Fish Oil Enriched n-3 Polyunsaturated Fatty Acids Improve Ketogenic Low-Carbohydrate/High-Fat Diet-Caused Dyslipidemia, Excessive Fat Accumulation, and Weight Control in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
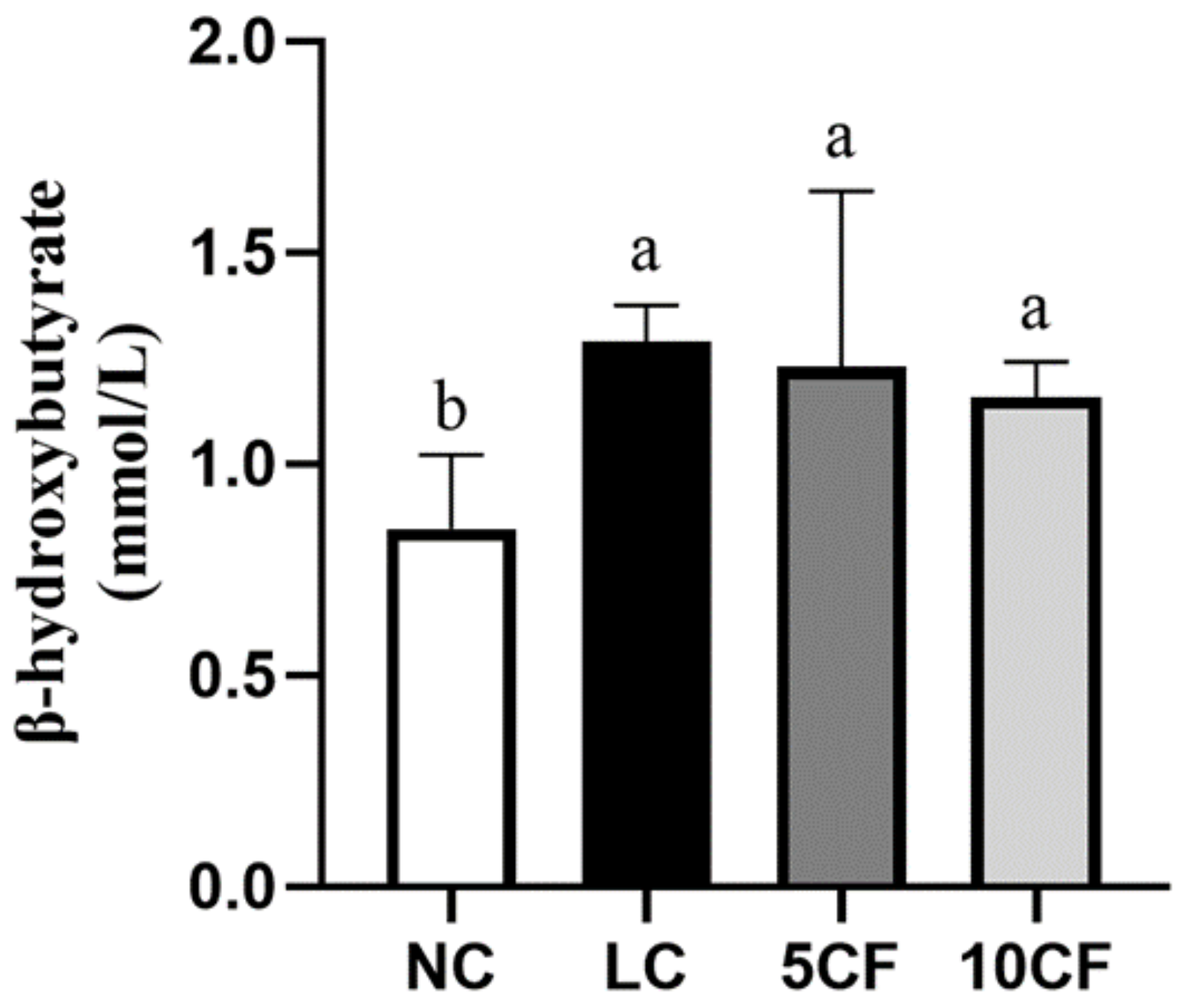
2.2. Analysis of Ketosis
2.3. Measurement of Blood Lipids, Circulating Cytokines, and Metabolic Parameters
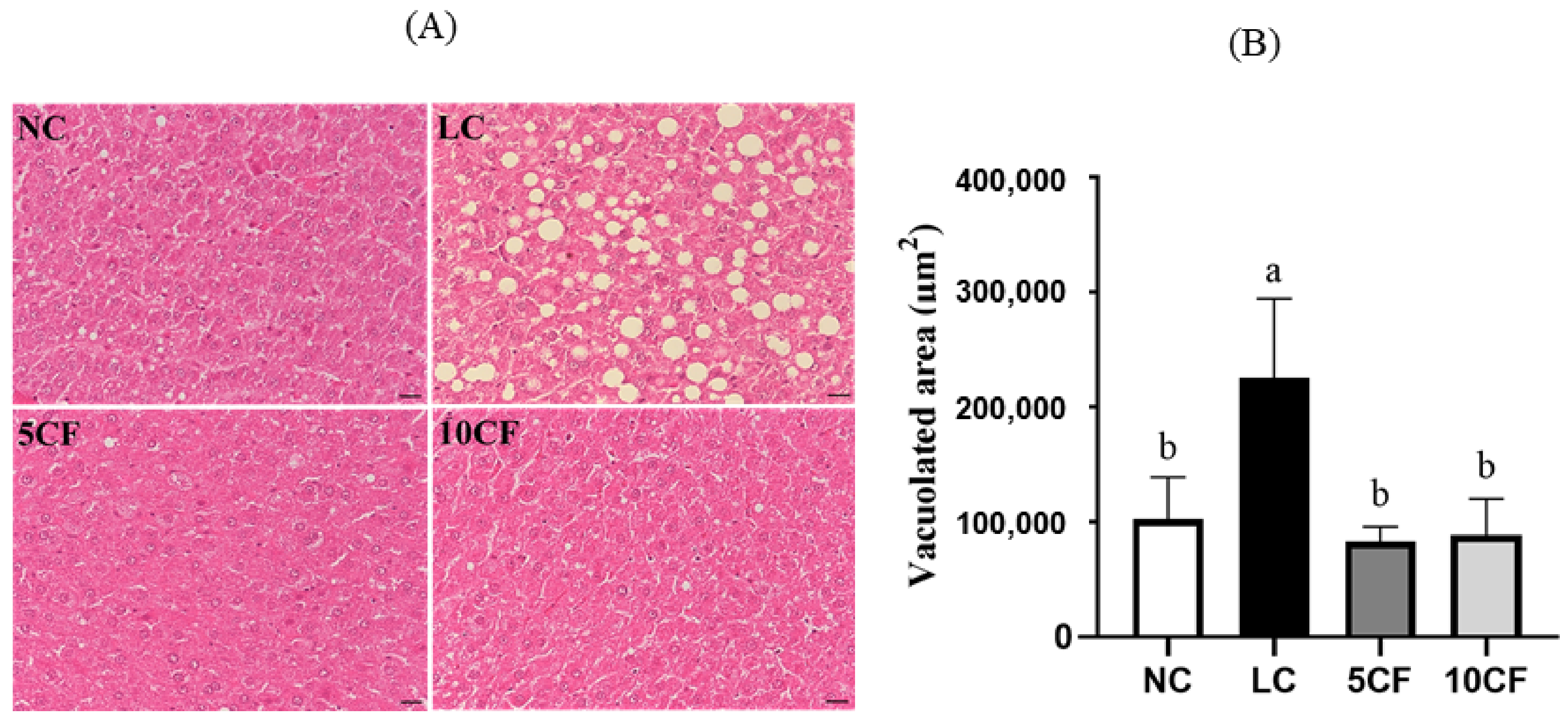
2.4. Assessment of Liver Histology
2.5. Detection of Liver Lipid Content
2.6. Data Management and Statistical Analysis
3. Results
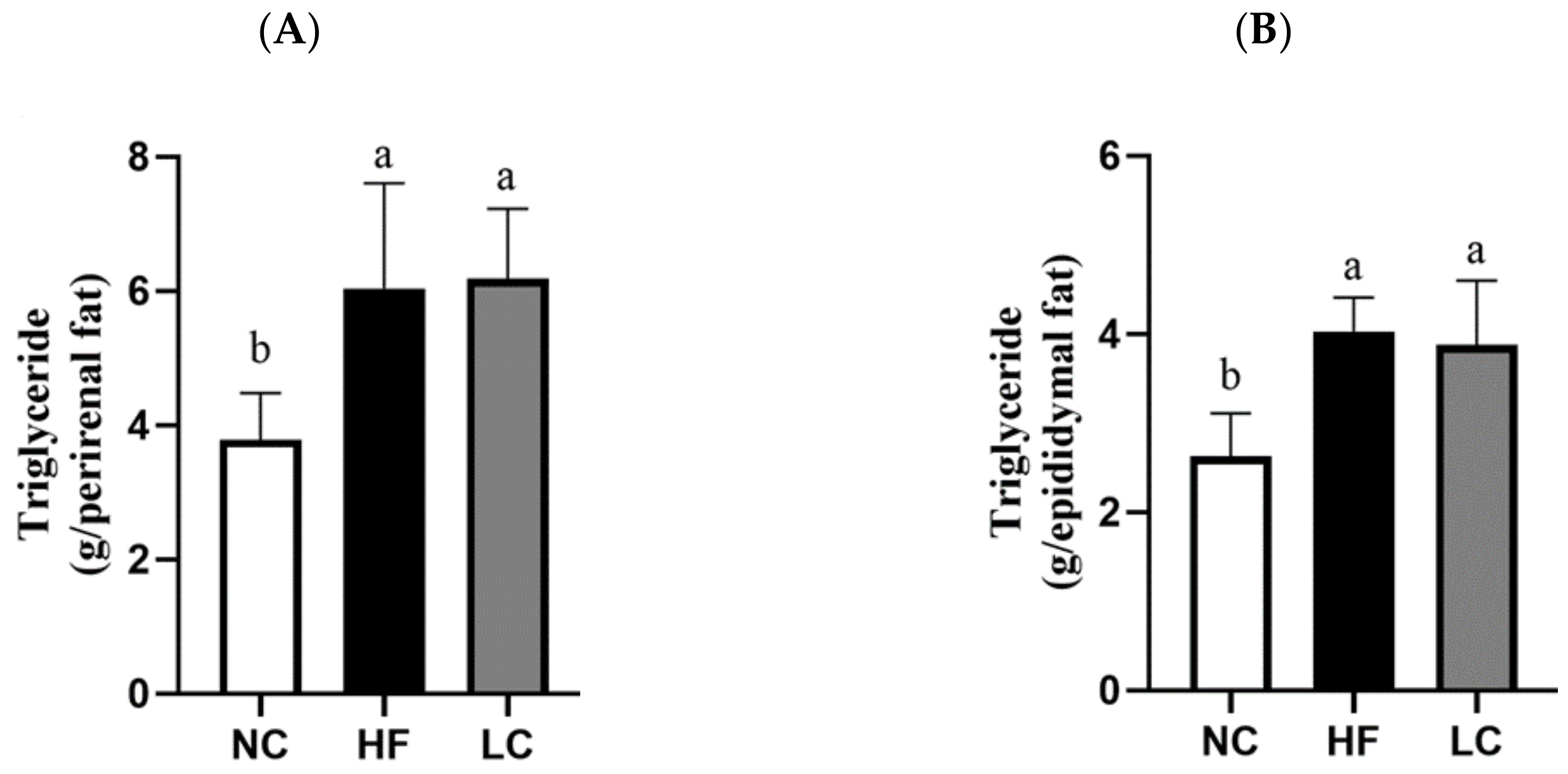
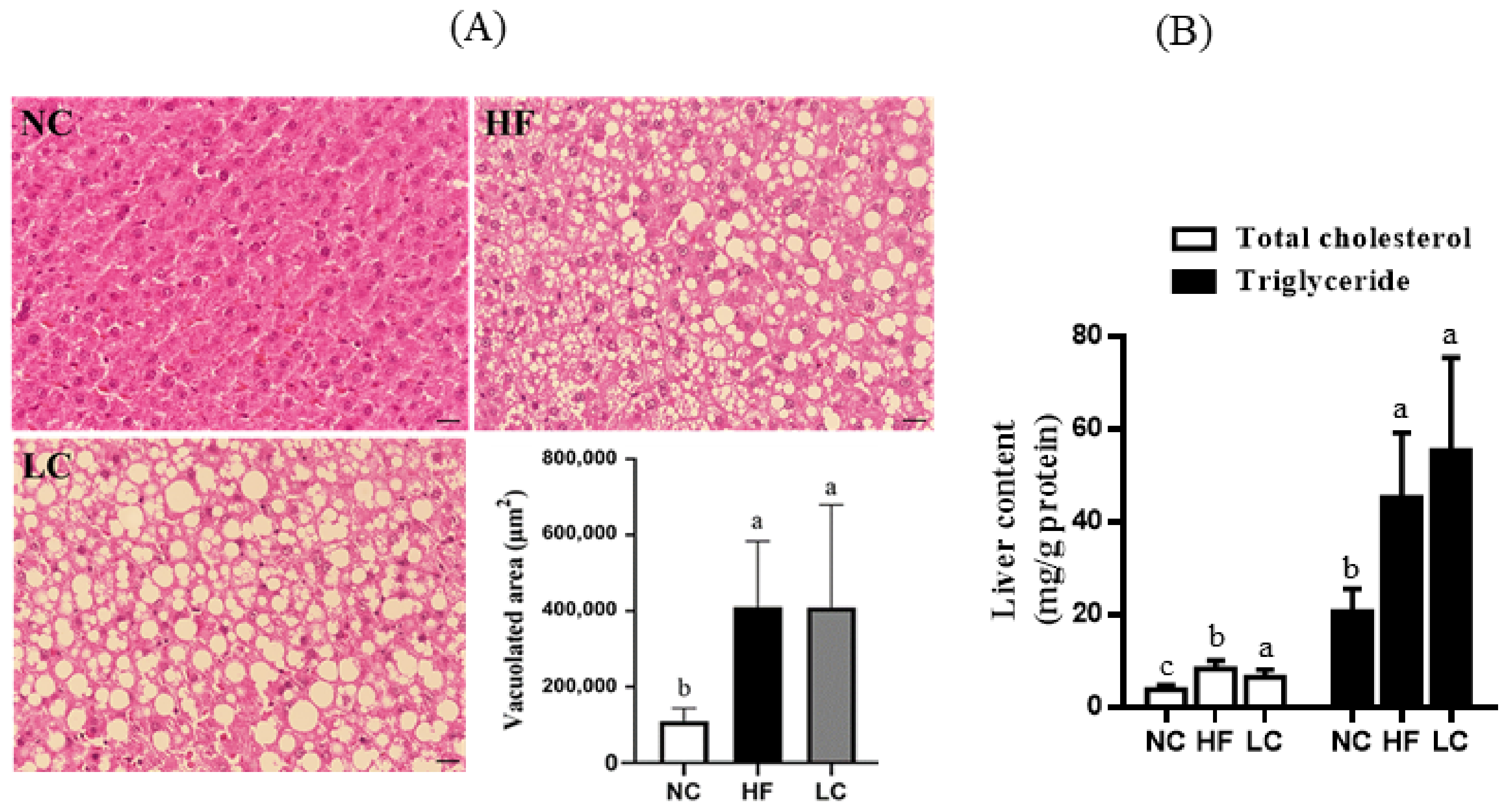
3.1. Ketogenic Low-Carbohydrate and High-Fat Diet Has No Effect on Body Weight Control but Leads to Proinflammatory State, Dyslipidemia, Visceral Fat Deposition, and Hepatic Steatosis
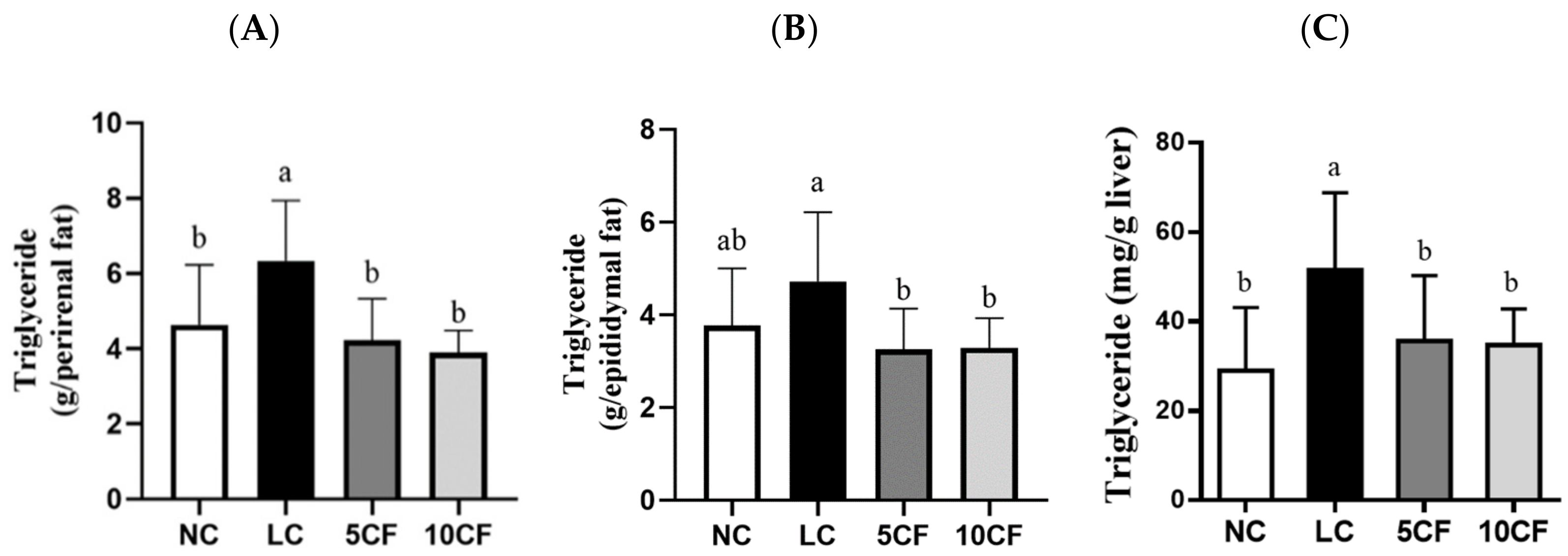
3.2. Fish Oil (FO) Substitution Ameliorates the Ketogenic Low-Carbohydrate and High-Fat Diet-Caused Metabolic Disorder
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Guo, X.; Chen, L.; Chen, T.; Yu, J.; Wu, C.; Zheng, J. Ketogenic diets and cardio-metabolic diseases. Front. Endocrinol. 2021, 12, 753039. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Swaminathan, A.; Paseka, J.; Hanson, C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-A review. Nutrients 2020, 12, 1809. [Google Scholar] [CrossRef] [PubMed]
- Dowis, K.; Banga, S. The potential health benefits of the ketogenic diet: A narrative review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Santos, F.L.; Esteves, S.S.; da Costa Pereira, A.; Yancy, W.S., Jr.; Nunes, J.P. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef]
