Associations of Maternal Consumption of Dairy Products during Pregnancy with Perinatal Fatty Acids Profile in the EDEN Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
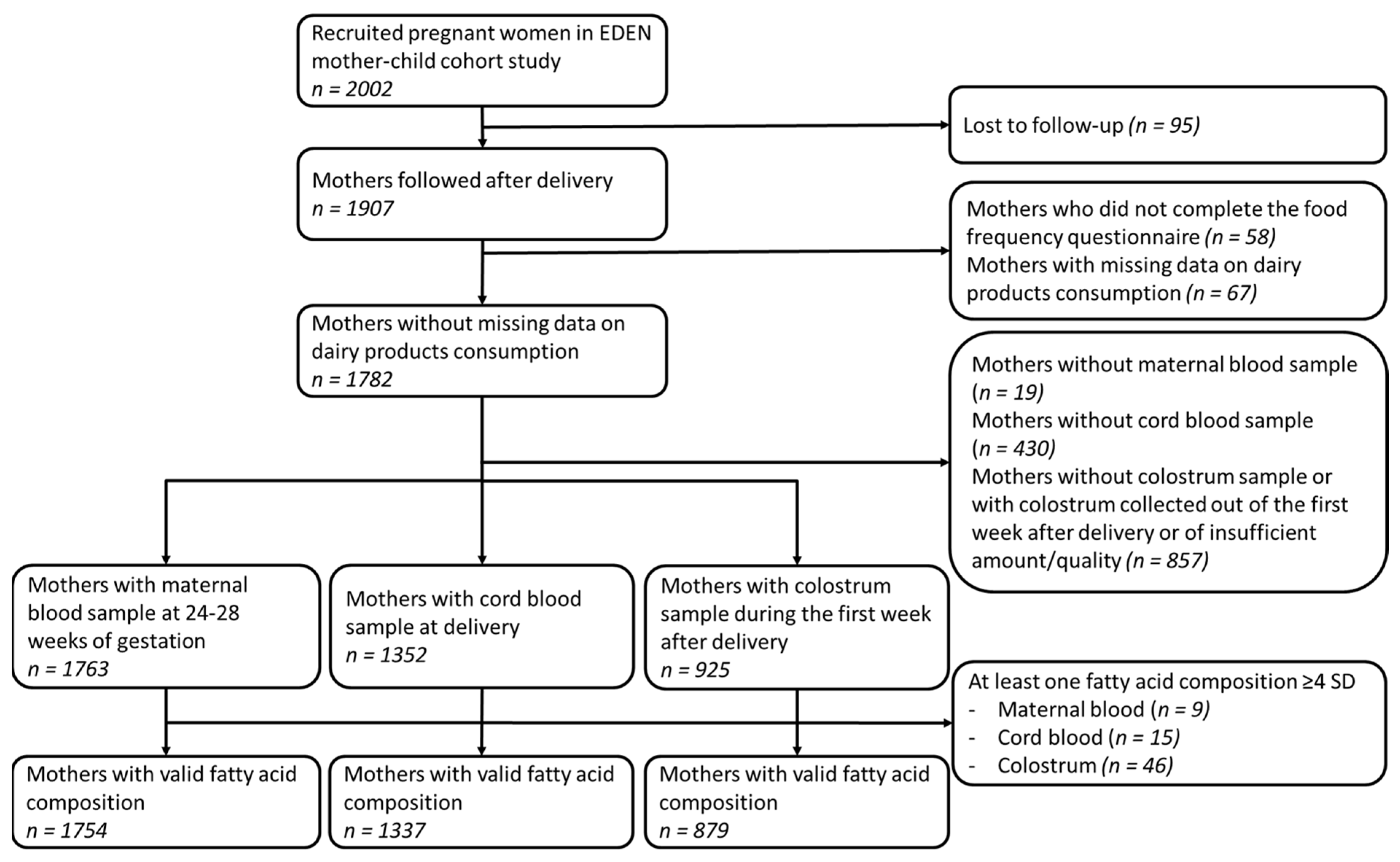
2.1. Study Population
2.2. Maternal DP Consumption
2.3. Biofluids Fatty Acids Composition Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
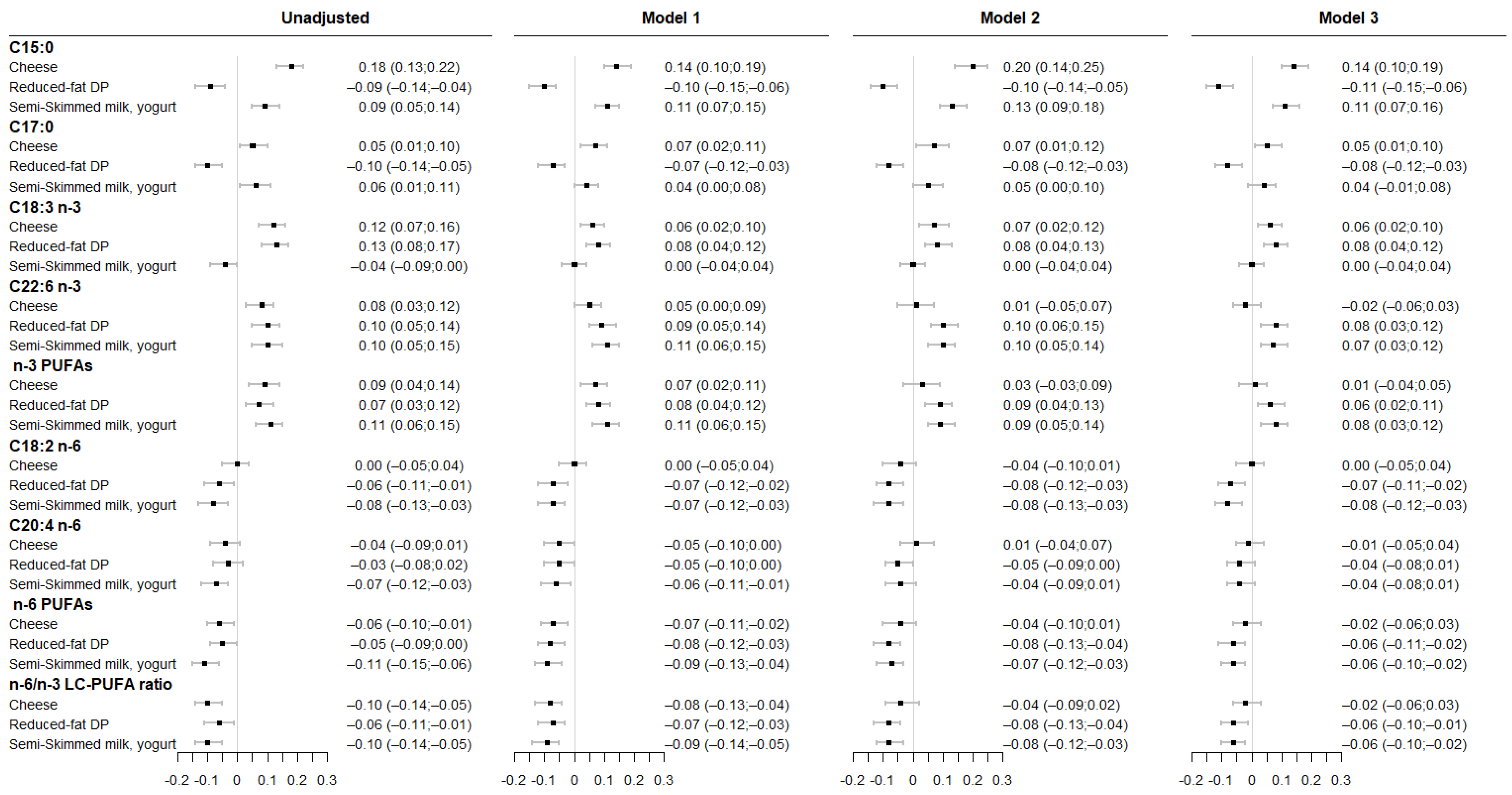
3.1. Associations between Maternal DP Consumption and Fatty Acids Levels in Maternal RBC Membrane
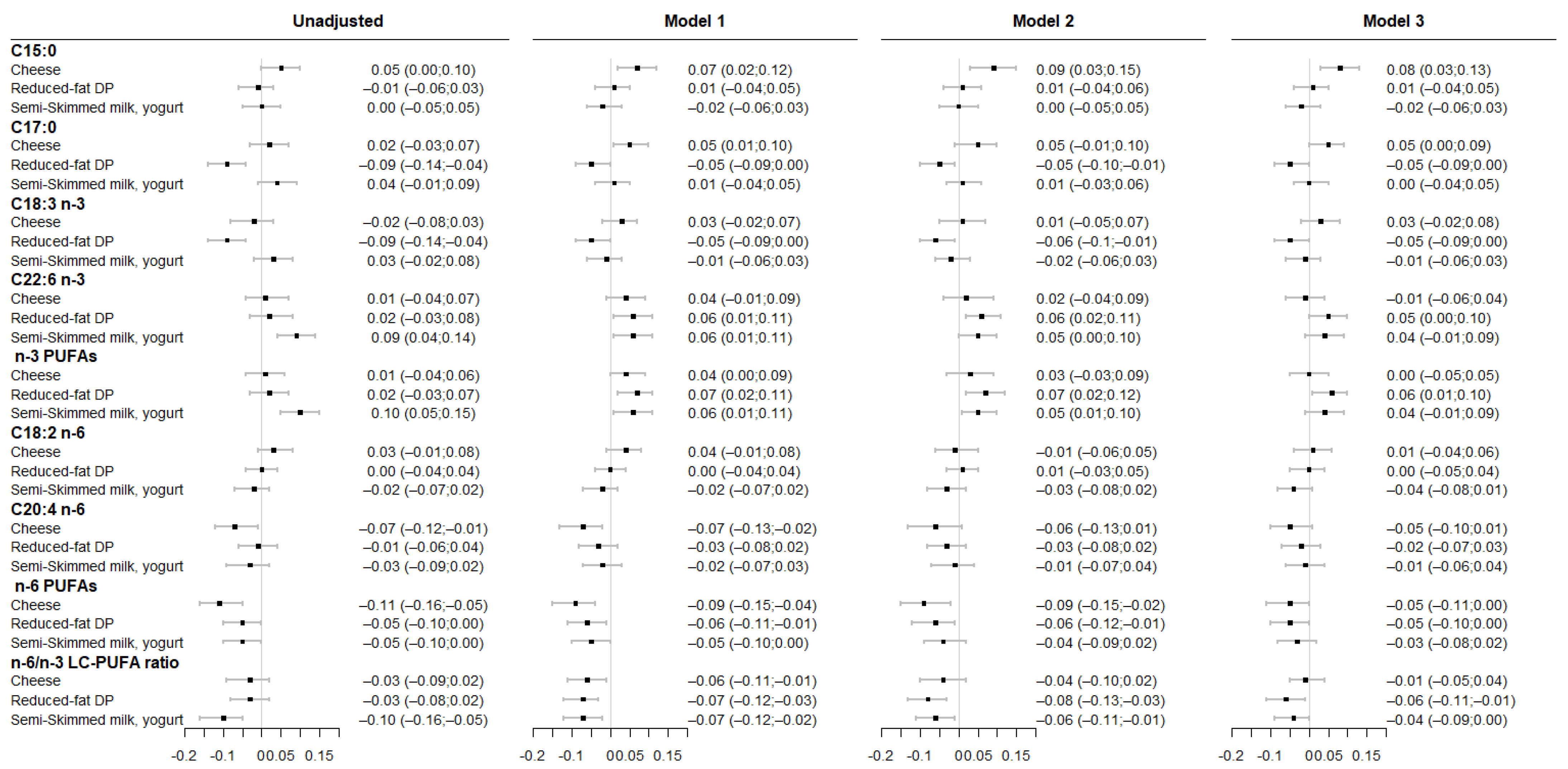
3.2. Associations between Maternal DP Patterns and Fatty Acid Levels in Cord RBC Membrane
3.3. Associations between Maternal DP Consumption and Fatty Acids Level in Colostrum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 39–103. