Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities
Abstract
1. Introduction
2. Research Methodology
3. Production and Season
4. Traditional Uses of L. domesticum
5. Nutritional Value of L. domesticum
6. L. domesticum Enzymes
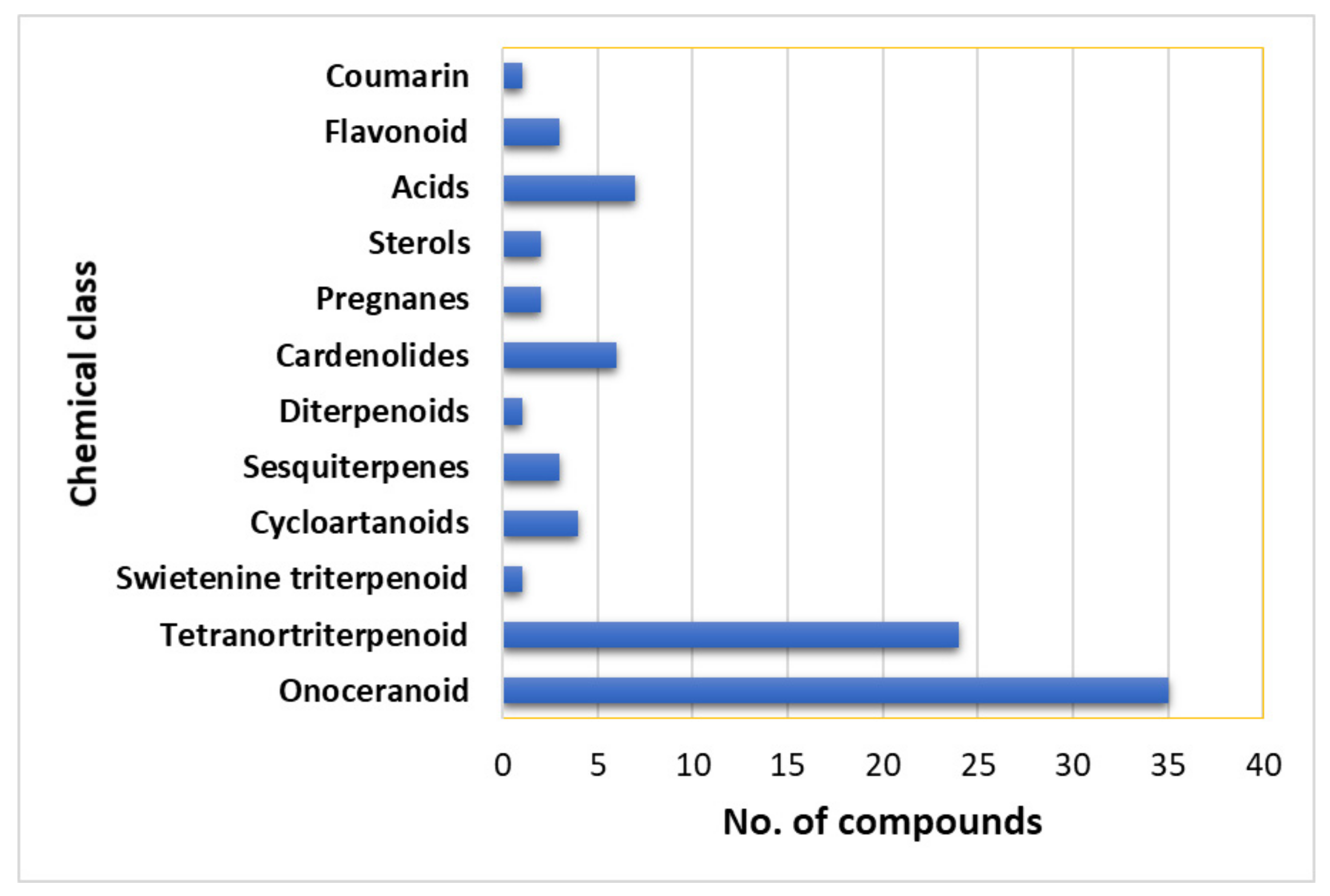
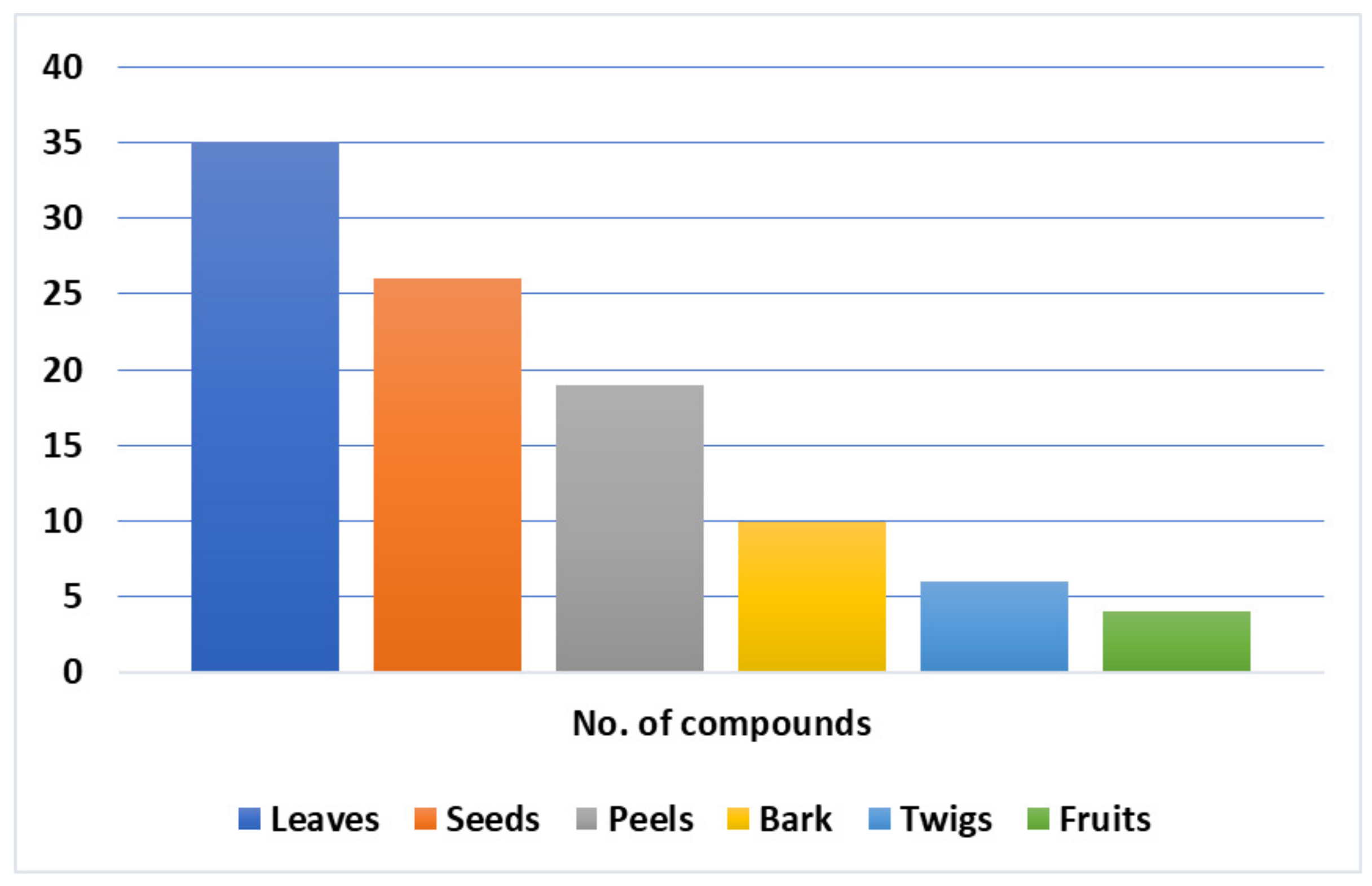
7. Phytoconstituents of L. domesticum
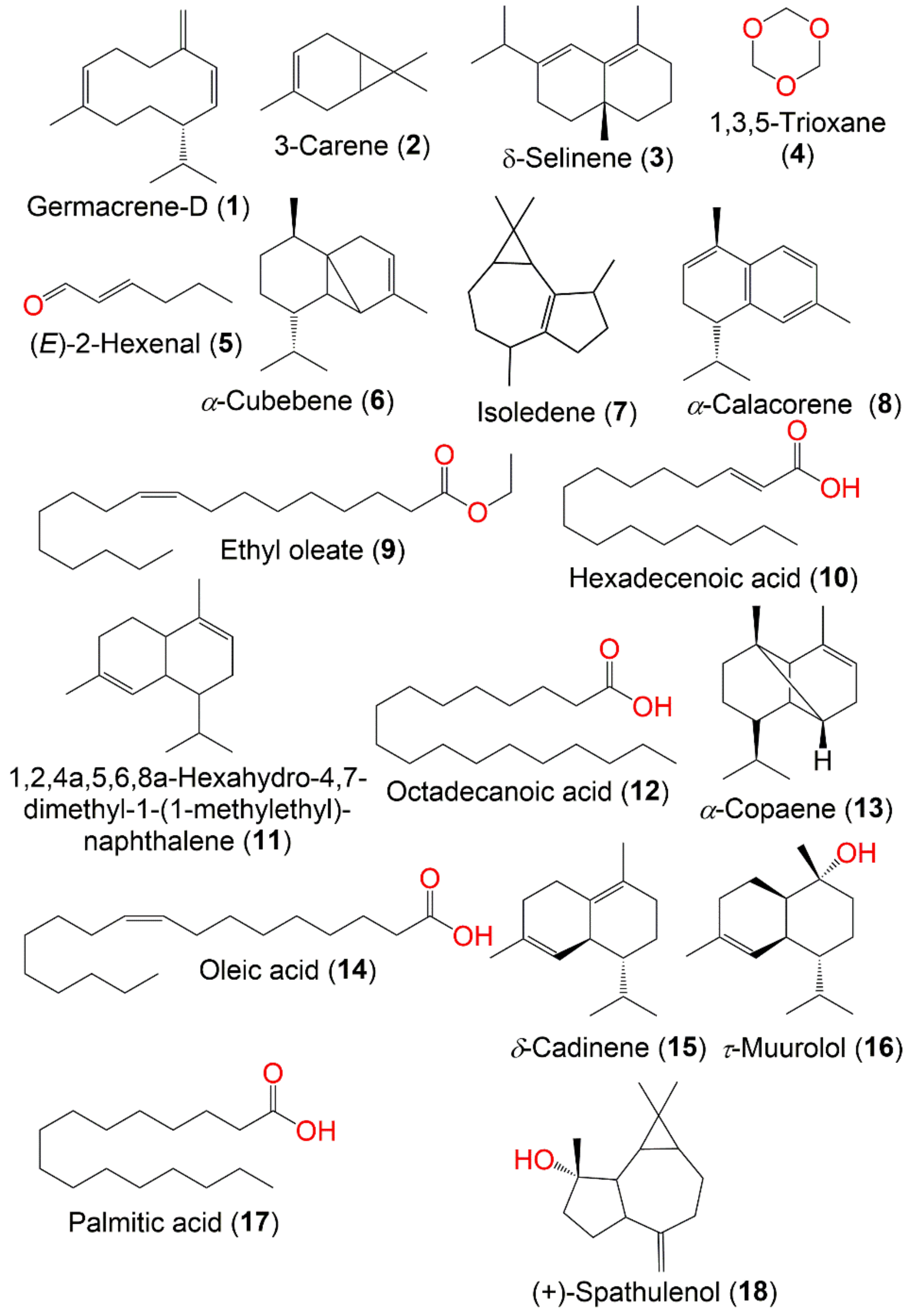
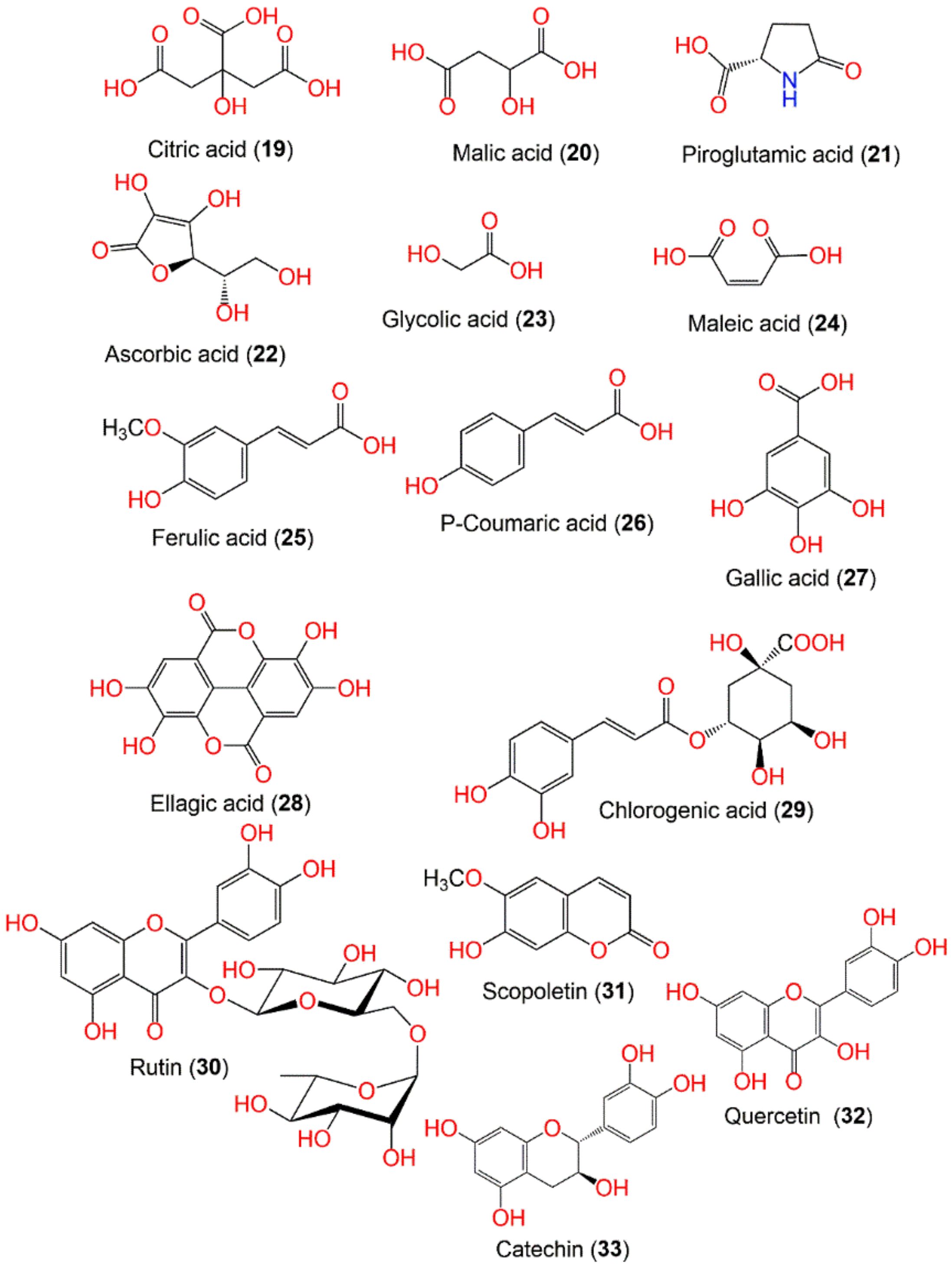
7.1. Volatile Organic Compounds and Organic Acids
| Compound Name | Chemical Class | Plant Part | Extract/ Fraction | Mol. Wt. | Mol. Formula | City, Country | Ref. |
|---|---|---|---|---|---|---|---|
| Germacrene D (1) | Sesquiterpene | Fruits | Essential oil | 204 | C15H24 | Seepoa village, Narathiwat, Thailand | [57] |
| Fruits | Essential oil | - | - | Penang, Malaysia | [11] | ||
| Seeds | CH2Cl2/acetone | 204 | C15H24 | Laguna, Philippine | [58] | ||
| 3-Carene (2) | Monterpene | Fruits | Juice | 136 | C10H16 | Eastern Thailand | [56] |
| δ-Selinene (3) | Sesquiterpene | Fruits | Juice | 204 | C15H24 | Eastern Thailand | [56] |
| 1,3,5-Trioxane (4) | Organic compound | Fruits | Juice | 90 | C3H6O3 | Eastern Thailand | [56] |
| (E)-2-Hexenal (5) | Aldehyde | Fruits | Juice | 98 | C6H10O | Eastern Thailand | [56] |
| α-Cubebene (6) | Sesquiterpene | Young fruit | CHCl3 | 204 | C15H24 | Narathiwat, Satun, and Yala, Thailand | [43] |
| Fruits | Juice | - | - | Eastern Thailand | [56] | ||
| Isoledene (7) | Sesquiterpene | Fruits | Juice | 204 | C15H24 | Eastern Thailand | [56] |
| α-Calacorene (8) | Sesquiterpene | Fruits | Juice | 200 | C15H20 | Eastern Thailand | [56] |
| Ethyl oleate (9) | Fatty acid ester | Young fruit | CHCl3 | 310 | C20H38O2 | Narathiwat, Satun, and Yala, Thailand | [43] |
| Hexadecenoic acid (10) | Fatty acid | Young fruit | CHCl3 | 254 | C16H30O2 | Narathiwat, Satun, and Yala, Thailand | [43] |
| 1,2,4a,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene (11) | Sesquiterpene | Young fruit | CHCl3 | 204 | C15H24 | Narathiwat, Satun, and Yala, Thailand | [43] |
| Octadecanoic acid (12) | Fatty acid | Young fruit | CHCl3 | 284 | C18H36O2 | Narathiwat, Satun, and Yala, Thailand | [43] |
| α-Copaene (13) | Sesquiterpene | Fruits | Essential oil | 204 | C15H24 | Seepoa village, Narathiwat, Thailand | [57] |
| Oleic acid (14) | Fatty acid | Fruits | MeOH | 282 | C18H34O2 | Seepoa village, Narathiwat, Thailand | [57] |
| δ-Cadinene (15) | Sesquiterpene | Fruits | Essential oil | 204 | C15H24 | Seepoa village, Narathiwat, Thailand | [57] |
| τ-Muurolol (16) | Sesquiterpene | Fruits | Essential oil | 222 | C15H26O | Seepoa village, Narathiwat, Thailand | [57] |
| Palmitic acid (17) | Fatty acid | Fruits | MeOH | 256 | C16H32O2 | Seepoa village, Narathiwat, Thailand | [57] |
| (+)-Spathulenol (18) | Sesquiterpene | Fruits | Essential oil | 220 | C15H24O | Seepoa village, Narathiwat, Thailand | [57] |
| Citric acid (19) | Organic acid | Fruits | H2O | 192 | C6H8O7 | North Sulawesi, Indonesia | [36] |
| Malic acid (20) | Organic acid | Fruits | H2O | 134 | C4H6O5 | North Sulawesi, Indonesia | [36] |
| Piroglutamic acid (21) | Organic acid | Fruits | H2O | 129 | C5H7NO3 | North Sulawesi, Indonesia | [36] |
| Ascorbic acid (22) | Organic acid | Fruits | H2O | 176 | C6H8O6 | North Sulawesi, Indonesia | [36] |
| Glycolic acid (23) | Organic acid | Fruits | MeOH | 76 | C2H4O3 | Seepoa village, Narathiwat province, Thailand | [57] |
| Maleic acid (24) | Organic acid | Fruits | MeOH | 116 | C4H4O4 | Seepoa village, Narathiwat province, Thailand | [57] |
| Ferulic acid (25) | Phenolic acid | Fruits | MeOH | 194 | C10H10O4 | Singapore | [59] |
| P-Coumaric acid (26) | Phenolic acid | Fruits | MeOH | 164 | C9H8O3 | Singapore | [59] |
| Gallic acid (27) | Phenolic acid | Fruits | MeOH | 170 | C7H6O5 | Singapore | [59] |
| Ellagic acid (28) | Phenolic acid | Fruits | MeOH | 302 | C14H6O8 | Singapore | [20] |
