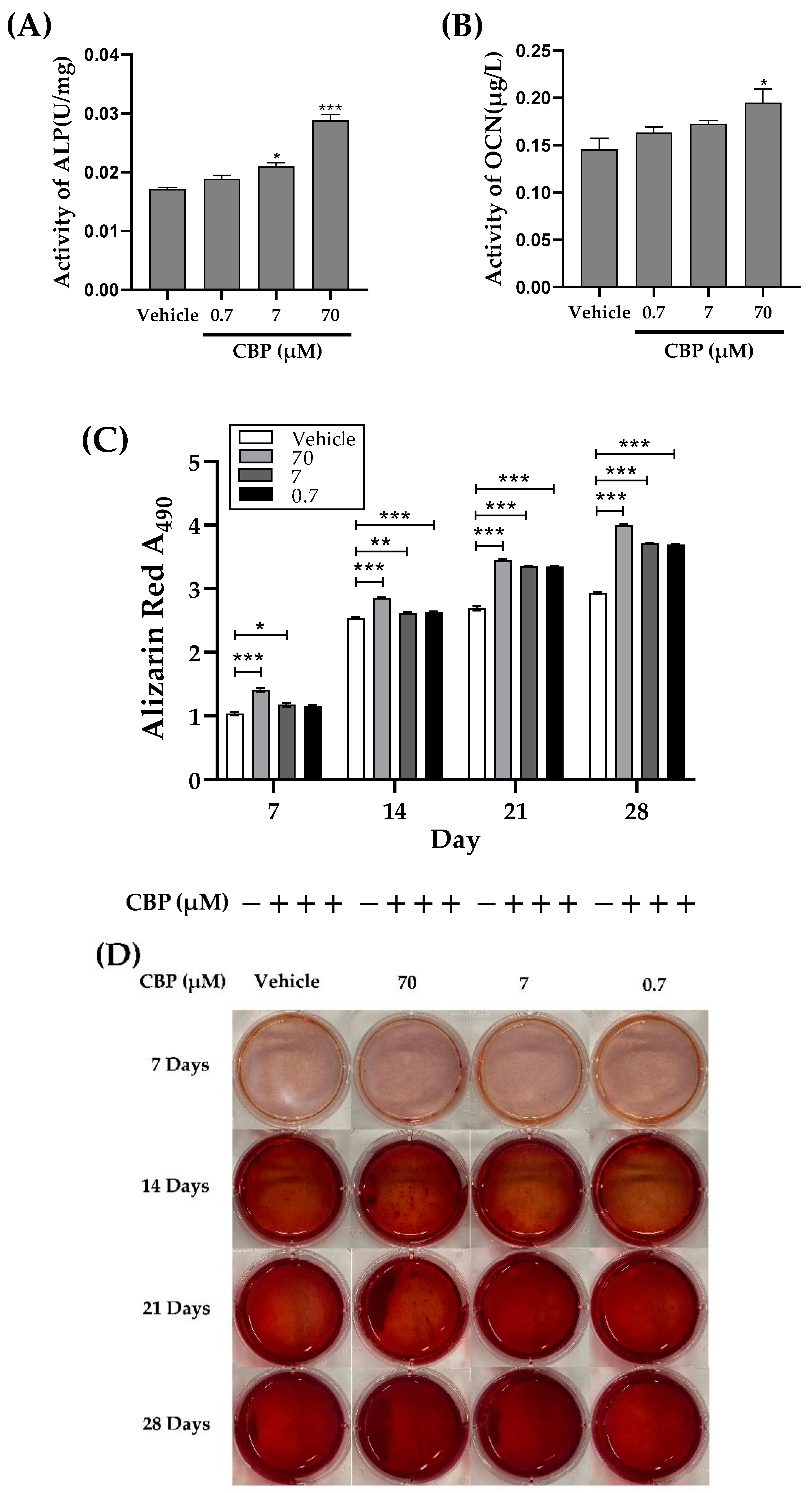
2.5. Differentiation and Mineralization Assay of MC3T3-E1
2.5.1. Analysis of ALP Activity
2.5.2. Analysis of OCN Activity
2.5.3. Analysis of Mineralization
2.6. Effect of CBP on the mRNA Expression of Osteoblastic Markers
2.7. Western Blotting Analysis
2.8. Calcium Ion Measurement
2.9. Assessment of Antiosteoporosis Effects in a Zebrafish Model of GIOP
2.9.1. Zebrafish Husbandry and Maintenance
2.9.2. Calcein Staining and Image Acquisition
2.9.3. Behavioral Analysis
2.10. Statistical Analysis
3. Results
3.1. CBP Stimulated Cell Proliferation in MC3T3-E1 Cells
3.2. CBP Stimulated Differentiation and Mineralization in MC3T3-E1 Cells
3.3. CBP Increased mRNA Expression of Osteoblastic Markers
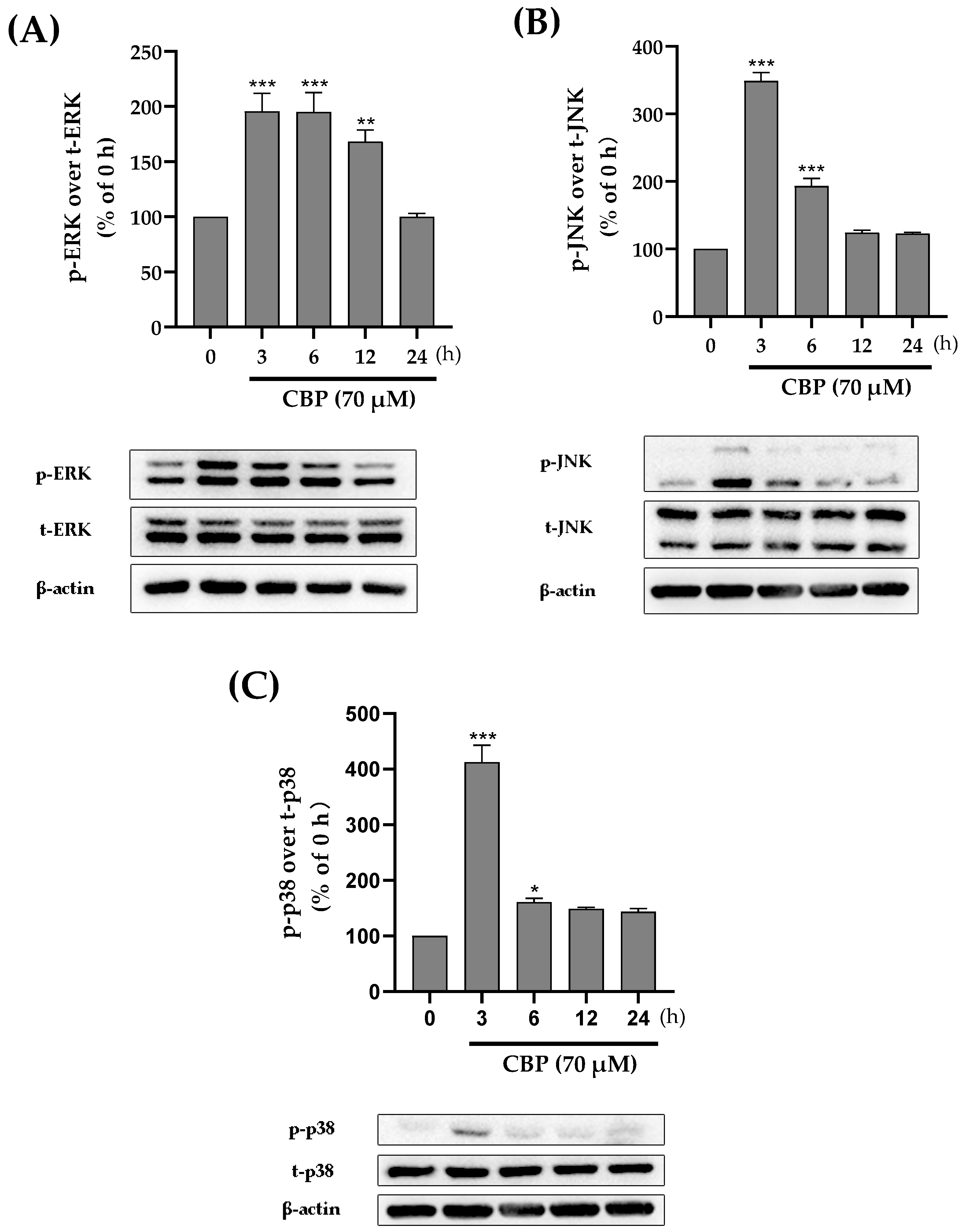
3.4. CBP Promoted Osteoblast Differentiation by Activating the MAPK Pathway
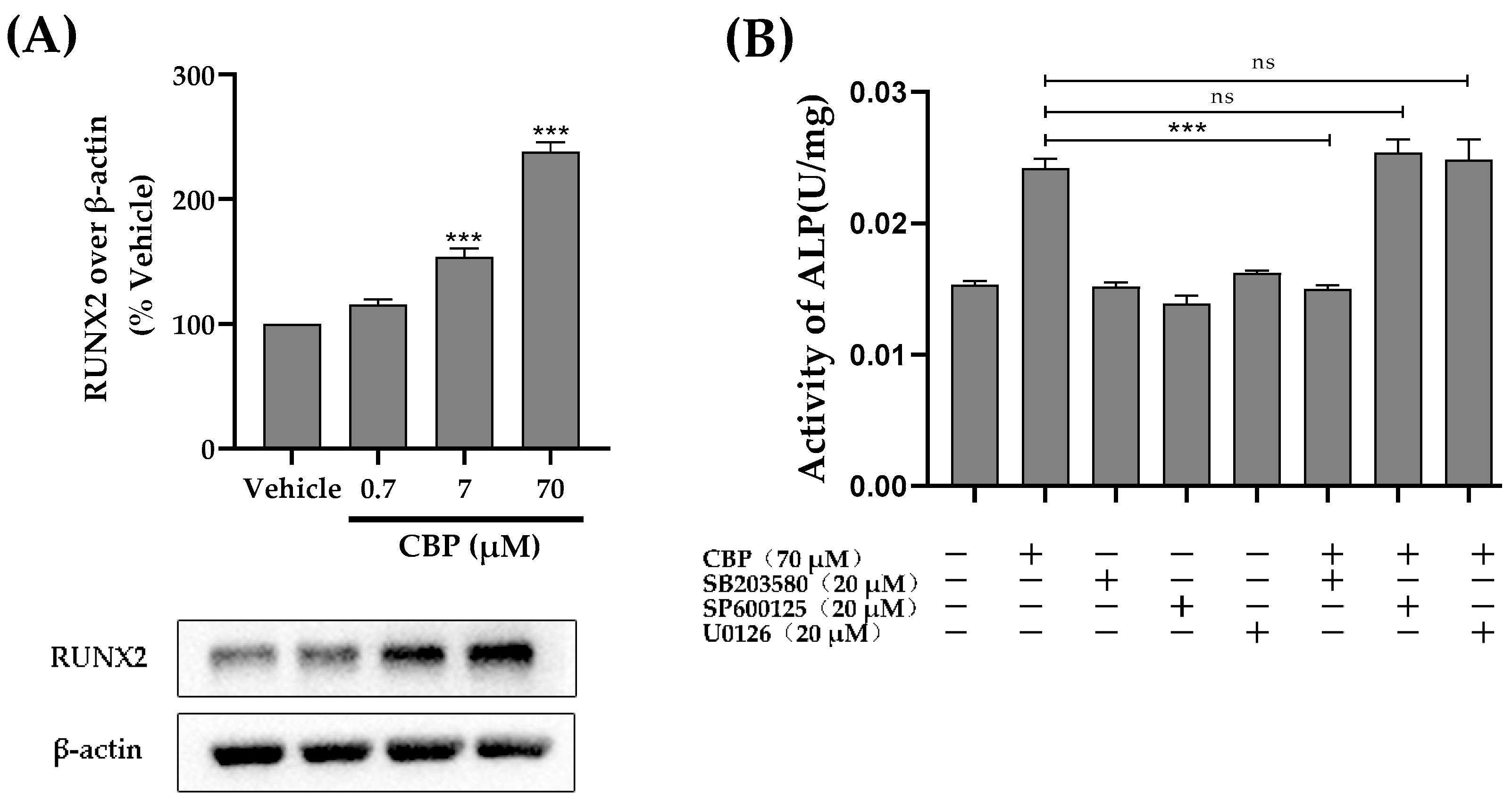
3.5. CBP Stimulated Osteoblastic Differentiation Activity through the TβRI-p38-MAPK-Mediated Activation of RUNX2 Pathway
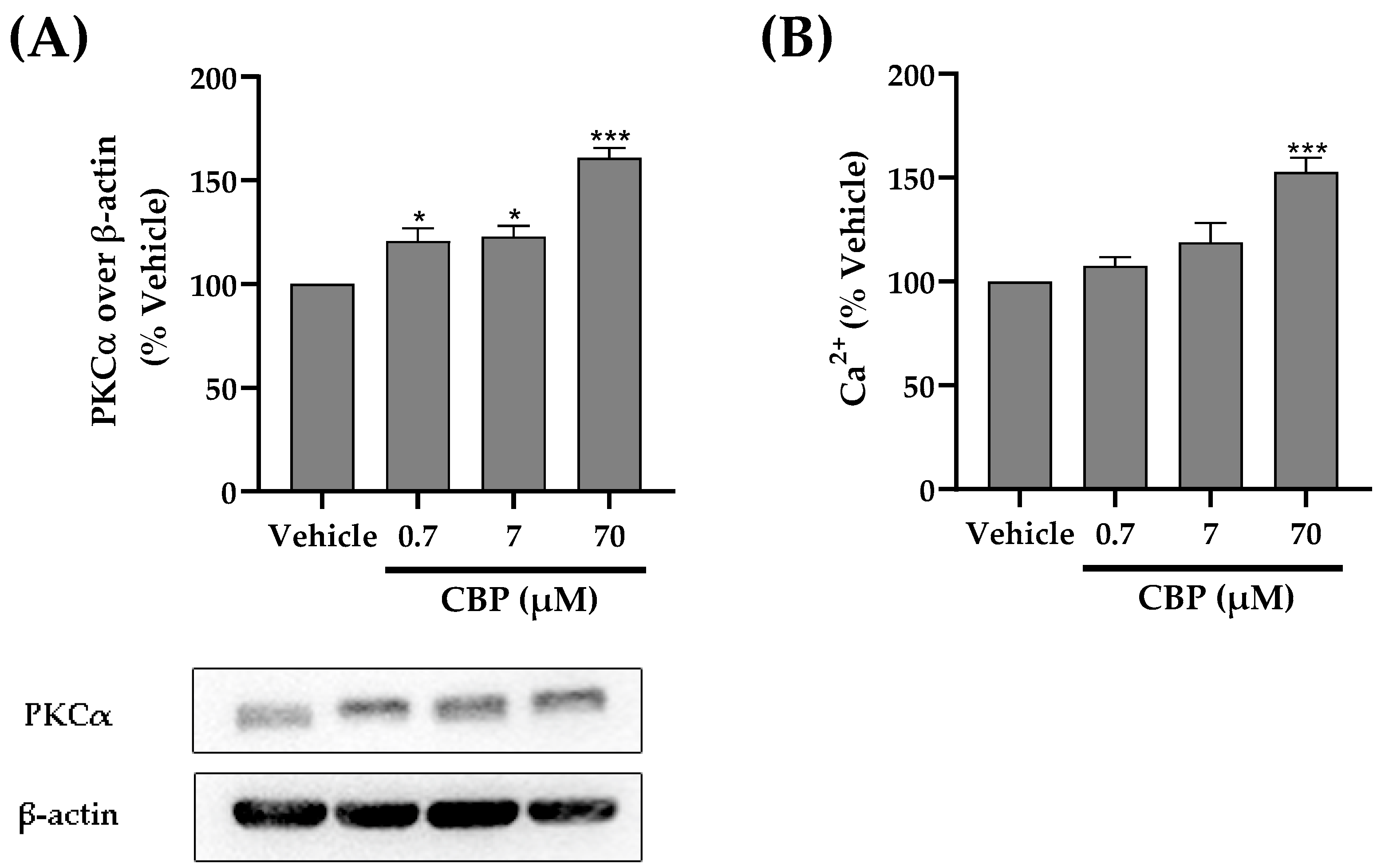
3.6. CBP Activated the PKCα and Ca2+ Pathway
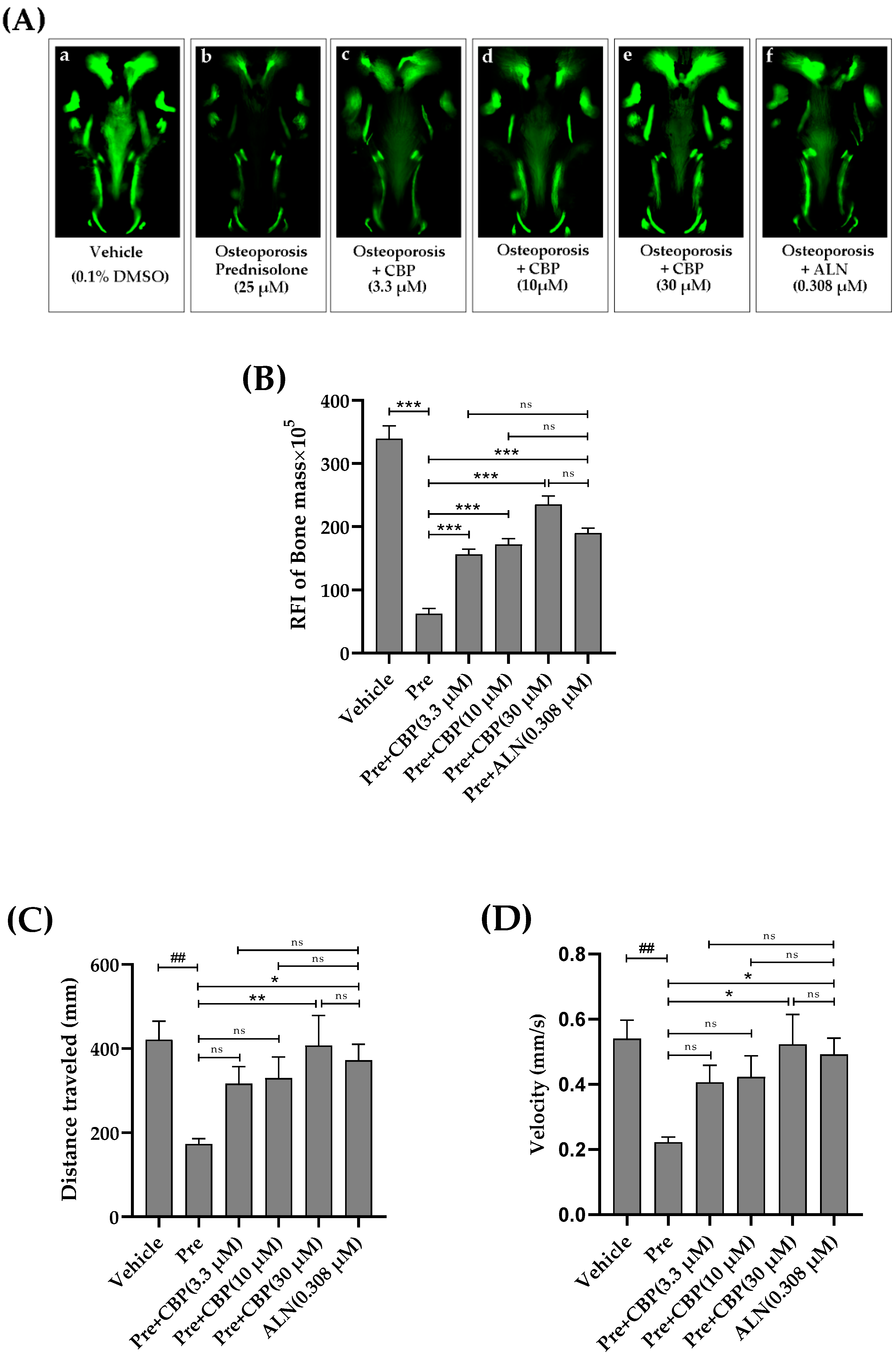
3.7. The Prevention of Osteoporosis In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBP | soy peptide |
