Effect of Extract-Added Water Derived from Deep-Sea Water with Different Hardness on Cognitive Function, Motor Ability and Serum Indexes of Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Water Preparation
2.3. Behavioral Assessment
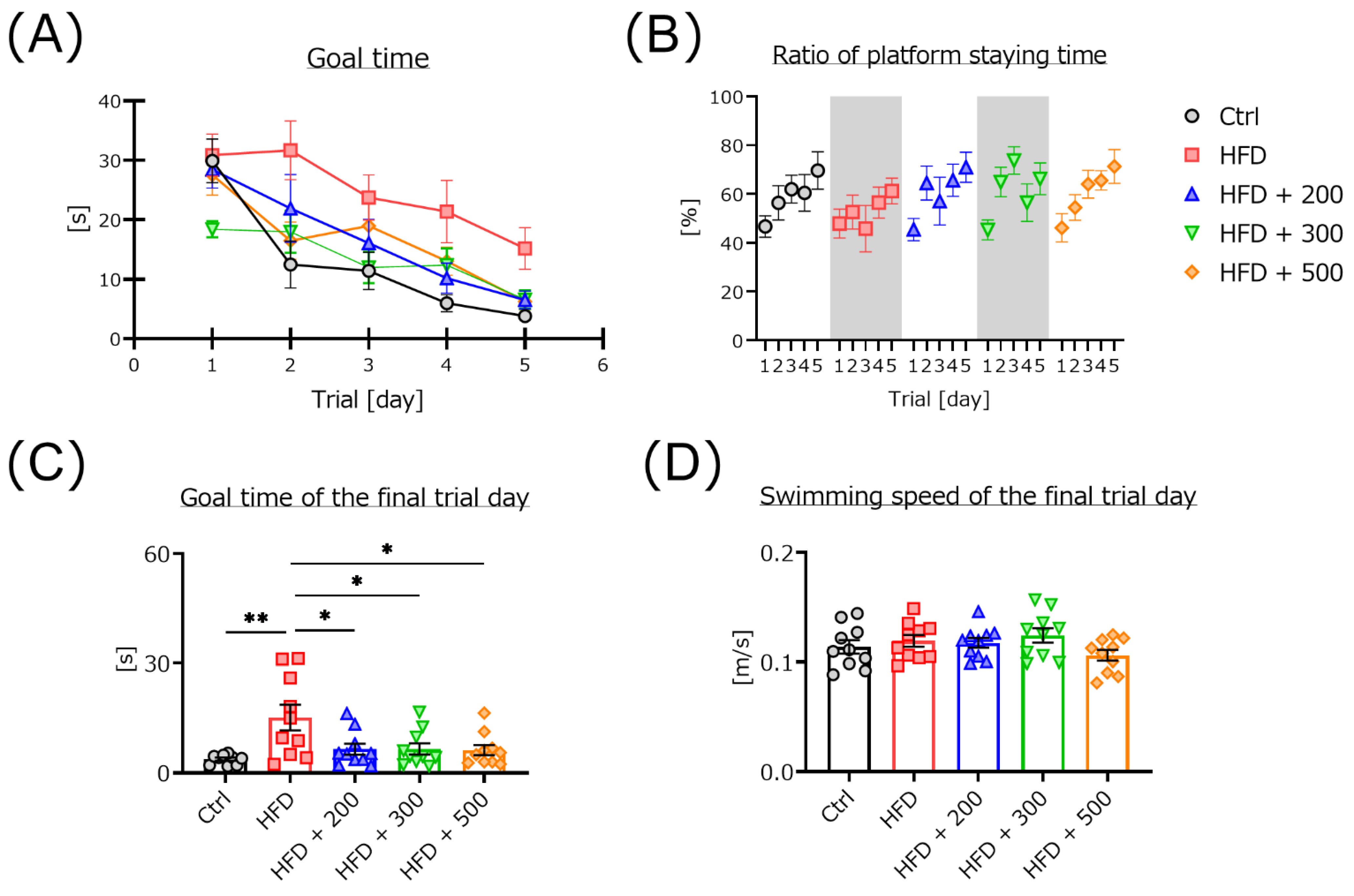
2.3.1. Morris Water Maze
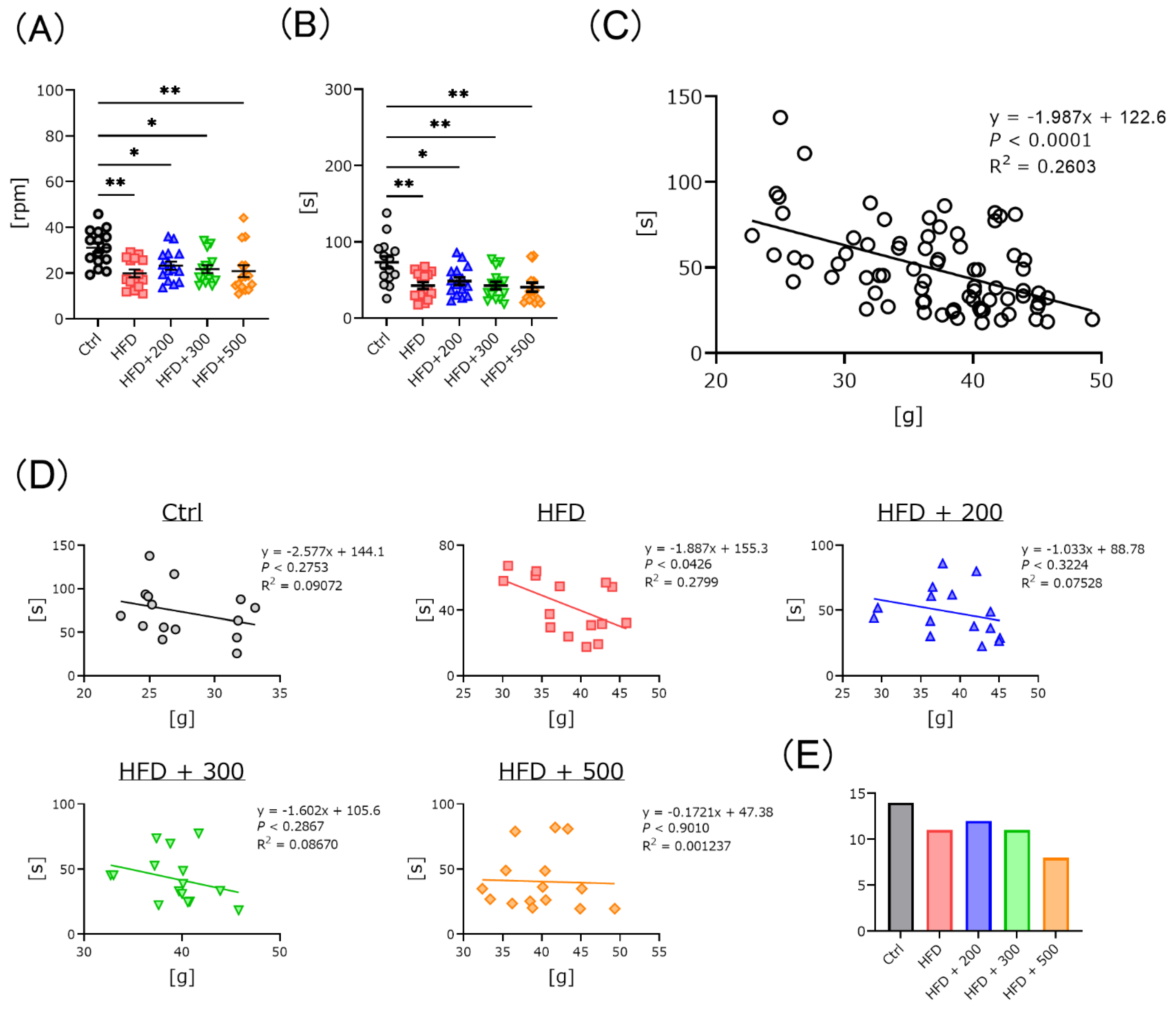
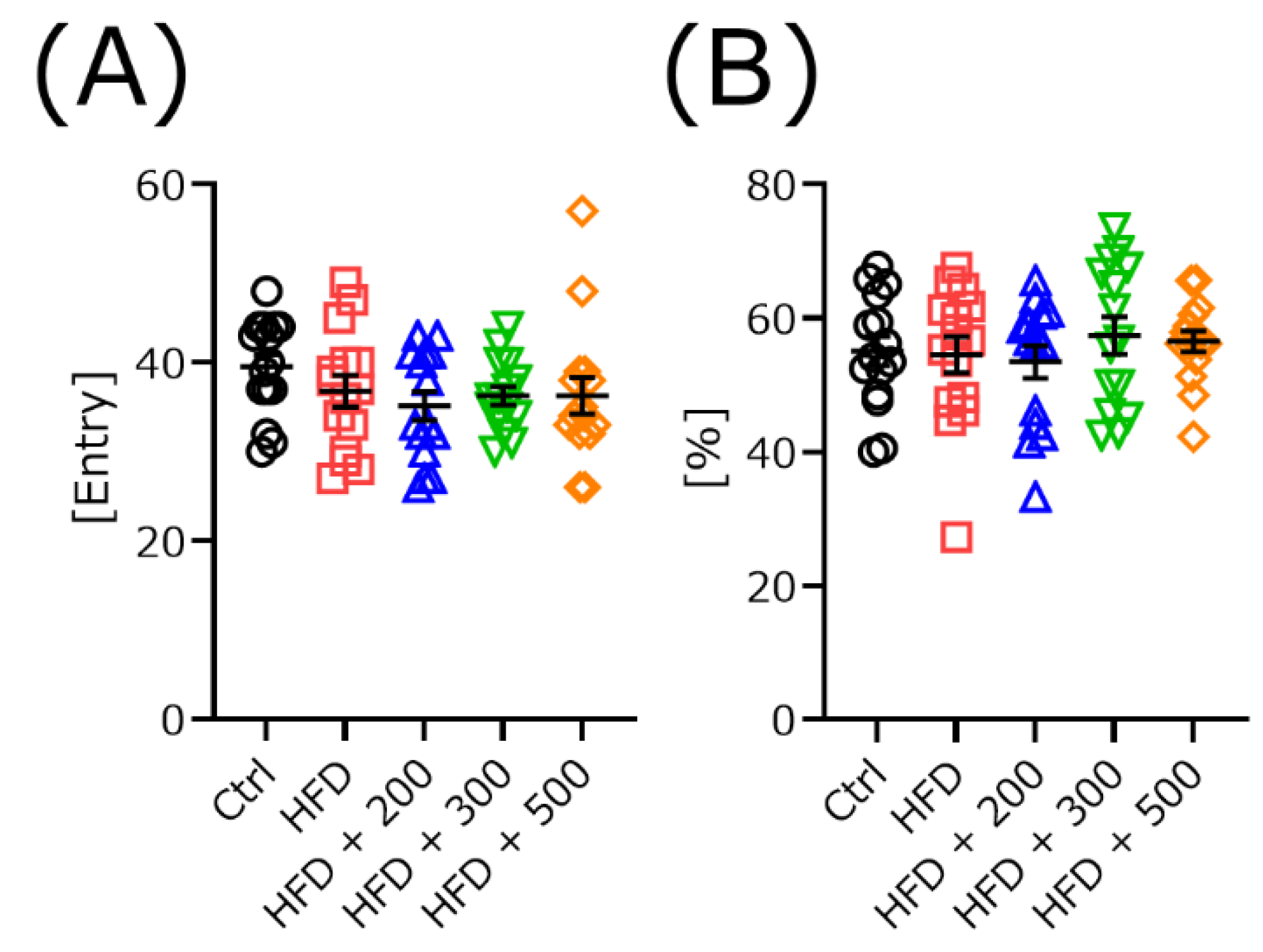
2.3.2. Rota-Rod and Y-Maze Test
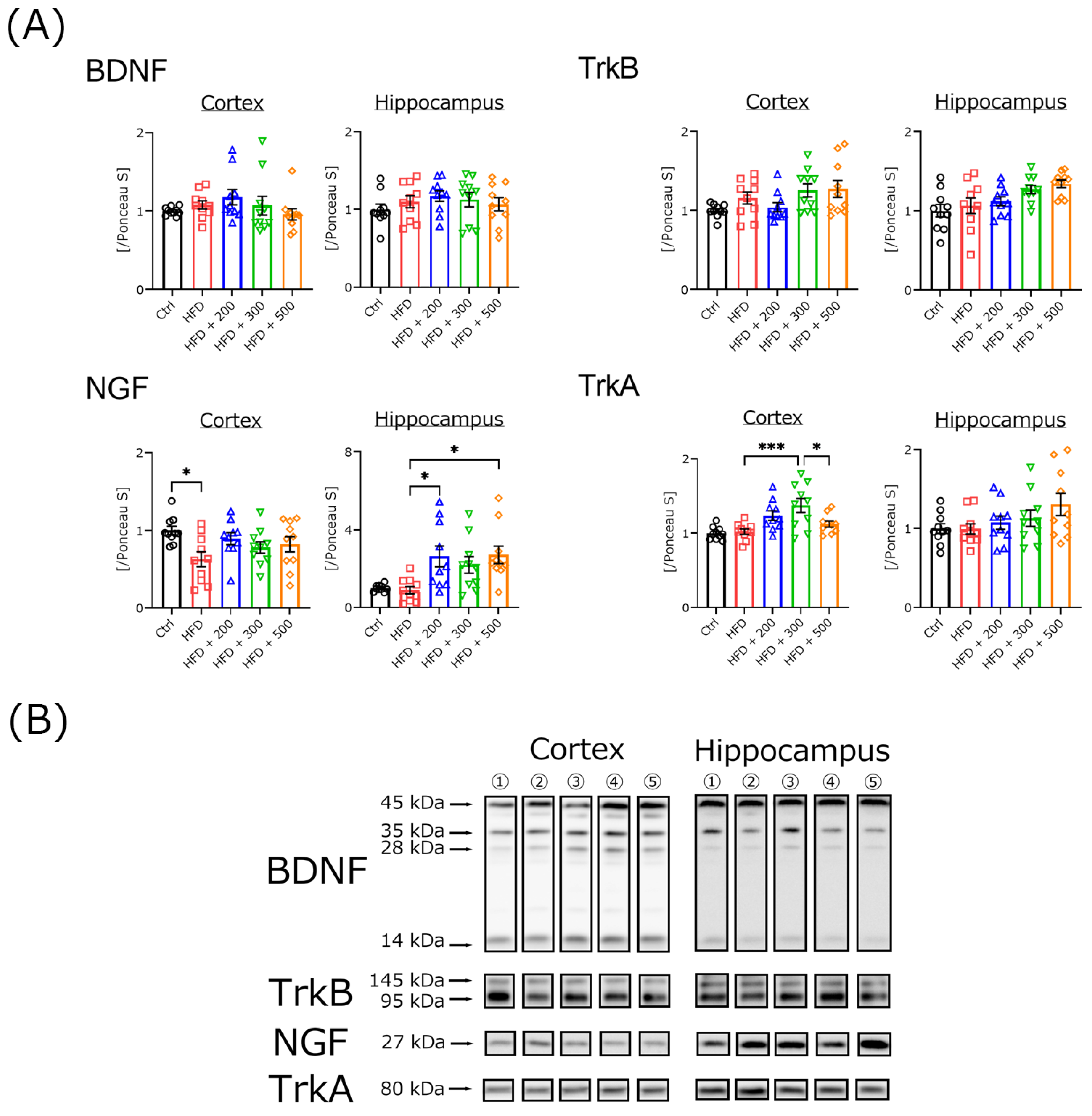
2.4. Western Blotting
2.5. Quantitative Analysis of DSW-Extract Added Water
2.6. Statistical Analysis
3. Results
3.1. DSW-Extract-Added Water Did Not Change Body Weights in HFD-Treated Mice
