The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases
Abstract
:1. Overview of Liver Diseases and Polysaccharides
2. Polysaccharides in Different Liver Diseases
2.1. Nonalcoholic Fatty Liver Disease and Ethanol-induced Liver Disease
2.2. Hepatic Fibrosis
2.3. Hepatocellular Carcinoma (HCC)
2.4. Drug-Induced Liver Injury (DILI)
| Polysaccharide | Source | Types of Liver Disease Treated | Cell/Animal Models | Effects and Mechanisms | References |
|---|---|---|---|---|---|
| Acidic polysaccharides from carrot (CPS) | Carrot | ALD | Mice | Reducing lipid droplets | [46] |
| Aconitum coreanum polysaccharide | Aconitum coreanum | HCC | H22 cells/mice | Inducing apoptosis by suppressing P13K/Akt and activating p38 | [86] |
| alkalic-extractable polysaccharides from Coprinus comatus (APCC) | Coprinus comatus | ALD | Mice | Inhibiting inflammation and ROS. Improving alcohol metabolism. | [58] |
| Angelica sinensis polysaccharide (ASP) | The dry roots of Angelica sinensis | NAFLD | Mice | Inhibiting ROS. Increasing PPARγ and SIRT1-AMPK signaling. | [45] |
| Hepatic fibrosis | Mice | Inhibiting inflammation. Decreasing ECM accumulation | [66] | ||
| HCC | Mice | Drug delivery nanoparticles | [98,99] | ||
| DILI | Hepatocytes/rats | Inhibiting ROS and apoptosis | [101] | ||
| Asparagus polysaccharide | Asparagus | HCC | SK-Hep1 and Hep-3B cells | Suppressing MAPK/PI3K and HIF-1α/VEGF signaling pathway | [83,84] |
| Astragalus polysaccharides (APS) | Astragalus | HCC | Mice | Inducing apoptosis by increasing Bax and decreasing Bcl-2 | [79] |
| Bletilla striata polysaccharide | Bletilla striata | NAFLD | Mice | Regulating fatty acids and arachidonic acid metabolism | [41] |
| Chicory polysaccharide (CP) | Chicory | NAFLD | Zebrafish and rats | Inhibiting ROS and lipogenesis. Promoting lipolysis and AMPK. | [30,37,39] |
| Cordyceps sinensis polysaccharide (CSP) | Cordyceps Sinensis | NAFLD | Mice | Modulating lipid metabolism and gut microbiota | [28] |
| Coriolus versicolor mycelia polysaccharide (CVMP) | Coriolus versicolor mycelia | ALD | Mice | Inhibiting inflammation and ROS. Regulating lipid metabolism | [34] |
| Crude monkshood polysaccharide | Monkshood | HCC | Hepa1-6 cells/mice | Enhancing the immunocyte to kill the tumor | [97] |
| Dandelion polysaccharide | Dandelion | HCC | HepG2, Hepa1-6, H22 cells/mice | Suppressing the HIF-1α/VEGF signaling pathway | [82] |
| Dendrobium huoshanense polysaccharide (DHP) | Dendrobium huoshanense | ALD | Mice | Correcting the abnormal hepatic methionine metabolism pathway and decreasing the hepatic methylglyoxal level | [49] |
| Dendrobium officinale polysaccharide (DOP) | Dendrobium officinale | ALD | L02 cells/rats | Inhibiting TLR4/NF-κB signaling | [56] |
| Hepatic fibrosis | Rats | Inhibiting the TLR4-NF-κB pathway | [75] | ||
| Dictyophora polysaccharides | Dictyophora | Hepatic fibrosis | Rats | Decreasing ECM accumulation | [65] |
| Echinacea purpurea polysaccharide (EPP) | Echinacea purpurea | ALD | Mice | Activation of the Nrf2/HO-1 pathway | [50] |
| Enteromorpha prolifera polysaccharide | Enteromorp-ha prolifera | NAFLD | Rats | Reducing serum lipid levels by increasing H2S production | [31] |
| Fucoidan | Brown algae | HCC | MHCC97H, Hep3B cells/mice | Inducing apoptosis by increasing lncRNA LINC00261 expression | [87] |
| DILI | HL7702 cells/mice | Inhibiting ROS by Nrf2 signaling | [105] | ||
| Fucoidan–fucoxanthin mix (FFM) | Sargassum hemiphyllum | NAFLD | HepaRG cells/mice/patients | Inhibiting inflammation. Modulating the leptin–adiponectin axis | [32] |
| Ganoderma lucidum polysaccharide (GLP) | Ganoderma lucidum | NAFLD | HepG2 cells/mice | Modulating bile acid synthesis through the FXR-SHP/FGF pathway | [38] |
| DILI | Mice | Inhibiting nitric oxide production and inflammation | [108] | ||
| Ganoderma lucidum spore polysaccharide (GLSP) | The spores of Ganoderma lucidum | HCC | Mice | Promoting the polarization of primary macrophages to the M1 type | [80] |
| Garlic polysaccharide (GP) | Garlic | ALD | Mice | Regulating gut microbiota | [59] |
| Ginger polysaccharide | Ginger | HCC | HepG2 cells | Inducing apoptosis | [89] |
| Grifola frondose polysaccharide | Grifola frondosa | HCC | H22 and HepG2 cells | Inducing the mitochondrial apoptotic pathway | [90] |
| Lycium barbarum polysaccharide (LBP) | Lycii Fructus | NAFLD | Rats/humans | Inhibiting inflammation and regulating host gut microbiota | [71,74] |
| ALD | BRL-3A cells/mice | Inhibiting TXNIP and activating AMPK. Inhibiting inflammation, ROS, and apoptosis. | [33,55] | ||
| Miltiorrhiza bunge polysaccharide | Salvia miltiorrhiza | NAFLD | Mice | Modulating gut microbiota and improving insulin resistance | [73] |
| Modified polysaccharides from Coprinus comatus (MPCC) | Coprinus comatus | ALD | Mice | Inhibiting inflammation and ROS. Reducing serum lipid levels. Promoting alcohol metabolism. | [48] |
| Mussel polysaccharide α-D-glucan (MP-A) | Mytilus coruscus | NAFLD | Rats | Inhibiting inflammation. Increasing short-chain fatty acids. Inhibiting PPAR signaling. | [36] |
| Neutral polysaccharide from Panax notoginseng | Panax notoginseng | HCC | Mice | Enhancing the anti-tumor effect of cyclophosphamide | [96] |
| O. lanpingensis polysaccharides (OLP) | Ophiocordyceps lanpingensis | Hepatic fibrosis | Mice | Inhibiting inflammation, ROS, and apoptosis | [64] |
| Ophiopogon japonicus polysaccharide (MDG-1) | Ophiopogon | NAFLD | Mice | Inhibiting inflammation. Modulating the gut–liver axis and hepatic lipid metabolism. | [35] |
| Phellinus linteus mycelia polysaccharide (PL-N1) | Phellinus linteus mycelia | DILI | Mice | Decreasing cytochrome P450 2E1 expression and hepatic release of cytokines | [103] |