- Harvey, C.; Schofield, G.M.; Zinn, C.; Thornley, S.J.; Crofts, C.; Merien, F.L.R. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: A randomised clinical trial. PeerJ 2019, 7, e6273. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic diets and chronic disease: Weighing the benefits against the risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Ellenbroek, J.H.; van Dijck, L.; Tons, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.; de Koning, E.J. Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef]
- Grandl, G.; Straub, L.; Rudigier, C.; Arnold, M.; Wueest, S.; Konrad, D.; Wolfrum, C. Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J. Physiol. 2018, 596, 4597–4609. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhang, J.; Jiang, T.; Zhang, Z.; Wang, Z.; Gong, M.; Zhao, L.; Zhang, C. Ketogenic diets induced glucose intolerance and lipid accumulation in mice with alterations in gut microbiota and metabolites. mBio 2021, 12, e03601-20. [Google Scholar] [CrossRef] [PubMed]
- Garbow, J.R.; Doherty, J.M.; Schugar, R.C.; Travers, S.; Weber, M.L.; Wentz, A.E.; Ezenwajiaku, N.; Cotter, D.G.; Brunt, E.M.; Crawford, P.A. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G956–G967. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparros, E.; Navas-Carrillo, D.; Marin, F.; Orenes-Pinero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Bender, N.; Portmann, M.; Heg, Z.; Hofmann, K.; Zwahlen, M.; Egger, M. Fish or n3-PUFA intake and body composition: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 657–665. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. Omega-3 and omega-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Buckley, J.D.; Howe, P.R. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity-a review. Nutrients 2010, 2, 1212–1230. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Medrikova, D.; van Schothorst, E.M.; Pavlisova, J.; Kuda, O.; Hensler, M.; Bardova, K.; Flachs, P.; Stankova, B.; Vecka, M.; et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta 2014, 1841, 267–278. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Wang, L.P.; Liu, S.H.; Chiang, M.T. Fish oil supplementation alleviates the altered lipid homeostasis in blood, liver, and adipose tissues in high-fat diet-fed rats. J. Agric. Food Chem. 2018, 66, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Bielohuby, M.; Menhofer, D.; Kirchner, H.; Stoehr, B.J.; Muller, T.D.; Stock, P.; Hempel, M.; Stemmer, K.; Pfluger, P.T.; Kienzle, E.; et al. Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E65–E76. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The impact of macronutrient intake on non-alcoholic fatty liver disease (NAFLD): Too much fat, too much carbohydrate, or just too many calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Rohling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.C.; Chitto, A.L.; Muller, A.P.; Rocha, J.K.; Castro da Silva, M.; Quincozes-Santos, A.; Nardin, P.; Rotta, L.N.; Ziegler, D.R.; Goncalves, C.A.; et al. Ketogenic diet-fed rats have increased fat mass and phosphoenolpyruvate carboxykinase activity. Mol. Nutr. Food Res. 2008, 52, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Akagiri, S.; Naito, Y.; Ichikawa, H.; Mizushima, K.; Takagi, T.; Handa, O.; Kokura, S.; Yoshikawa, T. A mouse model of metabolic syndrome; increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta Mice. J. Clin. Biochem. Nutr. 2008, 42, 150–157. [Google Scholar] [CrossRef]
- De Meijer, V.E.; Le, H.D.; Meisel, J.A.; Akhavan Sharif, M.R.; Pan, A.; Nose, V.; Puder, M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 2010, 59, 1092–1105. [Google Scholar] [CrossRef]
- Holland, A.M.; Kephart, W.C.; Mumford, P.W.; Mobley, C.B.; Lowery, R.P.; Shake, J.J.; Patel, R.K.; Healy, J.C.; McCullough, D.J.; Kluess, H.A.; et al. Effects of a ketogenic diet on adipose tissue, liver, and serum biomarkers in sedentary rats and rats that exercised via resisted voluntary wheel running. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R337–R351. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef]