| Chlorogenic acid (29) | Phenolic acid | Fruit peels | LDSK50-EA LDSK50-H2O | 354 | C16H18O9 | Prathumthani, Thailand | [51] |
| Rutin (30) | Flavonoid | Fruit peels | LDSK50-EA LDSK50-H2O | 610 | C27H30O16 | Prathumthani, Thailand | [51] |
| Scopoletin (31) | Coumarin | Fruit peels | LDSK50-EA LDSK50-H2O | 192 | C10H8O4 | Prathumthani, Thailand | [51] |
| Quercetin (32) | Flavonoid | Fruit peels | LDSK50-EA LDSK50-H2O | 302 | C15H10O7 | Prathumthani, Thailand | [51] |
| Catechin (33) | Flavonoid | Fruit peels | LDSK50-EA LDSK50-H2O | 290 | C15H14O6 | Prathumthani, Thailand | [51] |
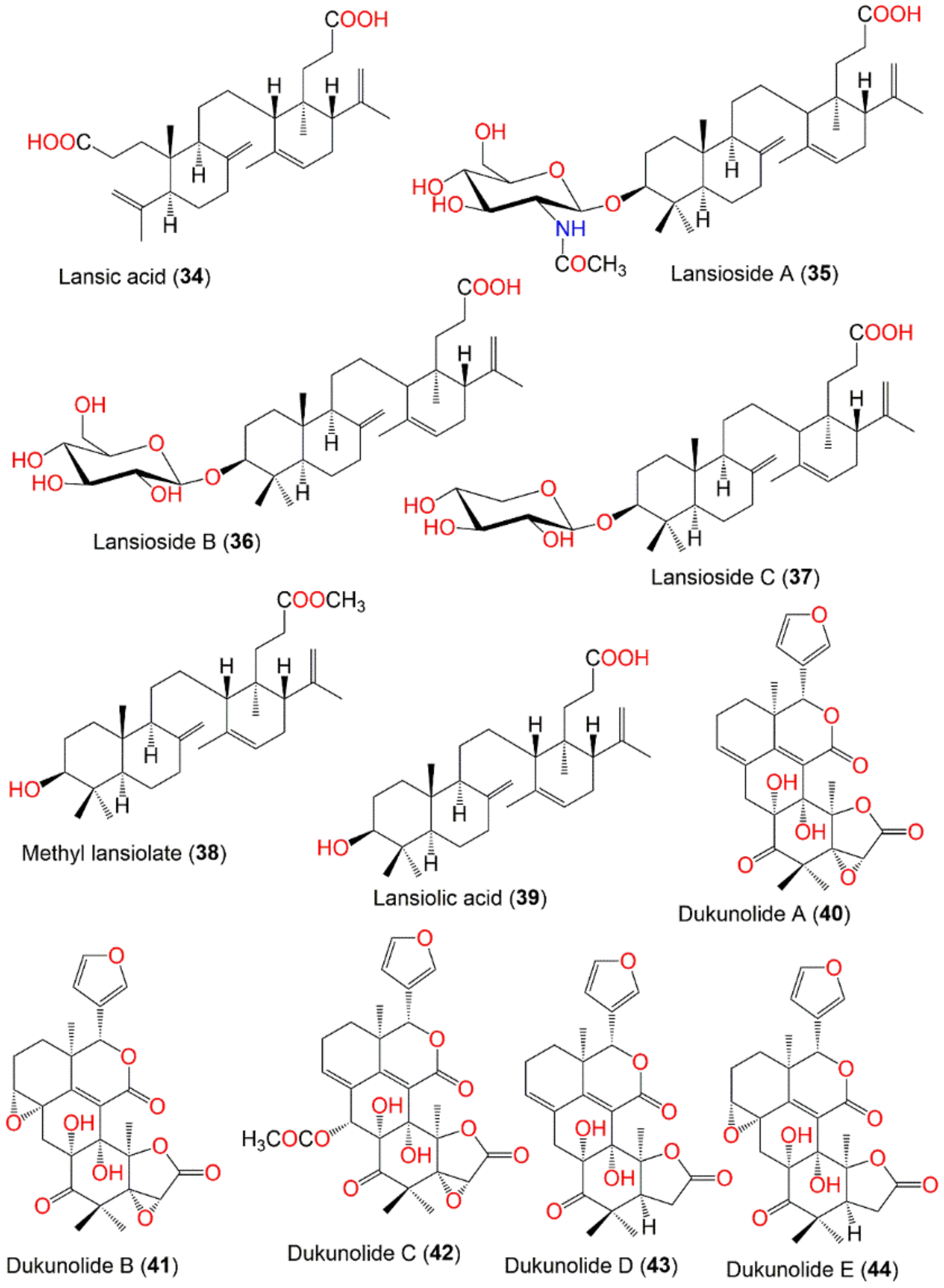
| Lansic acid (34) | Onoceranoid triterpenoid | Fruit peels | EtOH/CH2Cl2 | 470 | C30H46O4 | Bogor, Indonesis | [19,60] |
| Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | - | - | Khon Kaen, Thailand | [61] | |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | |
| Lansioside A (35) | Onoceranoid triterpenoid | Fruit peels | EtOH/CH2Cl2 | 659 | C38H61NO8 | Bogor, Indonesia | [19,60,63] |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | |
| Onoceranoid triterpenoid | Fruit peels | EtOH | - | - | Japan | [64] | |
| Lansioside B (36) | Onoceranoid triterpenoid | Fruit peels | EtOH/CH2Cl2 | 618 | C36H58O8 | Bogor, Indonesia | [19,60] |
| Onoceranoid triterpenoid | Fruit peels | EtOH | - | - | Japan | [64] | |
| Onoceranoid triterpenoid | Seeds | CH2Cl2/EtOAc | - | - | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] | |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | |
| Lansioside C (37) | Onoceranoid triterpenoid | Fruit peels | EtOH/CH2Cl2 | 588 | C35H56O7 | Bogor, Indonesia | [19,60] |
| Onoceranoid triterpenoid | Fruit peels | EtOH | - | - | Japan | [64] | |
| Onoceranoid triterpenoid | Fruit peels | CH2Cl2/EtOAc | - | - | Laguna, Philippine | [58] | |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | |
| Methyl lansiolate (38) | Onoceranoid triterpenoid | Fruit peels | EtOH/CH2Cl2 | 470 | C31H50O3 | Bogor, Indonesia | [19] |
| Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | - | - | Ra-ngae, Narathiwat, Thailand | [66] | |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | |
| Lansiolic acid (39) | Onoceranoid triterpenoid | Fruit peels | EtOH/CH2Cl2 | 456 | C30H43O3 | Bogor, Indonesia | [19] |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 454 | C30H46O3 | Indonesia | [67] | |
| Onoceranoid triterpenoid | Fruit peels and seeds | CH2Cl2/EtOAc | - | - | Laguna, Philippine | [58] | |
| Onoceranoid triterpenoid | Seeds | CH2Cl2/EtOAc | - | - | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] | |
| Onoceranoid triterpenoid | Bark | EtOH/EtOAc | - | - | Apo Kayan Indonesia | [68] | |
| Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | - | - | Ra-ngae, Narathiwat, Thailand | [66] | |
| Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | |
| Dukunolide A (40) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane (Duku) | 482 | C26H26O9 | Bogor, Indonesia | [69,70] |
| Seeds | MeOH/EtOAc | - | - | Pontianak, West Kalimantan, Indonesia | [71] | ||
| Dukunolide B (41) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 498 | C26H26O10 | Bogor, Indonesia | [70] |
| Seeds | CH2Cl2/EtOAc | - | - | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] | ||
| Seeds | MeOH/EtOAc | - | - | Pontianak, West Kalimantan, Indonesia | [71] | ||
| Dukunolide C (42) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 540 | C28H28O11 | Bogor, Indonesia | [70] |
| Seeds | CH2Cl2/EtOAc | - | - | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] | ||
| Seeds | MeOH/EtOAc | - | - | Pontianak, West Kalimantan, Indonesia | [71] | ||
| Dukunolide D (43) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 468 | C26H28O8 | Bogor, Indonesia | [72] |
| Seeds | CH2Cl2/EtOAc | - | - | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] | ||
| Seeds | MeOH/EtOAc | - | - | Pontianak, West Kalimantan, Indonesia | [71] | ||