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef] [Green Version]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.C.; Bistrian, B.R. Essential fatty acids and their derivatives. Curr. Opin. Gastroenterol. 2005, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sanchez, A.; Larque, E.; Demmelmair, H.; Acien, M.I.; Faber, F.L.; Parrilla, J.J.; Koletzko, B. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am. J. Clin. Nutr. 2010, 92, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.G. Lipids in human milk. Lipids 1999, 34, 1243–1271. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Human milk and formula fatty acids. J. Pediatr. 1992, 120, S56–S61. [Google Scholar] [CrossRef]
- Emken, E.A.; Adlof, R.O.; Rakoff, H.; Rohwedder, W.K. Metabolism of deuterium-labeled linolenic, linoleic, oleic, stearic and palmitic acid in human subjects. Agric. Res. Serv. 1988, 210, 713–716. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Armand, M.; Bernard, J.Y.; Forhan, A.; Heude, B.; Charles, M.A.; Group Em-ccs. Maternal nutritional determinants of colostrum fatty acids in the EDEN mother-child cohort. Clin. Nutr. 2018, 37, 2127–2136. [Google Scholar] [CrossRef]
- Reddy, S.; Sanders, T.A.; Obeid, O. The influence of maternal vegetarian diet on essential fatty acid status of the newborn. World Rev. Nutr. Diet. 1994, 75, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Anses. Etude Individuelle Nationale des Consommations Alimentaires 3 (INCA 3); Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail: Maisons-Alfort, France, 2017.