| DEDEQIPSHPPR | Asp-Glu-Asp-Glu-Gln-IIe-Pro-Ser-His-Pro-Pro-Arg |
| ALP | Alkaline phosphatase |
Appendix A
References
- Li, J.; Chen, X.; Lu, L.; Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Shang, N.; Bhullar, K.S.; Wu, J. Pea protein-derived tripeptide LRW shows osteoblastic activity on MC3T3-E1 cells via the activation of the Akt/Runx2 pathway. Food Funct. 2020, 11, 7197–7207. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 2008, 473, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-C.; Cao, S.; Dong, X.; Law, M.-C.; Chan, T.-H.; Wong, M.-S. (−)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro. Nutrients 2017, 9, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Ju, W.-C.; Yeo, J.-H.; Kim, J.Y.; Seo, H.S.; Uchida, Y.; Cho, Y. Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int. J. Mol. Med. 2013, 33, 178–184. [Google Scholar] [CrossRef]
- Zhao, M.; Li, S.; Ahn, D.U.; Huang, X. Phosvitin phosphopeptides produced by pressurized hea-trypsin hydrolysis promote the differentiation and mineralization of MC3T3-E1 cells via the OPG/RANKL signaling pathways. Poult. Sci. 2021, 100, 527–536. [Google Scholar] [CrossRef]
