The Development of the Davis Food Glycopedia—A Glycan Encyclopedia of Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Foods for Inclusion in the Glycopedia
2.2. Sources of Materials
2.3. Preparation of Food and Quality Control (QC) Samples
2.4. Monosaccharide Analysis of Food Samples
2.5. Mass Spectrometry Instrumental Analysis
2.6. Data Analysis
2.7. Assigning Food Groups to Glycopedia Foods
3. Results
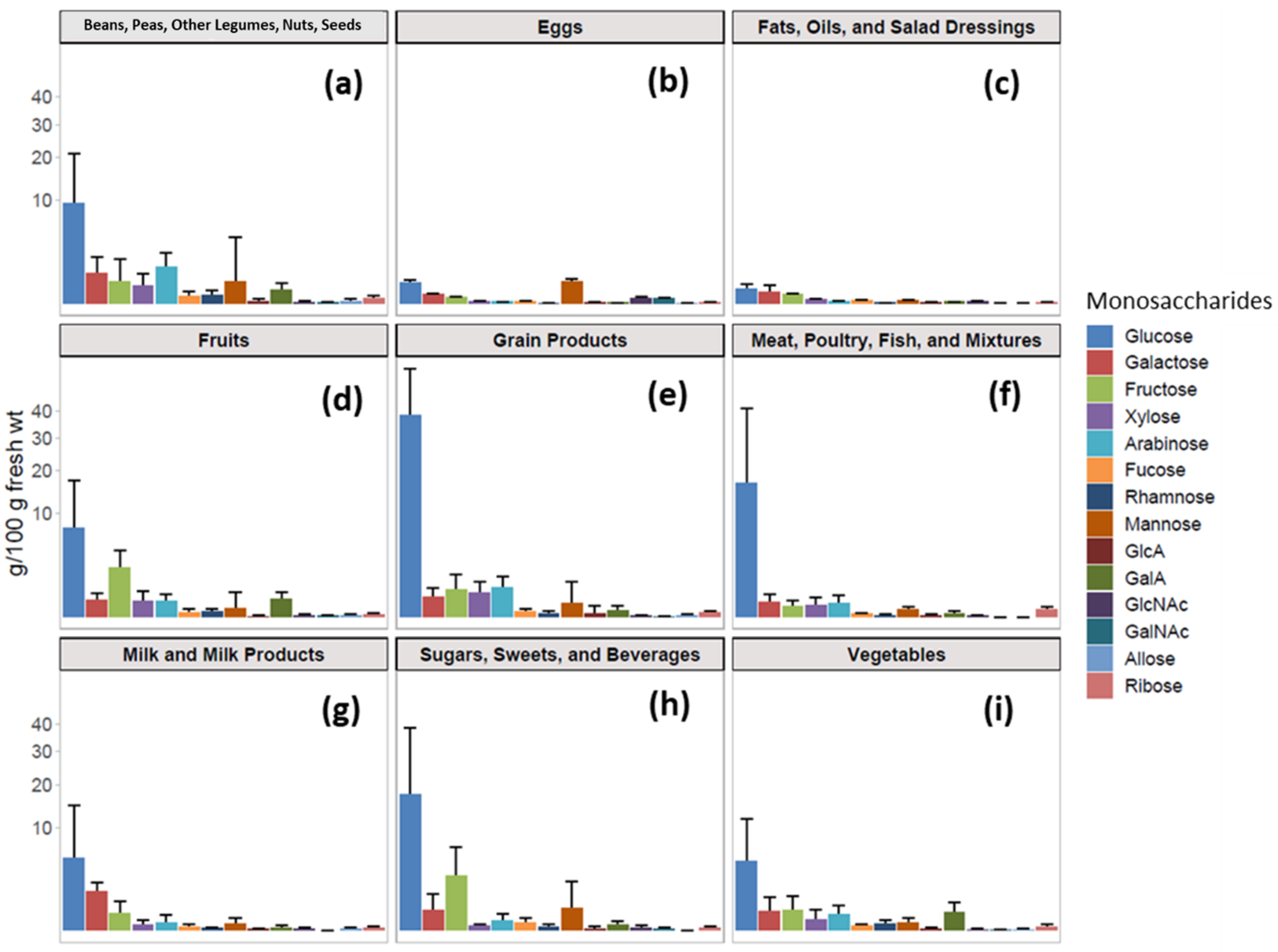
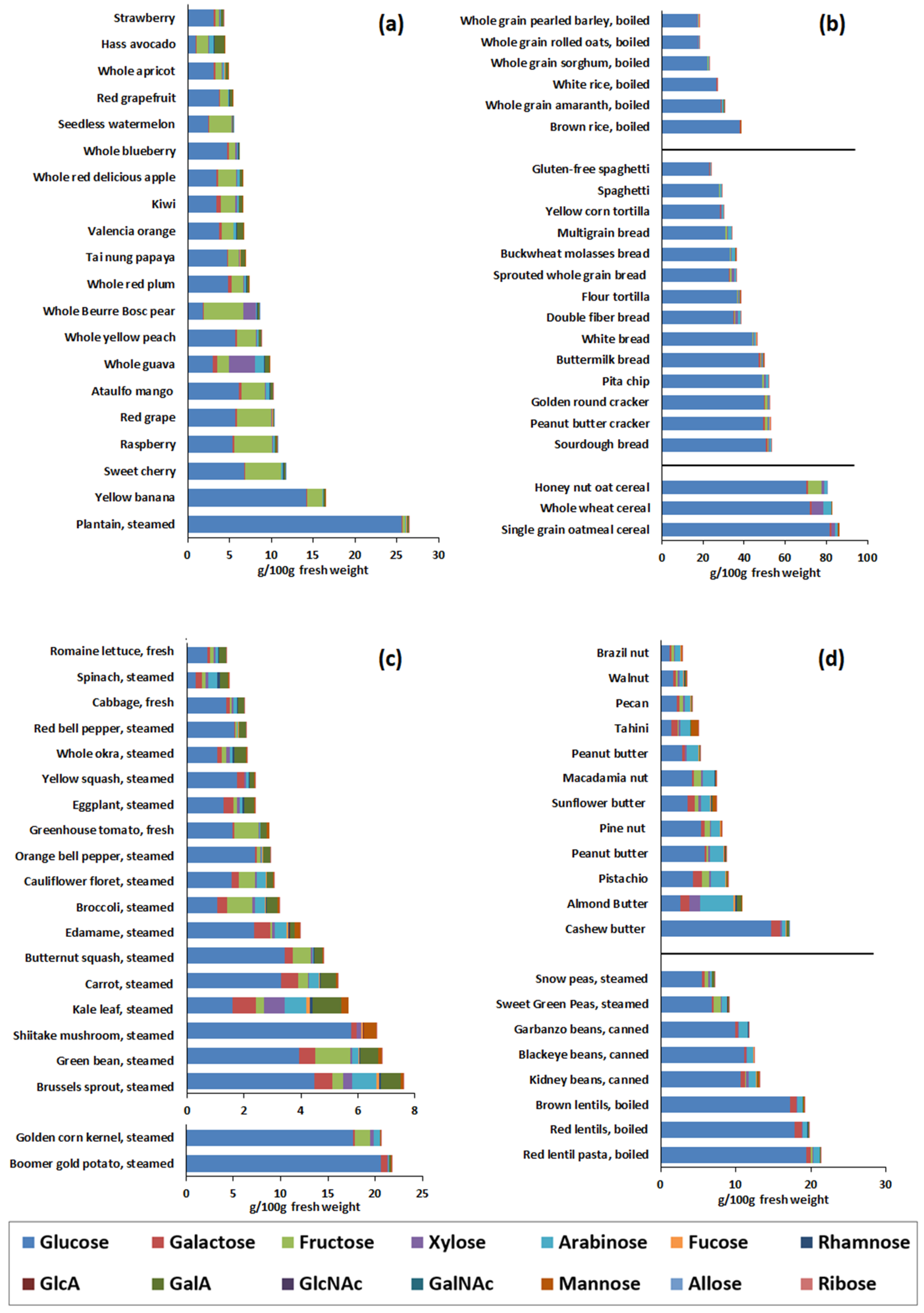
3.1. Monosaccharide Compositional Analysis of Foods
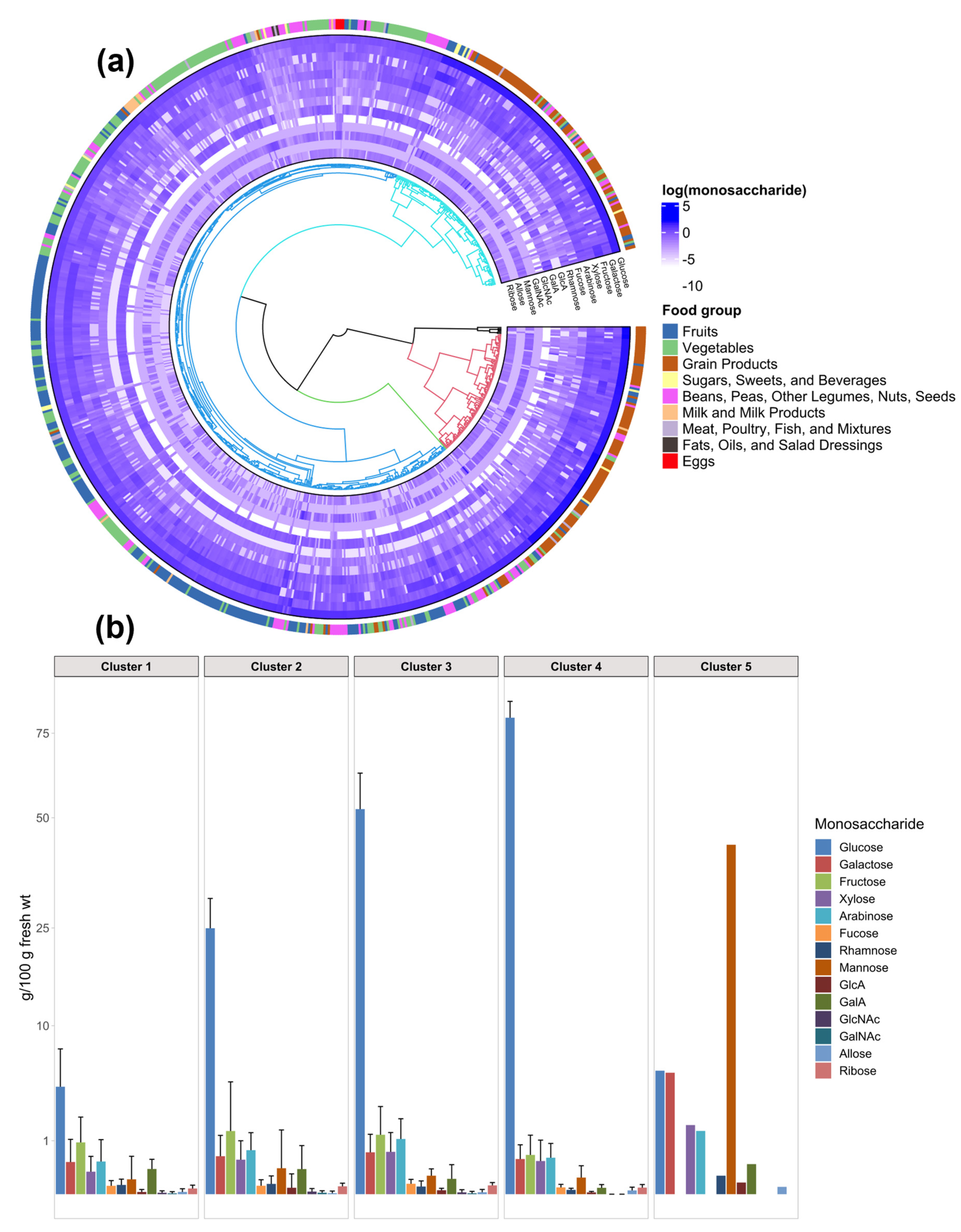
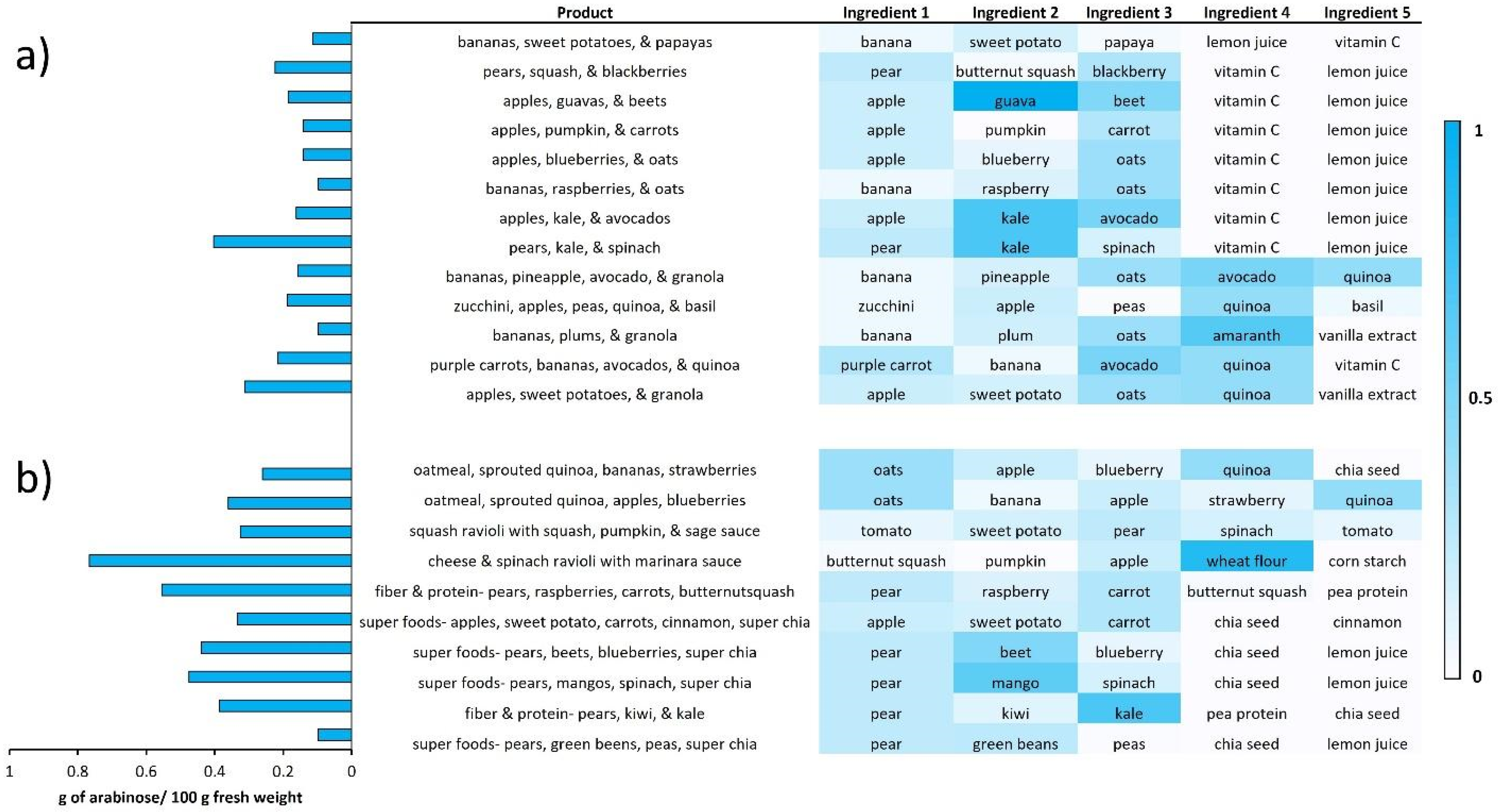
3.2. Clustering Analysis Yields New Combinations of Foods
3.3. Creation of Diets Based on Monosaccharide Compositions
