Immunonutrition and SARS-CoV-2 Infection in Children with Obesity
Abstract
:1. Introduction
2. Methods
3. Obesity and SARS-CoV-2 Infection in Children
3.1. Epidemiological Data
3.2. SARS-CoV-2 Infection in Children and Immune Function
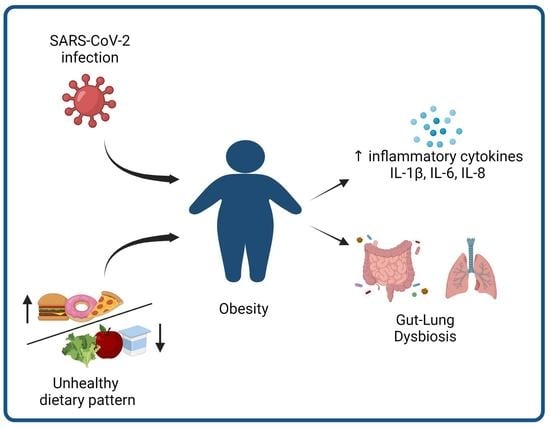
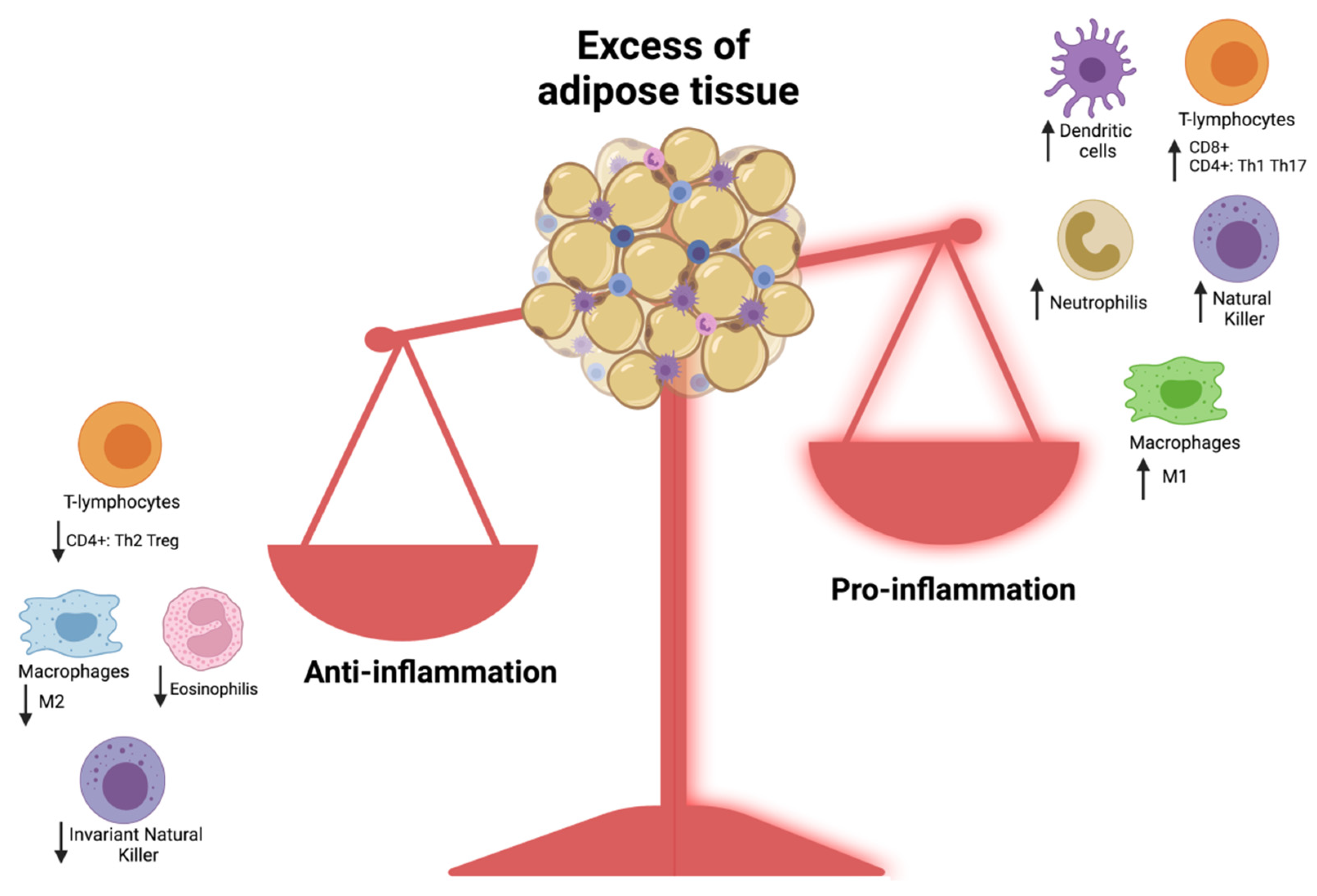
3.3. Obesity as a Risk Factor for Severe COVID-19 Infection in Children: What Is the Link?
4. Nutrition and Immune Function
5. Dietary Patterns and COVID-19
6. Nutritional Supplements and COVID-19
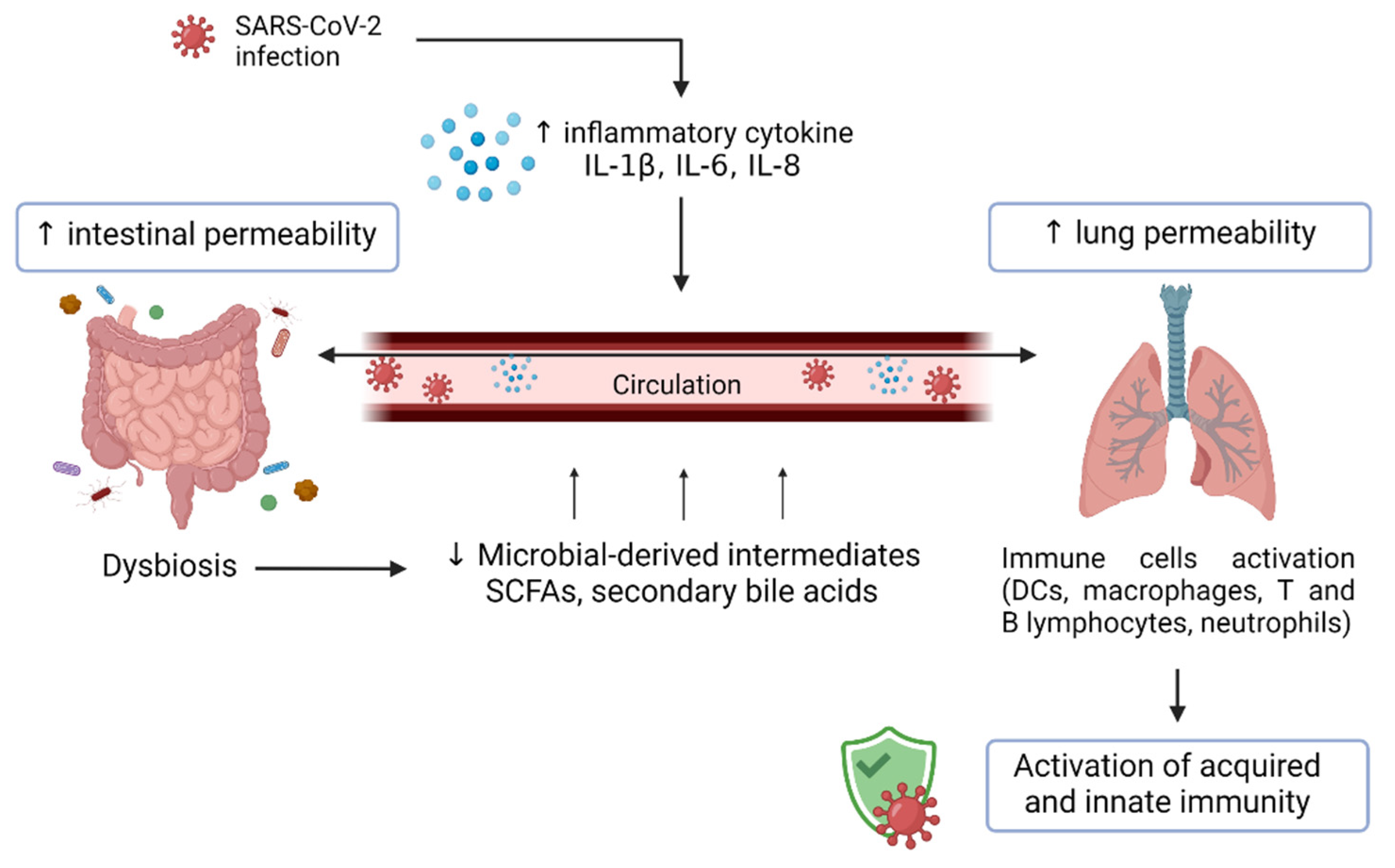
7. The Gut–Lung Axis, Dysbiosis, and Dietary Immunomodulation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 March 2022).
- Zuccotti, G.V. Immunonutrition in Pediatrics. Minerva Pediatr. 2021, 73, 95–97. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef]
- Zapatera, B.; Prados, A.; Gómez-Martínez, S.; Marcos, A. Immunonutrition: Methodology and Applications. Nutr. Hosp. 2015, 31 (Suppl. 3), 145–154. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, F.; de Punder, K.; van Heijningen, M.; van Doesburg, F. Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review. Cells 2021, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Bena, J.; Pantalone, K.M.; Burguera, B. Association of Obesity with Postacute Sequelae of COVID-19. Diabetes Obes. Metab. 2021, 23, 2183–2188. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut Microbiota Phenotypes of Obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [Green Version]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 13 April 2022).
- Siebach, M.K.; Piedimonte, G.; Ley, S.H. COVID-19 in Childhood: Transmission, Clinical Presentation, Complications and Risk Factors. Pediatr. Pulmonol. 2021, 56, 1342–1356. [Google Scholar] [CrossRef]
- Chen, P.Z.; Bobrovitz, N.; Premji, Z.A.; Koopmans, M.; Fisman, D.N.; Gu, F.X. SARS-CoV-2 Shedding Dynamics across the Respiratory Tract, Sex, and Disease Severity for Adult and Pediatric COVID-19. eLife 2021, 10, e70458. [Google Scholar] [CrossRef] [PubMed]
- AAP and CHA—Children and COVID-19 State Data Report 7.30.20. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ (accessed on 13 April 2022).