- Nardone, V.; D’Asta, F.; Brandi, M.L. Pharmacological management of osteogenesis. Clinics 2014, 69, 438–446. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Pepe, J.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.J. Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS. J. Clin. Endocrinol. Metab. 2022, 107, 1441–1460. [Google Scholar] [CrossRef]
- Brent, M.B.; Stoltenborg, F.E.; Brüel, A.; Thomsen, J.S. Teriparatide and Abaloparatide Have a Similar Effect on Bone in Mice. Front. Endocrinol. 2021, 12, 628994. [Google Scholar] [CrossRef] [PubMed]
- Bégin, M.-J.; Ste-Marie, L.-G.; Coupal, L.; Ethier, J.; Räkel, A. Hypomagnesemia During Teriparatide Treatment in Osteoporosis: Incidence and Determinants. J. Bone Miner. Res. 2018, 33, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G.; et al. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Liu, W.; Zhang, X.; Zhao, M.; Zhu, B.; Hou, T.; He, H. Duck Egg White–Derived Peptide VSEE (Val-Ser-Glu-Glu) Regulates Bone and Lipid Metabolisms by Wnt/β-Catenin Signaling Pathway and Intestinal Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900525. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Fan, F.; Chen, H.; Xu, Z.; Cheng, S.; Lu, W.; Du, M. A bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J. Dairy Sci. 2020, 103, 3950–3960. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Guo, Y. Cell Growth Stimulation, Cell Cycle Alternation, and Anti-Apoptosis Effects of Bovine Bone Collagen Hydrolysates Derived Peptides on MC3T3-E1 Cells Ex Vivo. Molecules 2020, 25, 2305. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Heaney, R.-P.; Dowell, M.-S.; Rafferty, K.; June, B. Bioavailability of the calcium in fortified soy imitation milk, with some observations on method. Am. J. Clin. Nutr. 2000, 71, 1166. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Wang, C.; Tang, Q.; Yang, F.; Xu, Y. Possible mechanisms of prednisolone-induced osteoporosis in zebrafish larva. Biomed. Pharmacother. 2018, 101, 981–987. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Ko, S.-C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Wang, Z.; Fan, F.; Shi, P.; Tu, M.; Du, M. Isolation and Characterization of Peptides from Mytilus edulis with Osteogenic Activity in Mouse MC3T3-E1 Preosteoblast Cells. J. Agric. Food Chem. 2019, 67, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Wu, J. Egg White Ovotransferrin Shows Osteogenic Activity in Osteoblast Cells. J. Agric. Food Chem. 2018, 66, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-R.; Leung, W.N.; Li, G.; Kong, S.K.; Lu, X.; Wong, Y.M.; Chan, C.W. Osthole Enhances Osteogenesis in Osteoblasts by Elevating Transcription Factor Osterix via