Changes in Parenteral Nutrition Requirements and BMI in Patients with Parenteral Nutrition-Dependent Short Bowel Syndrome after Stopping Teduglutide—9 Years of Follow-Up
Abstract
:1. Introduction
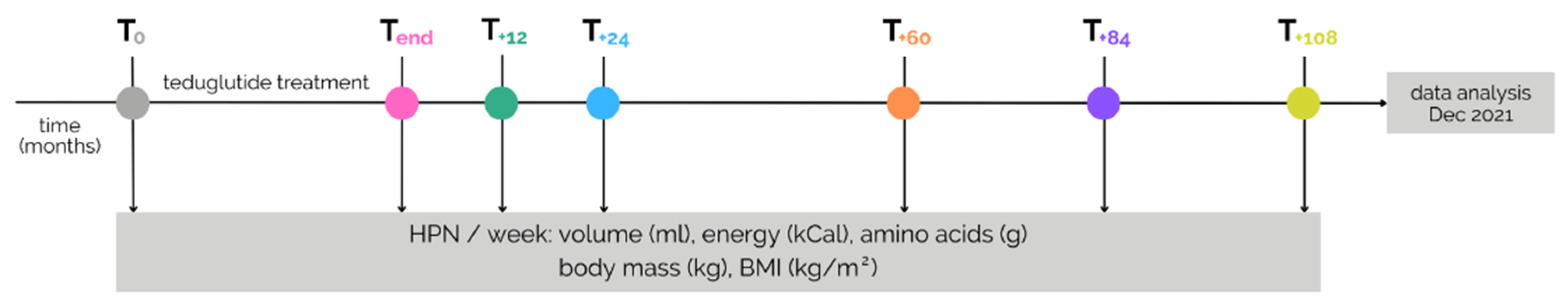
2. Materials and Methods
2.1. Patient Population
2.2. Data Collection
2.3. Study Design and Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Group Characteristics
Predrug Values and Drug Response
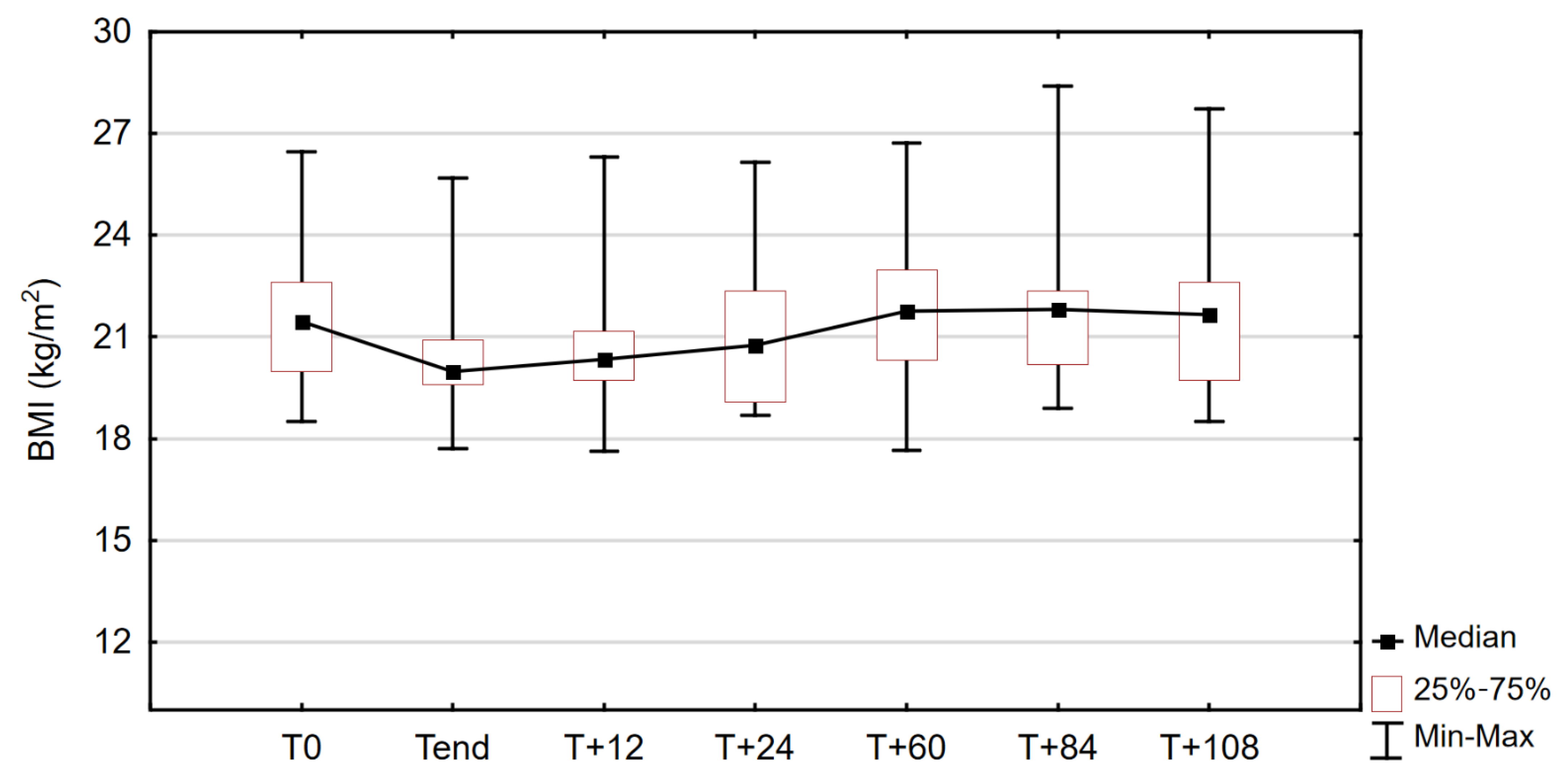
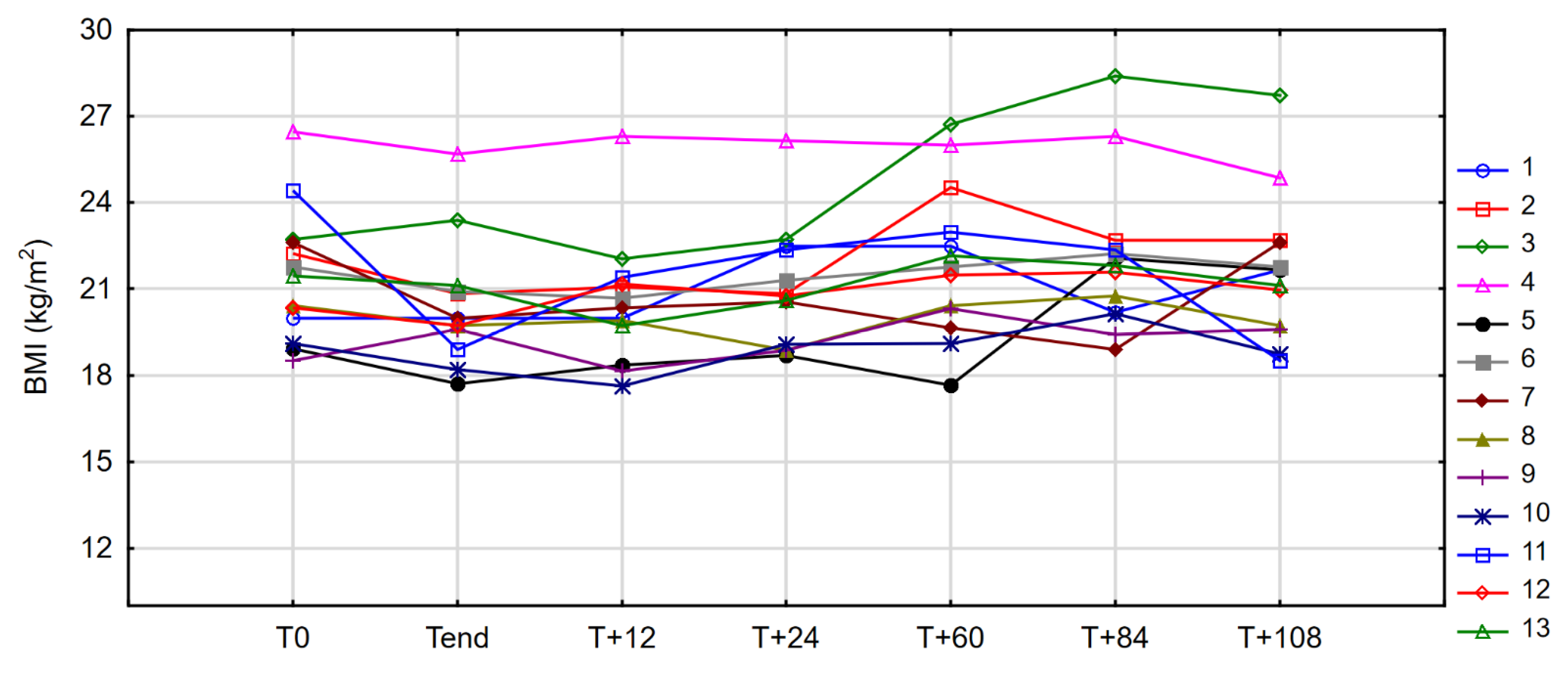
3.2. Anthropometric Measures
3.3. Parenteral Nutrition Requirements
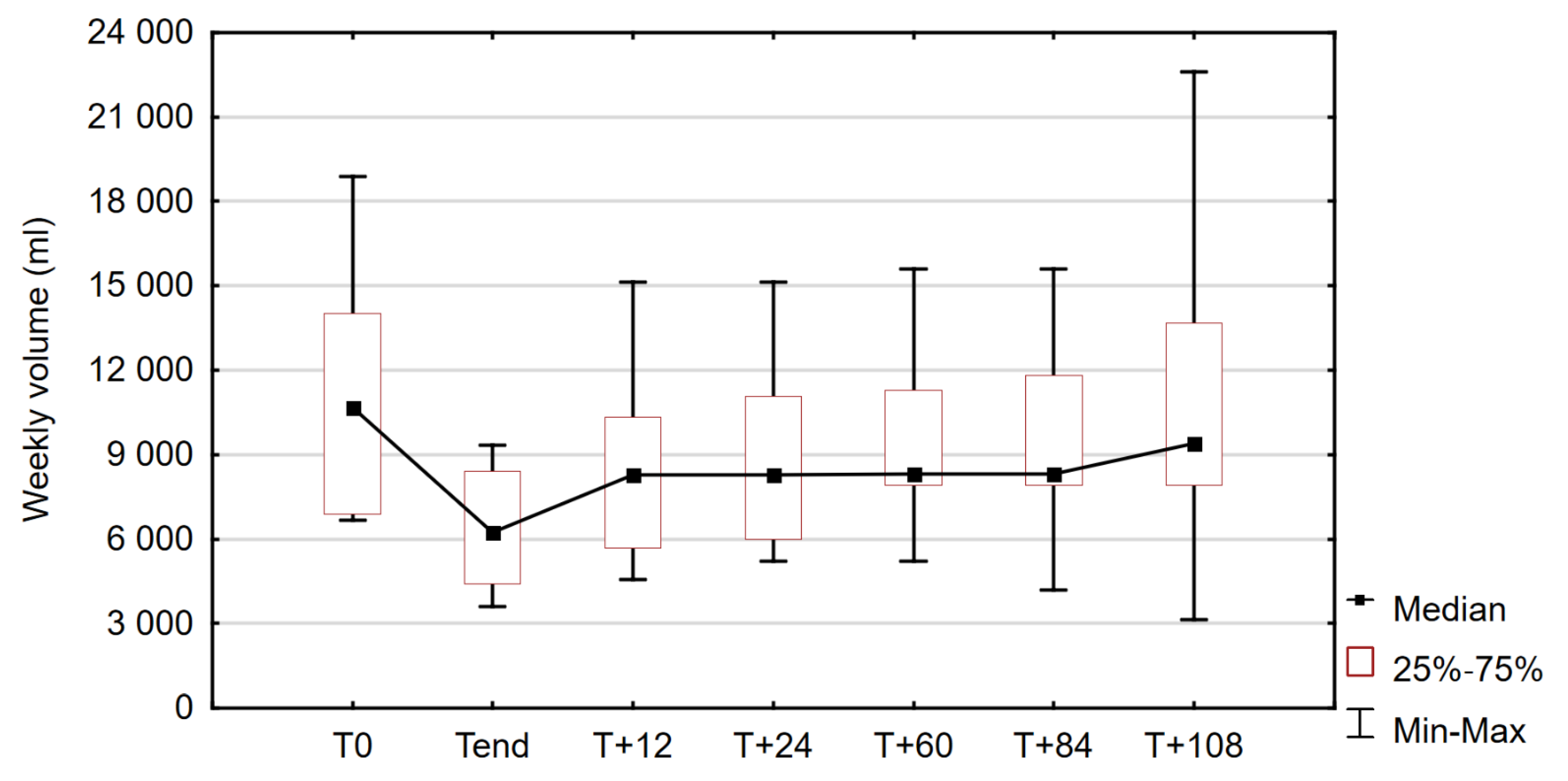
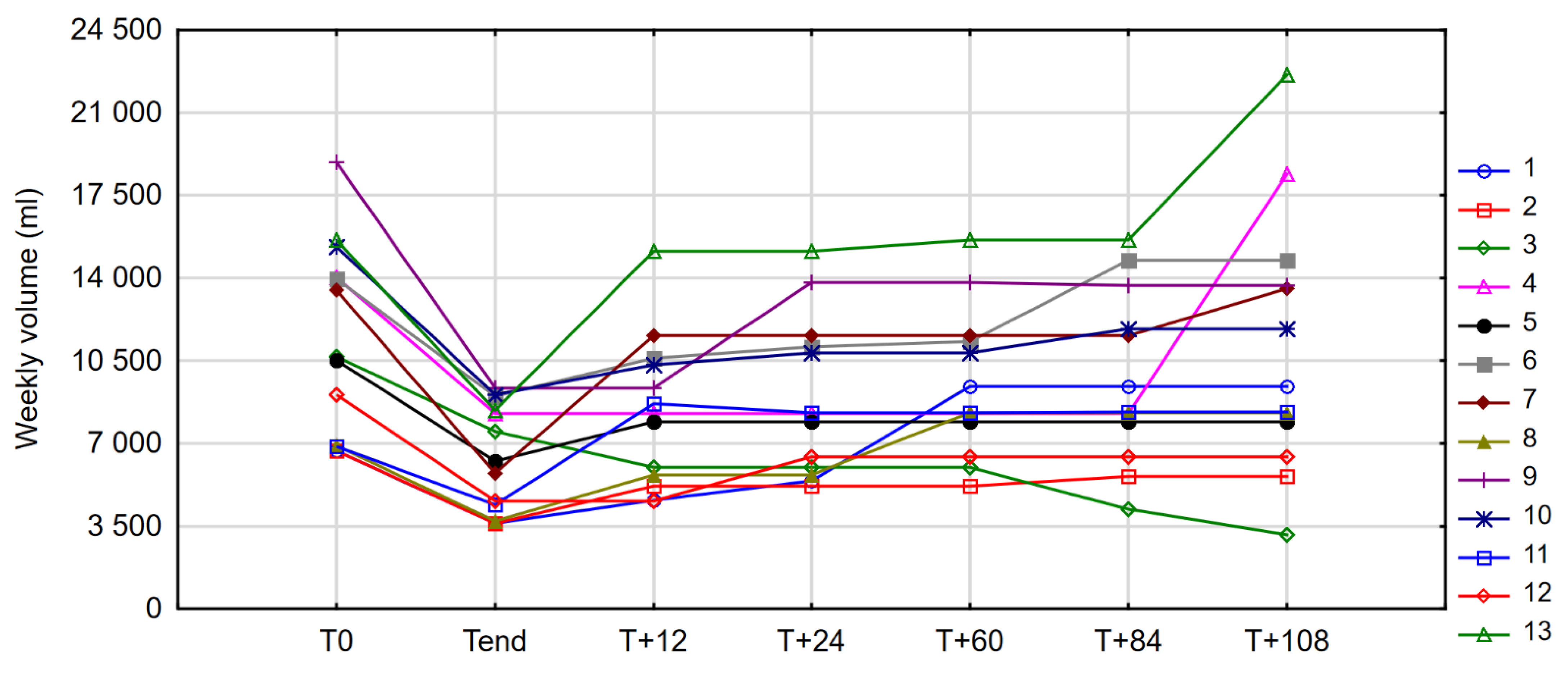
3.3.1. PN Volume
3.3.2. PN Energy and Amino Acid Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Keefe, S.J.; Buchman, A.L.; Fishbein, T.M.; Jeejeebhoy, K.N.; Jeppesen, P.B.; Shaffer, J. Short bowel syndrome and intestinal failure: Consensus definitions and overview. Clin. Gastroenterol. Hepatol. 2006, 4, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Spectrum of short bowel syndrome in adults: Intestinal insufficiency to intestinal failure. JPEN J. Parenter. Enter. Nutr. 2014, 38, 8S–13S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [Green Version]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Amiot, A.; Messing, B.; Corcos, O.; Panis, Y.; Joly, F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin. Nutr. 2013, 32, 368–374. [Google Scholar] [CrossRef]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Mühlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN guideline on home parenteral nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef]
- Cuerda, C.; Pironi, L.; Arends, J.; Bozzetti, F.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin. Nutr. 2021, 40, 5196–5220. [Google Scholar] [CrossRef]