- Munro, I.A.; Garg, M.L. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: A double-blinded randomised controlled trial. Food Funct. 2013, 4, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Moro, T.; Bosco, G.; Bianco, A.; Grimaldi, K.A.; Camporesi, E.; Mangar, D. Effects of n-3 polyunsaturated fatty acids (omega-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar. Drugs 2015, 13, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Aydin Cil, M.; Ghosi Ghareaghaji, A.; Bayir, Y.; Buyuktuncer, Z.; Besler, H.T. Efficacy of krill oil versus fish oil on obesity-related parameters and lipid gene expression in rats: Randomized controlled study. PeerJ 2021, 9, e12009. [Google Scholar] [CrossRef] [PubMed]
- Hainault, I.; Carolotti, M.; Hajduch, E.; Guichard, C.; Lavau, M. Fish oil in a high lard diet prevents obesity, hyperlipemia, and adipocyte insulin resistance in rats. Ann. N. Y. Acad. Sci. 1993, 683, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Belzung, F.; Raclot, T.; Groscolas, R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am. J. Physiol. 1993, 264, R1111–R1118. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Tillander, V.; Bjørndal, B.; Burri, L.; Bohov, P.; Skorve, J.; Berge, R.K.; Alexson, S.E. Fish oil and krill oil supplementations differentially regulate lipid catabolic and synthetic pathways in mice. Nutr. Metab. 2014, 11, 20. [Google Scholar] [CrossRef]
- Spahis, S.; Alvarez, F.; Dubois, J.; Ahmed, N.; Peretti, N.; Levy, E. Plasma fatty acid composition in French-Canadian children with non-alcoholic fatty liver disease: Effect of n-3 PUFA supplementation. Prostaglandins Leukot. Essent. Fatty Acids 2015, 99, 25–34. [Google Scholar] [CrossRef]