cAMP/CREB Signaling In Vitro and In Vivo. Nutrients 2017, 9, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Wang, Y.; Patel, G.; Xue, Q.; Njateng, G.S.S.; Cai, S.; Cheng, G.; Kai, G. In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-kappaB and MAPK signaling pathways. J. Ethnopharmacol. 2020, 261, 113105. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Bhullar, K.S.; Wu, J. Ovotransferrin Exhibits Osteogenic Activity Partially via LDL Receptor-related Protein 1 (LRP1) Activation in MC3T3-E1 Cells. J. Agric. Food Chem. 2020, 68, 9427–9435. [Google Scholar] [CrossRef]
- Barrett, R.; Chappell, C.; Quick, M.; Fleming, A. A rapid, high content, in vivo model of glucocorticoid-induced osteoporosis. Biotechnol. J. 2006, 1, 651–655. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, S.; Wang, C.; Zhang, Q. A fructooligosaccharide from Achyranthes bidentata inhibits osteoporosis by stimulating bone formation. Carbohydr. Polym. 2019, 210, 110–118. [Google Scholar] [CrossRef]
- Wang, W.; Olson, D.; Cheng, B.; Guo, X.; Wang, K. Sanguis Draconis resin stimulates osteoblast alkaline phosphatase activity and mineralization in MC3T3-E1 cells. J. Ethnopharmacol. 2012, 142, 168–174. [Google Scholar] [CrossRef]