| Pinus koraiensis pine nut polysaccharide (PNP80b) | Pine nut | ALDDILI | Mice | Inhibiting inflammation and ROS by Nrf2 signaling | [52] |
| Pleurotus citrinipileatus polysaccharide | Pleurotus citrinipileatus | Hepatic fibrosis | Mice | Reducing the level of cytokine TGF-β1 | [69] |
| Polysaccharide from Lachnum sp. (LSP) | Lachnum sp. | HCC | HepG2, SMMC7721, H22 and L02 cells/mice | Inducing apoptosis by inhibiting the MEK and PI3K pathways | [94,95] |
| Polysaccharide from Lentinus | Lentinus edodes | HCC | HepG2 and H22 cells/mice | Inducing the mitochondrial apoptotic pathway and inhibiting NF-κB, Stat3, and survivin signaling | [93] |
| Polysaccharide from Maca (MP) | Maca (Lepidium meyenii) | ALD | HepG2 cells/ mice | Reducing ROS and serum lipid levels | [57] |
| Polysaccharide from Pleurotus geesteranus mycelium | The mycelium of Pleurotus geesteranus | ALD | Mice | Inhibiting inflammation and ROS. Regulating alcohol metabolism. Reducing serum lipid levels. | [53,60] |
| Polysaccharide from Pleurotus geesteranus (PFP-1) | The fruiting body of Pleurotus geesteranus | ALD | Mice | Activating Nrf2 signaling and inhibiting the TLR4-mediated NF-κB signal pathways | [54] |
| Polysaccharide from Pleurotus ostreatus | Pleurotus ostreatus | HCC | HepG2 and HCCLM3 cells/ mice | Inducing apoptosis. Downregulation of regenerative genes and secretion of immunological factors. | [78] |
| Polysaccharide from the residue of Panax notoginseng (PNPS) | the residue of Panax notoginseng | ALD | Mice | Inhibiting inflammation and ROS by Nrf2 signaling. Reducing serum lipid levels. | [47] |
| Pomelo fruitlet polysaccharide (YZW-A) | Pomelo fruitlet | NAFLD | Mice | Promoting hepatic AMPK and Nrf2 signaling. | [40] |
| Pumpkin polysaccharide (PPPF) | Pumpkin | HCC | HepG2 cells | Inducing apoptosis by inhibiting the JAK2/STAT3 pathway | [88] |
| Rhizopus Nigrum polysaccharide | Rhizopus Nigrum | HCC | HepG2 and Huh7 cells/mice | Inducing apoptosis | [85] |
| Sagittaria sagittifolia L. polysaccharide | The root tubers of S. sagittifolia | DILI | Mice | Inhibiting ROS by Nrf2 | [107] |
| Schisandra chinensis caulis polysaccharide (SCP) | Schisandra chinensis Caulis | DILI | Mice | Inhibiting inflammation, ROS, and apoptosis | [102] |
| NAFLD | Rats | Inhibiting ROS. Regulating glucose and lipid metabolism. | [29] | ||
| Seabuckthorn berry polysaccharide (SP) | The berries of seabuckthorn (Hippophae rhamnoides L.) | DILI | Mice | Inhibiting ROS and apoptosis by Nrf2/HO1/SOD signaling | [104] |
| Triticum aestivum sprout-derived polysaccharide (TASP) | Triticum aestivum | ALD | Mice | Inhibiting inflammation, ROS, and apoptosis by Nrf2 signaling. Reducing serum lipid levels. | [51] |
| Walnut green husk polysaccharides (WGHP) | Walnut green husk | NAFLD | Rats | Improving gut microbiota and short-chain fatty acids | [72] |
| Yulangsan polysaccharide | The root of Millettia pulchra | DILI | Mice | Inhibiting ROS | [106] |
3. Cell Death in Liver Diseases
3.1. Polysaccharides Regulating Apoptosis
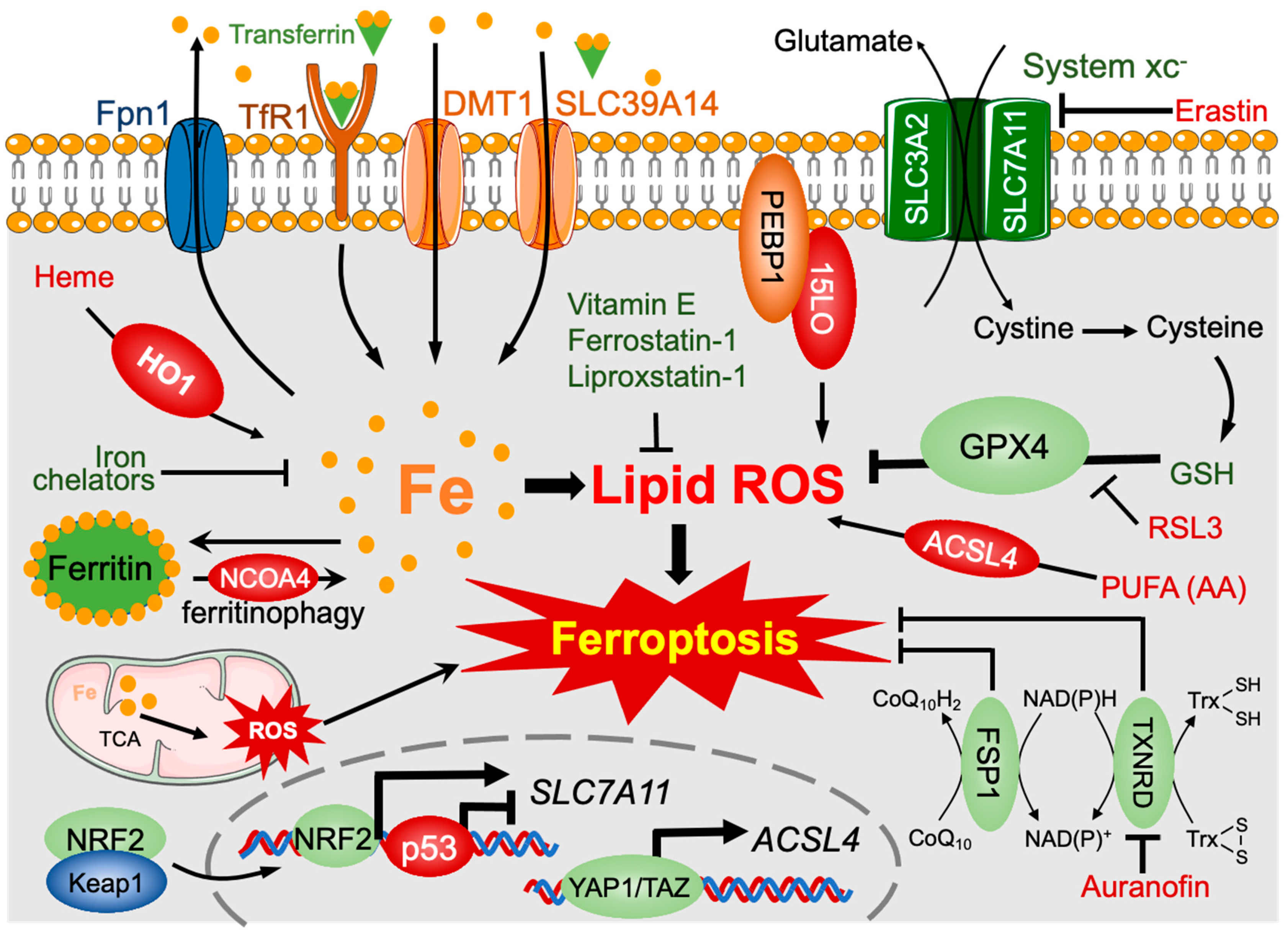
3.2. Polysaccharides and Other Phytochemicals Regulating Ferroptosis
| Agonist/Inhibitor | Phytochemicals | Types of Phytochemicals | Types of Diseases Treated | Cell/Animal Models | Mechanisms | References |
|---|---|---|---|---|---|---|
| Agonist | Artemether | Terpene | Liver fibrosis | LX2 cells/mice | Activiting p53 signaling. Accumulating IRP2 | [148,151] |
| Agonist | Artesunate | Terpene | Liver fibrosis | Mice | Promoting ferritinophagy | [145] |
| HCC | Huh7, SNU-449, SNU-182 HCC cells | Promoting ferritin degradation and decreasing GSH | [156] | |||
| Agonist | Chrysophanol | Quinone | Liver fibrosis | Mice | Promoting ER stress | [146] |
| Agonist | Dihydroartemisinin (DHA) | Terpene | Liver fibrosis | Rats, mice | Promoting ferritinophagy | [150,152] |
| HCC | Hep3B, HepG2, and Huh7 cells/mice | Promoting ER stress and PEBP1/15-LO formation | [153,154,155] | |||
| Agonist | Heteronemin | Terpene | HCC | HA22T, HA59T cells | Increasing ROS | [158] |
| Agonist | Lycium barbarum polysaccharide (LBP) | Polysaccharide | Breast cancer | MCF-7 and MDA-MB-231 cells | Triggering ferroptosis by downregulating SLC7A11 and GPX4 | [134] |
| Agonist | Magnesium isoglycyrrhizinate | Terpene | Liver fibrosis | Rats | Increasing HO-1 expression | [147] |
| Agonist | Red ginseng polysaccharide | Polysaccharide | Lung and breast cancer | A549 and MDA-MB-231 cells | Triggering ferroptosis by inhibiting GPX4 | [136] |
| Agonist | Solasonine | Alkaloid | HCC | HepG2, HepRG cells | Inhibiting GPX4 and GSH synthetase | [157] |
| Agonist | Wild bitter melon extract | Liver fibrosis | Mice | Inhibiting GPX4 and SLC7A11 | [144] | |
| Agonist | Alkaloid berberine | Alkaloid | Liver fibrosis | Mice | Blocking the autophagy–lysosome pathway and increasing ROS | [149] |
| Inhibitor | Astragalus polysaccharide (APS) | Polysaccharide | Colitis | Caco-2 cells/DSS-challenged mice | Decreasing lipid ROS | [137] |
| Inhibitor | Baicalein | Flavonoid | Acute liver injury | HepG2 cells/mice | Inhibiting the NF-κB pathway and ALOX12 | [164] |
| Inhibitor | Clausenamide | Pyrrolidone | DILI | Hepa RG and HepG2 cells/mice | Activating the Keap1-Nrf2 pathway | [161] |
| Inhibitor | Dehydroabietic acid | Terpene | NAFLD | HEK293T and HL7702 cells/mice | Activating the Nrf2-ARE pathway | [159] |
| Inhibitor | Fucoidans | Polysaccharide | Retinal disease | ARPE-19 and OMM-1 cells | Inhibiting ferroptosis by increasing GPX4 | [138] |
| Inhibitor | Ginkgolide B | Terpene | NAFLD | HepG2 cells/ mice | Activating Nrf2 signaling | [160] |