3.2. DSW-Extract-Added Water Significantly Improved HFD-Induced Cognitive Dysfunction
3.3. DSW-Extract-Added Water Significantly Improved HFD-Induced Coordination Disability
3.4. DSW-Extract-Added Water Did Not Change Exploratory Behavior in HFD-Treated Mice
3.5. DSW-Extract-Added Water Did Not Change Neurotrophic Factor Secretion in HFD-Treated Mouse Brains
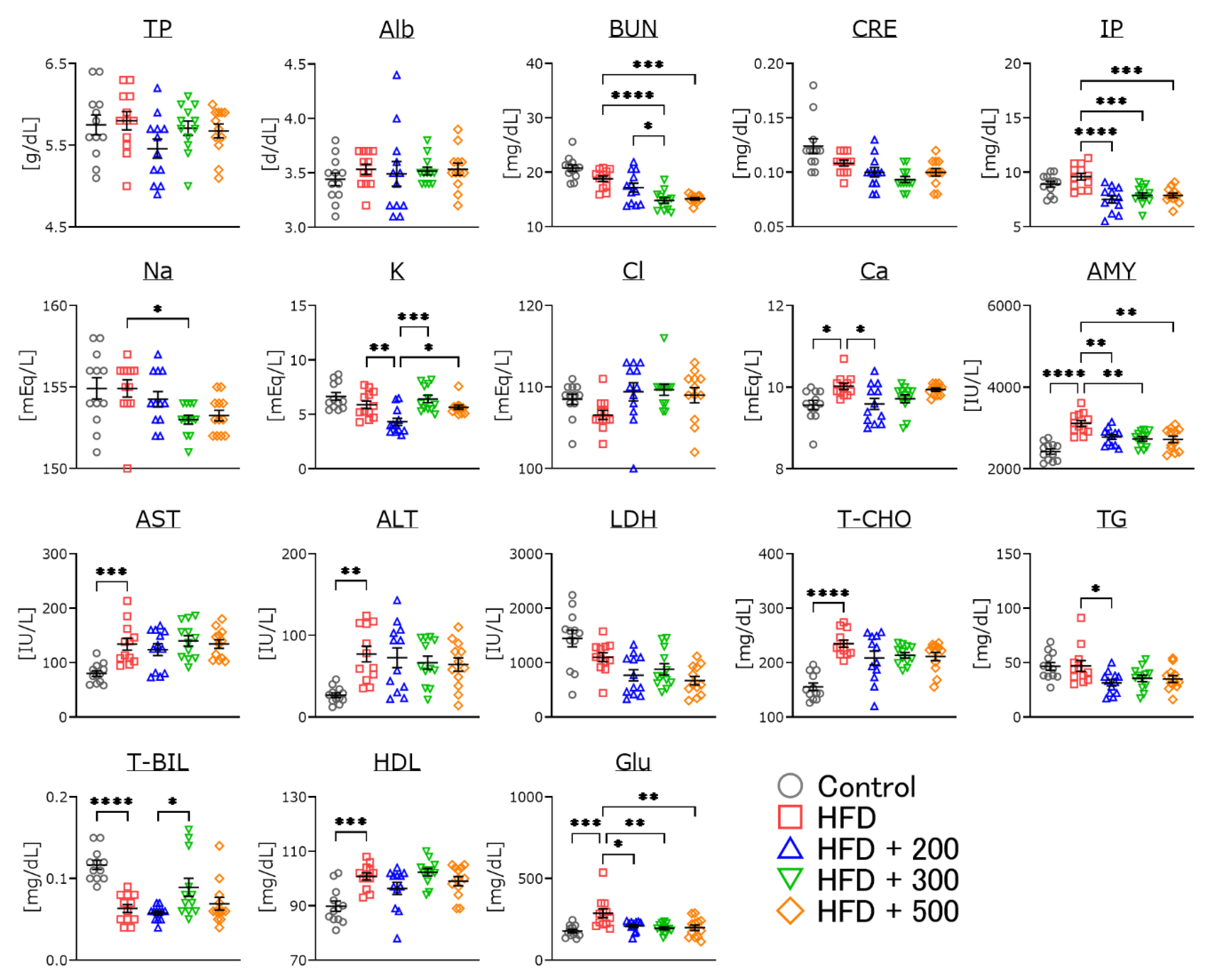
3.6. Changes in Serum Parameters of DSW-Extract-Added Water-Treated Obese Mice
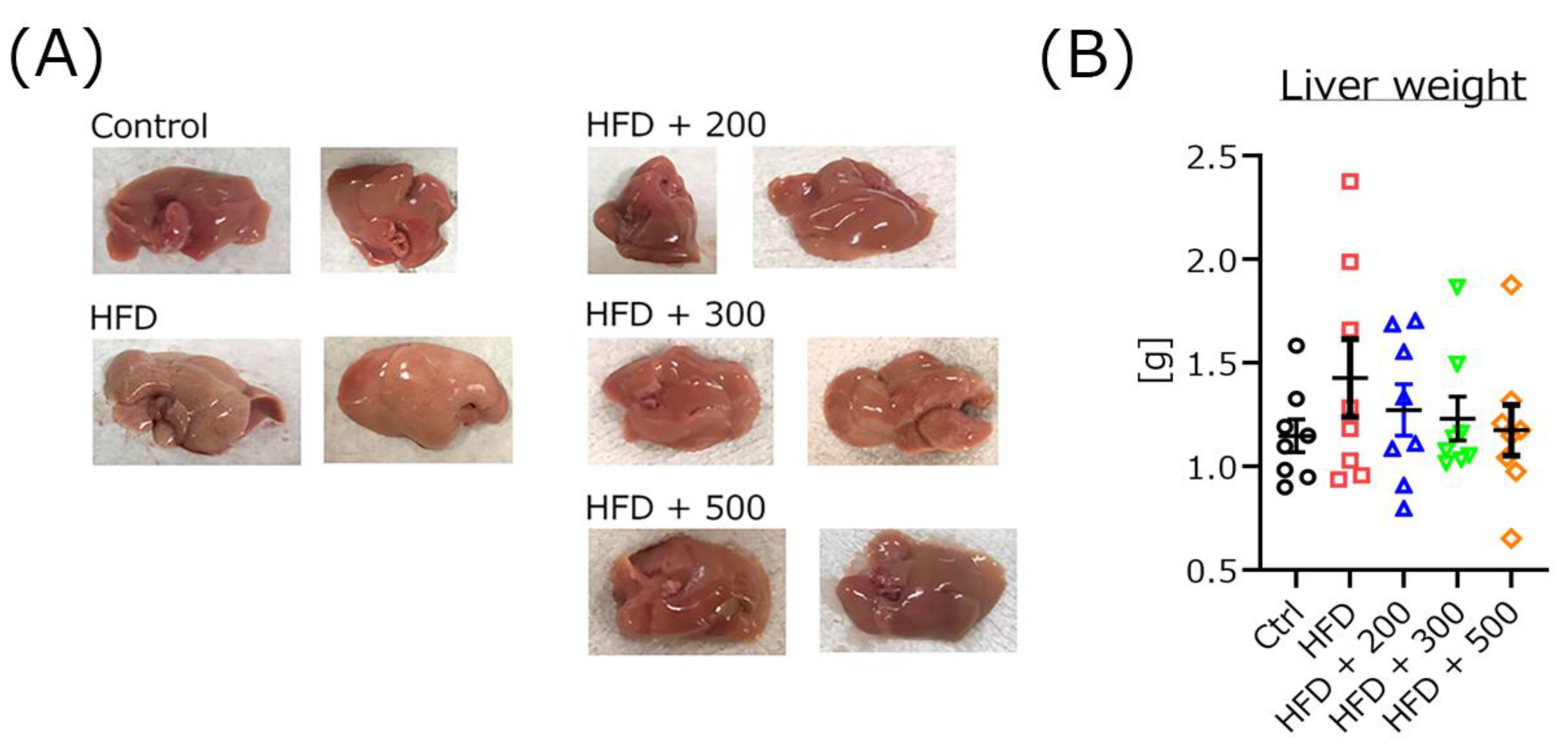
3.7. DSW-Extract-Added Water Improved Fatty Liver but Not Liver Weight in HFD-Treated Mice
