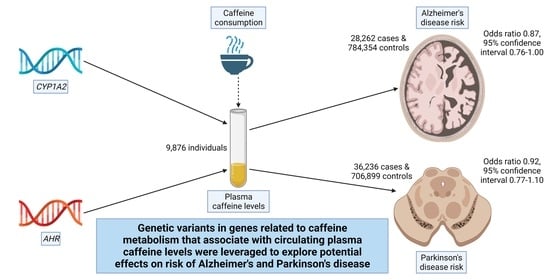
Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
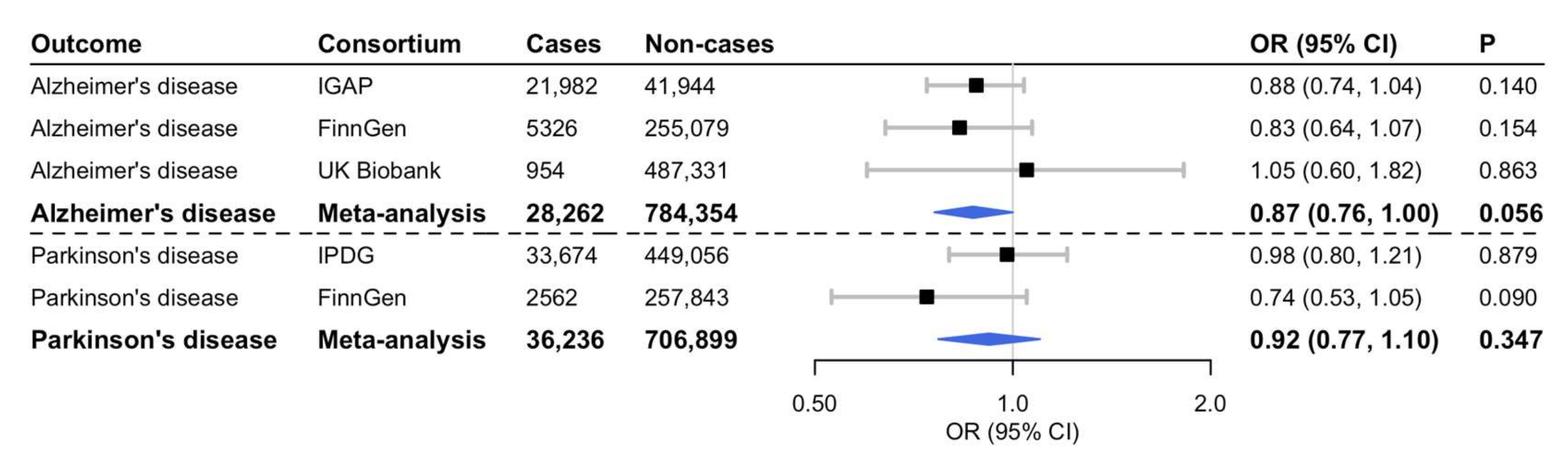
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera-Oliver, M.; Diaz-Rios, M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: A review. Life Sci. 2014, 101, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Orsini, N. Coffee Consumption and Risk of Dementia and Alzheimer’s Disease: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 2018, 10, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.; Lunet, N.; Santos, C.; Santos, J.; Vaz-Carneiro, A. Caffeine exposure and the risk of Parkinson’s disease: A systematic review and meta-analysis of observational studies. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S221–S238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelis, M.C.; Kacprowski, T.; Menni, C.; Gustafsson, S.; Pivin, E.; Adamski, J.; Artati, A.; Eap, C.B.; Ehret, G.; Friedrich, N.; et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum. Mol. Genet. 2016, 25, 5472–5482. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- FinnGen Study. Data Freeze 6 Results and Summary Statistics. Available online: https://finngen.gitbook.io/documentation/ (accessed on 1 February 2022).