| Inhibitor | Glycyrrhizin | Terpene | Acute liver injury | L02 cells/mice | Promoting the Nrf2/HO-1/HMGB1 pathway | [162] |
| Inhibitor | Holly (Ilex latifolia Thunb.) polyphenols | Polyphenol | Acute liver injury | Piglet | Decreasing lipid ROS | [163] |
| Inhibitor | Polysaccharide of atractylodes macrocephala Koidz | Polysaccharide | Spleen injury in infections | Goslings | Inhibiting ferroptosis by restoring the expression and distribution of GPX4 | [139] |
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | alcoholic liver disease |
| AMPK | AMP-activated protein kinase |
| APAP | acetaminophen |
| APCC | alkalic-extractable polysaccharides from Coprinus comatus |
| APS | Astragalus polysaccharides |
| ASP | Angelica sinensis polysaccharide |
| CLD | chronic liver disease |
| CP | chicory polysaccharide |
| CPS | carrot polysaccharide |
| CSP | Cordyceps sinensis polysaccharide |
| CTX | cyclophosphamide |
| CVMP | polysaccharide from Coriolus versicolor mycelia |
| DHA | dihydroartemisinin |
| DILI | drug-induced liver injury |
| DOP | Dendrobium officinale polysaccharide |
| ECM | extracellular matrix |
| EPP | Echinacea purpurea polysaccharide |
| FFM | fucoidan and fucoxanthin mix |
| GLP | Ganoderma lucidum polysaccharide |
| GLSP | Ganoderma lucidum spore polysaccharide |
| GP | garlic polysaccharide |
| GPX | glutathione-glutathione peroxidases |
| GSH | glutathione |
| HCC | hepatocellular carcinoma |
| HDL-C | high-density lipoprotein cholesterol |
| HIF | hypoxia-inducible factor |
| HIF-1a | hypoxia-inducible factor 1a |
| HSC | hepatic stellate cells |
| LBP | Lycium barbarum polysaccharide |
| LDL-C | low-density lipoprotein cholesterol |
| LSP | polysaccharide from Lachnum sp. |
| MDG-1 | Ophiopogon japonicus polysaccharide |
| MP | maca (Lepidium meyenii) polysaccharide |
| MP-A | mussel polysaccharide α-D-glucan |
| MPCC | modified polysaccharides from Coprinus comatus |
| mTOR | mammalian target of rapamycin |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| NF-κB | nuclear factor kappa-B |
| Nrf2 | nuclear factor E2-related factor 2 |
| OLP | O. lanpingensis polysaccharides |
| PE | phosphatidylethanolamine |
| PEBP1 | PE-binding protein 1 |
| PFP-1 | Pleurotus geesteranus polysaccharide |
| PNP80b-2 | Pinus koraiensis pine nut polysaccharide |
| PNPS | polysaccharide from the residue of Panax notoginseng |
| PPPF | polysaccharide from pumpkin fruit |
| PUFAs | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SCP | Schisandra chinensis caulis polysaccharide |
| TAMs | tumor-associated macrophages |
| TASP | Triticum aestivum sprout-derived polysaccharide |
| Trx | thioredoxin |
| TXNIP | thioredoxin-interacting protein |
| TXNRD | thioredoxin reductase |
| VEGFs | vascular endothelial growth factors |
| WGHP | walnut green husk polysaccharides |
| YZW-A | polysaccharide extract from pomelo fruitlet |
References
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver. Int. 2018, 38 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.C.S.; Huang, J.L.W.; George, J.; Huang, J.; Leung, C.; Eslam, M.; Chan, H.L.Y.; Ng, S.C. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 57–73. [Google Scholar] [CrossRef]
- Udompap, P.; Kim, D.; Kim, W.R. Current and Future Burden of Chronic Nonmalignant Liver Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 2031–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asrani, S.K.; Hall, L.; Hagan, M.; Sharma, S.; Yeramaneni, S.; Trotter, J.; Talwalkar, J.; Kanwal, F. Trends in Chronic Liver Disease-Related Hospitalizations: A Population-Based Study. Am. J. Gastroenterol. 2019, 114, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, R.; van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Adib Ridzuan, N.R.; Abdul Jalil, N.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef]
- Dhahri, M.; Alghrably, M.; Mohammed, H.A.; Badshah, S.L.; Noreen, N.; Mouffouk, F.; Rayyan, S.; Qureshi, K.A.; Mahmood, D.; Lachowicz, J.I.; et al. Natural Polysaccharides as Preventive and Therapeutic Horizon for Neurodegenerative Diseases. Pharmaceutics 2021, 14, 1. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, M.; Li, Y.; Li, Y.; Ji, H.; Hu, Q. Cardiovascular Protective Effects of Plant Polysaccharides: A Review. Front. Pharmacol. 2021, 12, 783641. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review. Mar. Drugs 2021, 19, 608. [Google Scholar] [CrossRef]
- Yuan, Y.; Che, L.; Qi, C.; Meng, Z. Protective effects of polysaccharides on hepatic injury: A review. Int. J. Biol. Macromol. 2019, 141, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Qiu, Y.; Liu, Y.; Zhu, R.; Chen, Y.; El-Seedi, H.R.; Chen, X.; Zhao, C. Cancer-fighting potentials of algal polysaccharides as nutraceuticals. Food Res. Int. 2021, 147, 110522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, F.; Zhang, J.; Wang, W.; Li, L.; Yan, J. Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.; Xiong, Z.; Lu, G.; Ma, W.; Lv, Q.; Wang, L.; Jia, X.; Feng, L. A review on the applications of Traditional Chinese medicine polysaccharides in drug delivery systems. Chin. Med. 2022, 17, 12. [Google Scholar] [CrossRef]
- Xie, M.; Tao, W.; Wu, F.; Wu, K.; Huang, X.; Ling, G.; Zhao, C.; Lv, Q.; Wang, Q.; Zhou, X.; et al. Anti-hypertensive and cardioprotective activities of traditional Chinese medicine-derived polysaccharides: A review. Int. J. Biol. Macromol. 2021, 185, 917–934. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 15080. [Google Scholar] [CrossRef]