3.8. Quantitative Analysis of the DSW-Extract-Added Water
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Colotti, G.; Ilari, A.; Boffi, A.; Morea, V. Metals and metal derives in medicine. Mini-Rev. Med. Chem. 2013, 13, 211–221. [Google Scholar] [PubMed]
- Kacar, B.; Garcia, A.K.; Anbar, A.D. Evolutionary history of bioessential elements can guide the search for life in the universe. Chembiochem 2021, 22, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Biochemistry, Nutrients. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554545/ (accessed on 21 December 2021).
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekkan, P.; Sadler, P.J. The elements of life and medicines. Philos. Trans. A Math. Phys. Eng. Sci. 2015, 373, 20140182. [Google Scholar]
- Overview of the Dietary Reference Intakes for Japanese. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000862500.pdf (accessed on 20 December 2021).
- Gil, A.; Ortega, R.M. Introduction and executive summary of the supplement, role of milk and dairy products in health and prevention of noncommunicable chronic diseases: A series of systemic reviews. Adv. Nutr. 2019, 10, S67–S73. [Google Scholar] [CrossRef]
- Hasegawa, M.; Endo, S. Consideration about comparison and sales strategy of mineral water products in the Japanese market. In Proceedings of the National Conference of Japan Society for Management Information, Osaka, Japan, 20–21 October 2018. [Google Scholar]
- Hori, M.; Shozugawa, K.; Sugimori, K.; Watanabe, Y. A survey of monitoring tap water hardness in Japan and its distribution patterns. Sci. Rep. 2021, 11, 13546. [Google Scholar] [CrossRef]
- The Mineral Water Association of Japan. Changes in Per Capita Consumption of Mineral Water. Available online: https://minekyo.net/publics/index/5/ (accessed on 21 December 2021).