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Drouin, G.; Catheline, D.; Sinquin, A.; Baudry, C.; Le Ruyet, P.; Rioux, V.; Legrand, P. Incorporation of Dairy Lipids in the Diet Increased Long-Chain Omega-3 Fatty Acids Status in Post-weaning Rats. Front. Nutr. 2018, 5, 42. [Google Scholar] [CrossRef]
- Park, Y.; McGuire, M.K.; Behr, R.; McGuire, M.A.; Evans, M.A.; Shultz, T.D. High-fat dairy product consumption increases delta 9c,11t-18:2 (rumenic acid) and total lipid concentrations of human milk. Lipids 1999, 34, 543–549. [Google Scholar] [CrossRef]
- Yahvah, K.M.; Brooker, S.L.; Williams, J.E.; Settles, M.; McGuire, M.A.; McGuire, M.K. Elevated dairy fat intake in lactating women alters milk lipid and fatty acids without detectible changes in expression of genes related to lipid uptake or synthesis. Nutr. Res. 2015, 35, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.; Barman, M.; Moberg, S.; Sjoberg, A.; Brekke, H.K.; Hesselmar, B.; Johansen, S.; Wold, A.E.; Sandberg, A.S. Fat intake and breast milk fatty acid composition in farming and nonfarming women and allergy development in the offspring. Pediatr. Res. 2016, 79, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Otto, M.C.; Nettleton, J.A.; Lemaitre, R.N.; Steffen, L.M.; Kromhout, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R.; Mozaffarian, D. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the Multi-ethnic Study of Atherosclerosis. J. Am. Heart. Assoc. 2013, 2, e000092. [Google Scholar] [CrossRef] [Green Version]
- Warensjo, E.; Jansson, J.H.; Cederholm, T.; Boman, K.; Eliasson, M.; Hallmans, G.; Johansson, I.; Sjogren, P. Biomarkers of milk fat and the risk of myocardial infarction in men and women: A prospective, matched case-control study. Am. J. Clin. Nutr. 2010, 92, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Hotamisligil, G.; Tsai, M.Y.; Siscovick, D.S.; Nettleton, J.A. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2013, 97, 854–861. [Google Scholar] [CrossRef]
- Wang, D.H.; Jackson, J.R.; Twining, C.; Rudstam, L.G.; Zollweg-Horan, E.; Kraft, C.; Lawrence, P.; Kothapalli, K.; Wang, Z.; Brenna, J.T. Saturated Branched Chain, Normal Odd-Carbon-Numbered, and n-3 (Omega-3) Polyunsaturated Fatty Acids in Freshwater Fish in the Northeastern United States. J. Agric. Food Chem. 2016, 64, 7512–7519. [Google Scholar] [CrossRef]
- Allen, N.E.; Grace, P.B.; Ginn, A.; Travis, R.C.; Roddam, A.W.; Appleby, P.N.; Key, T. Phytanic acid: Measurement of plasma concentrations by gas-liquid chromatography-mass spectrometry analysis and associations with diet and other plasma fatty acids. Br. J. Nutr. 2008, 99, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; Pfeiffer, A.F.; Klaus, S. Odd-chain fatty acids as a biomarker for dietary fiber intake: A novel pathway for endogenous production from propionate. Am. J. Clin. Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertiwi, K.; Kupers, L.K.; Wanders, A.J.; de Goede, J.; Zock, P.L.; Geleijnse, J.M. Associations of dairy and fiber intake with circulating odd-chain fatty acids in post-myocardial infarction patients. Nutr. Metab. 2019, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Kornsteiner, M.; Singer, I.; Elmadfa, I. Very low n-3 long-chain polyunsaturated fatty acid status in Austrian vegetarians and vegans. Ann. Nutr. Metab. 2008, 52, 37–47. [Google Scholar] [CrossRef]