- Noelting, J.; Gramlich, L.; Whittaker, S.; Armstrong, D.; Marliss, E.; Jurewitsch, B.; Raman, M.; Duerksen, D.R.; Stevenson, D.; Lou, W.; et al. Survival of Patients with Short-Bowel Syndrome on Home Parenteral Nutrition: A Prospective Cohort Study. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1083–1088. [Google Scholar] [CrossRef]
- Buchman, A.L. Intestinal Failure and Rehabilitation. Gastroenterol. Clin. N. Am. 2018, 47, 327–340. [Google Scholar] [CrossRef]
- Rubin, D.C.; Levin, M.S. Mechanisms of intestinal adaptation. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, B.W. The Pathogenesis of Resection-Associated Intestinal Adaptation. Cell Mol. Gastroenterol. 2016, 2, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, U.-F.; Maasberg, S.; Pascher, A. Pharmacological strategies to enhance adaptation in intestinal failure. Curr. Opin. Organ. Translant. 2016, 21, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Gut hormones in the treatment of short-bowel syndrome and intestinal failure. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Billiauws, L.; Thomas, M.; Le Beyec-Le Bihan, J.; Joly, F. Intestinal adaptation in short bowel syndrome. What is new? Nutr. Hosp. 2018, 35, 731–737. [Google Scholar] [CrossRef]
- Høyerup, P.; Hellström, P.M.; Schmidt, P.T.; Brandt, C.F.; Askov-Hansen, C.; Mortensen, P.B.; Jeppesen, P.B. Glucagon-like peptide-2 stimulates mucosal microcirculation measured by laser Doppler flowmetry in end-jejunostomy short bowel syndrome patients. Regul. Pept. 2013, 180, 12–16. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Hartmann, B.; Thulesen, J.; Graff, J.; Lohmann, J.; Hansen, B.S.; Tofteng, F.; Poulsen, S.S.; Madsen, J.L.; Holst, J.J. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 2001, 120, 806–815. [Google Scholar] [CrossRef]
- Lei, Q.; Bi, J.; Chen, H.; Tian, F.; Gao, X.; Li, N.; Wang, X. Glucagon-like peptide-2 improves intestinal immune function and diminishes bacterial translocation in a mouse model of parenteral nutrition. Nutr. Res. 2018, 49, 56–66. [Google Scholar] [CrossRef]