- Nani, S.Z.M.; Majid, F.A.; Jaafar, A.B.; Mahdzir, A.; Musa, M.N. Potential health benefits of deep sea water: A review. Evid. Based Complement Altern. Med. 2016, 2016, 6520475. [Google Scholar]
- Takeuchi, H.; Higuchi, K.; Yoshikane, Y.; Takagi, R.; Tokuhiro, S.; Takenkaka, K.; Oboshi, W.; Kimura, A.; Islam, J.M.; Kaneko, A.; et al. Drinking refined deep-sea water improves the gut ecosystem with beneficial effects on intestinal health in humans: A randomized double-blind controlles trial. Nutrients 2020, 12, 2646. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A.; Lee, S.H.; Yun, J.W. Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar. Biotechnol. 2009, 11, 531–539. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chang, Y.Y.; Chiu, C.H.; Yang, K.T.; Wang, Y.; Fu, S.G.; Chen, Y.C. Cardiovascular protection of deep-seawater drinking water in high-fat/cholesterol fed hamsters. Food Chem. 2011, 127, 1146–1152. [Google Scholar] [CrossRef]
- Sheu, M.J.; Chou, P.Y.; Lin, W.H.; Pan, C.H.; Chien, Y.C.; Chung, Y.L.; Liu, F.C.; Wu, C.H. Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: An in vivo study. Mar. Drugs 2013, 11, 2183–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, K.; Shirai, M.; Ninuma, T.; Kato, Y. Anti-obesity effects of tocotrienols and bran in high-fat diet-treated mice. Nutrients 2019, 11, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, K.; Onodera, K.; Shinkai, T.; Suzuki, S.; Urano, S. Impairment of learning and memory in rats caused by oxidative stress and aging and changes in antioxidative defense systems. Ann. N. Y. Acad. Sci. 2001, 928, 168–175. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Kato, Y.; Aoki, Y.; Fukui, K. Tocotrienols influence body weight gain and brain protein expression in long-term high-fat diet-treated mice. Int. J. Mol. Sci. 2020, 21, 4533. [Google Scholar] [CrossRef]
- Kato, Y.; Uchiumi, H.; Usami, R.; Takatsu, H.; Aoki, Y.; Yanai, S.; Endo, S.; Fukui, K. Tocotrienols reach the brain and play roles in the attenuation of body weight gain and improvement of cognitive function in high-fat diet-treated mice. J. Clin. Biochem. Nutr. 2021, 69, 256–264. [Google Scholar] [CrossRef]
- Fukui, K.; Okihiro, S.; Ohfuchi, Y.; Hashimoto, M.; Kato, Y.; Yoshida, N.; Mochizuki, K.; Tsumoto, H.; Miura, Y. Proteomic study on neurite responses to oxidative stress: Search for differentially expressed proteins in isolated neurites of N1E-115 cells. J. Clin. Biochem. Nutr. 2019, 64, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, B.G.; Park, J.E.; Shin, E.J.; Shon, Y.H. Effects of balanced deep-sea water on adipocyte hypertrophy and liver steatosis in high-fat, diet-induced obese mice. Obes. Silver Spring 2014, 22, 1669–1678. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Watanabe, A.; Fujisawa, N.; Kaneko, T.; Ishizu, T.; Fujimoto, T.; Nakamura, K.; Yamamoto, M. Effects of desalted deep seawater on hematologic and blood chemical values in mice. Tohoku J. Exp. Med. 2004, 203, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.S.; Chang, Y.Y.; Hsu, C.L.; Lin, H.W.; Chang, M.H.; Chen, J.W.; Chen, S.S.; Chen, Y.C. Alleviative effects of deep-seawater drinking water on hepatic lipid accumulation and oxidation induced by a high-fat diet. J. Chin. Med. Assoc. 2013, 76, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Tan, Z.; Hua, Y.; Huang, X.; Gao, Y.; Wu, Y.; Liu, B.; Zhou, Y. Deep sea water improves exercise and inhibits oxidative stress in a physical fatigue mouse model. Biomed. Rep. 2016, 4, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Miyamura, M.; Yoshioka, S.; Hamada, A.; Takuma, D.; Yokota, J.; Kusunose, M.; Kyotani, S.; Kawakita, H.; Odai, K.; Tsutsui, Y.; et al. Difference between Deep Seawater and Surface Seawater in the Preventive Effect of Atherosclerosis. Biol. Pharm. Bull. 2004, 27, 1784–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaseth, J.; Skalny, A.V.; Roos, P.M.; Alezander, J.; Aschner, M.; Tinkov, A.A. Copper, Iron, Selenium and lipo-glycemic dysmetabolism in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 9461. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I.; Abumaria, N.; Wu, L.J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.G.; Zhuo, M.; et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Kawatani, T.; Fukui, K.; Koike, T. Cellular Zinc2+ chelators cause “dying-back” neurite degeneration associated with energy impairment. J. Neurosci. Res. 2007, 85, 2844–2855. [Google Scholar] [CrossRef]
- Ronzano, R.; Roux, T.; Thetiot, M.; Aigrot, M.S.; Richard, L.; Lejeune, F.X.; Mazuir, E.; Vallat, J.M.; Lubetzki, C.; Desmazieres, A. Microglia-neuron interaction at node of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nat. Coomun. 2021, 12, 5219. [Google Scholar] [CrossRef]
- He, S.; Hao, J.; Peng, W.; Qiu, P.; Li, C.; Guan, H. Modulation of lipid metabolism by deep-sea water in cultured human liver (HepG2) cells. Mar. Biotechnol. 2014, 16, 219–229. [Google Scholar] [CrossRef]
- Lee, K.S.; Chun, S.Y.; Kwon, Y.S.; Kim, S.; Nam, K.S. Deep sea water improves hypercholesterolemia and hepatic lipid accumulation through the regulation of hepatic metabolic gene expression. Mol. Med. Rep. 2017, 15, 2814–2822. [Google Scholar] [CrossRef]
- Aida, C.H.R.; Tanaka, S.; Kamada, C.; Tanaka, S.; Yoshimura, Y.; Kodera, R.; Fujihara, K.; Kawasaki, R.; Moriya, T.; Yamashita, H.; et al. Sodium intake and incidence of diabetes complications in elderly patients with type-2 diabetes-analysis of data from the Japanese elderly diabetes intervention study (J-EDIT). Nutrients 2021, 13, 689. [Google Scholar]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morten, D.G.; et al. DASH-Sodium Collaborative Research Group. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, F.; Vora, J.; Fenici, P.; Solini, A. Cardiovascular protection with sodium-glucose co-transporter-2 inhibitors type 2 diabetes: Does it apply to all patients? Diabetes Obes. Metab. 2020, 22, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
| (A) | Body Weight | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | [Week] | ||
| Ctrl | 18.97 ± 0.90 | 21.64 ± 0.87 | 23.18 ± 0.87 | 24.81 ± 1.06 | 25.83 ± 1.08 | 26.86 ± 1.13 | 27.40 ± 1.14 | 27.42 ± 1.01 | 27.84 ± 0.90 | ||
| HFD | 18.73 ± 0.74 | 22.74 ± 0.68 | 24.79 ± 0.76 | 27.19 ± 1.01 | 29.74 ± 1.08 | 32.11 ± 1.26 * | 34.45 ± 1.35 ** | 36.80 ± 1.34 *** | 38.46 ± 1.39 *** | ||
| HFD + 200 | 18.80 ± 0.86 | 23.08 ± 0.64 | 25.37 ± 0.86 | 27.94 ± 1.04 | 30.69 ± 1.13 * | 33.29 ± 1.33 ** | 36.02 ± 1.47 *** | 37.92 ± 1.46 *** | 39.69 ± 1.46 *** | ||
| HFD + 300 | 18.45 ± 0.73 | 22.65 ± 0.35 | 25.06 ± 0.52 | 27.61 ± 0.64 | 30.16 ± 0.66 * | 32.56 ± 0.73 ** | 35.54 ± 0.77 *** | 37.89 ± 0.93 *** | 39.56 ± 0.96 *** | ||
| HFD + 500 | 19.05 ± 0.87 | 23.27 ± 0.75 | 25.86 ± 0.98 | 28.41 ± 1.13 | 30.55 ± 1.12 * | 33.04 ± 1.34 * | 35.33 ± 1.34 ** | 37.06 ± 1.36 *** | 38.98 ± 1.46 *** | [g] | |
| (B) | Food Intake Volume | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | [Week] | ||
| Ctrl | 3.06 ± 0.08 | 2.66 ± 0.14 | 2.94 ± 0.15 | 2.70 ± 0.13 | 2.71 ± 0.08 | 3.03 ± 0.09 | 2.89 ± 0.18 | 3.10 ± 0.16 | 2.98 ± 0.15 | ||
| HFD | 3.31 ± 0.16 | 2.49 ± 0.13 | 2.70 ± 0.15 | 2.74 ± 0.13 | 2.71 ± 0.11 | 2.74 ± 0.10 | 2.98 ± 0.12 | 2.89 ± 0.12 | 3.05 ± 0.09 | ||
| HFD + 200 | 3.33 ± 0.20 | 2.59 ± 0.11 | 2.59 ± 0.09 | 2.69 ± 0.09 | 2.74 ± 0.14 | 2.92 ± 0.08 | 3.01 ± 0.10 | 3.05 ± 0.14 | 3.04 ± 0.09 | ||
