Vitamin B12 Levels, Substance Use Patterns and Clinical Characteristics among People with Severe Substance Use Disorders: A Cohort Study from Western Norway
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics; Design, Population, Data Collection and Study Sample
2.2. Measuring Serum Vitamin B12; Laboratory Assays and Definitions
2.3. Study Variables, Baseline, Clinical and Sociodemographic Factors
3. Statistical Analyses
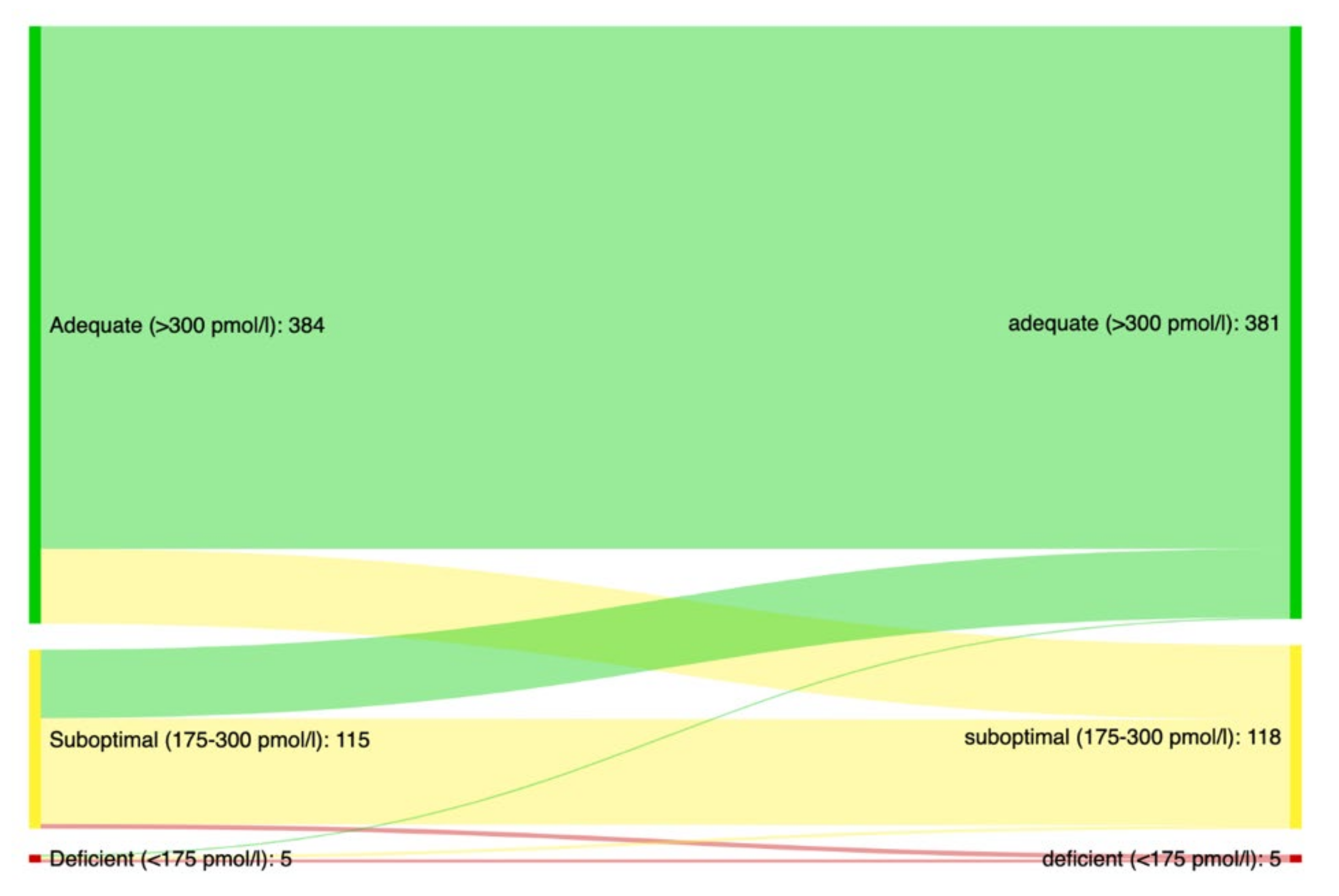
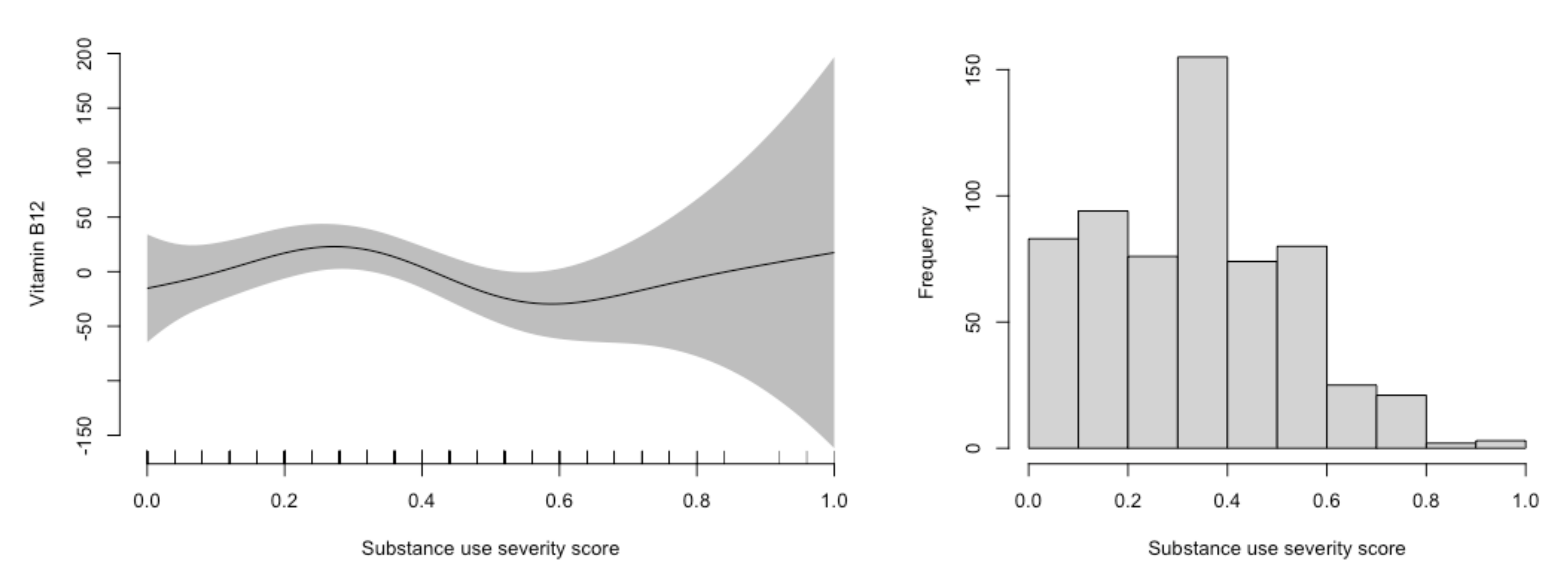
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahboub, N.; Rizk, R.; Karavetian, M.; de Vries, N. Nutritional status and eating habits of people who use drugs and/or are undergoing treatment for recovery: A narrative review. Nutr. Rev. 2021, 79, 627–635. [Google Scholar] [CrossRef]
- Ruiz, M.K. Nutritional Concerns in Substance Use Disorders. University of Washington: Washington, DC, USA, 2021. [Google Scholar]
- Neale, J.; Nettleton, S.; Pickering, L.; Fischer, J. Eating patterns among heroin users: A qualitative study with implications for nutritional interventions. Addiction 2012, 107, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Saeland, M.; Haugen, M.; Eriksen, F.-L.; Wandel, M.; Smehaugen, A.; Böhmer, T.; Oshaug, A. High sugar consumption and poor nutrient intake among drug addicts in Oslo, Norway. Br. J. Nutr. 2011, 105, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, A.; Kheradmand, A. Nutritional status in patients under methadone maintenance treatment. J. Subst. Use 2020, 25, 173–176. [Google Scholar] [CrossRef]
- El-Nakah, A.; Frank, O.; Louria, D.B.; Quinones, M.A.; Baker, H. A vitamin profile of heroin addiction. Am. J. Public Health 1979, 69, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Czernichow, S.; Raphaöl, M.; Roussel, C.; Chadefaux-Vekemans, B.; Morineau, G.; Giraudier, S.; Tibi, A.; Henry, O.; Vayssiere, M. Very low oral doses of vitamin B-12 increase serum concentrations in elderly subjects with food-bound vitamin B-12 malabsorption. J. Nutr. 2007, 137, 373–378. [Google Scholar] [CrossRef][Green Version]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H. Vitamin B 12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 1–20. [Google Scholar]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Baker, H.; Leevy, C.B.; DeAngelis, B.; Frank, O.; Baker, E.R. Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. J. Am. Coll. Nutr. 1998, 17, 235–238. [Google Scholar] [CrossRef]