- Xia, M.; Wang, X.; Xu, J.; Qian, Q.; Gao, M.; Wang, H. Tris (1-chloro-2-propyl) phosphate exposure to zebrafish causes neurodevelopmental toxicity and abnormal locomotor behavior. Sci. Total Environ. 2021, 758, 143694. [Google Scholar] [CrossRef]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef]
- N’Deh, K.P.U.; Yoo, H.-S.; Chung, K.-H.; Lee, K.-J.; Kim, D.-H.; A Yoon, J.; An, J.H. Collagen Extract Derived from Yeonsan Ogye Chicken Increases Bone Microarchitecture by Suppressing the RANKL/OPG Ratio via the JNK Signaling Pathway. Nutrients 2020, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Bhullar, K.S.; Hubbard, B.P.; Wu, J. Tripeptide IRW initiates differentiation in osteoblasts via the RUNX2 pathway. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Chang, Y.-H.; Wei, B.-L.; Huang, Y.-L.; Chiou, W.-F. Betulinic Acid Stimulates the Differentiation and Mineralization of Osteoblastic MC3T3-E1 Cells: Involvement of BMP/Runx2 and β-Catenin Signals. J. Agric. Food Chem. 2010, 58, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-L.; Yuan, Y.; Tu, J.; Zou, G.-M.; Li, Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 5, e1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, J.; Ren, F.; Zhang, W.; Zhang, H.; Zhao, L.; Zhang, M.; Cui, W.; Wang, X.; Guo, H. Lactoferrin Promotes Osteogenesis