- Gozzo, S.; Di Felice, M.; Salvati, S. Ontogenetic alterations of the cerebral cortex in rat caused by a diet containing a lipid fraction extracted from yeast (Candida lipolytica) grown on N-alkanes. Int. J. Neurosci. 1981, 13, 219–227. [Google Scholar] [CrossRef]
- Gozzo, S.; Oliverio, A.; Salvati, S.; Serlupi-Crescenzi, G.; Tagliamonte, B.; Tomassi, G. Effects of dietary phospholipids and odd-chain fatty acids on the behavioural maturation of mice. Food Chem. Toxicol. 1982, 20, 153–157. [Google Scholar] [CrossRef]
- Heude, B.; Forhan, A.; Slama, R.; Douhaud, L.; Bedel, S.; Saurel-Cubizolles, M.J.; Hankard, R.; Thiebaugeorges, O.; De Agostini, M.; Annesi-Maesano, I.; et al. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int. J. Epidemiol. 2016, 45, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, V.; de Lauzon-Guillain, B.; Lafay, L.; Borys, J.M.; Charles, M.A.; Romon, M. Reproducibility and relative validity of a food-frequency questionnaire among French adults and adolescents. Eur. J. Clin. Nutr. 2009, 63, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Hercberg, S.; Deheeger, M.; Preziosi, P. SU.VI.MAX. Portions Alimentaires: Manuel Photos Pour L’estimation des Quantités; Polytechnica Edn: Paris, France, 2002. [Google Scholar]
- Garbi, A.; Armand, M.; Beltran-Anzola, A.-A.; Sarté, C.; Brévaut-Malaty, V.; Tosello, B.; Gire, C. Effect of Massage with Oil Balanced in Essential Fatty Acids on Development and Lipid Parameters in Very Premature Neonates: A Randomized, Controlled Study. Children 2022, 9, 463. [Google Scholar] [CrossRef]
- Yuan, W.L.; Nicklaus, S.; Lioret, S.; Lange, C.; Forhan, A.; Heude, B.; Charles, M.A.; de Lauzon-Guillain, B. Early factors related to carbohydrate and fat intake at 8 and 12 months: Results from the EDEN mother-child cohort. Eur. J. Clin. Nutr. 2017, 71, 219–226. [Google Scholar] [CrossRef]
- Warensjo, E.; Ohrvall, M.; Vessby, B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Folsom, A.R.; Zheng, Z.J.; Pankow, J.S.; Eckfeldt, J.H.; Investigators, A.S. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokor, S.; Koletzko, B.; Decsi, T. Systematic review of fatty acid composition of human milk from mothers of preterm compared to full-term infants. Ann. Nutr. Metab. 2007, 51, 550–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobinski, R.; Mikulska, M.; Mojska, H.; Simon, M. Comparison of the fatty acid composition of maternal blood and cord blood of mothers who delivered healthy full-term babies, preterm babies, and full-term small for gestational age infants. J. Matern. Fetal. Neonatal. Med. 2013, 26, 96–102. [Google Scholar] [CrossRef]
- Yuan, W.L.; Bernard, J.Y.; Armand, M.; Sarté, C.; Charles, M.A.; Heude, B. Associations between Maternal Diet during Pregnancy and Fatty Acids Composition in Maternal Blood, Cord Blood and Colostrum Samples. Available online: https://osf.io/cb8ud/ (accessed on 31 January 2022).