| Dukunolide E (44) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 484 | C26H28O9 | Bogor, Indonesia | [72] |
| Dukunolide F (45) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 484 | C26H28O9 | Bogor, Indonesia | [72] |
| Seeds | MeOH/EtOAc | - | - | Pontianak, West Kalimantan, Indonesia | [71] | ||
| Seco-Dukunolide F (46) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 500 | C27H32O9 | Nakhon Si Thammarat, Thailand | [73] |
| Seeds | MeOH/EtOAc | - | - | Pontianak, West Kalimantan, Indonesia | [71] | ||
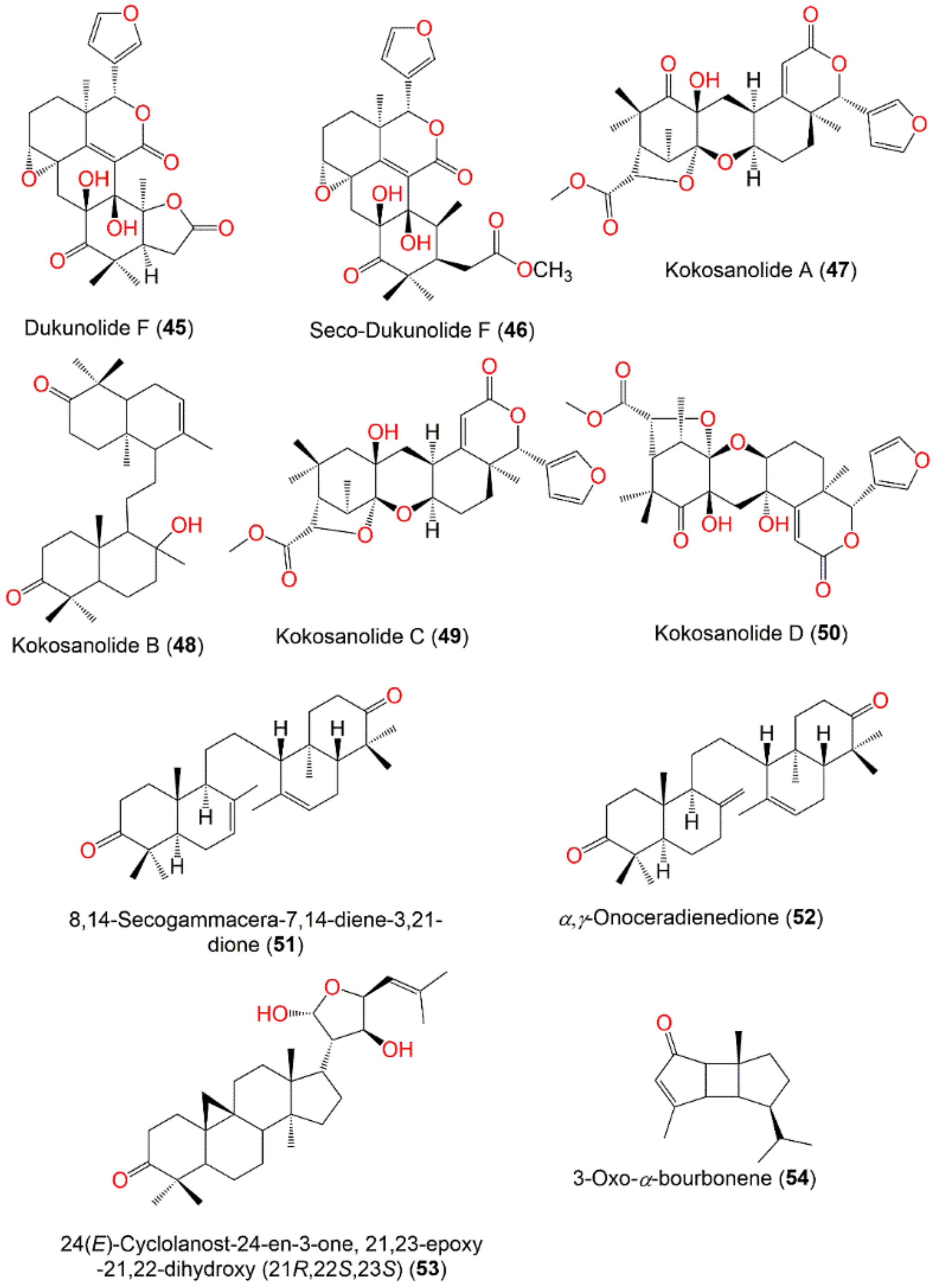
| Kokosanolide A (47) | Tetranortriterpenoid | Seeds | CH2Cl2/n-Hexane | 500 | C27H32O9 | Malaysia | [74] |
| Seeds | MeOH/n-Hexane | - | - | Cililin, Bandung, Indonesia | [75] | ||
| Kokosanolide B (48) | Tetranortriterpenoid | Bark | MeOH/n-Hexane | 456 | C30H48O3 | Cililin, Bandung, Indonesia | [76] |
| Bark | MeOH/EtOAc | - | - | Cililin, Bandung, Indonesia | [75] | ||
| Bark | MeOH/EtOAc | - | - | Cililin, Bandung, Indonesia | [77] | ||
| Kokosanolide C (49) | Tetranortriterpenoid | Seeds | MeOH/n-Hexane | 486 | C27H34O8 | Cililin, Bandung, Indonesia | [75] |
| Kokosanolide D (50) | Tetranortriterpenoid | Fruit peels | MeOH/n-BuOH (Kokossan) | 516 | C27H32O10 | Cililin, Bandung, Indonesia | [78] |
| 8,14-Secogammacera-7,14-diene-3,21-dione (51) | Tetranortriterpenoid | Bark | MeOH/n-Hexane | 438 | C30H46O2 | Cililin, Bandung, Indonesia | [79] |
| Bark | MeOH/EtOAc | - | - | Cililin, Bandung, Indonesia | [75] | ||
| Bark | MeOH/EtOAc | - | - | Cililin, Bandung, Indonesia | [77] | ||
| Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | ||
| α,γ-Onoceradienedione = 8,14-Secogammacera-7,14(27)-diene-3,21-dione (52) | Onoceranoid triterpenoid | Fruit peels | CH2Cl2/EtOAc | 438 | C30H46O2 | Laguna, Philippine | [58] |
| Bark | EtOH/EtOAc | - | - | Apo Kayan Indonesia | [34,68] | ||
| Bark | MeOH/n-Hexane | 438 | C30H46O2 | Cililin, Bandung, Indonesia | [79] | ||
| Fruit peels | MeOH/n-Hexane | - | - | Nganjuk, East Java, Indonesia | [80] | ||
| 24(E)-Cyclolanost-24-en-3-one, 21,23-epoxy-21,22-dihydroxy (21R,22S,23S) (53) | Cycloartane triterpenoid | Leaves | MeOH/EtOAc (kokossan) | 470 | C30H46O4 | Cililin, Bandung, Indonesia | [81] |
| Bark | MeOH/EtOAc | - | - | Cililin, Bandung, Indonesia | [75] | ||
| Leaves | MeOH/EtOAc | - | - | Cililin, Bandung, Indonesia | [77] | ||
| 3-Oxo-α-bourbonene (54) | Sesquiterpene | Fruit peels | - | 218 | C15H22O | - | [82] |
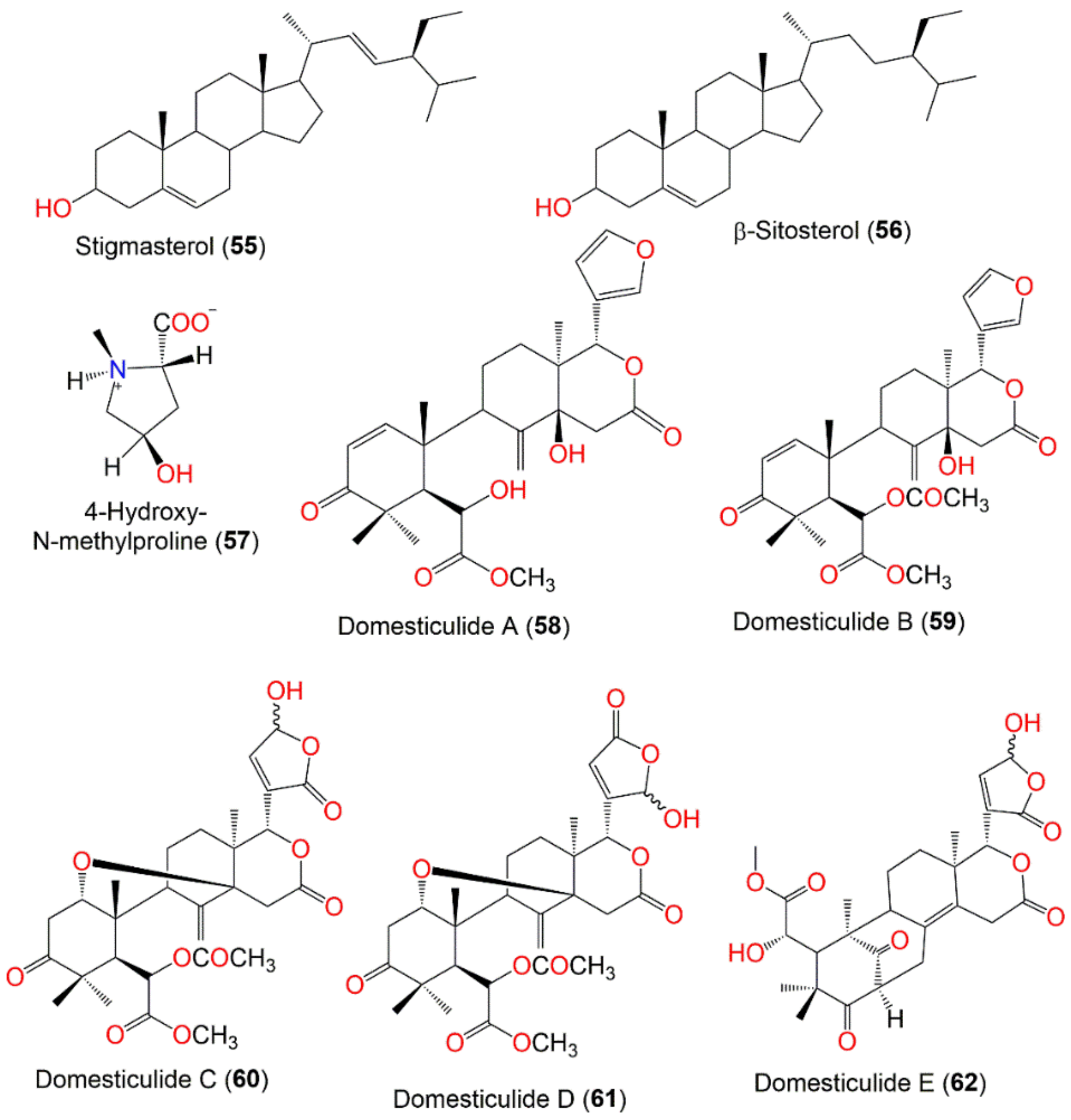
| Stigmasterol (55) | Sterol | Peel | Hexane/CH2Cl2 | 412 | C29H48O | - | [83] |
| β-Sitosterol (56) | Sterol | Peel | Hexane/CH2Cl2 | 414 | C29H50O | - | [83] |
| 4-Hydroxy-N-methylproline (57) | Nitrogenous compound | Fruit peels | MeOH | 145 | C6H11NO3 | Bogor, Indonesis | [84] |
| Domesticulide A (58) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 486 | C27H34O8 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Domesticulide B (59) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 528 | C29H36O9 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Domesticulide C (60) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 560 | C29H36O11 | Thumbon Nopitum, Nakhon Si Thammasat, Thailand | [65] |
| Domesticulide D (61) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 560 | C29H36O11 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Domesticulide E (62) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 516 | C27H32O10 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
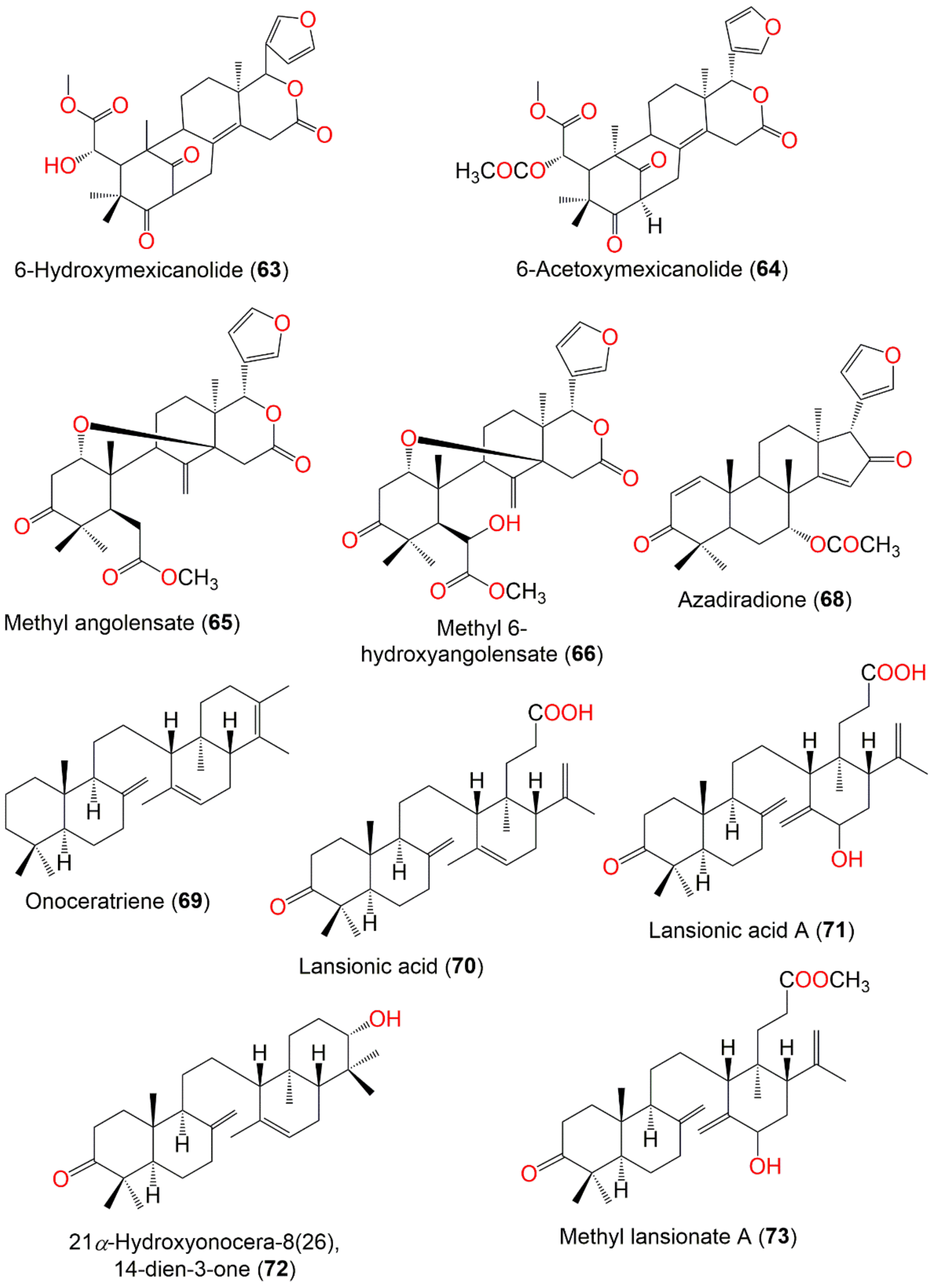
| 6-Hydroxymexicanolide (63) | Swietenine triterpenoid | Seeds | CH2Cl2/n-Hexane | 484 | C27H32O8 | Thailand | [85] |
| Seeds | CH2Cl2/EtOAc | - | - | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] | ||
| 6-Acetoxymexicanolide = Ekeberin C3 (64) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 526 | C29H34O9 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Leaves | EtOH/EtOAc | Menglun town of Yunnan, China | [86] | ||||