- Burness, C.B.; McCormack, P.L. Teduglutide: A review of its use in the treatment of patients with short bowel syndrome. Drugs 2013, 73, 935–947. [Google Scholar] [CrossRef]
- Jeppesen, P.B. Pharmacologic options for intestinal rehabilitation in patients with short bowel syndrome. JPEN J. Parenter. Enter. Nutr. 2014, 38, 45s–52s. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, P.; Gilroy, R.; Pertkiewicz, M.; Allard, J.; Messing, B.; O’keefe, S. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011, 60, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’keefe, S.J.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012, 143, 1473–1481.e1473. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Klag, T.; Wendler, J.; Bernhard, S.; Adolph, M.; Kirschniak, A.; Goetz, M.; Malek, N.; Wehkamp, J. GLP-2 analog teduglutide significantly reduces need for parenteral nutrition and stool frequency in a real-life setting. Ther. Adv. Gastroenterol. 2018, 11, 1756284818793343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, F.; Seguy, D.; Nuzzo, A.; Chambrier, C.; Beau, P.; Poullenot, F.; Thibault, R.; Armengol Debeir, L.; Layec, S.; Boehm, V.; et al. Six-month outcomes of teduglutide treatment in adult patients with short bowel syndrome with chronic intestinal failure: A real-world French observational cohort study. Clin. Nutr. 2020, 39, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Pevny, S.; Maasberg, S.; Rieger, A.; Karber, M.; Blüthner, E.; Knappe-Drzikova, B.; Thurmann, D.; Büttner, J.; Weylandt, K.-H.; Wiedenmann, B.; et al. Experience with teduglutide treatment for short bowel syndrome in clinical practice. Clin. Nutr. 2019, 38, 1745–1755. [Google Scholar] [CrossRef]