- Nabi, O.; Boursier, J.; Lacombe, K.; Mathurin, P.; de Ledinghen, V.; Goldberg, M.; Zins, M.; Serfaty, L. Comorbidities Are Associated with Fibrosis in NAFLD Subjects: A Nationwide Study (NASH-CO Study). Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef]
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol's Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Taylor, B.; Mohapatra, S.; Irving, H.; Baliunas, D.; Patra, J.; Roerecke, M. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol Rev. 2010, 29, 437–445. [Google Scholar] [CrossRef]
- Michalak, A.; Lach, T.; Cichoz-Lach, H. Oxidative Stress-A Key Player in the Course of Alcohol-Related Liver Disease. J. Clin. Med. 2021, 10, 3011. [Google Scholar] [CrossRef]
- Albano, E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Aspects Med. 2008, 29, 9–16. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Kaneider, N.C. Pathways of liver injury in alcoholic liver disease. J. Hepatol. 2011, 55, 1159–1161. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, L.; Wang, W.; Qiu, W.; Liu, L.; Ning, A.; Cao, J.; Huang, M.; Zhong, M. Polysaccharides isolated from Cordyceps Sinensis contribute to the progression of NASH by modifying the gut microbiota in mice fed a high-fat diet. PLoS ONE 2020, 15, e0232972. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Chen, C.; Lin, H.; Xu, G.; Li, H.; Wang, C.; Chen, J.; Sun, J. Study on the Hepatoprotection of Schisandra chinensis Caulis Polysaccharides in Nonalcoholic Fatty Liver Disease in Rats Based on Metabolomics. Front. Pharmacol. 2021, 12, 727636. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Ahmad, O.; Cao, Y.; Wang, B.; He, Q.; Li, J.; Yin, H.; Zhang, Y.; He, J.; et al. Lipid-modulate activity of Cichorium glandulosum Boiss. et Huet polysaccharide in nonalcoholic fatty liver disease larval zebrafish model. J. Pharmacol. Sci. 2018, 138, 257–262. [Google Scholar] [CrossRef]
- Ren, R.; Yang, Z.; Zhao, A.; Huang, Y.; Lin, S.; Gong, J.; Chen, J.; Zhu, P.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera increases hydrogen sulfide production and attenuates non-alcoholic fatty liver disease in high-fat diet rats. Food Funct. 2018, 9, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Shiue, S.J.; Chen, C.N.; Cheng, S.W.; Lin, H.Y.; Wu, L.W.; Wu, M.S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tipoe, G.L.; Yang, C.; Nanji, A.A.; Hao, X.; So, K.F.; Xiao, J. Lycium barbarum Polysaccharide Supplementation Improves Alcoholic Liver Injury in Female Mice by Inhibiting Stearoyl-CoA Desaturase 1. Mol. Nutr. Food Res. 2018, 62, e1800144. [Google Scholar] [CrossRef]
- Wang, K.L.; Lu, Z.M.; Mao, X.; Chen, L.; Gong, J.S.; Ren, Y.; Geng, Y.; Li, H.; Xu, H.Y.; Xu, G.H.; et al. Structural characterization and anti-alcoholic liver injury activity of a polysaccharide from Coriolus versicolor mycelia. Int. J. Biol. Macromol. 2019, 137, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, L.; Wang, X.; Feng, Y.; Wang, Y. MDG-1, an Ophiopogon polysaccharide, restrains process of non-alcoholic fatty liver disease via modulating the gut-liver axis. Int. J. Biol. Macromol. 2019, 141, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, H.; Zhang, J.; Ying, Y.; Cheng, Y.; Zhao, D.; Dou, X.; Lv, H.; Li, S.; Liu, F.; et al. Mussel polysaccharide alpha-d-glucan (MP-A) protects against non-alcoholic fatty liver disease via maintaining the homeostasis of gut microbiota and regulating related gut-liver axis signaling pathways. Int. J. Biol. Macromol. 2019, 130, 68–78. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, F.; Jiang, H.; Wang, Z.; Hua, C.; Zhang, Y. Chicory (Cichorium intybus L.) polysaccharides attenuate high-fat diet induced non-alcoholic fatty liver disease via AMPK activation. Int. J. Biol. Macromol. 2018, 118, 886–895. [Google Scholar] [CrossRef]
- Zhong, D.; Xie, Z.; Huang, B.; Zhu, S.; Wang, G.; Zhou, H.; Lin, S.; Lin, Z.; Yang, B. Ganoderma Lucidum Polysaccharide Peptide Alleviates Hepatoteatosis via Modulating Bile Acid Metabolism Dependent on FXR-SHP/FGF. Cell Physiol. Biochem. 2018, 49, 1163–1179. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Wu, Y.; Jiang, H.; Zhou, F.; Xie, X.; Wang, R.; Hua, C. Untargeted metabonomics reveals intervention effects of chicory polysaccharide in a rat model of non-alcoholic fatty liver disease. Int. J. Biol. Macromol. 2019, 128, 363–375. [Google Scholar] [CrossRef]
- Zou, C.; Fang, Y.; Lin, N.; Liu, H. Polysaccharide extract from pomelo fruitlet ameliorates diet-induced nonalcoholic fatty liver disease in hybrid grouper (Epinephelus lanceolatusmale symbol x Epinephelus fuscoguttatusfemale symbol). Fish Shellfish Immunol. 2021, 119, 114–127. [Google Scholar] [CrossRef]
- Hu, B.; Yang, H.; Chen, G.; Sun, X.; Zou, X.; Ma, J.; Yao, X.; Liang, Q.; Liu, H. Structural characterization and preventive effect on non-alcoholic fatty liver disease of oligosaccharides from Bletilla striata. Food Funct. 2022, 13, 4757–4769. [Google Scholar] [CrossRef] [PubMed]
- Hasenour, C.M.; Berglund, E.D.; Wasserman, D.H. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol. Cell Endocrinol. 2013, 366, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Cao, P.; Wang, H.; Tang, Z.; Wang, N.; Wang, J.; Zhang, Y. Chronic administration of Angelica sinensis polysaccharide effectively improves fatty liver and glucose homeostasis in high-fat diet-fed mice. Sci. Rep. 2016, 6, 26229. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Liang, W.; Li, X.; Qiu, M.; Xu, W.; Chen, H. Characterization of an Acidic Polysaccharides from Carrot and Its Hepatoprotective Effect on Alcoholic Liver Injury in Mice. Chem. Biodivers 2021, 18, e2100359. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Liu, S.; Guo, X.; Qu, Y.; Gao, M.; Cui, X.; Yang, Y. A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice. Int. J. Biol. Macromol. 2020, 149, 1084–1097. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Lai, Q.; Yang, Q.; Dong, Y.; Liu, X.; Wang, W.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of modified polysaccharides from Coprinus comatus in mice with alcohol-induced liver injury. Int. J. Biol. Macromol. 2019, 127, 476–485. [Google Scholar] [CrossRef]