| Methyl angolensate (65) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 470 | C27H34O7 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Methyl 6-hydroxyangolensate (66) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 486 | C27H34O8 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Methyl 6-acetoxyangolensate (67) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 528 | C29H36O9 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Azadiradione (68) | Tetranortriterpenoid | Seeds | CH2Cl2/EtOAc | 450 | C28H34O5 | Thumbon Nopitum, Nakhon Si Thammarat, Thailand | [65] |
| Onoceratriene (69) | Onoceranoid triterpenoid | Bark | EtOH/EtOAc | 408 | C30H48 | Apo Kayan Indonesis | [34,68] |
| Lansionic acid = 3-Ketolansiolic acid (70) | Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | 454 | C30H46O3 | Khon Kaen, Thailand | [61] |
| Fruit peels | CH2Cl2/EtOAc | - | - | Laguna, Philippine | [58] | ||
| Bark | EtOH/EtOAc | - | - | Apo Kayan Indonesis | [34,68] | ||
| Fruit peels | MeOH/EtOAc | - | - | Ra-ngae, Narathiwat, Thailand | [66] | ||
| Lansionic acid A = Lansiolic acid A (71) | Onoceranoid triterpenoid | Bark | EtOH/EtOAc | 470 | C30H46O4 | Apo Kayan Indonesis | [34,68] |
| Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | ||
| 21α-Hydroxyonocera-8(26),14-dien-3-one = 3-keto-22-hydroxyonoceradiene (72) | Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | 440 | C30H48O2 | Khon Kaen, Thailand | [61] |
| Bark | EtOH/EtOAc | - | - | Apo Kayan Indonesis | [34,68] | ||
| Methyl lansionate A = methyl lansiolate A (73) | Onoceranoid triterpenoid | Bark | EtOH/EtOAc | 484 | C31H48O4 | Apo Kayan Indonesis | [68] |
| 8,14-Secogammacera-14-hydroxy-7-ene-3,21-dione (74) | Tetranortriterpenoid | Bark | MeOH/EtOAc | 456 | C30H48O3 | Cililin, Bandung, Indonesia | [75] |
| Lansium acid I (75) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 470 | C30H46O4 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid II (76) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 486 | C30H46O5 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid III (77) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 468 | C30H44O4 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid IV (78) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 470 | C30H46O4 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid V (79) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 504 | C30H48O6 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid VI (80) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 604 | C35H56O8 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid VII (81) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 620 | C35H56O9 | Ba’kelalan, Sarawak, Malaysia | [62] |
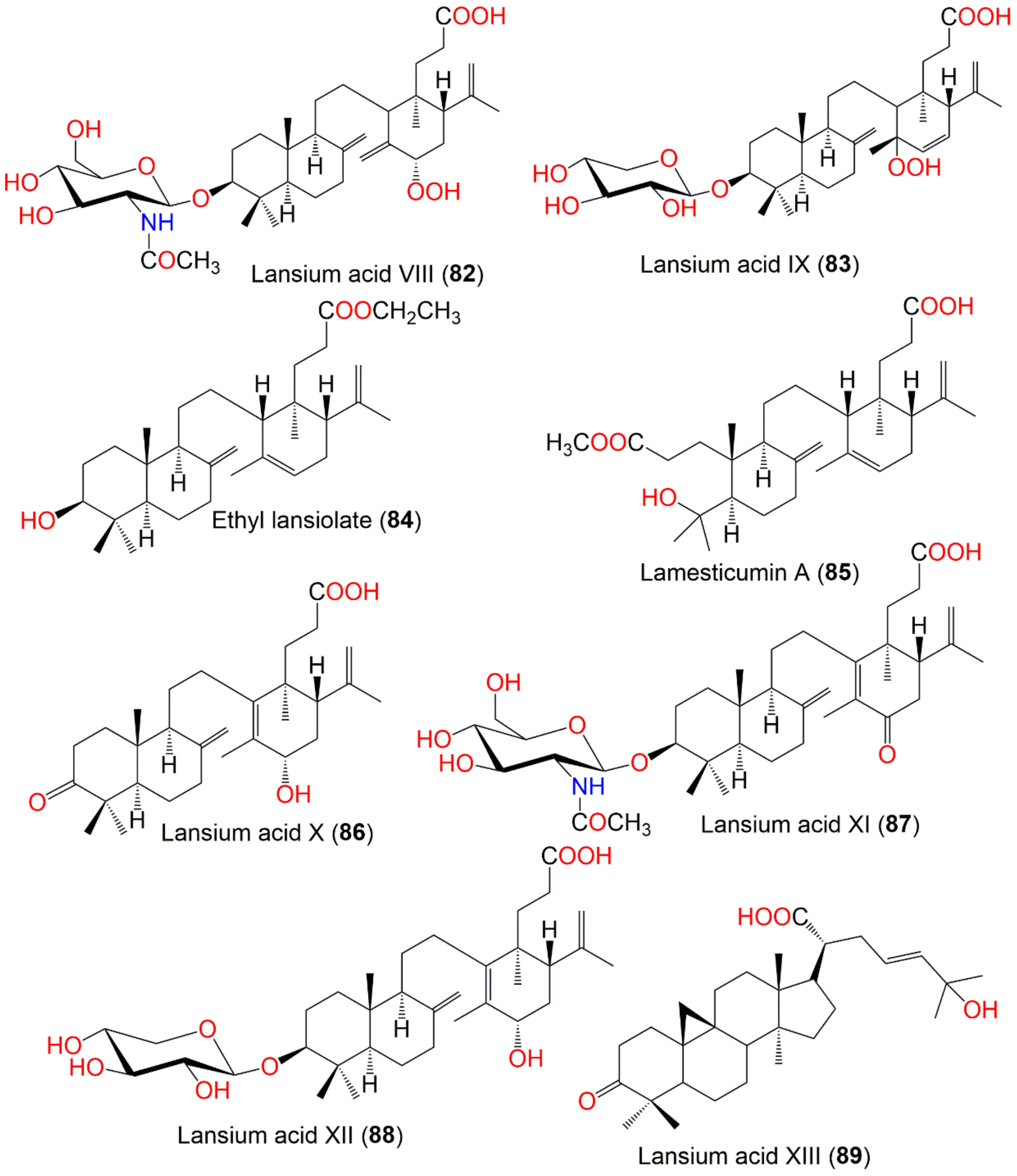
| Lansium acid VIII (82) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 691 | C38H61NO10 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lansium acid IX (83) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 620 | C35H56O9 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Ethyl lansiolate (84) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 484 | C32H52O3 | Ba’kelalan, Sarawak, Malaysia | [62] |
| Lamesticumin A (85) | Onoceranoid triterpenoid | Twigs | EtOH/EtOAc | 502 | C31H50O5 | Xishuangbanna, Mengla, Yunnan, China | [87] |
| Leaves | MeOH/EtOAc | - | - | Ba’kelalan, Sarawak, Malaysia | [62] | ||
| Fruit peels | EtOAc/n-Hexane | - | - | Bantul, Yogyakarta, Indonesia | [35] | ||
| Lansium acid X (86) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 470 | C30H46O4 | Ba’kelalan, Sarawak, Malaysia | [88] |
| Lansium acid XI (87) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 673 | C38H59NO9 | Ba’kelalan, Sarawak, Malaysia | [88] |
| Lansium acid XII (88) | Onoceranoid triterpenoid | Leaves | MeOH/EtOAc | 604 | C35H56O8 | Ba’kelalan, Sarawak, Malaysia | [88] |