| HFD + 300 | 3.32 ± 0.13 | 2.49 ± 0.13 | 2.72 ± 0.10 | 2.69 ± 0.11 | 2.69 ± 0.12 | 2.94 ± 0.11 | 2.95 ± 0.08 | 2.84 ± 0.13 | 3.19 ± 0.07 | ||
| HFD + 500 | 3.41 ± 0.20 | 2.60 ± 0.11 | 2.64 ± 0.09 | 2.66 ± 0.08 | 2.75 ± 0.08 | 2.94 ± 0.08 | 3.03 ± 0.11 | 2.91 ± 0.11 | 3.07 ± 0.05 | [g] | |
| (C) | Water Intake Volume | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | [Week] | ||
| Ctrl | 3.25 ± 0.29 | 3.18 ± 0.25 | 3.26 ± 0.36 | 2.79 ± 0.20 | 2.84 ± 0.26 | 2.84 ± 0.24 | 3.11 ± 0.32 | 3.03 ± 0.22 | 3.06 ± 0.16 | ||
| HFD | 3.14 ± 0.21 | 2.87 ± 0.15 | 3.04 ± 0.23 | 2.86 ± 0.15 | 3.39 ± 0.39 | 2.86 ± 0.19 | 3.07 ± 0.23 | 3.04 ± 0.27 | 2.83 ± 0.18 | ||
| HFD + 200 | 2.72 ± 0.13 | 2.61 ± 0.17 | 2.51 ± 0.16 | 2.69 ± 0.19 | 2.74 ± 0.16 | 2.46 ± 0.11 | 2.59 ± 0.16 | 2.96 ± 0.34 | 2.65 ± 0.21 | ||
| HFD + 300 | 2.76 ± 0.12 | 2.74 ± 0.17 | 2.64 ± 0.14 | 2.79 ± 0.17 | 2.79 ± 0.15 | 2.56 ± 0.14 | 2.79 ± 0.18 | 3.01 ± 0.28 | 2.76 ± 0.17 | ||
| HFD + 500 | 2.93 ± 0.10 | 2.84 ± 0.15 | 2.69 ± 0.13 | 2.83 ± 0.21 | 3.10 ± 0.27 | 2.88 ± 0.17 | 3.14 ± 0.28 | 3.04 ± 0.29 | 2.91 ± 0.26 | [mL] | |
| Element | Estimated Concentration [ng/mL] | Lower Limited | |||
|---|---|---|---|---|---|
| Filtered Tap Water from Saitama City | Filtered Well Water from Kochi | H300 DSW Extract-Added Water * | [ng/mL] | ||
| Li | Litium | N.D. | N.D. | 10 | 5 |
| B | Boron | 80 | N.D. | 200 | 10 |
| Na | Sodium | 20,000 | 500 | 50,000 | 200 |
| Mg | Manesium | 6000 | N.D. | 70,000 | 10 |
| Si | Silicon | 8000 | N.D. | 70 | 50 |
| S | Sulfer | 10,000 | N.D. | 300 | 100 |
| K | Potassium | 3000 | N.D. | 20,000 | 2000 |
| Ca | Calcium | 30,000 | N.D. | 20,000 | 10 |
| V | Vanadium | 0.7 | N.D. | N.D. | 0.1 |
| Cr | Chromium | 0.1 | N.D. | 0.1 | 0.1 |
| Mn | Manganese | N.D. | N.D. | 0.1 | 0.1 |
| Fe | Iron | 0.3 | 0.5 | 0.1 | 0.1 |
| Ni | Nickel | 0.8 | N.D. | 0.6 | 0.1 |
| Cu | Copper | 0.6 | N.D. | 2 | 0.1 |
| Zn | Zinc | 4 | N.D. | 2 | 0.5 |
| Ga | Gallium | 2 | N.D. | 0.2 | 0.1 |
| Ge | Germanium | N.D. | N.D. | 0.6 | 0.2 |
| As | Arsenic | 0.4 | N.D. | N.D. | 0.2 |
| Br | Bromine | 30 | N.D. | 4000 | 5 |
| Rb | Rubidium | 3 | N.D. | 6 | 0.1 |
| Sr | Strontium | 100 | N.D. | 300 | 10 |
| Mo | Molybdenum | 0.9 | N.D. | N.D. | 0.1 |
| Sb | Antimony | 0.1 | N.D. | N.D. | 0.1 |
| I | Iodine | 9 | N.D. | 4 | 1 |
| Cs | Cesium | 0.1 | N.D. | N.D. | 0.1 |
| Ba | Barium | 10 | N.D. | 0.8 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukui, K.; Suzuki, Y.; Kato, Y.; Takeuchi, N.; Takenaka, H.; Kohno, M. Effect of Extract-Added Water Derived from Deep-Sea Water with Different Hardness on Cognitive Function, Motor Ability and Serum Indexes of Obese Mice. Nutrients 2022, 14, 1794. https://doi.org/10.3390/nu14091794
Fukui K, Suzuki Y, Kato Y, Takeuchi N, Takenaka H, Kohno M. Effect of Extract-Added Water Derived from Deep-Sea Water with Different Hardness on Cognitive Function, Motor Ability and Serum Indexes of Obese Mice. Nutrients. 2022; 14(9):1794. https://doi.org/10.3390/nu14091794
Chicago/Turabian StyleFukui, Koji, Yuki Suzuki, Yugo Kato, Nozomu Takeuchi, Hirotsugu Takenaka, and Masahiro Kohno. 2022. "Effect of Extract-Added Water Derived from Deep-Sea Water with Different Hardness on Cognitive Function, Motor Ability and Serum Indexes of Obese Mice" Nutrients 14, no. 9: 1794. https://doi.org/10.3390/nu14091794
APA StyleFukui, K., Suzuki, Y., Kato, Y., Takeuchi, N., Takenaka, H., & Kohno, M. (2022). Effect of Extract-Added Water Derived from Deep-Sea Water with Different Hardness on Cognitive Function, Motor Ability and Serum Indexes of Obese Mice. Nutrients, 14(9), 1794. https://doi.org/10.3390/nu14091794