- CDC COVID-19 Response Team; CDC COVID-19 Response Team; Chow, N.; Fleming-Dutra, K.; Gierke, R.; Hall, A.; Hughes, M.; Pilishvili, T.; Ritchey, M.; Roguski, K.; et al. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions among Patients with Coronavirus Disease 2019—United States, February 12–March 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 382–386. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Erratum for Ramasamy and Subbian, “Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis”. Clin. Microbiol. Rev. 2021, 34, e0016321. [Google Scholar] [CrossRef] [PubMed]
- Luzi, L.; Radaelli, M.G. Influenza and Obesity: Its Odd Relationship and the Lessons for COVID-19 Pandemic. Acta Diabetol. 2020, 57, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Zachariah, P.; Johnson, C.L.; Halabi, K.C.; Ahn, D.; Sen, A.I.; Fischer, A.; Banker, S.L.; Giordano, M.; Manice, C.S.; Diamond, R.; et al. Epidemiology, Clinical Features, and Disease Severity in Patients with Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020, 174, e202430. [Google Scholar] [CrossRef]
- Ealey, K.N.; Phillips, J.; Sung, H.-K. COVID-19 and Obesity: Fighting Two Pandemics with Intermittent Fasting. Trends Endocrinol. Metab. TEM 2021, 32, 706–720. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme-Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble Angiotensin-Converting Enzyme 2: A Potential Approach for Coronavirus Infection Therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Epelman, S.; Tang, W.H.W.; Chen, S.Y.; Van Lente, F.; Francis, G.S.; Sen, S. Detection of Soluble Angiotensin-Converting Enzyme 2 in Heart Failure: Insights into the Endogenous Counter-Regulatory Pathway of the Renin-Angiotensin-Aldosterone System. J. Am. Coll. Cardiol. 2008, 52, 750–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Boechat, J.L.; Wandalsen, G.F.; Kuschnir, F.C.; Delgado, L. COVID-19 and Pediatric Asthma: Clinical and Management Challenges. Int. J. Environ. Res. Public. Health 2021, 18, 1093. [Google Scholar] [CrossRef]
- Schmaier, A.H. The Plasma Kallikrein-Kinin System Counterbalances the Renin-Angiotensin System. J. Clin. Invest. 2002, 109, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Su, J.B. Different Cross-Talk Sites between the Renin-Angiotensin and the Kallikrein-Kinin Systems. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2014, 15, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.M.; Murrow, J.R.; Ozkor, M.A.; Kavtaradze, N.; Lin, J.; De Staercke, C.; Hooper, W.C.; Manatunga, A.; Hayek, S.; Quyyumi, A.A. Endothelium-Derived Hyperpolarizing Factor Mediates Bradykinin-Stimulated Tissue Plasminogen Activator Release in Humans. J. Vasc. Res. 2014, 51, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.-N.; Yu, C.-H.; Pan, M.-X.; Zhang, Y.; Zheng, B.-J.; Yang, Q.-J.; Zheng, Z.-M.; Meng, Y. Author Correction: Mir-21 Mediates the Inhibitory Effect of Ang (1–7) on AngII-Induced NLRP3 Inflammasome Activation by Targeting Spry1 in Lung Fibroblasts. Sci. Rep. 2020, 10, 21896. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Tang, T.; Lv, L.; Liu, H.; Ma, K.; Liu, B. NLRP3 Inflammasome Activation Is Involved in Ang II-Induced Kidney Damage via Mitochondrial Dysfunction. Oncotarget 2016, 7, 54290–54302. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Yu, X.-Q.; Wang, P.-H. Inflammasome Activation and Th17 Responses. Mol. Immunol. 2019, 107, 142–164. [Google Scholar] [CrossRef]
- Zhuang, M.-W.; Cheng, Y.; Zhang, J.; Jiang, X.-M.; Wang, L.; Deng, J.; Wang, P.-H. Increasing Host Cellular Receptor-Angiotensin-Converting Enzyme 2 Expression by Coronavirus May Facilitate 2019-NCoV (or SARS-CoV-2) Infection. J. Med. Virol. 2020, 92, 2693–2701. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Li, F.; Qin, S.; Song, X.; Wu, Y.; Zeng, Q.; et al. A Comprehensive Investigation of the MRNA and Protein Level of ACE2, the Putative Receptor of SARS-CoV-2, in Human Tissues and Blood Cells. Int. J. Med. Sci. 2020, 17, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for Potential Pathogenesis of SARS-CoV-2 Infection-a Review of Immune Changes in Patients with Viral Pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Onabajo, O.O.; Banday, A.R.; Stanifer, M.L.; Yan, W.; Obajemu, A.; Santer, D.M.; Florez-Vargas, O.; Piontkivska, H.; Vargas, J.M.; Ring, T.J.; et al. Interferons and Viruses Induce a Novel Truncated ACE2 Isoform and Not the Full-Length SARS-CoV-2 Receptor. Nat. Genet. 2020, 52, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, Diagnostics and Drug Development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on Erythrocytes, Platelets and Clot Viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef]
- Fossiez, F.; Djossou, O.; Chomarat, P.; Flores-Romo, L.; Ait-Yahia, S.; Maat, C.; Pin, J.J.; Garrone, P.; Garcia, E.; Saeland, S.; et al. T Cell Interleukin-17 Induces Stromal Cells to Produce Proinflammatory and Hematopoietic Cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef] [Green Version]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe Immunosuppression and Not a Cytokine Storm Characterizes COVID-19 Infections. JCI Insight 2020, 5, 140329. [Google Scholar] [CrossRef]
- Bastolla, U.; Chambers, P.; Abia, D.; Garcia-Bermejo, M.-L.; Fresno, M. Is Covid-19 Severity Associated With ACE2 Degradation? arXiv 2021, arXiv:2102.13210. [Google Scholar] [CrossRef]