- Seidner, D.L.; Fujioka, K.; Boullata, J.I.; Iyer, K.; Lee, H.M.; Ziegler, T.R. Reduction of Parenteral Nutrition and Hydration Support and Safety with Long-Term Teduglutide Treatment in Patients With Short Bowel Syndrome-Associated Intestinal Failure: STEPS-3 Study. Nutr. Clin. Pract. 2018, 33, 520–527. [Google Scholar] [CrossRef]
- Klek, S.; Kunecki, M.; Sobocki, J.; Matysiak, K.; Karwowska, K.; Urbanowicz, K. The Polish Intestinal Failure Centres’ consensus on the use of teduglutide for the treatment of short bowel syndrome. Nutrition 2017, 38, 28–33. [Google Scholar] [CrossRef]
- Daoud, D.C.; Joly, F. The new place of enterohormones in intestinal failure. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 344–349. [Google Scholar] [CrossRef]
- Raghu, V.K.; Rudolph, J.A.; Smith, K.J. Cost-effectiveness of teduglutide in pediatric patients with short bowel syndrome: Markov modeling using traditional cost-effectiveness criteria. Am. J. Clin. Nutr. 2020, 113, 172–178. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual. 2007. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 1 March 2022).
- Compher, C.; Gilroy, R.; Pertkiewicz, M.; Ziegler, T.R.; Ratcliffe, S.J.; Joly, F.; Rochling, F.; Messing, B. Maintenance of parenteral nutrition volume reduction, without weight loss, after stopping teduglutide in a subset of patients with short bowel syndrome. JPEN J. Parenter. Enter. Nutr. 2011, 35, 603–609. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Lund, P.; Gottschalck, I.B.; Nielsen, H.B.; Holst, J.J.; Mortensen, J.; Poulsen, S.S.; Quistorff, B.; Mortensen, P.B. Short bowel patients treated for two years with glucagon-like Peptide 2: Effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol. Res. Pract. 2009, 2009, 616054. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Sanguinetti, E.L.; Buchman, A.; Howard, L.; Scolapio, J.S.; Ziegler, T.R.; Gregory, J.; Tappenden, K.A.; Holst, J.; Mortensen, P.B. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005, 54, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bioletto, F.; D’Eusebio, C.; Merlo, F.D.; Aimasso, U.; Ossola, M.; Pellegrini, M.; Ponzo, V.; Chiarotto, A.; De Francesco, A.; Ghigo, E.; et al. Efficacy of Teduglutide for Parenteral Support Reduction in Patients with Short Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 796. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.K.; O’keefe, S.J.; Fujioka, K.; Gabe, S.M.; Lamprecht, G.; Pape, U.-F.; Li, B.; Youssef, N.N.; Jeppesen, P.B. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin. Transl. Gastroenterol. 2016, 7, e142. [Google Scholar] [CrossRef] [Green Version]
- Puello, F.; Wall, E.; Herlitz, J.; Lozano, E.S.; Semrad, C.; Micic, D. Long-Term Outcomes with Teduglutide From a Single Center. JPEN J. Parenter. Enter. Nutr. 2021, 45, 318–322. [Google Scholar] [CrossRef]
- Iyer, K.R.; Kunecki, M.; Boullata, J.I.; Fujioka, K.; Joly, F.; Gabe, S.; Pape, U.F.; Schneider, S.M.; Virgili Casas, M.N.; Ziegler, T.R. Independence from parenteral nutrition and intravenous fluid support during treatment with teduglutide among patients with intestinal failure associated with short bowel syndrome. JPEN J. Parenter. Enter. Nutr. 2017, 41, 946–951. [Google Scholar] [CrossRef]
- Seidner, D.L.; Gabe, S.M.; Lee, H.M.; Olivier, C.; Jeppesen, P.B. Enteral autonomy and days off parenteral support with teduglutide treatment for short bowel syndrome in the STEPS trials. JPEN J. Parenter. Enter. Nutr. 2020, 44, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Harpain, F.; Schlager, L.; Hütterer, E.; Dawoud, C.; Kirchnawy, S.; Stift, J.; Krotka, P.; Stift, A. Teduglutide in short bowel syndrome patients: A way back to normal life? JPEN J. Parenter. Enter. Nutr. 2022, 46, 300–309. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D.; Jeppesen, P.B.; Gilroy, R.; Pertkiewicz, M.; Allard, J.P.; Messing, B. Safety and Efficacy of Teduglutide After 52 Weeks of Treatment in Patients with Short Bowel Intestinal Failure. Clin. Gastroenterol. Hepatol. 2013, 11, 815–823.e813. [Google Scholar] [CrossRef]