| Lansium acid XIII (89) | Cycloartane triterpenoid | Leaves | MeOH/EtOAc | 470 | C30H46O44 | Ba’kelalan, Sarawak, Malaysia | [88] |
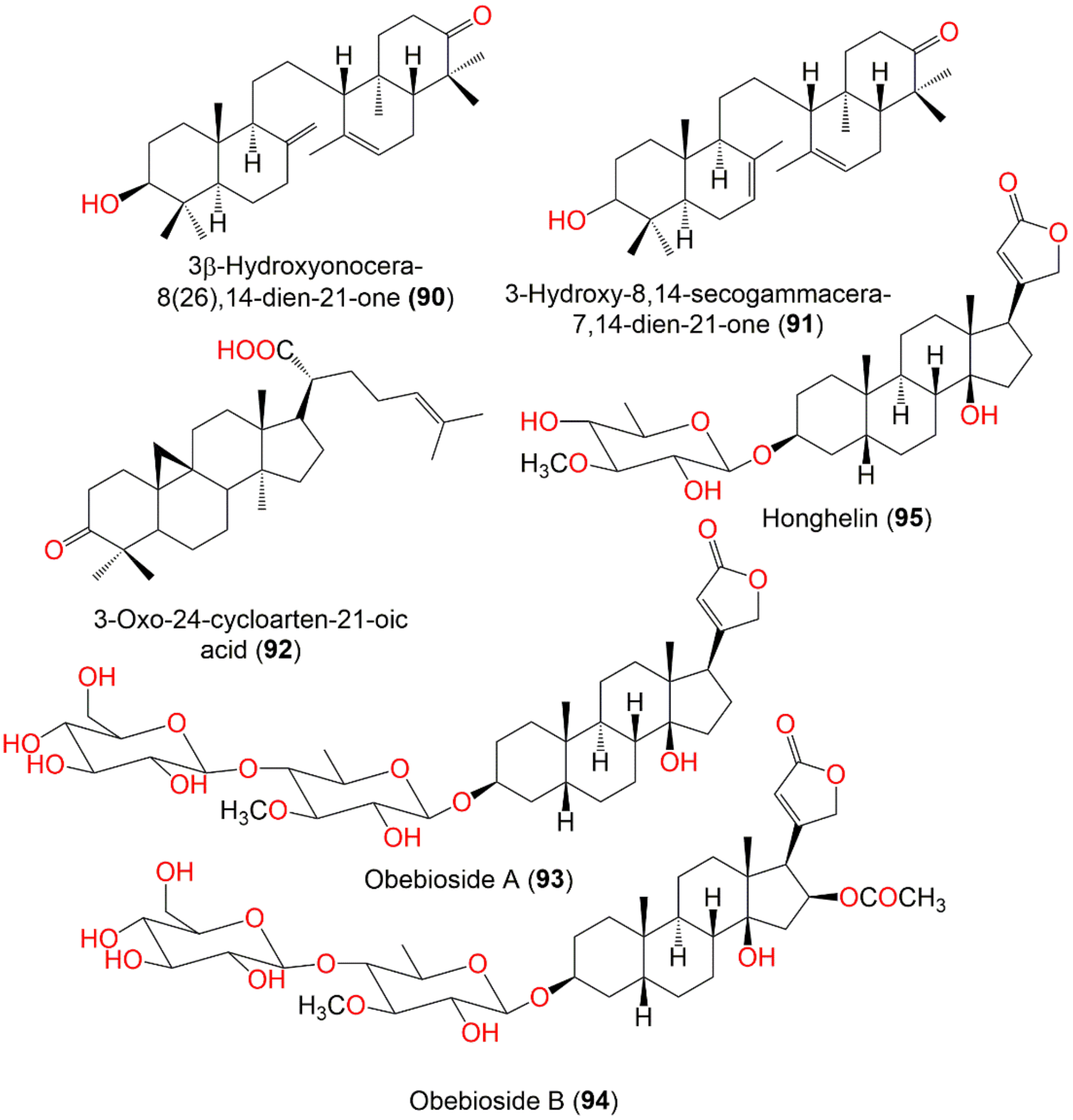
| 3β-Hydroxyonocera-8(26),14-dien-21-one (90) | Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | 440 | C30H48O2 | Khon Kaen, Thailand | [61] |
| Fruit peels | CH2Cl2/EtOAc | - | - | Laguna, Philippine | [58] | ||
| Fruit peels | MeOH/EtOAc | - | - | Ra-ngae, Narathiwat, Thailand | [66] | ||
| 3-Hydroxy-8,14-secogammacera-7,14-dien-21-one (91) | Onoceranoid triterpenoid | Fruit peels | n-Hexane/EtOAc | 440 | C30H48O2 | Cililin, West Java, Indonesia | [89] |
| 3-Oxo-24-cycloarten-21-oic acid (92) | Cycloartane triterpenoid | Leaves | MeOH/EtOAc | 454 | C30H46O3 | Indonesia | [67] |
| Obebioside A (93) | Cardenolide | Leaves | MeOH/EtOAc | 696 | C36H56O13 | Thailand | [90] |
| Obebioside B (94) | Cardenolide | Leaves | MeOH/EtOAc | 754 | C38H58O15 | Thailand | [90] |
| Honghelin (95) | Cardenolide | Leaves | MeOH/EtOAc | 534 | C30H46O8 | Thailand | [90] |
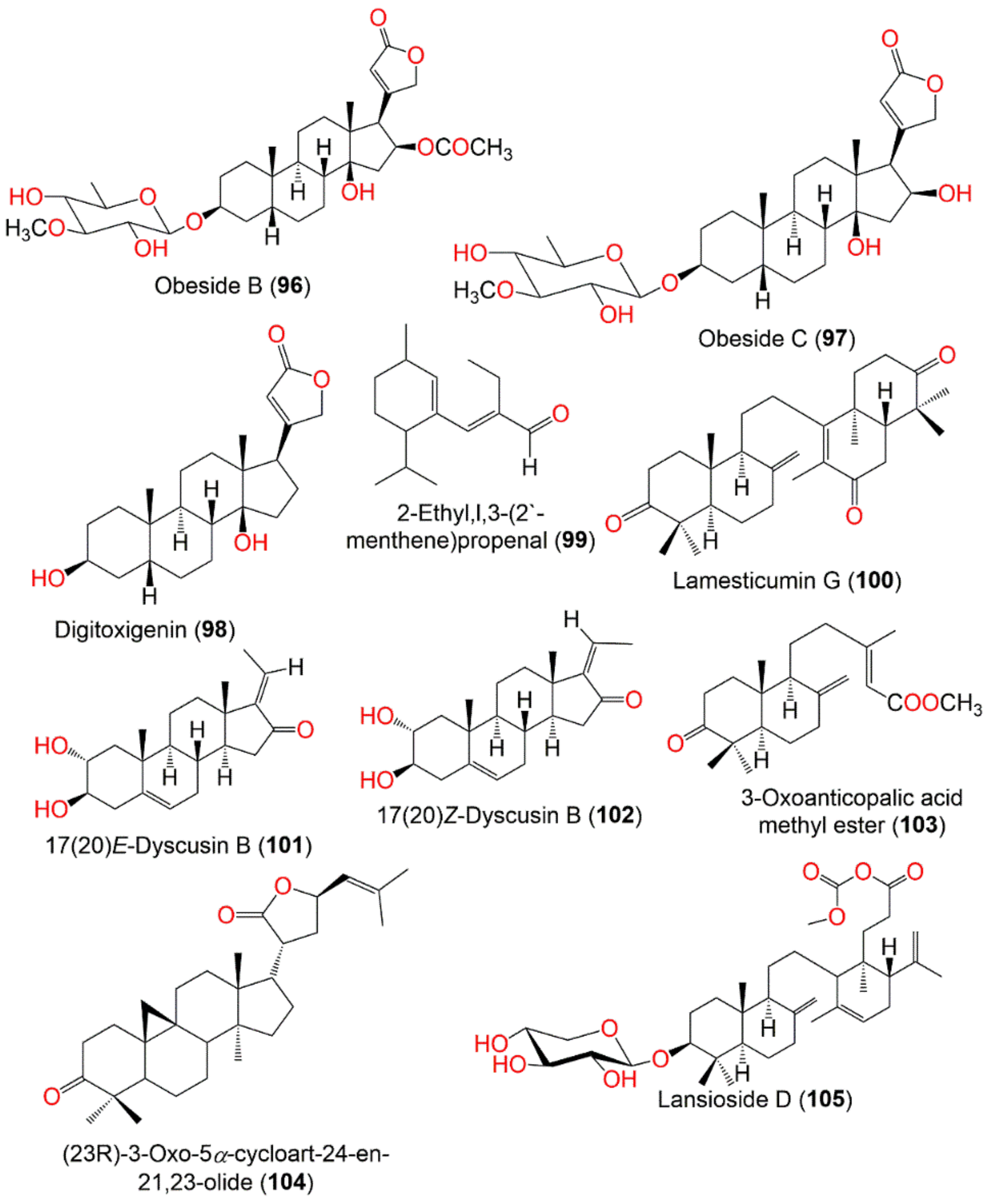
| Obeside B (96) | Cardenolide | Leaves | MeOH/EtOAc | 592 | C32H48O10 | Thailand | [90] |
| Obeside C (97) | Cardenolide | Leaves | MeOH/EtOAc | 550 | C30H46O9 | Thailand | [90] |
| Digitoxigenin (98) | Cardenolide | Leaves | MeOH/EtOAc | 374 | C23H34O4 | Thailand | [90] |
| 2-Ethyl,l,3-(2‘-menthene) propenal (99) | Sesquiterpene | Fruit peels | EtOAc/n-Hexane | 220 | C15H24O | Purbalingga, Central Java, Indonesia | [91] |
| Lamesticumin G (100) | Onoceranoid triterpenoid | Fruit peels | MeOH/EtOAc | 452 | C30H44O3 | Ra-ngae, Narathiwat, Thailand | [66] |
| 17(20)E-Dyscusin B (101) | Pregnane | Leaves | EtOH/EtOAc | 330 | C21H30O3 | Menglun town of Yunnan, China | [86] |
| 17(20)Z-Dyscusin B (102) | Pregnane | Leaves | EtOH/EtOAc | 330 | C21H30O3 | Menglun town of Yunnan, China | [86] |
| 3-Oxoanticopalic acid methyl ester (103) | Diterpene | Leaves | EtOH/EtOAc | 332 | C21H32O3 | Menglun town of Yunnan, China | [86] |
| (23R)-3-Oxo-5α-cycloart-24-en-21,23-olide (104) | Cycloartane triterpenoid | Leaves | MeOH/EtOAc | 452 | C30H44O3 | Menglun town of Yunnan, China | [86] |
| Lansioside D (105) | Onoceranoid triterpenoid | Fruit peels | EtOH/Acetone | 646 | C37H58O9 | Laguna, Philippines | [92] |
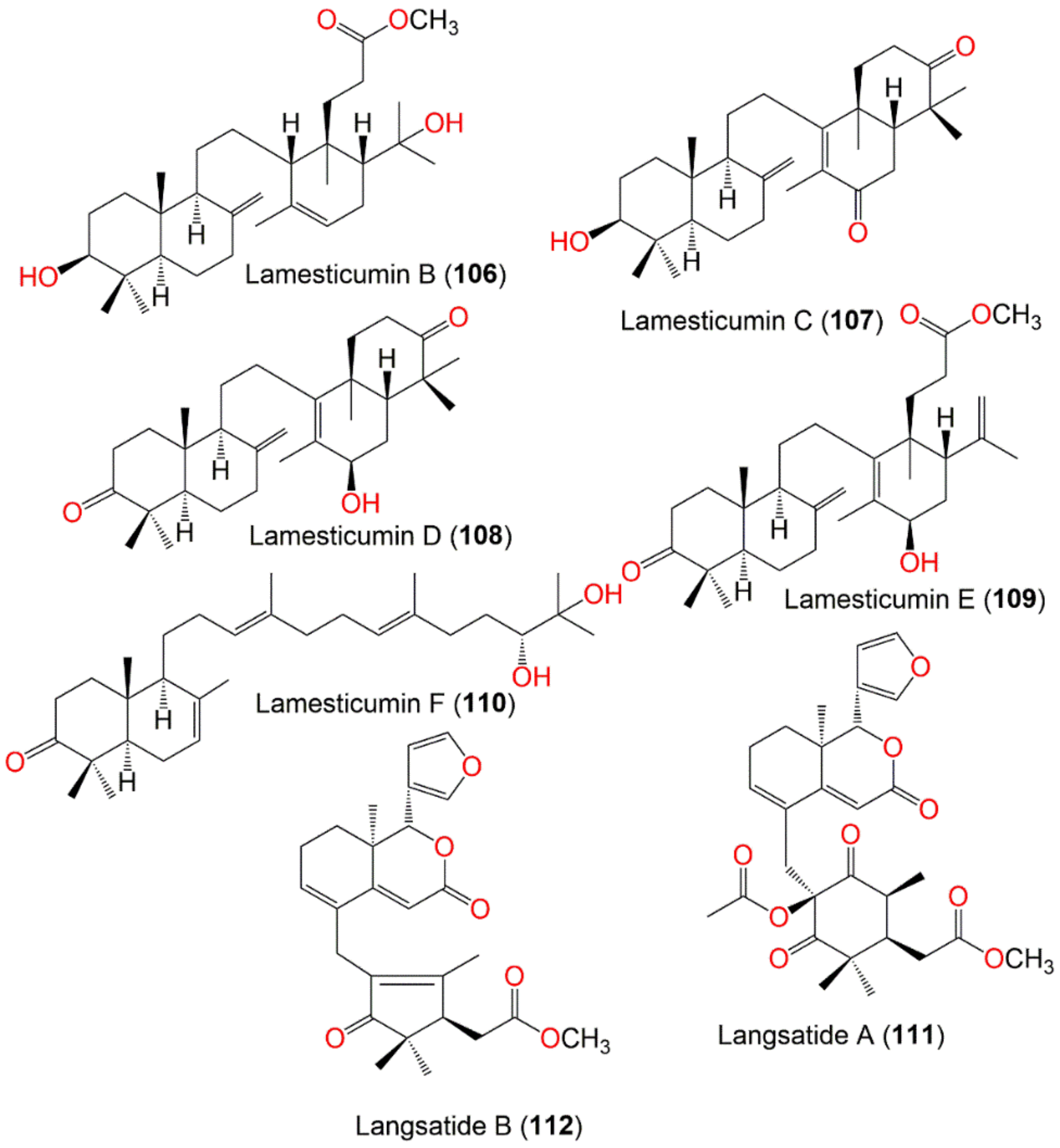
| Lamesticumin B (106) | Onoceranoid triterpenoid | Twigs | EtOH/EtOAc | 488 | C31H52O4 | Xishuangbanna, Mengla, Yunnan, China | [87] |
| Lamesticumin C (107) | Onoceranoid triterpenoid | Twigs | EtOH/EtOAc | 454 | C30H46O3 | Xishuangbanna, Mengla, Yunnan, China | [87] |
| Lamesticumin D (108) | Onoceranoid triterpenoid | Twigs | EtOH/EtOAc | 454 | C30H46O3 | Xishuangbanna, Mengla, Yunnan, China | [87] |
| Lamesticumin E (109) | Onoceranoid triterpenoid | Twigs | EtOH/EtOAc | 484 | C31H48O4 | Xishuangbanna, Mengla, Yunnan, China | [87] |
| Lamesticumin F (110) | Onoceranoid triterpenoid | Twigs | EtOH/EtOAc | 458 | C30H50O3 | Xishuangbanna, Mengla, Yunnan, China | [87] |
| Langsatide A (111) | Tetranortriterpenoid | Seeds | MeOH/EtOAc | 526 | C29H34O9 | Pontianak, West Kalimantan, Indonesia | [71] |
| Langsatide B (112) | Tetranortriterpenoid | Seeds | MeOH/EtOAc | 438 | C26H30O6 | Pontianak, West Kalimantan, Indonesia | [71] |
7.2. Phenolics
7.3. Terpenoids
7.4. Sterols
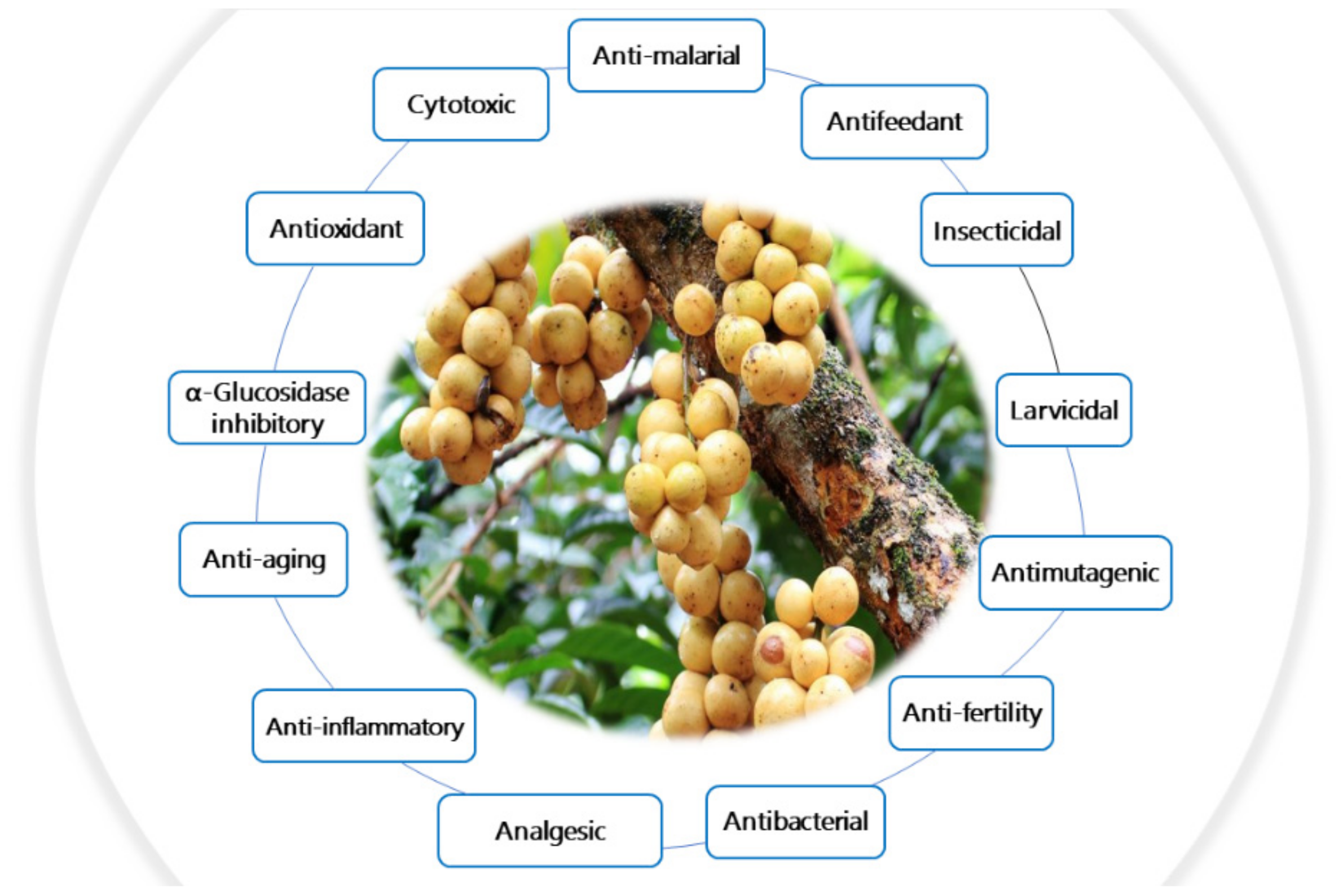
8. Biological Activities of L. domesticum Extracts and Isolated Compounds
8.1. Anti-Malarial Activity
8.2. Antifeedant, Insecticidal, and Larvicidal Activities
8.3. Anti-Fertility Activity
8.4. Antimutagenic Activity
8.5. Cytotoxic Activity
8.6. Antioxidant Activity
8.7. α-Glucosidase Inhibitory Activity
8.8. Anti-Aging Activity
8.9. Analgesic and Anti-Inflammatory Activities
8.10. Antibacterial Activity
8.11. 5α-Reductase Inhibitory Activity
8.12. Wound Healing Activity
9. Safety of L. domesticum