- Wang, X.Y.; Luo, J.P.; Chen, R.; Zha, X.Q.; Wang, H. The effects of daily supplementation of Dendrobium huoshanense polysaccharide on ethanol-induced subacute liver injury in mice by proteomic analysis. Food Funct. 2014, 5, 2020–2035. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Xu, W.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156. [Google Scholar] [CrossRef]
- Nepali, S.; Ki, H.H.; Lee, J.H.; Cha, J.Y.; Lee, Y.M.; Kim, D.K. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidant system in mice. Int. J. Mol. Med. 2017, 40, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, H.; Gao, X.; Wang, Z.Y.; Yi, J.J. Comparative study on hepatoprotection of pine nut (Pinus koraiensis Sieb. et Zucc.) polysaccharide against different types of chemical-induced liver injury models in vivo. Int. J. Biol. Macromol. 2020, 155, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Zhang, J.; Zhang, C.; Dong, Y.; Ren, Z.; Gao, Z.; Liu, M.; Zhao, H.; Jia, L. Antioxidative and hepatoprotective effects of enzymatic and acidic-hydrolysis of Pleurotus geesteranus mycelium polysaccharides on alcoholic liver diseases. Carbohydr. Polym. 2018, 201, 75–86. [Google Scholar] [CrossRef]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A polysaccharide of PFP-1 from Pleurotus geesteranus attenuates alcoholic liver diseases via Nrf2 and NF-kappaB signaling pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Liu, Y.; Tipoe, G.L.; Xing, F.; So, K.F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014, 69, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Zhan, L.; Lu, T.; Zhou, C.; Chen, X.; Dong, Y.; Lv, G.; Chen, S. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-kappaB signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Q.; Wang, L.; Zhao, M.; Zhao, B. Protective effect of polysaccharide from maca (Lepidium meyenii) on Hep-G2 cells and alcoholic liver oxidative injury in mice. Int. J. Biol. Macromol. 2017, 99, 63–70. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, J.; Liu, X.; Yang, Q.; Dong, Y.; Jia, L. The antioxidant activities of alkalic-extractable polysaccharides from Coprinus comatus on alcohol-induced liver injury in mice. Sci. Rep. 2018, 8, 11695. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, M.; Zhao, X.; Li, X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018, 56, 325–332. [Google Scholar] [CrossRef]
- Song, X.; Cui, W.; Meng, F.; Xia, Q.; Li, X.; Hou, M.; Jia, L.; Zhang, J. Glucopyranose from Pleurotus geesteranus prevent alcoholic liver diseases by regulating Nrf2/HO-1-TLR4/NF-kappaB signalling pathways and gut microbiota. Food Funct. 2022, 13, 2441–2455. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Karsdal, M.A.; Weiskirchen, R. Advances in the clinical use of collagen as biomarker of liver fibrosis. Expert Rev. Mol. Diagn. 2020, 20, 947–969. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Trautwein, C. Mechanisms of liver fibrosis resolution. J. Hepatol. 2015, 63, 1038–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying-Mei, K.E.; Min, J.; Shu-Bo, Z.; Hong, Y.U.; Juan, W.; Feng, G.E. Component analysis of Ophiocordyceps lanpingensis polysaccharides and study on alleviation of hepatic fibrosis in mice by polysaccharides. Zhongguo Zhong Yao Za Zhi 2020, 45, 5256–5264. [Google Scholar] [CrossRef]
- Wang, G.; Zuo, P.; Ding, K.; Zeng, Q.; Hu, T.; Wei, S.; Luo, P. Intervention Study of Dictyophora Polysaccharides on Arsenic-Induced Liver Fibrosis in SD Rats. Biomed. Res. Int. 2022, 2022, 7509620. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Song, M.; Wang, H.; Xia, N.; Zhang, Y. Angelica sinensis polysaccharide attenuates CCl4-induced liver fibrosis via the IL-22/STAT3 pathway. Int. J. Biol. Macromol. 2020, 162, 273–283. [Google Scholar] [CrossRef]
- Zhangdi, H.J.; Su, S.B.; Wang, F.; Liang, Z.Y.; Yan, Y.D.; Qin, S.Y.; Jiang, H.X. Crosstalk network among multiple inflammatory mediators in liver fibrosis. World J. Gastroenterol. 2019, 25, 4835–4849. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-kappaB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Pang, H.; Gao, Z.; Zhao, H.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of residue polysaccharides by Pleurotus citrinipileatus. Int. J. Biol. Macromol. 2019, 131, 315–322. [Google Scholar] [CrossRef]
- Nishimura, N.; Kaji, K.; Kitagawa, K.; Sawada, Y.; Furukawa, M.; Ozutsumi, T.; Fujinaga, Y.; Tsuji, Y.; Takaya, H.; Kawaratani, H.; et al. Intestinal Permeability Is a Mechanical Rheostat in the Pathogenesis of Liver Cirrhosis. Int. J. Mol. Sci. 2021, 22, 6921. [Google Scholar] [CrossRef]