- Pironi, L. Translation of Evidence into Practice with Teduglutide in the Management of Adults with Intestinal Failure due to Short-Bowel Syndrome: A Review of Recent Literature. JPEN J. Parenter. Enter. Nutr. 2020, 44, 968–978. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Pertkiewicz, M.; Forbes, A.; Pironi, L.; Gabe, S.M.; Joly, F.; Messing, B.; Loth, S.; Youssef, N.N.; Heinze, H.; et al. Quality of life in patients with short bowel syndrome treated with the new glucagon-like peptide-2 analogue teduglutide–Analyses from a randomised, placebo-controlled study. Clin. Nutr. 2013, 32, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Scolapio, J.S.; Savoy, A.D.; Kaplan, J.; Burger, C.D.; Lin, S.-C. Sleep patterns of cyclic parenteral nutrition, a pilot study: Are there sleepless nights? JPEN J. Parenter. Enter. Nutr. 2002, 26, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Sobocki, J.; Zaczek, Z.; Jurczak, P.; Lachowicz, K.; Kunecki, M.; Groszek, P.; Majewska, K.; Panczyk, M.; Forbes, A. Restricted v. unrestricted oral intake in high output end-jejunostomy patients referred to reconstructive surgery. Br. J. Nutr. 2021, 125, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mu, F.; Xie, J.; Kelkar, S.S.; Olivier, C.; Signorovitch, J.; Jeppesen, P.B. Impact of teduglutide on quality of life among patients with short bowel syndrome and intestinal failure. JPEN J. Parenter. Enter. Nutr. 2020, 44, 119–128. [Google Scholar] [CrossRef] [PubMed]
| Female (n = 7) | Male (n = 6) | z | p-Value * | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Range) | IQR/2 | Median (Range) | IQR/2 | Median (Range) | IQR/2 | CV% | |||