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ibrahim, S.R.; Mohamed, G.A. Litchi chinensis: Medicinal uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2015, 174, 492–513. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.E.S. Genus Hylocereus: Beneficial phytochemicals, nutritional importance, and biological relevance—A review. J. Food Biochem. 2018, 42, e12491. [Google Scholar] [CrossRef]
- Chiruvella, K.K.; Mohammad, M.; Thammineni, C.; Paritala, V.; Ghanta, G. In vitro propagation, phytochemical investigations and biological activities of an endemic medicinal plant, Indian red wood (Meliaceae): A Review. Int. J. Med. Plants 2014, 107, 558–571. [Google Scholar]
- Mabberley, D. Florae malesianae praecursores LXVII. Meliaceae (Divers genera). Blumea 1985, 31, 129–152. [Google Scholar]
- Lim, T. Edible Medicinal Plant 3th Vol Fruits; Springer: New York, NY, USA, 2012. [Google Scholar]
- Backer, C.A.; Bakhuizen Van Den Brink, R. Flora of Java (Spermatophytes Only) Vol. 2. Angiospermae, Families; N.V.P. Noordhoff: Groningen, The Netherlands, 1965; pp. 111–160. [Google Scholar]
- Song, B.K.; Clyde, M.M.; Wickneswari, R.; Normah, M.N. Genetic relatedness among lansium domesticum accessions using RAPD markers. Ann. Bot. 2000, 86, 299–307. [Google Scholar] [CrossRef]
- Hanum, L.; Kasiamdari, R.S.; Santosa, S.; Rugayah, R. The phylogenetic relationship among varieties of Lansium domesticum correa based on ITS rDNA sequences (Similarity). Indones. J. Biotechnol. 2013, 18, 123–132. [Google Scholar] [CrossRef]
- Brown, B.I.; Lizada, M.C.C. Modified atmospheres and deterioration of lanzones (Lansium domesticum Correa.). Postharvest Res. Notes 1985, 1, 36–39. [Google Scholar]
- Tilaar, M.; Wih, W.L.; Ranti, A.S.; Wasitaatmadja, S.M.; Junardy, F. Review of Lansium domesticum corrêa and its use in cosmetics. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2008, 7, 183–189. [Google Scholar]
- Wong, K.; Wong, S.; Siew, S.; Tie, D. Volatile constituents of the fruits of Lansium domesticum correa (Duku and langsat) and Baccaurea motleyana (Muell. Arg.) Muell. Arg. (Rambai). Flavour Fragr. J. 1994, 9, 319–324. [Google Scholar] [CrossRef]
- Venkatachalam, K. Postharvest physiology and handling of longkong fruit: A review. Fruits 2016, 71, 289–298. [Google Scholar] [CrossRef]
- Sapii, A.T.; Yunus, N.; Muda, P.; Lin, T.S. Postharvest quality changes in dokong (Lansium domesticum Corr.) harvested at different stages of ripeness. In Proceedings of the ACIAR Proceedings, Bruce, Australia, 23 January 2000; pp. 201–205. [Google Scholar]
- Ochse, J.J. Fruits and Fruitculture in the Dutch East Indies; G. Kolff & Co.: Jakarta, Indonesia, 1931. [Google Scholar]
- Mabberley, D.J.; Pannell, C.M. Meliaceae. In Tree Flora of Malaysia; Ng, F.S.P., Ed.; Longman: Kuala Lumpur, Malaysia, 1989; Volume 4, pp. 199–260. [Google Scholar]
- Heyne, K. Tumbuhan Berguna Indonesia. In Badan Penelitian dan Pengembangan Kehutanan, Departemen Kehutanan; Sarana Wana Jaya Foundation: Jakarta, Indonesia, 1987; Volume 2, pp. 1188–1189. [Google Scholar]
- Yapp, D.T.; Yap, S. Lansium domesticum: Skin and leaf extracts of this fruit tree interrupt the lifecycle of Plasmodium falciparum, and are active towards a chloroquine-resistant strain of the parasite (T9) in vitro. J. Ethnopharmacol. 2003, 85, 145–150. [Google Scholar] [CrossRef]
- Morton, J.L. Fruit of Warm Climates; Julia F. Morton: Miami, FL, USA, 1987; pp. 201–204. [Google Scholar]
- Nishizawa, M.; Nishide, H.; Kosela, S.; Hayashi, Y. Structure of lansiosides: Biologically active new triterpene glycosides from Lansium domesticum. J. Org. Chem. 1983, 48, 4462–4466. [Google Scholar] [CrossRef]
- Techavuthiporn, C. Langsat—Lansium domesticum. In Exotic Fruits; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–283. [Google Scholar]
- Wijayanti, R.; Saleh, E.; Hanum, H.; Aprianti, N. Climate change analysis (Monthly rainfall) on palembang duku production (Lansium domesticum Corr). Sriwij. J. Env. 2020, 5, 120–126. [Google Scholar] [CrossRef]