- Ermens, A.; Vlasveld, L.; Lindemans, J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin. Biochem. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Mechie, N.-C.; Goralzcyk, A.D.; Reinhardt, L.; Mihm, S.; Amanzada, A. Association of serum vitamin B 12 levels with stage of liver fibrosis and treatment outcome in patients with chronic hepatitis C virus genotype 1 infection: A retrospective study. BMC Res. Notes 2015, 8, 260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fragasso, A.; Mannarella, C.; Ciancio, A.; Sacco, A. Functional vitamin B12 deficiency in alcoholics: An intriguing finding in a retrospective study of megaloblastic anemic patients. Eur. J. Intern. Med. 2010, 21, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, L.T.; Aas, C.F.; Vold, J.H.; Ohldieck, C.; Leiva, R.A.; Chalabianloo, F.; Skurtveit, S.; Lygren, O.J.; Dalgård, O.; Vickerman, P. Integrated treatment of hepatitis C virus infection among people who inject drugs: Study protocol for a randomised controlled trial (INTRO-HCV). BMC Infect. Dis. 2019, 19, 943. [Google Scholar] [CrossRef] [PubMed]
- ATLAS4LAR: Kartlegging og Behandling av Lungesykdom i Legemiddelassistert Behandling. Available online: https://helse-bergen.no/avdelinger/rusmedisin/rusmedisin-seksjon-forsking/bar/atlas4lar-kartlegging-og-behandling-av-lungesykdom-i-legemiddelassistert-behandling (accessed on 2 February 2022).
- Kobalamin. Available online: https://analyseoversikten.no/analyser/112 (accessed on 2 February 2022).
- Hagve, T.; Brun, A.; Hov, G.; Lindberg, M.; Asberg, A. Nasjonal brukerhåndbok i medisinsk biokjemi. In Oslo; Q-Base Publishing: Oslo, Norway, 2014. [Google Scholar]
- Stott, D.J.; Langhorne, P.; Hendry, A.; McKAY, P.J.; Holyoake, T.; Macdonald, J.; Lucie, N. Prevalence and haemopoietic effects of low serum vitamin B12 levels in geriatric medical patients. Br. J. Nutr. 1997, 78, 57–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klee, G.G. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clin. Chem. 2000, 46, 1277–1283. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Enders, C.K. Applied Missing Data Analysis. Guilford Press: New York, NY, USA, 2010. [Google Scholar]