3.4. Personalized Nutrition Based on Specific Monosaccharide Abundances
3.5. Processed Foods—Monosaccharide Composition in Commercial Complementary Foods
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Couteur, D.G.; Solon-Biet, S.; Wahl, D.; Cogger, V.C.; Willcox, B.J.; Willcox, D.C.; Raubenheimer, D.; Simpson, S.J. New Horizons: Dietary protein, ageing and the Okinawan ratio. Age Ageing 2016, 45, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Liu, J. Fiber consumption and all-cause, cardiovascular, and cancer mortalities: A systematic review and meta-analysis of cohort studies. Mol. Nutr. Food Res. 2015, 59, 139–146. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Amicucci, M.J.; Nandita, E.; Galermo, A.G.; Castillo, J.J.; Chen, S.; Park, D.; Smilowitz, J.T.; German, J.B.; Mills, D.A.; Lebrilla, C.B. A nonenzymatic method for cleaving polysaccharides to yield oligosaccharides for structural analysis. Nat. Commun. 2020, 11, 3963. [Google Scholar] [CrossRef]
- Phillips, K.M.; Haytowitz, D.B.; Pehrsson, P.R. Implications of two different methods for analyzing total dietary fiber in foods for food composition databases. J. Food Compos. Anal. 2019, 84, 103253. [Google Scholar] [CrossRef] [Green Version]
- Barratt, M.J.; Lebrilla, C.; Shapiro, H.Y.; Gordon, J.I. The Gut Microbiota, Food Science, and Human Nutrition: A Timely Marriage. Cell Host Microbe 2017, 22, 134–141. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Baldiviez, L.M.; Keim, N.L.; Laugero, K.D.; Hwang, D.H.; Huang, L.; Woodhouse, L.R.; Burnett, D.J.; Zerofsky, M.S.; Bonnel, E.L.; Allen, L.H.; et al. Design and implementation of a cross-sectional nutritional phenotyping study in healthy US adults. BMC Nutr. 2017, 3, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA Agricultural Research Service. What We Eat in America (WWEIA) Food Categories; USDA Agricultural Research Service: Beltsville, MD, USA, 2020.
- USDA Agricultural Research Service. Food and Nutrient Database for Dietary Studies (FNDDS) 2017–2018; USDA Agricultural Research Service: Beltsville, MD, USA, 2019.
- NIH Division of Cancer Control and Population Sciences. Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool; NIH Division of Cancer Control and Population Sciences: Bethesda, MD, USA, 2013.
- Xu, G.; Amicucci, M.J.; Cheng, Z.; Galermo, A.G.; Lebrilla, C.B. Revisiting monosaccharide analysis-quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst 2017, 143, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Amicucci, M.J.; Galermo, A.G.; Nandita, E.; Vo, T.T.T.; Liu, Y.Y.; Lee, M.; Xu, G.G.; Lebrilla, C.B. A rapid-throughput adaptable method for determining the monosaccharide composition of polysaccharides. Int. J. Mass Spectrom. 2019, 438, 22–28. [Google Scholar] [CrossRef]
- USDA Agricultural Research Service. Food and Nutrient Database for Dietary Studies (FNDDS) 2015–2016; USDA Agricultural Research Service: Beltsville, MD, USA, 2017.