- Krachler, B.; Norberg, M.; Eriksson, J.W.; Hallmans, G.; Johansson, I.; Vessby, B.; Weinehall, L.; Lindahl, B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 503–510. [Google Scholar] [CrossRef]
- Zhu, Y.; Tsai, M.Y.; Sun, Q.; Hinkle, S.N.; Rawal, S.; Mendola, P.; Ferrara, A.; Albert, P.S.; Zhang, C. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am. J. Clin. Nutr. 2018, 107, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; Fretts, A.; Marklund, M.; Ardisson Korat, A.V.; Yang, W.S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.A.; Wong, K.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.T.; Johnson, S.B.; Kokmen, E. Deficiencies of polyunsaturated fatty acids and replacement by nonessential fatty acids in plasma lipids in multiple sclerosis. Proc. Natl. Acad. Sci. USA 1989, 86, 4720–4724. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.T.; Adams, C.E.; Nelson, R.A.; Grater, S.J.; Jaskiewicz, J.A.; Johnson, S.B.; Erdman, J.W., Jr. Patients with anorexia nervosa demonstrate deficiencies of selected essential fatty acids, compensatory changes in nonessential fatty acids and decreased fluidity of plasma lipids. J. Nutr. 1995, 125, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Cipolla, M.; Chiang, J.; Arakaki, X.; Harrington, M.G. Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PLoS ONE 2014, 9, e100519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Ma, J.; Campos, H.; Hu, F.B. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am. J. Clin. Nutr. 2007, 86, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevik, A.; Veierod, M.B.; Drevon, C.A.; Andersen, L.F. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur. J. Clin. Nutr. 2005, 59, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Golley, R.K.; Hendrie, G.A. Evaluation of the relative concentration of serum fatty acids C14:0, C15:0 and C17:0 as markers of children’s dairy fat intake. Ann. Nutr. Metab. 2014, 65, 310–316. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. [Google Scholar] [CrossRef]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot Essent Fat. Acids 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Rudolph, M.C.; Neville, M.C.; Anderson, S.M. Lipid synthesis in lactation: Diet and the fatty acid switch. J. Mammary Gland Biol. Neoplasia 2007, 12, 269–281. [Google Scholar] [CrossRef]
- Steffen, B.T.; Steffen, L.M.; Tracy, R.; Siscovick, D.; Jacobs, D.; Liu, K.; He, K.; Hanson, N.Q.; Nettleton, J.A.; Tsai, M.Y. Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the Multi-Ethnic Study of Atherosclerosis (MESA). Eur. J. Clin. Nutr. 2012, 66, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, J.Y.; Armand, M.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; Group EM-CCS. The association between linoleic acid levels in colostrum and child cognition at 2 and 3 y in the EDEN cohort. Pediatr. Res. 2015, 77, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; Group EM-CCS. Breastfeeding, Polyunsaturated Fatty Acid Levels in Colostrum and Child Intelligence Quotient at Age 5–6 Years. J. Pediatr. 2017, 183, 43–50.e43. [Google Scholar] [CrossRef] [PubMed]

| Emmental, Gruyère, Comté, Beaufort in pieces | 0.67 | 0.03 | −0.11 |
| Bonbel®, Babybel®, Gouda, Edam, Cantal, Tommes, Saint-nectaire, Reblochon | 0.67 | 0.10 | −0.20 |
| Brie, camembert, pont-l’évêque, Munster, Vacherin, Saint-marcellin, Caprice des Dieux® | 0.58 | −0.02 | −0.26 |
| Emmental, Gruyère, Comté, Beaufort grated | 0.49 | −0.15 | 0.08 |
| Goat cheese | 0.49 | −0.07 | −0.27 |