- Vold, J.H.; Chalabianloo, F.; Aas, C.F.; Løberg, E.-M.; Johansson, K.A.; Fadnes, L.T. Changes in substance use during outpatient treatment for substance use disorders: A prospective Norwegian cohort study from 2016 to 2020. Subst. Abus. Treat. Prev. Policy 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Baik, H.; Russell, R. Vitamin B12 deficiency in the elderly. Annu. Rev. Nutr. 1999, 19, 357–377. [Google Scholar] [CrossRef]
- Malik, E.; Rozner, L.; Adelson, M.; Schreiber, S.; Peles, E. The Relation between Changes in Vitamin D and Vitamin B12 Levels, Body Mass Index and Outcome in Methadone Maintenance Treatment Patients. J. Psychoact. Drugs 2021, 53, 55–64. [Google Scholar] [CrossRef]
- Zhai, C.; Cui, M.; Cheng, X.; Ao, X.; Zhao, T.; Wu, W.; Shao, Q.; Ge, D.; Song, H.; Qi, F. Vitamin B12 levels in methamphetamine addicts. Front. Behav. Neurosci. 2018, 12, 320. [Google Scholar] [CrossRef]
- Himmerich, H.; Anghelescu, I.; Klawe, C.; Szegedi, A. Vitamin B12 and hepatic enzyme serum levels correlate in male alcohol-dependent patients. Alcohol Alcohol. 2001, 36, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Liappas, I.A.; Nicolaou, C.; Chatzipanagiotou, S.; Tzavellas, E.O.; Piperi, C.; Papageorgiou, C.; Boufidou, F.; Bagos, P.; Soldatos, C.R. Vitamin B12 and hepatic enzyme serum levels correlate with interleukin-6 in alcohol-dependent individuals without liver disease. Clin. Biochem. 2007, 40, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, T.; Koda, M.; Okamoto, T.; Miyoshi, K.; Matono, T.; Oyama, K.; Hosho, K.; Okano, J.-I.; Isomoto, H.; Murawaki, Y. Falsely elevated serum vitamin B12 levels were associated with the severity and prognosis of chronic viral liver disease. Yonago Acta Med. 2017, 60, 31. [Google Scholar]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015, 53, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Podell, E.R.; Margell, P.D.; Stabler, S.P.; Allen, R.H. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N. Engl. J. Med. 1988, 318, 1720–1728. [Google Scholar] [CrossRef]
- Healton, E.B.; Savage, D.G.; Brust, J.; Garrett, T.; Lindenbaum, J. Neurologic aspects of cobalamin deficiency. Medicine 1991, 70, 229–245. [Google Scholar] [CrossRef]
- Oosterhuis, W.P.; Niessen, R.W.; Bossuyt, P.M.; Sanders, G.T.; Sturk, A. Diagnostic value of the mean corpuscular volume in the detection of vitamin B12 deficiency. Scand. J. Clin. Lab. Investig. 2000, 60, 9–18. [Google Scholar] [CrossRef]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood J. Am. Soc. Hematol. 2017, 129, 2603–2611. [Google Scholar] [CrossRef]