- Kim, M.G.; Yang, I.; Lee, H.S.; Lee, J.Y.; Kim, K. Lipid-modifying effects of krill oil vs fish oil: A network meta-analysis. Nutr. Rev. 2020, 78, 699–708. [Google Scholar] [CrossRef]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers-An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Świderska-Kołacz, G.; Jefimow, M.; Klusek, J.; Rączka, N.; Zmorzyński, S.; Wojciechowska, A.; Stanisławska, I.; Łyp, M.; Czerwik-Marcinkowska, J. Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney. Nutrients 2021, 13, 1996. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Kullberg, J.; Stahlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated Fat: A randomized trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef]
- Sato, A.; Kawano, H.; Notsu, T.; Ohta, M.; Nakakuki, M.; Mizuguchi, K.; Itoh, M.; Suganami, T.; Ogawa, Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: Importance of hepatic lipogenesis. Diabetes 2010, 59, 2495–2504. [Google Scholar] [CrossRef]
- Galmiche, G.; Huneau, J.F.; Mathe, V.; Mourot, J.; Simon, N.; Le Guillou, C.; Hermier, D. n-3 Fatty acids preserve muscle mass and insulin sensitivity in a rat model of energy restriction. Br. J. Nutr. 2016, 116, 1141–1152. [Google Scholar] [CrossRef][Green Version]
- Di Girolamo, F.G.; Situlin, R.; Mazzucco, S.; Valentini, R.; Toigo, G.; Biolo, G. Omega-3 fatty acids and protein metabolism: Enhancement of anabolic interventions for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 145–150. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Aspects Med. 2018, 64, 135–146. [Google Scholar] [CrossRef]
- Gray, B.; Steyn, F.; Davies, P.S.; Vitetta, L. Omega-3 fatty acids: A review of the effects on adiponectin and leptin and potential implications for obesity management. Eur. J. Clin. Nutr. 2013, 67, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Munzberg, H.; Morrison, C.D. Structure, production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, J.S.; Jo, M.J.; Cho, E.; Ahn, S.Y.; Kwon, Y.J.; Ko, G.J. The roles and associated mechanisms of adipokines in development of metabolic syndrome. Molecules 2022, 27, 334. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Panchali, T.; Paul, B.; Khatun, A.; Rao Jarapala, S.; Mondal, K.C.; Ghosh, K.; Chakrabarti, S. Anti-obesity potentiality of Tapra fish (Opisthopterus tardoore) oil. J. Food Biochem. 2020, 44, e13448. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, A.; Hall, B.; Stolecka-Warzecha, A.; Stanula, A.; Sadowska-Krepa, E. The effect of omega-3 fatty acid supplementation on serum adipocytokines, lipid profile and biochemical markers of inflammation in recreational runners. Nutrients 2021, 13, 456. [Google Scholar] [CrossRef]

| Ingredient (%) | NC | HF | LC | 5CF | 10CF |
|---|---|---|---|---|---|
| Corn starch | 64.8 | 52.8 | 32.8 | 32.8 | 32.8 |
| Casein | 20 | 20 | 20 | 20 | 20 |
| Soybean oil | 2 | 2 | 2 | 2 | 2 |
| Lard | 3 | 15 | 35 | 30 | 25 |
| Fish oil | 0 | 0 | 0 | 5 | 10 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamins 1 | 1 | 1 | 1 | 1 | 1 |
| Minerals 2 | 4 | 4 | 4 | 4 | 4 |
| Cellulose | 5 | 5 | 5 | 5 | 5 |
| Total calories (kcal/100 g) | 394.2 | 454.2 | 554.2 | 554.2 | 554.2 |
| Carbohydrate (%) | 68.290 | 48.701 | 25.478 | 25.478 | 25.478 |
| Protein (%) | 20.294 | 17.613 | 14.435 | 14.435 | 14.435 |
| Fat (%) | 11.416 | 33.686 | 60.087 | 60.087 | 60.087 |
| Total (%) | 100 | 100 | 100 | 100 | 100 |
| NC | HF | LC | |
|---|---|---|---|
| Initial body weight (g) | 162.3 ± 8.76 a | 164.2 ± 7.80 a | 164.1 ± 8.12 a |
| Final body weight (g) | 525.3 ± 32.9 b | 591.1 ± 32.5 a | 564.6 ± 38.4 ab |
| Body weight gain (g) | 363.0 ± 35.5 b | 426.9 ± 32.6 a | 400.6 ± 34.4 ab |
| Food intake (g/day) | 28.1 ± 1.21 a | 24.6 ± 1.53 b | 19.1 ± 1.05 c |
| Calorie intake (kcal/day) | 110.0 ± 4.82 ab | 114.5 ± 6.4 a | 107.5 ± 6.85 b |
| NC | HF | LC | |
|---|---|---|---|
| Total cholesterol (mg/dL) | 93.77 ± 15.58 a | 78.95 ± 20.34 ab | 66.2 ± 13.91 b |