| Age at T0 (y) | 58.0 (26.0–78.0) | 13.5 | 49.5 (25.0–73.0) | 6.0 | 0.930 | 0.366 | 52.0 (25.0–78.0) | 9.0 | 31.8 |
| Age at Tend (y) | 60.0 (28.0–80.0) | 13.5 | 52.5 (28.0–75.0) | 6.0 | 0.863 | 0.366 | 55.0 (28.0–80.0) | 8.5 | 29.9 |
| Predrug duration of HPN (mo.) | 57.0 (20.0–127.0) | 37.0 | 57.0 (20.0–161.0) | 67.0 | 0.000 | 0.945 | 57.0 (20.0–161.0) | 37.0 | 67.0 |
| Duration of TED treatment (mo.) | 30.0 (25.0–37.0) | 0.5 | 36.0 (25.0–37.0) | 3.0 | −0.878 | 0.366 | 31.0 (25.0–37.0) | 3.0 | 13.3 |
| Before Treatment (T0) | After Treatment (Tend) | z | p * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mdn | IQR/2 | Min | Max | Mdn | IQR/2 | Min | Max | |||
| PN Volume (ml/week) | 10,680 | 3557.5 | 6680 | 18,900 | 6240 | 1990 | 3600 | 9345 | 3.180 | 0.001 |
| PN Energy (kCal/week) | 6050.0 | 2265.5 | 3258.5 | 13,258.0 | 5440.0 | 1996.0 | 2700.0 | 10,122.0 | 3.180 | 0.001 |
| PN Amino acids (g/week) | 212.50 | 90.00 | 104.13 | 437.50 | 170.00 | 56.25 | 89.25 | 437.50 | 2.521 | 0.012 |
| Body weight (kg) | 54.70 | 6.25 | 47.00 | 82.00 | 51.20 | 5.60 | 45.00 | 79.60 | 2.040 | 0.041 |
| BMI (kg/m2) | 21.45 | 1.31 | 18.51 | 26.47 | 19.97 | 0.66 | 17.72 | 25.70 | 2.197 | 0.028 |
| PN Volume (mL/Week) | ||||
|---|---|---|---|---|
| Time Point | Median | IQR/2 | Min | Max |
| 12 months off drug (T+12) | 8270.00 | 2318.75 | 4575 | 15,120 |
| 24 months off drug (T+24) | 8270.00 | 2537.00 | 5200 | 15,120 |
| 60 months off drug (T+60) | 8305.00 | 1695.75 | 5200 | 15,610 |
| 84 months off drug (T+84) | 8315.00 | 1960.00 | 4200 | 15,610 |
| 108 months off drug (T+108) | 9400.00 | 2877.00 | 3150 | 22,610 |
| PN Energy (kCal/Week) | PN Amino Acids (g/Week) | |||||
|---|---|---|---|---|---|---|
| Time Point | Median | IQR/2 | Range | Median | IQR/2 | Range |
| 12 months off drug (T+12) | 5950 | 2156 | 2760–12,229 | 212.50 | 111.25 | 102–437.5 |