| Roquefort, blue cheese | 0.47 | −0.12 | −0.19 |
| Cottage cheese such as Tartare® or Kiri® | 0.42 | 0.20 | 0.14 |
| Strained yogurt 0% fat | 0.18 | 0.60 | 0.17 |
| Reduced-fat butter | 0.07 | 0.59 | 0.05 |
| Reduced-fat cream | 0.15 | 0.40 | 0.14 |
| Skimmed milk | −0.02 | 0.38 | −0.29 |
| Full-fat milk | 0.12 | −0.16 | −0.15 |
| Cream | 0.23 | −0.52 | 0.11 |
| Butter (added to a dish) | 0.24 | −0.54 | 0.26 |
| Strained yogurt 20%, 40% fat | 0.32 | 0.16 | 0.57 |
| Yogurts (plain, flavoured, with fruits) | 0.34 | 0.06 | 0.48 |
| Semi-skimmed milk | 0.01 | −0.14 | 0.47 |
| % explained variance | 15 | 10 | 8 |
| Component label | “Cheese” | “Reduced-fat DP” | “Semi-skimmed milk, yogurt” |
| Study Populations | ||||
|---|---|---|---|---|
| EDEN Full Cohort (n = 2002) | Maternal Blood (n = 1754) | Cord Blood (n = 1337) | Colostrum (n = 879) | |
| Age at delivery (years) | 29 (5) | 29 (5) | 29 (5) | 29 (5) |
| Educational attainment,% | ||||
| <high school diploma | 29 (549) | 27 (478) | 27 (357) | 22 (189) |
| high school diploma | 18 (340) | 18 (314) | 17 (232) | 15 (135) |
| 2-year university degree | 22 (414) | 22 (384) | 23 (305) | 25 (214) |
| >2-year university degree | 32 (607) | 32 (565) | 33 (432) | 38 (334) |
| Monthly household income, % | ||||
| <1500 € | 17 (327) | 16 (287) | 17 (225) | 13 (111) |
| 1500–2300 € | 30 (568) | 29 (506) | 29 (390) | 27 (240) |
| 2301–3000 € | 26 (501) | 27 (466) | 27 (354) | 27 (237) |
| >3000 € | 27 (517) | 28 (485) | 27 (358) | 33 (285) |
| BMI before pregnancy (kg/m2), % | ||||
| <18.5 | 9 (161) | 8 (146) | 9 (120) | 10 (83) |
| 18.5–24.9 | 65 (1227) | 66 (1134) | 66 (866) | 70 (602) |
| 25.0–29.9 | 18 (330) | 17 (298) | 17 (221) | 14 (120) |
| ≥30.0 | 9 (166) | 9 (149) | 8 (103) | 7 (61) |
| Gestational diabetes, % | 6 (123) | 6 (110) | 6 (85) | 6 (49) |
| Hypertensive disorders during pregnancy, % | ||||
| Gestational hypertension | 3 (56) | 3 (51) | 3 (36) | 3 (30) |
| Preeclampsia | 2 (40) | 2 (37) | 2 (26) | 1 (12) |
| Smoking during pregnancy, % | 26 (484) | 26 (450) | 27 (351) | 23 (199) |
| Any breastfeeding duration (months), % | ||||
| Never | 27 (514) | 27 (477) | 29 (384) | 0 (1) |
| <3 | 27 (502) | 27 (464) | 26 (346) | 34 (296) |
| 3–5 | 23 (435) | 23 (398) | 23 (301) | 32 (284) |
| ≥6 | 23 (440) | 23 (410) | 23 (303) | 34 (294) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.L.; Bernard, J.Y.; Armand, M.; Sarté, C.; Charles, M.A.; Heude, B. Associations of Maternal Consumption of Dairy Products during Pregnancy with Perinatal Fatty Acids Profile in the EDEN Cohort Study. Nutrients 2022, 14, 1636. https://doi.org/10.3390/nu14081636
Yuan WL, Bernard JY, Armand M, Sarté C, Charles MA, Heude B. Associations of Maternal Consumption of Dairy Products during Pregnancy with Perinatal Fatty Acids Profile in the EDEN Cohort Study. Nutrients. 2022; 14(8):1636. https://doi.org/10.3390/nu14081636
Chicago/Turabian StyleYuan, Wen Lun, Jonathan Y. Bernard, Martine Armand, Catherine Sarté, Marie Aline Charles, and Barbara Heude. 2022. "Associations of Maternal Consumption of Dairy Products during Pregnancy with Perinatal Fatty Acids Profile in the EDEN Cohort Study" Nutrients 14, no. 8: 1636. https://doi.org/10.3390/nu14081636
APA StyleYuan, W. L., Bernard, J. Y., Armand, M., Sarté, C., Charles, M. A., & Heude, B. (2022). Associations of Maternal Consumption of Dairy Products during Pregnancy with Perinatal Fatty Acids Profile in the EDEN Cohort Study. Nutrients, 14(8), 1636. https://doi.org/10.3390/nu14081636