| Triglyceride (mg/dL) | 87.14 ± 18.51 a | 60.02 ± 18.27 b | 31.63 ± 16.98 c |
| Leptin (ng/mL) | 4.06 ± 1.39 a | 6.99 ± 2.65 ab | 8.15 ± 2.74 b |
| TNF-α (pg/mL) | 4.27 ± 1.72 a | 13.75 ± 4.10 b | 8.75 ± 2.77 c |
| IL-6 (pg/mL) | 66.57 ± 8.02 a | 66.93 ± 11.35 a | 62.65 ± 12.94 a |
| AST (U/L) | 20.3 ± 4.44 a | 19.5 ± 2.47 a | 20.3 ± 2.39 a |
| ALT (U/L) | 15.2 ± 5.20 a | 14.5 ± 3.92 a | 16.3 ± 7.13 a |
| Liver weight (g) | 17.0 ± 2.28 a | 16.8 ± 1.41 a | 15.1 ± 1.11 a |
| Relative liver weight (g/100 g BW) | 3.22 ± 0.28 a | 2.84 ± 0.23 b | 2.68 ± 0.11 b |
| Perirenal adipose weight (g) | 14.3 ± 2.62 b | 21.9 ± 5.75 a | 22.6 ± 3.65 a |
| Epididymal adipose weight (g) | 9.59 ± 1.81 b | 14.7 ± 1.34 a | 14.0 ± 2.50 a |
| Small intestine weight (g) | 7.97 ± 1.46 a | 8.50 ± 0.92 a | 8.59 ± 0.39 a |
| Small intestine length (cm) | 122.9 ± 11.4 a | 116.4 ± 8.32 a | 120.0 ± 3.27 a |
| NC | LC | 5CF | 10CF | |
|---|---|---|---|---|
| Initial body weight (g) | 170.4 ± 12.4 a | 171.3 ± 12.3 a | 172.9 ± 11.1 a | 172.5 ± 9.5 a |
| Mid-term body weight (g) | 456.4 ± 34.8 ab | 465.8± 31.5 a | 427.7 ± 14.7 b | 438.9 ± 19.1 ab |
| Final body weight (g) | 555.1 ± 50.8 ab | 577.6 ± 52.1 a | 534.0 ± 23.4 b | 553.5 ± 28.2 ab |
| Body weight gain (g) | 384.8 ± 48.7 a | 406.3 ± 51.3 a | 361.1 ± 27.1 a | 381.0 ± 27.6 a |
| Food intake (g/day) | 26.7 ± 2.02 a | 19.2 ± 1.71 b | 17.1 ± 0.54 c | 18.2 ± 0.68 bc |
| Calorie intake (kcal/day) | 105.4 ± 6.18 a | 106.2 ± 6.41 a | 94.53 ± 5.27 b | 100.9 ± 5.34 ab |
| Total cholesterol (mg/dL) | 120.3 ± 30.4 a | 76.9 ± 23.8 b | 44.8 ± 20.1 c | 32.8 ± 11.3 c |
| Triglyceride (mg/dL) | 126.7 ± 53.4 a | 38.2 ± 15.7 b | 23.9 ± 18.6 b | 16.4 ± 9.65 b |
| Leptin (ng/mL) | 8.53 ± 4.18 b | 13.04 ± 4.63 a | 6.97 ± 2.73 b | 8.53 ± 4.18 b |
| AST (U/L) | 32.2 ± 18.5 a | 23.9 ± 10.6 a | 19.3 ± 8.79 a | 27.6 ± 14.9 a |