- Inde, Z.; Yapp, C.; Joshi, G.N.; Spetz, J.; Fraser, C.; Deskin, B.; Ghelfi, E.; Sodhi, C.; Hackam, D.J.; Kobzik, L.; et al. Age-Dependent Regulation of SARS-CoV-2 Cell Entry Genes and Cell Death Programs Correlates with COVID-19 Disease Severity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Huang, L.; Zhang, C.; Luo, R.; Zeng, L.; Liang, H.; Li, Q.; Lu, X.; Wang, X.; et al. Distinct Disease Severity Between Children and Older Adults With Coronavirus Disease 2019 (COVID-19): Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e4154–e4165. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous Expression of the SARS-Coronavirus-2 Receptor ACE2 in the Human Respiratory Tract. EBioMedicine 2020, 60, 102976. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Bungau, S.; Kumar, A.; Uddin, M.S.; Kumar, C.; Pal, G.; Sahil; Shrivastava, K.; Zengin, G.; et al. The Dual Impact of ACE2 in COVID-19 and Ironical Actions in Geriatrics and Pediatrics with Possible Therapeutic Solutions. Life Sci. 2020, 257, 118075. [Google Scholar] [CrossRef]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 Inflammation Modulates ACE2 and TMPRSS2 in Airway Epithelial Cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e8. [Google Scholar] [CrossRef]
- Sokolowska, M.; Lukasik, Z.M.; Agache, I.; Akdis, C.A.; Akdis, D.; Akdis, M.; Barcik, W.; Brough, H.A.; Eiwegger, T.; Eljaszewicz, A.; et al. Immunology of COVID-19: Mechanisms, Clinical Outcome, Diagnostics, and Perspectives-A Report of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy 2020, 75, 2445–2476. [Google Scholar] [CrossRef]
- Carsetti, R.; Quintarelli, C.; Quinti, I.; Piano Mortari, E.; Zumla, A.; Ippolito, G.; Locatelli, F. The Immune System of Children: The Key to Understanding SARS-CoV-2 Susceptibility? Lancet Child Adolesc. Health 2020, 4, 414–416. [Google Scholar] [CrossRef]
- Cristiani, L.; Mancino, E.; Matera, L.; Nenna, R.; Pierangeli, A.; Scagnolari, C.; Midulla, F. Will Children Reveal Their Secret? The Coronavirus Dilemma. Eur. Respir. J. 2020, 55, 2000749. [Google Scholar] [CrossRef] [Green Version]
- Varghese, L.; Zachariah, P.; Vargas, C.; LaRussa, P.; Demmer, R.T.; Furuya, Y.E.; Whittier, S.; Reed, C.; Stockwell, M.S.; Saiman, L. Epidemiology and Clinical Features of Human Coronaviruses in the Pediatric Population. J. Pediatr. Infect. Dis. Soc. 2018, 7, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.-N.; Louwen, F.; Yuan, J. Obesity and COVID-19: Molecular Mechanisms Linking Both Pandemics. Int. J. Mol. Sci. 2020, 21, 5793. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.; Razieh, C.; Zaccardi, F.; Davies, M.J.; Khunti, K. Obesity and Risk of COVID-19: Analysis of UK Biobank. Prim. Care Diabetes 2020, 14, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Fore, H.H.; Dongyu, Q.; Beasley, D.M.; Ghebreyesus, T.A. Child Malnutrition and COVID-19: The Time to Act Is Now. Lancet Lond. Engl. 2020, 396, 517–518. [Google Scholar] [CrossRef]
- Kang, H.M.; Jeong, D.C.; Suh, B.K.; Ahn, M.B. The Impact of the Coronavirus Disease-2019 Pandemic on Childhood Obesity and Vitamin D Status. J. Korean Med. Sci. 2021, 36, e21. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Poma, A.M.; Bonuccelli, D.; Proietti, A.; Macerola, E.; Ugolini, C.; Torregrossa, L.; Giannini, R.; Vignali, P.; Basolo, F.; et al. Adipose Tissue in COVID-19: Detection of SARS-CoV-2 in Adipocytes and Activation of the Interferon-Alpha Response. J. Endocrinol. Investig. 2022, 45, 1021–1029. [Google Scholar] [CrossRef]
- Couturier, J.; Lewis, D.E. HIV Persistence in Adipose Tissue Reservoirs. Curr. HIV/AIDS Rep. 2018, 15, 60–71. [Google Scholar] [CrossRef]
- Bassendine, M.F.; Bridge, S.H.; McCaughan, G.W.; Gorrell, M.D. COVID-19 and Comorbidities: A Role for Dipeptidyl Peptidase 4 (DPP4) in Disease Severity? J. Diabetes 2020, 12, 649–658. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Hosoya, Y.; Yamashita, H.; Fujita, H.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. Adipogenesis in Obesity Requires Close Interplay between Differentiating Adipocytes, Stromal Cells, and Blood Vessels. Diabetes 2007, 56, 1517–1526. [Google Scholar] [CrossRef] [Green Version]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose Tissue Hypoxia in Obesity and Its Impact on Adipocytokine Dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Ye, J. Emerging Role of Adipose Tissue Hypoxia in Obesity and Insulin Resistance. Int. J. Obes. 2005 2009, 33, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mraz, M.; Haluzik, M. The Role of Adipose Tissue Immune Cells in Obesity and Low-Grade Inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curat, C.A.; Miranville, A.; Sengenès, C.; Diehl, M.; Tonus, C.; Busse, R.; Bouloumié, A. From Blood Monocytes to Adipose Tissue-Resident Macrophages: Induction of Diapedesis by Human Mature Adipocytes. Diabetes 2004, 53, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.A.; Favelyukis, S.; Nguyen, A.-K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A Subpopulation of Macrophages Infiltrates Hypertrophic Adipose Tissue and Is Activated by Free Fatty Acids via Toll-like Receptors 2 and 4 and JNK-Dependent Pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [Green Version]
- Croce, S.; Avanzini, M.A.; Regalbuto, C.; Cordaro, E.; Vinci, F.; Zuccotti, G.; Calcaterra, V. Adipose Tissue Immunomodulation and Treg/Th17 Imbalance in the Impaired Glucose Metabolism of Children with Obesity. Children 2021, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- O’Garra, A.; Vieira, P. Regulatory T Cells and Mechanisms of Immune System Control. Nat. Med. 2004, 10, 801–805. [Google Scholar] [CrossRef]