| 24 months off drug (T+24) | 5950 | 2156 | 3240–12,229 | 212.50 | 100.00 | 102–437.5 |
| 60 months off drug (T+60) | 6500 * | 1981 | 3240–12,712 | 250.00 | 90.00 | 102–437.5 |
| 84 months off drug (T+84) | 6500 * | 1631 | 2940–12,712 | 250.00 | 90.00 | 102–437.5 |
| 108 months off drug (T+108) | 7245 * | 1763 | 1935–13,636 | 297.50 | 93.75 | 93.75–437.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaczek, Z.; Jurczak-Kobus, P.; Panczyk, M.; Braszczyńska-Sochacka, J.; Majewska, K.; Kunecki, M.; Dąbrowska, K.; Sobocki, J. Changes in Parenteral Nutrition Requirements and BMI in Patients with Parenteral Nutrition-Dependent Short Bowel Syndrome after Stopping Teduglutide—9 Years of Follow-Up. Nutrients 2022, 14, 1634. https://doi.org/10.3390/nu14081634
Zaczek Z, Jurczak-Kobus P, Panczyk M, Braszczyńska-Sochacka J, Majewska K, Kunecki M, Dąbrowska K, Sobocki J. Changes in Parenteral Nutrition Requirements and BMI in Patients with Parenteral Nutrition-Dependent Short Bowel Syndrome after Stopping Teduglutide—9 Years of Follow-Up. Nutrients. 2022; 14(8):1634. https://doi.org/10.3390/nu14081634
Chicago/Turabian StyleZaczek, Zuzanna, Paulina Jurczak-Kobus, Mariusz Panczyk, Joanna Braszczyńska-Sochacka, Krystyna Majewska, Marek Kunecki, Karolina Dąbrowska, and Jacek Sobocki. 2022. "Changes in Parenteral Nutrition Requirements and BMI in Patients with Parenteral Nutrition-Dependent Short Bowel Syndrome after Stopping Teduglutide—9 Years of Follow-Up" Nutrients 14, no. 8: 1634. https://doi.org/10.3390/nu14081634
APA StyleZaczek, Z., Jurczak-Kobus, P., Panczyk, M., Braszczyńska-Sochacka, J., Majewska, K., Kunecki, M., Dąbrowska, K., & Sobocki, J. (2022). Changes in Parenteral Nutrition Requirements and BMI in Patients with Parenteral Nutrition-Dependent Short Bowel Syndrome after Stopping Teduglutide—9 Years of Follow-Up. Nutrients, 14(8), 1634. https://doi.org/10.3390/nu14081634