- Gao, L.L.; Ma, J.M.; Fan, Y.N.; Zhang, Y.N.; Ge, R.; Tao, X.J.; Zhang, M.W.; Gao, Q.H.; Yang, J.J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, X.; Wang, J.; Zhong, D.; Zhang, R.; Zhang, Y.; Feng, L.; Zhang, Y. Walnut green husk polysaccharides prevent obesity, chronic inflammatory responses, nonalcoholic fatty liver disease and colonic tissue damage in high-fat diet fed rats. Int. J. Biol. Macromol. 2021, 182, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, A.L.; Li, Z.C.; Li, Y.; Xu, S.F.; Sang, H.C.; Zhi, F. Combination of Probiotics and Salvia miltiorrhiza Polysaccharide Alleviates Hepatic Steatosis via Gut Microbiota Modulation and Insulin Resistance Improvement in High Fat-Induced NAFLD Mice. Diabetes Metab. J. 2020, 44, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.L.; Li, Y.X.; Ma, J.M.; Guo, Y.Q.; Li, L.; Gao, Q.H.; Fan, Y.N.; Zhang, M.W.; Tao, X.J.; Yu, J.Q.; et al. Effect of Lycium barbarum polysaccharide supplementation in non-alcoholic fatty liver disease patients: Study protocol for a randomized controlled trial. Trials 2021, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, X.; Wu, Z.; Wang, H.; Li, Q.; Mei, H.; You, R.; Zhang, Y. Dendrobium officinale Polysaccharide Protected CCl4-Induced Liver Fibrosis Through Intestinal Homeostasis and the LPS-TLR4-NF-kappaB Signaling Pathway. Front. Pharmacol. 2020, 11, 240. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Application of Immunotherapy in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 699060. [Google Scholar] [CrossRef]
- Khinsar, K.H.; Abdul, S.; Hussain, A.; Ud Din, R.; Lei, L.; Cao, J.; Abbasi, M.; Ur Rehman, A.; Farooqui, N.; Yi, X.; et al. Anti-tumor effect of polysaccharide from Pleurotus ostreatus on H22 mouse Hepatoma ascites in-vivo and hepatocellular carcinoma in-vitro model. AMB Express 2021, 11, 160. [Google Scholar] [CrossRef]
- Lai, X.; Xia, W.; Wei, J.; Ding, X. Therapeutic Effect of Astragalus Polysaccharides on Hepatocellular Carcinoma H22-Bearing Mice. Dose Response 2017, 15, 1559325816685182. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Li, Z.H.; Gu, H.S.; Tang, R.Y.; Zhang, R.; Zhu, Y.L.; Liu, J.L.; Zhang, J.J.; Wang, L.Y. Ganoderma lucidum Spore Polysaccharide Inhibits the Growth of Hepatocellular Carcinoma Cells by Altering Macrophage Polarity and Induction of Apoptosis. J. Immunol. Res. 2021, 2021, 6696606. [Google Scholar] [CrossRef]
- Parmar, D.; Apte, M. Angiopoietin inhibitors: A review on targeting tumor angiogenesis. Eur. J. Pharmacol. 2021, 899, 174021. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wu, K.; Yang, Y.; Yang, Y.; Wang, Y.; Li, J. Dandelion Polysaccharide Exerts Anti-Angiogenesis Effect on Hepatocellular Carcinoma by Regulating VEGF/HIF-1alpha Expression. Front. Pharmacol. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Cheng, Z.; Weng, L.; Xing, D.; Zhang, M. Asparagus Polysaccharide inhibits the Hypoxia-induced migration, invasion and angiogenesis of Hepatocellular Carcinoma Cells partly through regulating HIF1alpha/VEGF expression via MAPK and PI3K signaling pathway. J. Cancer 2021, 12, 3920–3929. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Cheng, Z.; Xing, D.; Zhang, M. Asparagus Polysaccharide Suppresses the Migration, Invasion, and Angiogenesis of Hepatocellular Carcinoma Cells Partly by Targeting the HIF-1alpha/VEGF Signalling Pathway In Vitro. Evid Based Complement. Alternat. Med. 2019, 2019, 3769879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, G.; Miao, Y.; Huang, K.; Song, H.; Liu, L. Role and Mechanism of Rhizopus Nigrum Polysaccharide EPS1-1 as Pharmaceutical for Therapy of Hepatocellular Carcinoma. Front. Bioeng. Biotechnol. 2020, 8, 509. [Google Scholar] [CrossRef]
- Liang, M.; Liu, J.; Ji, H.; Chen, M.; Zhao, Y.; Li, S.; Zhang, X.; Li, J. A Aconitum coreanum polysaccharide fraction induces apoptosis of hepatocellular carcinoma (HCC) cells via pituitary tumor transforming gene 1 (PTTG1)-mediated suppression of the P13K/Akt and activation of p38 MAPK signaling pathway and displays antitumor activity in vivo. Tumour Biol. 2015, 36, 7085–7091. [Google Scholar] [CrossRef]
- Ma, D.; Wei, J.; Chen, S.; Wang, H.; Ning, L.; Luo, S.H.; Liu, C.L.; Song, G.; Yao, Q. Fucoidan Inhibits the Progression of Hepatocellular Carcinoma via Causing lncRNA LINC00261 Overexpression. Front. Oncol. 2021, 11, 653902. [Google Scholar] [CrossRef]
- Shen, W.; Chen, C.; Guan, Y.; Song, X.; Jin, Y.; Wang, J.; Hu, Y.; Xin, T.; Jiang, Q.; Zhong, L. A pumpkin polysaccharide induces apoptosis by inhibiting the JAK2/STAT3 pathway in human hepatoma HepG2 cells. Int. J. Biol. Macromol. 2017, 104, 681–686. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Song, R.; Cai, J.; Xu, J.; Tang, X.; Li, N. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2019, 123, 81–90. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Ji, H.Y.; Liu, A.J. The caspases-dependent apoptosis of hepatoma cells induced by an acid-soluble polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2020, 159, 364–372. [Google Scholar] [CrossRef]
- Fabregat, I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J. Gastroenterol. 2009, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ke, F.; Duan, C.; Lan, H.; Li, J.; Gao, C.; Li, J.; Zhong, Z. Mannan-conjugated adenovirus enhanced gene therapy effects on murine hepatocellular carcinoma cells in vitro and in vivo. Bioconjug. Chem. 2013, 24, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Wang, J.; Cheng, F.; Huang, X.; Cheng, Y.; Wang, K. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016, 377, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Li, J.; Yang, L.; Huang, Q.; Hou, G.; Ye, Z.; Ye, M. Mechanism of bioactive polysaccharide from Lachnum sp. acts synergistically with 5-fluorouracil against human hepatocellular carcinoma. J. Cell Physiol. 2019, 234, 15548–15562. [Google Scholar] [CrossRef]
- Zong, S.; Li, J.; Yang, L.; Huang, Q.; Ye, Z.; Hou, G.; Ye, M. Synergistic antitumor effect of polysaccharide from Lachnum sp. in combination with cyclophosphamide in hepatocellular carcinoma. Carbohydr. Polym. 2018, 196, 33–46. [Google Scholar] [CrossRef]