- Tao, L.; Liu, H.; Gong, Y. Role and Mechanism of the Th17/Treg Cell Balance in the Development and Progression of Insulin Resistance. Mol. Cell. Biochem. 2019, 459, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, V.; Croce, S.; Vinci, F.; De Silvestri, A.; Cordaro, E.; Regalbuto, C.; Zuccotti, G.V.; Mameli, C.; Albertini, R.; Avanzini, M.A. Th17 and Treg Balance in Children With Obesity and Metabolically Altered Status. Front. Pediatr. 2020, 8, 591012. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E. Cytokine-Producing B Lymphocytes-Key Regulators of Immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 Immunity: Contemporary Insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, C.; Petri, W.A. Leptin Regulation of Immune Responses. Trends Mol. Med. 2016, 22, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the Past Two Decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.N.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F. American Society for Parenteral and Enteral Nutrition Board of Directors Defining Pediatric Malnutrition: A Paradigm Shift toward Etiology-Related Definitions. JPEN J. Parenter. Enteral Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef] [Green Version]
- Verduci, E.; D’Auria, E.; Bosetti, A.; DI Profio, E.; Vizzuso, S.; Milanta, C.; Pendezza, E.; Borsani, B.; Zuccotti, G.V. Immunomodulatory Diet in Pediatric Age. Minerva Pediatr. 2021, 73, 128–149. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C. Nutritionally Mediated Programming of the Developing Immune System. Adv. Nutr. 2011, 2, 377–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.H.; O’Connor, T.G.; Roth, C.; Susser, E.; Bjørke-Monsen, A.-L. The Influence of Maternal Prenatal and Early Childhood Nutrition and Maternal Prenatal Stress on Offspring Immune System Development and Neurodevelopmental Disorders. Front. Neurosci. 2013, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Niers, L.; Stasse-Wolthuis, M.; Rombouts, F.M.; Rijkers, G.T. Nutritional Support for the Infant’s Immune System. Nutr. Rev. 2007, 65, 347–360. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C. Modification of Immune Responses to Exercise by Carbohydrate, Glutamine and Anti-Oxidant Supplements. Immunol. Cell Biol. 2000, 78, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.M.; Candlish, J.K. Carnosine and Anserine as Modulators of Neutrophil Function. Clin. Lab. Haematol. 1998, 20, 239–244. [Google Scholar] [CrossRef]
- Dubai, D.J. The Role of Nutritional Status in Immunity of Infants and Young Children. IJSR 2013, 4, 3. [Google Scholar]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected Vitamins and Trace Elements Support Immune Function by Strengthening Epithelial Barriers and Cellular and Humoral Immune Responses. Br. J. Nutr. 2007, 98 (Suppl. 1), S29–S35. [Google Scholar] [CrossRef]
- Pai, U.A.; Chandrasekhar, P.; Carvalho, R.S.; Kumar, S. The Role of Nutrition in Immunity in Infants and Toddlers: An Expert Panel Opinion. Clin. Epidemiol. Glob. Health 2018, 6, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Venter, C.; Greenhawt, M.; Meyer, R.W.; Agostoni, C.; Reese, I.; du Toit, G.; Feeney, M.; Maslin, K.; Nwaru, B.I.; Roduit, C.; et al. EAACI Position Paper on Diet Diversity in Pregnancy, Infancy and Childhood: Novel Concepts and Implications for Studies in Allergy and Asthma. Allergy 2020, 75, 497–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Noncommunicable Diseases and COVID-19. Available online: https://www.who.int/teams/noncommunicable-diseases/covid-19 (accessed on 20 February 2022).
- Costa, D.; Barbalho, M.C.; Miguel, G.P.S.; Forti, E.M.P.; Azevedo, J.L.M.C. The Impact of Obesity on Pulmonary Function in Adult Women. Clinics 2008, 63, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- De Onis, M.; Habicht, J.P. Anthropometric Reference Data for International Use: Recommendations from a World Health Organization Expert Committee. Am. J. Clin. Nutr. 1996, 64, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper From the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef]
- Moscatelli, F.; Sessa, F.; Valenzano, A.; Polito, R.; Monda, V.; Cibelli, G.; Villano, I.; Pisanelli, D.; Perrella, M.; Daniele, A.; et al. COVID-19: Role of Nutrition and Supplementation. Nutrients 2021, 13, 976. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Stachowska, E.; Folwarski, M.; Jamioł-Milc, D.; Maciejewska, D.; Skonieczna-Żydecka, K. Nutritional Support in Coronavirus 2019 Disease. Medicina 2020, 56, 289. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary Carbohydrate Intake and Mortality: A Prospective Cohort Study and Meta-Analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- De Faria Coelho-Ravagnani, C.; Corgosinho, F.C.; Sanches, F.L.F.Z.; Prado, C.M.M.; Laviano, A.; Mota, J.F. Dietary Recommendations during the COVID-19 Pandemic. Nutr. Rev. 2021, 79, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition, Immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Nutrition and Immunity: Lessons for COVID-19. Eur. J. Clin. Nutr. 2021, 75, 1309–1318. [Google Scholar] [CrossRef]
- Alkhatib, A. Antiviral Functional Foods and Exercise Lifestyle Prevention of Coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional Foods and the Immune System: A Review. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S29–S33. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef]
- Valenzano, A.; Polito, R.; Trimigno, V.; Di Palma, A.; Moscatelli, F.; Corso, G.; Sessa, F.; Salerno, M.; Montana, A.; Di Nunno, N.; et al. Effects of Very Low Calorie Ketogenic Diet on the Orexinergic System, Visceral Adipose Tissue, and ROS Production. Antioxidants 2019, 8, 643. [Google Scholar] [CrossRef] [Green Version]