- Carmel, R. Diagnosis and management of clinical and subclinical cobalamin deficiencies: Why controversies persist in the age of sensitive metabolic testing. Biochimie 2013, 95, 1047–1055. [Google Scholar] [CrossRef]
- Nexo, E.; Hoffmann-Lücke, E. Holotranscobalamin, a marker of vitamin B-12 status: Analytical aspects and clinical utility. Am. J. Clin. Nutr. 2011, 94, 359S–365S. [Google Scholar] [CrossRef]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M.; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279. [Google Scholar] [PubMed]
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Shipton, M.J.; Thachil, J. Vitamin B12 deficiency—A 21st century perspective. Clin. Med. 2015, 15, 145. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N (%) |
|---|---|
| Gender | |
| Male | 474 (71) |
| Female | 198 (29) |
| Age group | |
| <30 years | 78 (12) |
| 30–39 years | 192 (29) |
| 40–49 years | 204 (30) |
| 50–59 years | 157 (23) |
| ≥60 years | 41 (6) |
| Education level | |
| Not completed primary school | 39 (6) |
| Primary school (9 years) | 300 (45) |
| High school (12 years) | 269 (40) |
| ≤3 years higher education | 51 (8) |
| >3 years higher education | 13 (2) |
| Housing condition 1 | |
| Unstable | 81 (12) |
| Stable | 591 (88) |
| HCV infection 2 | 315 (53) |
| Fib-4 | |
| <1.45 | 452 (68) |
| >3.25 | 40 (6) |
| Injecting substances 3 | 325 (53) |
| Opioid agonist therapy | |
| Buprenorphine | 352 (53) |
| Methadone | 229 (35) |
| Not in OAT | 82 (12) |
| Weekly substance use 4 | |
| Alcohol | 151 (25) |
| Cannabis | 302 (49) |
| Stimulants 5 | 162 (26) |
| Benzodiazepines | 233 (38) |
| Non-prescribed opioids | 87 (14) |
| No weekly substance use | 145 (24) |
| Serum vitamin B12 | |
| Median pmol/L (IQR 6) | 396 (198) |
| % with suboptimal levels (CI 7) | 22 (19–25) |
| % with deficient levels (CI 7) | 1.2 (0.6–2.3) |
| Fixed Effects | ||||
|---|---|---|---|---|
| Partly Adjusted 1 | Adjusted | |||
| Effect Estimate | Time Trend (per Year) | Effect Estimate | Time Trend (per Year) | |
| Estimate (CI) | Slope (CI) | Estimate (CI) | Slope (CI) | |
| Vitamin B12 | 386 (331, 440) | 28 (5.2, 50) | ||
| Gender | ||||
| Male | 0 (reference) | |||
| Female | −7.3 (−37, 22) | |||
| Age | ||||
| <30 | 0 (reference) | |||