- Liu, Y.H.; Qin, H.Y.; Zhong, Y.Y.; Li, S.; Wang, H.J.; Wang, H.; Chen, L.L.; Tang, X.; Li, Y.L.; Qian, Z.Y.; et al. Neutral polysaccharide from Panax notoginseng enhanced cyclophosphamide antitumor efficacy in hepatoma H22-bearing mice. BMC Cancer 2021, 21, 37. [Google Scholar] [CrossRef]
- Yao, F.; Jiang, G.R.; Liang, G.Q.; Yuan, Q.; Zhu, Y.; Liu, M.; Zhang, L.R. The antitumor effect of the combination of aconitine and crude monkshood polysaccharide on hepatocellular carcinoma. Pak. J. Pharm. Sci. 2021, 34, 971–979. [Google Scholar]
- Zhang, Y.; Cui, Z.; Mei, H.; Xu, J.; Zhou, T.; Cheng, F.; Wang, K. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr. Polym. 2019, 219, 143–154. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef]
- Andrade, R.J.; Chalasani, N.; Bjornsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yang, S.; Qiao, Z.; Li, H.; Sun, J.; Zhuang, W.; Chen, J.; Wang, C. Schisandra chinensis acidic polysaccharide partialy reverses acetaminophen-induced liver injury in mice. J. Pharmacol. Sci. 2019, 140, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, X.; Qi, S.; A, C.P.D.; Yan, J.; Zhang, X. Hepatoprotective effect of Phellinus linteus mycelia polysaccharide (PL-N1) against acetaminophen-induced liver injury in mouse. Int. J. Biol. Macromol. 2020, 154, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhang, X.; Zhao, S.; Zou, K.; Xie, J.; Wang, X.; Liu, C.; Wang, J.; Wang, Y. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine 2018, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wei, J.G.; Tu, M.J.; Gu, J.G.; Zhang, W. Fucoidan Alleviates Acetaminophen-Induced Hepatotoxicity via Oxidative Stress Inhibition and Nrf2 Translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Huang, J.; Lin, X.; Zhang, S.; Jiao, Y.; Liang, T.; Chen, Z.; Huang, R. Hepatoprotective effects of Yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J. Ethnopharmacol. 2014, 152, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, W.; Li, B.; Lv, J.; Ke, X.; Ge, D.; Dong, R.; Wang, C.; Han, Y.; Zhang, C.; et al. Sagittaria sagittifolia polysaccharide protects against isoniazid- and rifampicin-induced hepatic injury via activation of nuclear factor E2-related factor 2 signaling in mice. J. Ethnopharmacol. 2018, 227, 237–245. [Google Scholar] [CrossRef]
- Zhang, G.L.; Wang, Y.H.; Ni, W.; Teng, H.L.; Lin, Z.B. Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice. World J. Gastroenterol. 2002, 8, 728–733. [Google Scholar] [CrossRef]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology 2014, 147, 765–783.e764. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2021, 23, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e626. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; An, P.; Xie, E.; Wu, Q.; Fang, X.; Gao, H.; Zhang, Z.; Li, Y.; Wang, X.; Zhang, J.; et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Shimada, K.; Hayano, M.; Pagano, N.C.; Stockwell, B.R. Cell-Line Selectivity Improves the Predictive Power of Pharmacogenomic Analyses and Helps Identify NADPH as Biomarker for Ferroptosis Sensitivity. Cell Chem. Biol. 2016, 23, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, H.; Yang, X.; Wu, Q.; An, P.; Jin, X.; Liu, W.; Huang, X.; Li, Y.; Yan, S.; et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal. Transduct. Target. Ther. 2020, 5, 138. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- He, S.; Li, R.; Peng, Y.; Wang, Z.; Huang, J.; Meng, H.; Min, J.; Wang, F.; Ma, Q. ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke. J. Cachexia Sarcopenia Muscle 2022. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, C.; Chen, Y.; Hu, W.; Yu, C.; Peng, C.; Feng, X.; Cheng, Q.; Wu, W.; Lu, Y.; et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Lett. 2021, 502, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, J.; Liu, L.; Xu, B.; Han, H.; Dai, W.; Pei, X.; Fu, X.; Hou, S. A novel anticancer property of Lycium barbarum polysaccharide in triggering ferroptosis of breast cancer cells. J. Zhejiang Univ. Sci. B 2022, 23, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.C.Y.; Yang, M. Lycium barbarum polysaccharides and ferroptosis: Jumping into the era of novel regulated cell death. Neural Regen. Res. 2022, 17, 1473–1474. [Google Scholar] [CrossRef]
- Zhai, F.G.; Liang, Q.C.; Wu, Y.Y.; Liu, J.Q.; Liu, J.W. Red ginseng polysaccharide exhibits anticancer activity through GPX4 downregulation-induced ferroptosis. Pharm. Biol. 2022, 60, 909–914. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Li, J.; Zhu, J.; Wang, R.; Xi, Q.; Wu, H.; Shi, T.; Chen, W. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur. J. Pharmacol. 2021, 911, 174518. [Google Scholar] [CrossRef]
- Dorschmann, P.; Apitz, S.; Hellige, I.; Neupane, S.; Alban, S.; Kopplin, G.; Ptak, S.; Frette, X.; Roider, J.; Zille, M.; et al. Evaluation of the Effects of Fucoidans from Fucus Species and Laminaria hyperborea against Oxidative Stress and Iron-Dependent Cell Death. Mar. Drugs 2021, 19, 557. [Google Scholar] [CrossRef]