- Moscatelli, F.; Valenzano, A.; Polito, R.; Francesco, S.; Montana, A.; Salerno, M.; Messina, A.; Monda, M.; Cibelli, G.; Monda, V.; et al. Ketogenic Diet and Sport Performance. Sport Mont 2020, 18, 91–94. [Google Scholar]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and Inflammation: Potential Implications for Severity of COVID-19. Ir. Med. J. 2020, 113, 81. [Google Scholar] [PubMed]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef]
- Kumar, R.; Rathi, H.; Haq, A.; Wimalawansa, S.J.; Sharma, A. Putative Roles of Vitamin D in Modulating Immune Response and Immunopathology Associated with COVID-19. Virus Res. 2021, 292, 198235. [Google Scholar] [CrossRef]
- Costagliola, G.; Spada, E.; Comberiati, P.; Peroni, D.G. Could Nutritional Supplements Act as Therapeutic Adjuvants in COVID-19? Ital. J. Pediatr. 2021, 47, 32. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-J.; Chang, H.-S.; Yang, Y.-P.; Lin, T.-W.; Lai, W.-Y.; Lin, Y.-Y.; Chang, C.-C. The Role of Micronutrient and Immunomodulation Effect in the Vaccine Era of COVID-19. J. Chin. Med. Assoc. JCMA 2021, 84, 821–826. [Google Scholar] [CrossRef]
- Trasino, S.E. A Role for Retinoids in the Treatment of COVID-19? Clin. Exp. Pharmacol. Physiol. 2020, 47, 1765–1767. [Google Scholar] [CrossRef]
- Jee, J.; Hoet, A.E.; Azevedo, M.P.; Vlasova, A.N.; Loerch, S.C.; Pickworth, C.L.; Hanson, J.; Saif, L.J. Effects of Dietary Vitamin A Content on Antibody Responses of Feedlot Calves Inoculated Intramuscularly with an Inactivated Bovine Coronavirus Vaccine. Am. J. Vet. Res. 2013, 74, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Sijtsma, S.R.; Kouwenhoven, B.; Rombout, J.H.; van der Zijpp, A.J. Epithelia-Damaging Virus Infections Affect Vitamin A Status in Chickens. J. Nutr. 1992, 122, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Goc, A.; Ivanova, S.; Niedzwiecki, A.; Rath, M. Inhibition of ACE2 Expression by Ascorbic Acid Alone and Its Combinations with Other Natural Compounds. Infect. Dis. 2021, 14, 1178633721994605. [Google Scholar] [CrossRef]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Gasmi Benahmed, A.; Bjørklund, G. Micronutrients as Immunomodulatory Tools for COVID-19 Management. Clin. Immunol. 2020, 220, 108545. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Risé, P.; Di Profio, E.; Fiori, L.; Vizzuso, S.; Dilillo, D.; Mannarino, S.; Zoia, E.; Calcaterra, V.; Pinna, C.; et al. Blood Fatty Acids Profile in MIS-C Children. Metabolites 2021, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 Pandemic: Can Maintaining Optimal Zinc Balance Enhance Host Resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.-Z. Lactoferrin as Potential Preventative and Adjunct Treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Fanos, V. Lactoferrin Is an Important Factor When Breastfeeding and COVID-19 Are Considered. Acta Paediatr. Oslo Nor. 1992 2020, 109, 2139–2140. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G. Viral Infections: Lactoferrin, a Further Arrow in the Quiver of Prevention. J. Pediatr. Neonatal Individ. Med. JPNIM 2020, 9, e090142. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and Selenoproteins in Viral Infection with Potential Relevance to COVID-19. Redox Biol. 2020, 37, 101715. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Dhar, D.; Mohanty, A. Gut Microbiota and COVID-19- Possible Link and Implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Lei, W.-T.; Shih, P.-C.; Liu, S.-J.; Lin, C.-Y.; Yeh, T.-L. Effect of Probiotics and Prebiotics on Immune Response to Influenza Vaccination in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 1175. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Koverech, A.; Zanetti, M. Nutrition Support in the Time of SARS-CoV-2 (COVID-19). Nutrition 2020, 74, 110834. [Google Scholar] [CrossRef]
- Arkin, N.; Krishnan, K.; Chang, M.G.; Bittner, E.A. Nutrition in Critically Ill Patients with COVID-19: Challenges and Special Considerations. Clin. Nutr. Edinb. Scotl. 2020, 39, 2327–2328. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.T.D. Nutrition in Times of Covid-19, How to Trust the Deluge of Scientific Information. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, S.; Mao, Z.; Wang, W.; Hu, H. Clinical Significance of Nutritional Risk Screening for Older Adult Patients with COVID-19. Eur. J. Clin. Nutr. 2020, 74, 876–883. [Google Scholar] [CrossRef]
- Budhwar, S.; Sethi, K.; Chakraborty, M. A Rapid Advice Guideline for the Prevention of Novel Coronavirus Through Nutritional Intervention. Curr. Nutr. Rep. 2020, 9, 119–128. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [Green Version]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [Green Version]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S150–S156. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, Metabolites, and the Gut-Lung Axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging Pathogenic Links between Microbiota and the Gut-Lung Axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Tan, J.-Y.; Tang, Y.-C.; Huang, J. Gut Microbiota and Lung Injury. Adv. Exp. Med. Biol. 2020, 1238, 55–72. [Google Scholar] [CrossRef]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the Gut and Lung Microbiome Has a Role in Asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef] [Green Version]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-Intestinal Cross-Talk in Mucosal Inflammatory Disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef] [Green Version]