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Burton, R.A.; Fincher, G.B. Evolution and development of cell walls in cereal grains. Front. Plant Sci. 2014, 5, 456. [Google Scholar] [CrossRef] [Green Version]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. Nbclust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Delannoy-Bruno, O.; Desai, C.; Raman, A.S.; Chen, R.Y.; Hibberd, M.C.; Cheng, J.Y.; Han, N.; Castillo, J.J.; Couture, G.; Lebrilla, C.B.; et al. Evaluating microbiome-directed fibre snacks in gnotobiotic mice and humans. Nature 2021, 595, 91–95. [Google Scholar] [CrossRef]
- Schutte, J.B.; Dejong, J.; Vanweerden, E.J.; Tamminga, S. Nutritional Implications of L-Arabinose in Pigs. Brit. J. Nutr. 1992, 68, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Osaki, S.; Kimura, T.; Sugimoto, T.; Hizukuri, S.; Iritani, N. L-arabinose feeding prevents increases due to dietary sucrose in lipogenic enzymes and triacylglycerol levels in rats. J. Nutr. 2001, 131, 796–799. [Google Scholar] [CrossRef]
- Marcotuli, I.; Hsieh, Y.S.; Lahnstein, J.; Yap, K.; Burton, R.A.; Blanco, A.; Fincher, G.B.; Gadaleta, A. Structural Variation and Content of Arabinoxylans in Endosperm and Bran of Durum Wheat (Triticum turgidum L.). J. Agric. Food Chem. 2016, 64, 2883–2892. [Google Scholar] [CrossRef]
- Perry, G.H.; Dominy, N.J.; Claw, K.G.; Lee, A.S.; Fiegler, H.; Redon, R.; Werner, J.; Villanea, F.A.; Mountain, J.L.; Misra, R.; et al. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 2007, 39, 1256–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides Protect the Intestinal Barrier by Maintaining the Tight Junction Network and Modulating the Inflammatory Responses after a Challenge with the Mycotoxin Deoxynivalenol in Human Caco-2 Cell Monolayers and B6C3F1 Mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natividad, J.M.; Rytz, A.; Keddani, S.; Bergonzelli, G.; Garcia-Rodenas, C.L. Blends of Human Milk Oligosaccharides Confer Intestinal Epithelial Barrier Protection in Vitro. Nutrients 2020, 12, 3047. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Li, S.; Chang, L.; Sun, P.; Lu, Y.; Yu, X.; Chen, S.; Wu, Z.; Xu, Z.; et al. Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-alpha induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019, 10, 2906–2913. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhang, J.W.; Zhong, X.Q.; Wei, J.A.; Han, L. Total polysaccharides of the Sijunzi decoction attenuate tumor necrosis factor-alpha-induced damage to the barrier function of a Caco-2 cell monolayer via the nuclear factor-kappaB-myosin light chain kinase-myosin light chain pathway. World J. Gastroenterol. 2018, 24, 2867–2877. [Google Scholar] [CrossRef]
- Barnett, A.M.; Roy, N.C.; McNabb, W.C.; Cookson, A.L. Effect of a Semi-Purified Oligosaccharide-Enriched Fraction from Caprine Milk on Barrier Integrity and Mucin Production of Co-Culture Models of the Small and Large Intestinal Epithelium. Nutrients 2016, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Tungland, B.C.; Meyer, D. Nondigestible Oligo- and Polysaccharides (Dietary Fiber): Their Physiology and Role in Human Health and Food. Compr. Rev. Food Sci. Food Saf. 2006, 1, 90–109. [Google Scholar] [CrossRef]
- Gonzalez, P.S.; O’Prey, J.; Cardaci, S.; Barthet, V.J.A.; Sakamaki, J.I.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Shi, J.L.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.Y.; Huang, Q.S.; Yang, H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Well. 2017, 6, 121–130. [Google Scholar] [CrossRef]
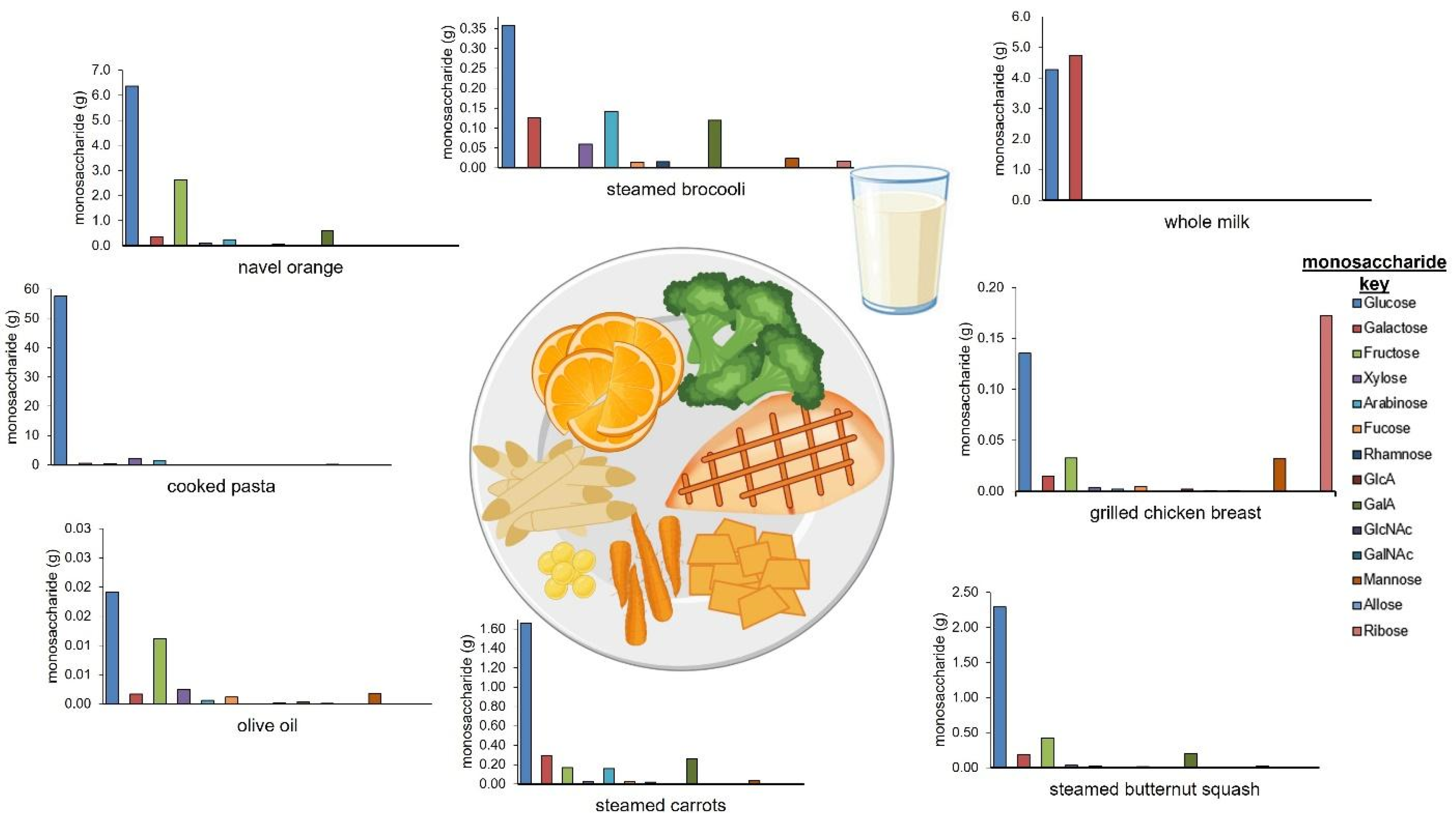

| Monosaccharide (g) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Food | Serving Amount | Amount (Grams) | Moisture (%) | Glc | Gal | Fruc | Xyl | Ara | Fuc | Rhm | GlcA | Gal A | GlcNAc | GalNAc | Man | All | Rib | Total |
| grilled chicken breast | 4 oz | 113 | 62.0 | 0.14 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.17 | 0.40 |
| steamed broccoli | 0.5 cups | 38 | 89.6 | 0.36 | 0.13 | 0.00 | 0.06 | 0.14 | 0.01 | 0.02 | 0.00 | 0.12 | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | 0.90 |
| steamed carrots | 0.33 cups | 50 | 87.1 | 1.66 | 0.29 | 0.17 | 0.03 | 0.16 | 0.02 | 0.02 | 0.00 | 0.26 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 2.70 |