- Li, W.; Zhou, X.; Xu, S.; Cao, N.; Li, B.; Chen, W.; Yang, B.; Yuan, M.; Xu, D. Lipopolysaccharide-induced splenic ferroptosis in goslings was alleviated by polysaccharide of atractylodes macrocephala koidz associated with proinflammatory factors. Poult. Sci. 2022, 101, 101725. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Wang, M.; Yu, H.M.; Han, F.X.; Wu, Q.S.; Cai, X.J.; Kurihara, H.; Chen, Y.X.; Li, Y.F.; He, R.R. Ferroptosis is involved in alcohol-induced cell death in vivo and in vitro. Biosci. Biotechnol. Biochem. 2020, 84, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kim, J.W.; Zhou, Z.; Lim, C.W.; Kim, B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, S.; Tsuchiya, Y.; Koumura, T.; Nakasone, M.; Sakamoto, T.; Matsuoka, M.; Imai, H.; Yuet-Yin Kok, C.; Okochi, H.; Nakano, H.; et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019, 10, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.H.; Huang, J.H.; Sun, M.S.; Tzeng, I.S.; Hsu, Y.C.; Kuo, C.Y. Wild Bitter Melon Extract Regulates LPS-Induced Hepatic Stellate Cell Activation, Inflammation, Endoplasmic Reticulum Stress, and Ferroptosis. Evid Based Complement. Alternat. Med. 2021, 2021, 6671129. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liu, R.; Cheng, Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 2019, 109, 2043–2053. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Chiu, V.; Hsieh, P.C.; Huang, C.Y.; Huang, S.J.; Tzeng, I.S.; Tsai, F.M.; Chen, M.L.; Liu, C.T.; Chen, Y.R. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J. Pharmacol. Sci. 2020, 144, 172–182. [Google Scholar] [CrossRef]
- Sui, M.; Jiang, X.; Chen, J.; Yang, H.; Zhu, Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 2018, 106, 125–133. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Li, M.; Wang, F.; Jia, Y.; Zhang, F.; Shao, J.; Chen, A.; Zheng, S. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life 2019, 71, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Wu, S.; Tan, S.; Qin, Y.; Wang, X.; Jiang, J.; Liu, H.; Wu, B. Berberine alleviates liver fibrosis through inducing ferrous redox to activate ROS-mediated hepatic stellate cells ferroptosis. Cell Death Discov. 2021, 7, 374. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Z.; Zhang, Z.; Cao, Y.; Wei, Z.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Dihydroartemisinin alleviates hepatic fibrosis through inducing ferroptosis in hepatic stellate cells. Biofactors 2021, 47, 801–818. [Google Scholar] [CrossRef]
- Li, Y.; Jin, C.; Shen, M.; Wang, Z.; Tan, S.; Chen, A.; Wang, S.; Shao, J.; Zhang, F.; Zhang, Z.; et al. Iron regulatory protein 2 is required for artemether -mediated anti-hepatic fibrosis through ferroptosis pathway. Free Radic. Biol. Med. 2020, 160, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Guo, M.; Li, Y.; Wang, Y.; Qiu, Y.; Shao, J.; Zhang, F.; Xu, X.; Yin, G.; Wang, S.; et al. m(6)A methylation is required for dihydroartemisinin to alleviate liver fibrosis by inducing ferroptosis in hepatic stellate cells. Free Radic. Biol. Med. 2022, 182, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, D.; Jin, C.; Li, Z.; Sun, S.; Xia, S.; Zhang, Y.; Zhang, Z.; Zhang, F.; Xu, X.; et al. Dihydroartemisinin Induces Ferroptosis in HCC by Promoting the Formation of PEBP1/15-LO. Oxid Med. Cell Longev. 2021, 2021, 3456725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein responseinduced upregulation of CHAC1 expression. Oncol. Rep. 2021, 46, 240. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, H.; Li, S.; Qin, T.; Shi, H.; Ma, J.; Li, L.; Yu, G.; Jiang, T.; Li, C. Dihydroartemisinin enhances the inhibitory effect of sorafenib on HepG2 cells by inducing ferroptosis and inhibiting energy metabolism. J. Pharmacol. Sci. 2022, 148, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Dai, H.Q.; Huang, X.W.; Feng, J.; Deng, J.H.; Wang, Z.X.; Yang, X.M.; Liu, Y.J.; Wu, Y.; Chen, P.H.; et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 2021, 42, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shi, C.; Li, T.; Wu, Y.; Hu, C.; Huang, G. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomed. Pharmacother. 2020, 129, 110282. [Google Scholar] [CrossRef]
- Chang, W.T.; Bow, Y.D.; Fu, P.J.; Li, C.Y.; Wu, C.Y.; Chang, Y.H.; Teng, Y.N.; Li, R.N.; Lu, M.C.; Liu, Y.C.; et al. A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid. Med. Cell Longev. 2021, 2021, 7689045. [Google Scholar] [CrossRef]
- Gao, G.; Xie, Z.; Li, E.W.; Yuan, Y.; Fu, Y.; Wang, P.; Zhang, X.; Qiao, Y.; Xu, J.; Holscher, C.; et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J. Nat. Med. 2021, 75, 540–552. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Gao, Q.; Shan, X.; Wang, J.; Lv, Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology 2020, 445, 152599. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C.Y.; Wang, T.; Yu, H.M.; Ouyang, S.H.; Wu, Y.P.; Gong, H.B.; Ma, X.H.; Jiao, G.L.; Fu, L.L.; et al. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. 2020, 11, 781. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Hua, H.; Tian, W.; Zhu, H.; Liu, Y.; Xu, X. Holly (Ilex latifolia Thunb.) Polyphenols Extracts Alleviate Hepatic Damage by Regulating Ferroptosis Following Diquat Challenge in a Piglet Model. Front. Nutr. 2020, 7, 604328. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Li, H.; Wang, Y.; Tang, S.; Velkov, T.; Shen, J. Inhibition of Oxidative Stress and ALOX12 and NF-kappaB Pathways Contribute to the Protective Effect of Baicalein on Carbon Tetrachloride-Induced Acute Liver Injury. Antioxidants 2021, 10, 976. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Li, S.; Song, Z.; Luo, Q.; Zhang, Y.; Wang, H. The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases. Nutrients 2022, 14, 2303. https://doi.org/10.3390/nu14112303
Ren Y, Li S, Song Z, Luo Q, Zhang Y, Wang H. The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases. Nutrients. 2022; 14(11):2303. https://doi.org/10.3390/nu14112303
Chicago/Turabian StyleRen, Yijing, Siyue Li, Zixuan Song, Qiuping Luo, Yingying Zhang, and Hao Wang. 2022. "The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases" Nutrients 14, no. 11: 2303. https://doi.org/10.3390/nu14112303
APA StyleRen, Y., Li, S., Song, Z., Luo, Q., Zhang, Y., & Wang, H. (2022). The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases. Nutrients, 14(11), 2303. https://doi.org/10.3390/nu14112303