- McAleer, J.P.; Kolls, J.K. Contributions of the Intestinal Microbiome in Lung Immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.-Y.; Zhang, Z.; Feng, Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut-Lung Axis: The Microbial Contributions and Clinical Implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The Role of the Lung Microbiota and the Gut-Lung Axis in Respiratory Infectious Diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grier, A.; McDavid, A.; Wang, B.; Qiu, X.; Java, J.; Bandyopadhyay, S.; Yang, H.; Holden-Wiltse, J.; Kessler, H.A.; Gill, A.L.; et al. Neonatal Gut and Respiratory Microbiota: Coordinated Development through Time and Space. Microbiome 2018, 6, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakansson, A.; Molin, G. Gut Microbiota and Inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric Obesity Is Associated with an Altered Gut Microbiota and Discordant Shifts in Firmicutes Populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in Gut Microbiota Composition between Obese and Lean Children: A Cross-Sectional Study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The Human Gut Microbiota: Metabolism and Perspective in Obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. CMLS 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation That Is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, L.G. Diet, Obesity, and Asthma. Ann. Am. Thorac. Soc. 2017, 14, S332–S338. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-Driven Allergic Lung Inflammation Is Ameliorated by Short-Chain Fatty Acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Macpherson, A.J. Immune Adaptations That Maintain Homeostasis with the Intestinal Microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Yi, H.; Yong, D.; Lee, K.; Cho, Y.-J.; Chun, J. Profiling Bacterial Community in Upper Respiratory Tracts. BMC Infect. Dis. 2014, 14, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, G.B.; van der Gast, C.J.; Serisier, D.J. Predominant Pathogen Competition and Core Microbiota Divergence in Chronic Airway Infection. ISME J. 2015, 9, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, H.T.; Higham, S.L.; Moffatt, M.F.; Cox, M.J.; Tregoning, J.S. Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio 2020, 11, e03236-19. [Google Scholar] [CrossRef] [Green Version]
- Bingula, R.; Filaire, M.; Radosevic-Robin, N.; Bey, M.; Berthon, J.-Y.; Bernalier-Donadille, A.; Vasson, M.-P.; Filaire, E. Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer. J. Oncol. 2017, 2017, 5035371. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Chen, P.-J.; Lee, Y.-T.; Ko, J.-L.; Lue, K.-H. Effects of Immunomodulatory Supplementation with Lactobacillus Rhamnosus on Airway Inflammation in a Mouse Asthma Model. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2016, 49, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Jing, W.; Wang, W. Bifidobacterium Lactis Ameliorates the Risk of Food Allergy in Chinese Children by Affecting Relative Percentage of Treg and Th17 Cells. Can. J. Infect. Dis. Med. Microbiol. J. Can. Mal. Infect. Microbiol. Med. 2018, 2018, 4561038. [Google Scholar] [CrossRef] [Green Version]
| Nutrient/Bioactive Compound | Positive Suggested Role against COVID-19 | References |
|---|---|---|
| Vitamin D | - Young children, elderly, and obese people are most at risk of hypovitaminosis D (caused by an insufficient sun exposure or by a diet low of VD-rich food). - In children, VD insufficiency (25-OH VD < 30 ng/mL) or deficiency (25-OH VD < 20 ng/mL) gives higher risk for respiratory infections. - VD supplementation lowers COVID-19 severity in hospitalized patients. - Promotes the production of antimicrobial molecules, activates defensive cells to destroy the virus, and decreases in vivo the production of inflammatory cytokines, preventing the cytokine storm. - Crucial regulator of the renin-angiotensin system (angiotensin converting enzyme 2, ACE2), useful for SARS-CoV-2 to move into the host cells. - Its supplementation can inhibit the transmission of the infection and avoid progression to severe disease. - In most studies in children, the intervention to prevent COVID-19 (daily intake 400–1200 IU for 6–12 months, considering higher doses in patients with VD deficiency or insufficiency, i.e., obese children and adolescents). | - Laird E et al., 2020 [116]. - Pecora F et al., 2020 [117]. - Entrenas Castillo M et al., 2020 [118]. - Giannini S et al., 2021 [119]. -Kumar R et al., 2021 [120]. - Costagliola G et al., 2021 [121]. - Martineau AR et al., 2017 [122]. |
| Vitamin A | - Regulates both innate immune response (through natural killer cells, macrophages, and neutrophils) and adaptive immunity - During the initial phase of SARS-CoV-2 infection, the innate immune system acts on it by releasing IFN-1. Retinoids (in particular retinoid acid), for its immune-modulating properties, may improve IFN-1 activities. - Vitamin A (VA) and retinoid could be tested as antiviral substances in preclinical trials for COVID-19 treatment. To date, limited human clinical trials are ongoing (IRCT20180520039738N2, IRCT20170117032004N3), but there is no direct evidence of the efficacy of VA supplementation in patients affected by COVID-19. | - Yu-Ju La et al., 2021 [123]. - Trasino SE., 2020 [124]. - Jee J et al., 2013 [125]. - West CE et al., 1992 [126]. |
| Vitamin C | - Influences functioning of the immune system (growth and function of both innate and adaptive immune cells, phagocytosis and microbial killing, antibody production, and generation of reactive oxygen species (ROS) and supportive epithelial barrier integrity). - VC deficiency is linked to an increased predisposition to severe respiratory infections such as pneumonia both in children and adults. - Ascorbic acid may inhibit the expression of ACE2 in human small alveolar epithelial cells, limiting the entrance of SARS-CoV-2. | - Maggini S et al., 2007 [92]. -Verduci E et al., 2021 [84]. - Pecora F et al., 2020 [117]. - Ivanov V et al., 2021 [127]. |