through TGF-β Receptor II Binding in Osteoblasts and Activation of Canonical TGF-β Signaling in MC3T3-E1 Cells and C57BL/6J Mice. J. Nutr. 2018, 148, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.Y.C.; Dong, H.; Quach, K.T.; Van Nguyen, P.N.; Chen, K.; Carethers, J.M. TGF-β mediates PTEN suppression and cell motility through calcium-dependent PKC-α activation in pancreatic cancer cells. Am. J. Physiol. Liver Physiol. 2008, 294, G899–G905. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J.; Kassem, M. Osteoblasts in osteoporosis: Past, emerging, and future anabolic targets. Eur. J. Endocrinol. 2011, 165, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef]
- Inubushi, T.; Kosai, A.; Yanagisawa, S.; Chanbora, C.; Miyauchi, M.; Yamasaki, S.; Sugiyama, E.; Ishikado, A.; Makino, T.; Takata, T. Bovine lactoferrin enhances osteogenesis through Smad2/3 and p38 MAPK activation. J. Oral Biosci. 2020, 62, 147–154. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Fan, F.; Shi, P.; Tu, M.; Wang, Z.; Du, M. Identification and mechanism evaluation of a novel osteogenesis promoting peptide from Tubulin Alpha-1C chain in Crassostrea gigas. Food Chem. 2019, 272, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Feng, C.-W.; Peng, M.-Y.; Chen, Y.-C.; Chan, T.