- Mitchell, R.; Elsworth, B.L.; Mitchell, R.; Raistrick, C.A.; Paternoster, L.; Hemani, G.; Gaunt, T.R. MRC IEU UK Biobank GWAS Pipeline Version 2, Faculty of Health Sciences. Available online: https://data.bris.ac.uk/data/dataset/pnoat8cxo0u52p6ynfaekeigi (accessed on 17 March 2022).
- Cao, C.; Loewenstein, D.A.; Lin, X.; Zhang, C.; Wang, L.; Duara, R.; Wu, Y.; Giannini, A.; Bai, G.; Cai, J.; et al. High blood caffeine levels in MCI linked to lack of progression to dementia. J. Alzheimers Dis. 2012, 30, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardener, S.L.; Rainey-Smith, S.R.; Villemagne, V.L.; Fripp, J.; Dore, V.; Bourgeat, P.; Taddei, K.; Fowler, C.; Masters, C.L.; Maruff, P.; et al. Higher coffee consumption is associated with slower cognitive decline and less cerebral Abeta-amyloid accumulation over 126 months: Data from the Australian Imaging, Biomarkers, and Lifestyle Study. Front. Aging Neurosci. 2021, 13, 744872. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Jeon, S.Y.; Jung, G.; Lee, H.N.; Sohn, B.K.; Lee, J.Y.; Kim, Y.K.; et al. Coffee intake and decreased amyloid pathology in human brain. Transl. Psychiatry 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaramelli, C.; Palmioli, A.; De Luigi, A.; Colombo, L.; Sala, G.; Riva, C.; Zoia, C.P.; Salmona, M.; Airoldi, C. NMR-driven identification of anti-amyloidogenic compounds in green and roasted coffee extracts. Food Chem. 2018, 252, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Polis, B.; Srikanth, K.D.; Elliott, E.; Gil-Henn, H.; Samson, A.O. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer’s Disease. Neurotherapeutics 2018, 15, 1036–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avitan, I.; Halperin, Y.; Saha, T.; Bloch, N.; Atrahimovich, D.; Polis, B.; Samson, A.O.; Braitbard, O. Towards a consensus on Alzheimer’s disease comorbidity? J. Clin. Med. 2021, 10, 4360. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Traylor, M.; Malik, R.; Dichgans, M.; Burgess, S.; Markus, H.S. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ 2017, 359, j5375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wang, M.; Yuan, S.; Cai, H.; Zhu, S.G.; Liu, X. Genetically Predicted Coffee Consumption and Risk of Alzheimer’s Disease and Stroke. J. Alzheimers Dis. 2021, 83, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, A.T.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Juul Rasmussen, I.; Bojesen, S.E. Self-reported and genetically predicted coffee consumption and smoking in dementia: A Mendelian randomization study. Atherosclerosis 2022, 348, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Munafo, M.R. Mendelian Randomization Studies of Coffee and Caffeine Consumption. Nutrients 2018, 10, 1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenighetti, C.; Sugier, P.E.; Sreelatha, A.A.K.; Schulte, C.; Grover, S.; Mohamed, O.; Portugal, B.; May, P.; Bobbili, D.R.; Radivojkov-Blagojevic, M.; et al. Mendelian Randomisation Study of Smoking, Alcohol, and Coffee Drinking in Relation to Parkinson’s Disease. J. Parkinsons Dis. 2022, 12, 267–282. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsson, S.C.; Woolf, B.; Gill, D. Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study. Nutrients 2022, 14, 1697. https://doi.org/10.3390/nu14091697
Larsson SC, Woolf B, Gill D. Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study. Nutrients. 2022; 14(9):1697. https://doi.org/10.3390/nu14091697
Chicago/Turabian StyleLarsson, Susanna C., Benjamin Woolf, and Dipender Gill. 2022. "Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study" Nutrients 14, no. 9: 1697. https://doi.org/10.3390/nu14091697
APA StyleLarsson, S. C., Woolf, B., & Gill, D. (2022). Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study. Nutrients, 14(9), 1697. https://doi.org/10.3390/nu14091697