| Vitamin E | - Antioxidant role (lowering the production of superoxides). -Supports T cell-mediated functions, optimization of Th1 response, and suppression of Th2 response. | - Yu-Ju Laia et al., 2021 [123]. - Gasmi A et al., 2020 [128]. |
| PUFAs and DHA | - DHA has anti-inflammatory and antioxidant properties when enzymatically converted to specialized pro-resolving mediators (SPMs) known as resolvins, protectins, and maresins and increases immune system activity by helping to resolve the inflammatory response. - In an observational study conducted in children affected by MIS-C (multisystemic inflammatory syndrome), COVID-19-related, evidence of fatty acid (FA) alterations has been shown, suggesting a significant contribution of ω-6 FAs (linoleic acid and arachidonic acid) to the observed inflammatory state and supporting a possible dietary intervention to re-establish an appropriate balance among the FAs capable of promoting the resolution of the observed inflammatory condition. | - Verduci E et al., 2021 [84]. - Verduci E et al., 2021 [129]. |
| Zinc | - Anti-inflammatory and antioxidant, reduces ROS in viral infections. - Direct role in antiviral activity by inhibiting viral replication on different pathogens, including SARS-CoV-2 and RSV, through the interference with the function of RNA-dependent RNA polymerase. - Promotes the proliferation and differentiation of T cells. - Activates the transcription factor FOXP3, implicated in the differentiation of Tregs (which produces anti-inflammatory cytokines such as IL-10), regulates the Th1/Th2 balance, the participates in the proliferation of Th17 cells. | - Calder PC et al., 2020 [102]. - Shakoor H et al., 2021 [130]. - Wessels I et al., 2020 [131]. - Razzaque MS. 2020 [132]. - Pal A et al., 2020 [133]. - Costagliola G et al., 2021 [121]. |
| Lactoferrin | - Antiviral effect with the impairment of viral anchoring on the cellular surface by preventing the interaction between the virus and heparin sulfate glycosaminoglycan, and the inhibition of viral replication. Facilitates the clearance of the infectious agent, enhancing the activity of macrophages, neutrophils, and natural killer cells. - Eases the antigen presentation to T cells and modulates the secretion of pro-inflammatory cytokines (IL-6) which has a pivotal role in the pathogenesis of ARDS and MIS-C in pediatric patients with COVID-19. Decreases the expression of different chemotactic factors and adhesion molecules. - Reduces the damage derived from the production of ROSs. - May represent one of the factors contributing to the lower incidence and severity of COVID-19, especially by decreasing incidence of clinically relevant disease in the newborn. | - Chang R et al., 2020 [134]. - Lang J et al., 2011 [135]. - Peroni DG, Fanos V. 2020 [136]. - Peroni DG. 2020 [137]. - Kruzel ML et al., 2017 [138]. -Rosa L et al., 2017 [139]. - Siqueiros-Cendón T et al., 2014 [140]. |
| Selenium | - Antioxidant role, ROS balance in inflammatory processes, immune cell function. | - Calder PC et al., 2020 [102]. - Shakoor H et al., 2021 [130]. - Bae M, Kim H. 2020 [141]. - Zhang J et al., 2020 [142]. |
| Probiotics | - Influence both the transmission of SARS-CoV-2 and the immune balance of the host. - Particularly in children, probiotics may interfere with this mechanism, reinforcing the gut epithelial barrier and directly competing with the proliferation of SARS-CoV-2. - Specific probiotics may enhance local and systemic immune response, creating a “gut–lung axis” which finally favors the clearance of the infectious agent. - Influence the gut microbiome increasing local and systemic production of different proinflammatory cytokines with antiviral activity. - Enhance the activity of innate and adaptive immune system including the increased function of toll-like receptors (TLR) and the influence on the function of antigen-presenting cells. - May moderate the systemic levels of pro-inflammatory cytokines associated with the “cytokine storm”, and cause a rise of serum anti-inflammatory cytokines such as IL-10. | - Baud D et al., 2020 [143]. - Dhar D, Mohanty A., 2020 [144]. - Lei WT et al., 2017 [145]. - Costagliola G et al., 2021 [121]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Auria, E.; Calcaterra, V.; Verduci, E.; Ghezzi, M.; Lamberti, R.; Vizzuso, S.; Baldassarre, P.; Pendezza, E.; Perico, V.; Bosetti, A.; et al. Immunonutrition and SARS-CoV-2 Infection in Children with Obesity. Nutrients 2022, 14, 1701. https://doi.org/10.3390/nu14091701
D’Auria E, Calcaterra V, Verduci E, Ghezzi M, Lamberti R, Vizzuso S, Baldassarre P, Pendezza E, Perico V, Bosetti A, et al. Immunonutrition and SARS-CoV-2 Infection in Children with Obesity. Nutrients. 2022; 14(9):1701. https://doi.org/10.3390/nu14091701
Chicago/Turabian StyleD’Auria, Enza, Valeria Calcaterra, Elvira Verduci, Michele Ghezzi, Rossella Lamberti, Sara Vizzuso, Paola Baldassarre, Erica Pendezza, Veronica Perico, Alessandra Bosetti, and et al. 2022. "Immunonutrition and SARS-CoV-2 Infection in Children with Obesity" Nutrients 14, no. 9: 1701. https://doi.org/10.3390/nu14091701
APA StyleD’Auria, E., Calcaterra, V., Verduci, E., Ghezzi, M., Lamberti, R., Vizzuso, S., Baldassarre, P., Pendezza, E., Perico, V., Bosetti, A., & Zuccotti, G. V. (2022). Immunonutrition and SARS-CoV-2 Infection in Children with Obesity. Nutrients, 14(9), 1701. https://doi.org/10.3390/nu14091701