- Loekitowati, P.; Hermansyah, H. Studi pemanfaatan biji duku (Lansium Domesticum. Jack.) untuk obat diare secara in vitro. J. Penelit. Sains 2017, 7, 41–48. [Google Scholar]
- Quisumbing, E. Medicinal Plants of the Philippines; Philippine Islands Technical Bulletin; Department of Agriculture and Commerce: Quezon, Philippines, 1951.
- Kusumadewi, A.P.; Supriyati, N. The analysis of chemical and physical properties of Lansium domesticum cortex, from Palu-central Sulawesi. J. Tumbuh. Obat Indones. 2019, 8, 41–46. [Google Scholar]
- Naito, Y. Medicinal Herb Index in Indonesia; PT. Eisei Indonesia: Jakarta, Indonesia, 1995. [Google Scholar]
- Paull, R.E.; Goo, T.; Chen, N.J. Growth and compositional changes during development of lanzone fruit. HortScience 1987, 22, 1252–1253. [Google Scholar]
- Kilala Tilaar, M.; Junardy, F.D.; Subroto, E.; Puspitosari, D. Safety and efficacy evaluation on combination of Lansium domesticum fruit extract and Hibiscus rosa-sinensis flower extract as lightening agent for cosmetic. Int. J. Pharm. Med. Biol. Sci. 2018, 7, 67–70. [Google Scholar]
- Alfonso, E.D.; Fernando, S.I.D.; Pineda, P.S.; Divina, C.C. Antibacterial activity and genotoxicity assays of lanzones (Lansium domesticum) seeds extract. Int. J. Agric. Technol. 2017, 13, 2427–2434. [Google Scholar]
- Burkill, I.H.; Birtwistle, W.; Foxworthy, F.W.; Scrivenor, J.B.; Watson, J.G. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture and Cooperatives: Kuala Lumpur, Malaysia, 1966.
- Leaman, D.J.; Arnason, J.T.; Yusuf, R.; Sangat-Roemantyo, H.; Soedjito, H.; Angerhofer, C.K.; Pezzuto, J.M. Malaria remedies of the Kenyah of the Apo Kayan, East Kalimantan, Indonesian Borneo: A quantitative assessment of local consensus as an indicator of biological efficacy. J. Ethnopharmacol. 1995, 49, 1–16. [Google Scholar] [CrossRef]
- Kurniati, R.; Dewi, H.; Hendra, M. Effect of water decoction of Langsat bark (Lansium domesticum Corr.) on estrous cycle and uterus weight in mice (Mus musculus L.). AIP Conf. Proc. 2017, 1844, 020003. [Google Scholar]
- Monzon, R.; Alvior, J.; Luczon, L.; Morales, A.; Mutuc, F. Larvicidal potential of five Philippine plants against Aedes aegypti (Linnaeus) and Culex quinquefasciatus (Say). Southeast Asian J. Trop. Med. Public Health 1994, 25, 755–759. [Google Scholar] [PubMed]
- Samuagam, L.; Sia, C.M.; Akowuah, G.A.; Okechukwu, P.N.; Yim, H.S. In vivo antioxidant potentials of rambutan, mangosteen, and langsat peel extracts and effects on liver enzymes in experimental rats. Food Sci. Biotechnol. 2015, 24, 191–198. [Google Scholar] [CrossRef]
- Omar, S.; Marcotte, M.; Fields, P.; Sanchez, P.; Poveda, L.; Mata, R.; Jimenez, A.; Durst, T.; Zhang, J.; MacKinnon, S. Antifeedant activities of terpenoids isolated from tropical Rutales. J. Stored Prod. Res. 2007, 43, 92–96. [Google Scholar] [CrossRef]
- Fadhilah, K.; Wahyuono, S.; Astuti, P. Fractions and isolated compounds from Lansium domesticum fruit peel exhibited cytotoxic activity against T-47D and HepG2 cell lines. Biodiversitas 2021, 22, 3743–3748. [Google Scholar] [CrossRef]
- Potoh, J.; Vanda, S.; Lungguk, P. Analysis of organic acid in langsat (Lansium domesticum var pubescens) and duku (Lansium domesticum var. domesticum) fruits by reversed phase HPLC technique. Int. J. Chemtech Res. 2015, 8, 238–242. [Google Scholar]
- Salma, I.; Razali, B. The reproductive biology of duku langsat, Lansium domesticum Corr. (Meliaceae). Peninsular Malaysia. MARDI Res. Bull. 1987, 15, 141–150. [Google Scholar]
- Arshad, R.; Ain Mustafa, K.; Abu Bakar, C.; Zakaria, A.; Nawawi, N.; Muda, S.; Anwar, N.; Mohamad, W. Proximate composition and mineral contents of Duku (Lansium domesticum) Fruit. Int. J. Food Sci. Nutr. 2021, 11, 20–26. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Ministry of Public Health. Nutritional Composition Table of Fruits in Thailand; Nutrition Division, Department of Health, Ministry of Public Heath: Bangkok, Thailand, 1987.