| 30–39 | 1.7 (−37, 40) | |||
| 40–49 | −38 (−80, 3.9) | |||
| 50–59 | −49 (−94, −4.7) | |||
| ≥60 | −91 (−153, −29) | |||
| Hepatic markers | ||||
| HCV | 20 (−7.4, 47) | |||
| Fib-4 | 31 (22, 40) | −4.1 (−8.6, 0.37) | 29 (20, 38) | −3.7 (−8.3, 0.9) |
| OAT dose ratio 2 | 30 (−1.1, 60) | −11 (−29, 5.8) | 19 (−12, 50) | −13 (−31, 5.2) |
| Injecting substances 3 | −23 (−54, 8.4) | −3.9 (−20, 12) | −12 (−46, 23) | −4.9 (−23, 13) |
| Weekly substance use 4 | ||||
| Alcohol | 21 (−14, 57) | −9.6 (−28, 8.4) | 10 (−25, 45) | −9.4 (−27, 9.1) |
| Cannabis | 27 (−3.4, 58) | −11 (27, 4.4) | 25 (−6.6, 56) | −11 (−27, 6.0) |
| Non-OAT opioids | −2.9 (−47, 42) | −4.0 (−28, 20) | 11 (−36, 57) | −4.3 (−30, 22) |
| Stimulants 5 | −36 (−72, −1.1) | −0.7 (−20, 18) | −35 (−74, 3.7) | 0.19 (−22, 21) |
| Benzodiazepines | 26 (−5.6, 58) | −3.7 (−20, 12) | 21 (−13, 55) | 2.0 (−16, 20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madebo, T.; Bemanian, M.; Vold, J.H.; Chowdhury, R.; Aas, C.F.; Druckrey-Fiskaaen, K.T.; Johansson, K.A.; Fadnes, L.T. Vitamin B12 Levels, Substance Use Patterns and Clinical Characteristics among People with Severe Substance Use Disorders: A Cohort Study from Western Norway. Nutrients 2022, 14, 1941. https://doi.org/10.3390/nu14091941
Madebo T, Bemanian M, Vold JH, Chowdhury R, Aas CF, Druckrey-Fiskaaen KT, Johansson KA, Fadnes LT. Vitamin B12 Levels, Substance Use Patterns and Clinical Characteristics among People with Severe Substance Use Disorders: A Cohort Study from Western Norway. Nutrients. 2022; 14(9):1941. https://doi.org/10.3390/nu14091941
Chicago/Turabian StyleMadebo, Tesfaye, Mitra Bemanian, Jørn Henrik Vold, Ranadip Chowdhury, Christer Frode Aas, Karl Trygve Druckrey-Fiskaaen, Kjell Arne Johansson, and Lars Thore Fadnes. 2022. "Vitamin B12 Levels, Substance Use Patterns and Clinical Characteristics among People with Severe Substance Use Disorders: A Cohort Study from Western Norway" Nutrients 14, no. 9: 1941. https://doi.org/10.3390/nu14091941
APA StyleMadebo, T., Bemanian, M., Vold, J. H., Chowdhury, R., Aas, C. F., Druckrey-Fiskaaen, K. T., Johansson, K. A., & Fadnes, L. T. (2022). Vitamin B12 Levels, Substance Use Patterns and Clinical Characteristics among People with Severe Substance Use Disorders: A Cohort Study from Western Norway. Nutrients, 14(9), 1941. https://doi.org/10.3390/nu14091941