-F. Antiosteoporosis effects of a marine antimicrobial peptide pardaxin via regulation of the osteogenesis pathway. Peptides 2021, 148, 170686. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Kong, X.; Du, M.; Yu, W.; Wang, Z.; Xu, B.; Yang, J.; Xu, J.; Liu, Z.; Cheng, Y.; et al. Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TβRI-p38-MAPK-Depending RUNX2 Activation. Nutrients 2022, 14, 1940. https://doi.org/10.3390/nu14091940
Wang K, Kong X, Du M, Yu W, Wang Z, Xu B, Yang J, Xu J, Liu Z, Cheng Y, et al. Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TβRI-p38-MAPK-Depending RUNX2 Activation. Nutrients. 2022; 14(9):1940. https://doi.org/10.3390/nu14091940
Chicago/Turabian StyleWang, Kuaitian, Xiao Kong, Mengdi Du, Wei Yu, Zhenhua Wang, Bo Xu, Jianrong Yang, Jingru Xu, Zhili Liu, Yongqiang Cheng, and et al. 2022. "Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TβRI-p38-MAPK-Depending RUNX2 Activation" Nutrients 14, no. 9: 1940. https://doi.org/10.3390/nu14091940
APA StyleWang, K., Kong, X., Du, M., Yu, W., Wang, Z., Xu, B., Yang, J., Xu, J., Liu, Z., Cheng, Y., & Gan, J. (2022). Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TβRI-p38-MAPK-Depending RUNX2 Activation. Nutrients, 14(9), 1940. https://doi.org/10.3390/nu14091940