| ALT (U/L) | 24.2 ± 11.3 a | 16.4 ± 5.77 a | 17.8 ± 7.61 a | 17.2 ± 3.92 a |
| Liver weight (g) | 17.5 ± 3.65 a | 14.5 ± 1.61 b | 15.0 ± 1.81 b | 16.2 ± 0.89 ab |
| Relative liver weight (g/100 g BW) | 3.12 ± 0.41 a | 2.52 ± 0.18 c | 2.80 ± 0.27 bc | 2.93 ± 0.23 ab |
| Perirenal adipose weight (g) | 15.3 ± 4.60 b | 21.5 ± 5.09 a | 13.7 ± 3.22 b | 12.9 ± 1.3 b |
| Epididymal adipose weight (g) | 11.7 ± 3.73 ab | 14.7 ± 4.58 a | 10.3 ± 2.68 b | 10.4 ± 1.89 b |
| Gastrocnemius muscle weight (g) | 6.08 ± 0.63 a | 5.96 ± 0.37 a | 5.77 ± 0.55 a | 5.94 ± 0.44 a |
| Soleus muscle weight (g) | 0.38 ± 0.05 a | 0.42 ± 0.04 a | 0.39 ± 0.04 a | 0.39 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Chen, Y.-X.; Tzeng, H.-P.; Chiang, M.-T. Fish Oil Enriched n-3 Polyunsaturated Fatty Acids Improve Ketogenic Low-Carbohydrate/High-Fat Diet-Caused Dyslipidemia, Excessive Fat Accumulation, and Weight Control in Rats. Nutrients 2022, 14, 1796. https://doi.org/10.3390/nu14091796
Liu S-H, Chen Y-X, Tzeng H-P, Chiang M-T. Fish Oil Enriched n-3 Polyunsaturated Fatty Acids Improve Ketogenic Low-Carbohydrate/High-Fat Diet-Caused Dyslipidemia, Excessive Fat Accumulation, and Weight Control in Rats. Nutrients. 2022; 14(9):1796. https://doi.org/10.3390/nu14091796
Chicago/Turabian StyleLiu, Shing-Hwa, Yu-Xuan Chen, Huei-Ping Tzeng, and Meng-Tsan Chiang. 2022. "Fish Oil Enriched n-3 Polyunsaturated Fatty Acids Improve Ketogenic Low-Carbohydrate/High-Fat Diet-Caused Dyslipidemia, Excessive Fat Accumulation, and Weight Control in Rats" Nutrients 14, no. 9: 1796. https://doi.org/10.3390/nu14091796
APA StyleLiu, S.-H., Chen, Y.-X., Tzeng, H.-P., & Chiang, M.-T. (2022). Fish Oil Enriched n-3 Polyunsaturated Fatty Acids Improve Ketogenic Low-Carbohydrate/High-Fat Diet-Caused Dyslipidemia, Excessive Fat Accumulation, and Weight Control in Rats. Nutrients, 14(9), 1796. https://doi.org/10.3390/nu14091796