| steamed butternut squash | 0.33 cups | 67 | 89.6 | 2.29 | 0.19 | 0.42 | 0.03 | 0.03 | 0.01 | 0.01 | 0.00 | 0.20 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 3.20 |
| cooked pasta | 0.75 cups | 150 | 54.7 | 57.60 | 0.51 | 0.45 | 2.10 | 1.51 | 0.00 | 0.02 | 0.01 | 0.03 | 0.00 | 0.00 | 0.15 | 0.00 | 0.03 | 62.40 |
| olive oil | 1 Tbsp | 14 | 0.8 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| navel orange | 1 medium orange | 165 | 87.3 | 6.37 | 0.37 | 2.64 | 0.12 | 0.24 | 0.04 | 0.06 | 0.00 | 0.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.40 |
| whole milk | 1 cup of milk | 245 | 89.0 | 4.27 | 4.74 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.01 | 9.10 |
| total | N/A | 842 | N/A | 72.70 | 6.25 | 3.72 | 2.35 | 2.09 | 0.09 | 0.13 | 0.01 | 1.21 | 0.00 | 0.00 | 0.30 | 0.00 | 0.25 | 89.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, J.J.; Couture, G.; Bacalzo, N.P., Jr.; Chen, Y.; Chin, E.L.; Blecksmith, S.E.; Bouzid, Y.Y.; Vainberg, Y.; Masarweh, C.; Zhou, Q.; et al. The Development of the Davis Food Glycopedia—A Glycan Encyclopedia of Food. Nutrients 2022, 14, 1639. https://doi.org/10.3390/nu14081639
Castillo JJ, Couture G, Bacalzo NP Jr., Chen Y, Chin EL, Blecksmith SE, Bouzid YY, Vainberg Y, Masarweh C, Zhou Q, et al. The Development of the Davis Food Glycopedia—A Glycan Encyclopedia of Food. Nutrients. 2022; 14(8):1639. https://doi.org/10.3390/nu14081639
Chicago/Turabian StyleCastillo, Juan J., Garret Couture, Nikita P. Bacalzo, Jr., Ye Chen, Elizabeth L. Chin, Sarah E. Blecksmith, Yasmine Y. Bouzid, Yael Vainberg, Chad Masarweh, Qingwen Zhou, and et al. 2022. "The Development of the Davis Food Glycopedia—A Glycan Encyclopedia of Food" Nutrients 14, no. 8: 1639. https://doi.org/10.3390/nu14081639
APA StyleCastillo, J. J., Couture, G., Bacalzo, N. P., Jr., Chen, Y., Chin, E. L., Blecksmith, S. E., Bouzid, Y. Y., Vainberg, Y., Masarweh, C., Zhou, Q., Smilowitz, J. T., German, J. B., Mills, D. A., Lemay, D. G., & Lebrilla, C. B. (2022). The Development of the Davis Food Glycopedia—A Glycan Encyclopedia of Food. Nutrients, 14(8), 1639. https://doi.org/10.3390/nu14081639