- Siong, T.E.; Asean, K.L.M.; Noor, M.I.; Azudin, M.N.; Idris, K. Nutrient Composition of Malaysian Foods; Kuala Lumpur (Malaysia) ASEAN Sub-Committee on Protein: Kuala Lumpur, Malaysia, 1988. [Google Scholar]
- Wong, K.C.S.K. Dimocarpus longan lour. In Edible Fruits and Nuts. Plant Resources of Southeast Asia; Verheij, E.W.M., Coronel, R.E., Eds.; Pudoc: Wageningen, The Netherlands, 1991; pp. 146–151. [Google Scholar]
- Manosroi, A.; Jantrawut, P.; Sainakham, M.; Manosroi, W.; Manosroi, J. Anticancer activities of the extract from longkong (Lansium domesticum) young fruits. Pharm. Biol. 2012, 50, 1397–1407. [Google Scholar] [CrossRef]
- Verheij, E.; Coronel, R. Plant Resources of South-East Asia No. 2. Edible Fruits and Nuts; Prosea Foundation: Bogor, Indonesia, 1992; pp. 186–190. [Google Scholar]
- Mokhtar, S.I.; Zakaria, F.; Ayob, M.A.; Siow, W.S.; AS, N.; Latiff, Z.A.A. Study on the nutritional values and customer acceptance of Lansium domesticum & Nephelium lappaceum newly fermented natural fruit vinegars in Malaysia. Asian Pac. J. Adv. Bus. Soc. Stud. 2016, 2, 402–413. [Google Scholar]
- Lichanporn, I.; Techavuthiporn, C. The effects of nitric oxide and nitrous oxide on enzymatic browning in longkong (Aglaia dookkoo Griff.). Postharvest Biol. Technol. 2013, 86, 62–65. [Google Scholar] [CrossRef]
- Lichanporn, I.; Srilaong, V.; Wongs-Aree, C.; Kanlayanarat, S. Postharvest physiology and browning of longkong (Aglaia dookkoo Griff.) fruit under ambient conditions. Postharvest Biol. Technol. 2009, 52, 294–299. [Google Scholar] [CrossRef]
- Chairin, T.; Petcharat, V. Induction of defense responses in longkong fruit (Aglaia dookkoo Griff.) against fruit rot fungi by Metarhizium guizhouense. Biol. Control 2017, 111, 40–44. [Google Scholar] [CrossRef]
- Venkatachalam, K. Changes in Quality and Enzymes of Longkong (Aglaia Dookkoo Griff.) Fruit during Storages as Affected by Maturation, Package and Methyl Jasmonate Treatment. Ph.D. Thesis, Prince of Songkla University, Songkla, Thailand, 2013. [Google Scholar]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Klungsupya, P.; Suthepakul, N.; Muangman, T.; Rerk-Am, U.; Thongdon, A.J. Determination of free radical scavenging, antioxidative DNA damage activities and phytochemical components of active fractions from Lansium domesticum Corr. fruit. Nutrients 2015, 7, 6852–6873. [Google Scholar] [CrossRef]
- Solidum, J.N. Potential nutritional and medicinal sources from fruit peels in Manila, Philippines. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 270. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Manosroi, W.; Manosroi, J. Anti-proliferative and matrix metalloproteinase-2 inhibition of Longkong (Lansium domesticum) extracts on human mouth epidermal carcinoma. Pharm. Biol. 2013, 51, 1311–1320. [Google Scholar] [CrossRef]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Kerdchoechuen, O.; Matta, F.B.; Silva, J.L.; Holmes, W.E. Postharvest survey of volatile compounds in five tropical fruits using headspace-solid phase microextraction (HS-SPME). HortScience 2007, 42, 309–314. [Google Scholar] [CrossRef]
- Chairgulprasert, V.; Krisornpornsan, B.; Hamad, A. Chemical constituents of the essential oil and organic acids from longkong (Aglaia dookkoo Griff.) fruits. Songklanakarin J. Sci. Technol. 2006, 28, 321–326. [Google Scholar]
- Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial terpenoids from Lansium domesticum. Philipp. Agric. Sci. 2006, 89, 101. [Google Scholar]
- Lee, P.R.; Tan, R.M.; Yu, B.; Curran, P.; Liu, S.Q. Sugars, organic acids, and phenolic acids of exotic seasonable tropical fruits. Nutr. Food Sci. 2013, 43, 267–276. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nishide, H.; Hayashi, Y.; Kosela, S. Biologically active constituents of Indonesian tropical plants. In Proceedings of the Symposium on the Chemistry of Natural Products, Surabaya, Indonesia, 28–30 September 2015; pp. 330–336. [Google Scholar]
- Tanaka, T.; Ishibashi, M.; Fujimoto, H.; Okuyama, E.; Koyano, T.; Kowithayakorn, T.; Hayashi, M.; Komiyama, K. New onoceranoid triterpene constituents from lansium domesticum. J. Nat. Prod. 2002, 65, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kitagawa, T.; Teo, S.; Anai, Y.; Ikeda, R.; Imahori, D.; Ahmad, H.S.b.; Watanabe, T. Structures and antimutagenic effects of onoceranoid-type triterpenoids from the leaves of Lansium domesticum. J. Nat. Prod. 2018, 81, 2187–2194. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nishide, H.; Hayashi, Y.; Kosela, S. The structure of Lansioside A: A novel triterpene glycoside with amino-sugar from lansium domesticum. Tetrahedron Lett. 1982, 23, 1349–1350. [Google Scholar] [CrossRef]
- Miyamoto, M.; Hamanaka, N. 5-α-reductase inhibitors from Lansium domesticum. Jpn. Kokai Tokkyo Koho 1985, 8, 1625–1630. [Google Scholar]
- Saewan, N.; Sutherland, J.D.; Chantrapromma, K. Antimalarial tetranortriterpenoids from the seeds of Lansium domesticum Corr. Phytochemistry 2006, 67, 2288–2293. [Google Scholar] [CrossRef]
- Potipiranun, T.; Worawalai, W.; Phuwapraisirisan, P. Lamesticumin G a new α-glucosidase inhibitor from the fruit peels of Lansium parasiticum. Nat. Prod. Res. 2018, 32, 1881–1886. [Google Scholar] [CrossRef]
- Nishizawa, M.; Emura, M.; Yamada, H.; Shiro, M.; Hayashi, Y.; Tokuda, H. Isolation of a new cycloartanoid triterpene from leaves of Lansium domesticum novel skin-tumor promotion inhibitors. Tetrahedron Lett. 1989, 30, 5615–5618. [Google Scholar] [CrossRef]
- Omar, S. Phytochemical Discovery of Antifeedant, Antimicrobial and Antimalarial Principles; University of Ottawa: Ottawa, ON, Canada, 2001. [Google Scholar]
- Nishizawa, M.; Nademoto, Y.; Sastrapradja, S.; Shiro, M.; Hayashi, Y. Structure of dukunolide A: A tetranortriterpenoid with a new carbon skeleton from Lansium domesticum. J. Chem. Soc. Chem. Commun. 1985, 7, 395–396. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nademoto, Y.; Sastrapradja, S.; Shiro, M.; Hayashi, Y. Structure of dukunolides, bitter principles of Lansium domesticum. J. Org. Chem. 1985, 50, 5487–5490. [Google Scholar] [CrossRef]
- Rudiyansyah Alimuddin, A.H.; Muharini, R.; Proksch, P. New tetranortriterpenoids, langsatides A and B from the seeds of Lansium domesticum Corr. (Meliaceae). Phytochem. Lett. 2018, 23, 90–93. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nademoto, Y.; Sastrapradja, S.; Shiro, M.; Hayashi, Y. Dukunolide D, E and F: New tetranortriterpenoids from the seeds of Lansium domesticum. Phytochemistry 1988, 27, 237–239. [Google Scholar] [CrossRef]
- Fun, H.-K.; Chantrapromma, S.; Boonnak, N.; Chaiyadej, K.; Chantrapromma, K.; Yu, X.-L. seco-Dukunolide F: A new tetranortriterpenoid from Lansium domesticum Corr. Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3725–o3727. [Google Scholar] [CrossRef]
- Mayanti, T.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Ng, S.W. Kokosanolide from the seed of Lansium domesticum Corr. Crystallogr. Sect. E Struct. Rep. Online 2009, 65, 750. [Google Scholar] [CrossRef]
- Mayanti, T.; Tjokronegoro, R.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Hadi, A.H.A. Antifeedant triterpenoids from the seeds and bark of Lansium domesticum cv Kokossan (Meliaceae). Molecules 2011, 16, 2785–2795. [Google Scholar] [CrossRef]
- Supratman, U.; Mayanti, T.; Awang, K.; Mukhtar, M.R.; Ng, S.W. 14-Hydroxy-8, 14-secogammacera-7-ene-3, 21-dione from the bark of Lansium domesticum Corr. Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o1621. [Google Scholar] [CrossRef]
- Mayanti, T.; Apriantini, Y.; Soidah, S.; Sianturi, J.; Darneti, D.; Julaeha, E.; Sumiarsa, D.N.M.; Maharani, R. Antibacterial triterpenoids from the bark and leaves of Lansium domesticum Corr cv. Kokossan (Meliaceae). J. Kim. 2018, 12, 54–58. [Google Scholar] [CrossRef]
- Fauzi, F.M.; Meilanie, S.R.; Farabi, K.; Herlina, T.; Al Anshori, J.; Mayanti, T. Kokosanolide D: A new tetranortriterpenoid from Fruit Peels of Lansium domesticum Corr. cv Kokossan. Molbank 2021, 2021, M1232. [Google Scholar] [CrossRef]
- Tjokronegero, R.-a.; Mayanti, T.; Supratman, U.; Mukhtar, M.R.; Ng, S.W. 8, 14-secogammacera-7, 14 (27)-diene-3, 21-dione–8, 14-secogammacera-7, 14-diene-3, 21-dione (1.5/0.5) from the bark of Lansium domesticum corr. Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o1448. [Google Scholar] [CrossRef] [PubMed]
- Labibah, Q.; Tun, K.; Aminah, N.; Kristanti, A.; Ramadhan, R.; Takaya, Y.; Abdullah, C.; Masarudin, M. Cytotoxic constituent un the fruit of Lansium domesticum. Rasayan J. Chem. 2021, 14, 1336–1340. [Google Scholar] [CrossRef]
- Mayanti, T.; Sianturi, J.; Harneti, D.; Supratman, U.; Rosli, M.M.; Fun, H.-K. 9, 19-Cyclolanost-24-en-3-one, 21, 23-epoxy-21, 22-dihydroxy (21R, 22S, 23S) from the Leaves of Lansium domesticum Corr cv Kokossan. Molbank 2015, 2015, M880. [Google Scholar] [CrossRef]
- Uyehara, T.; Suzuki, I.; Yamamoto, Y. Stereospecific synthesis of 3-oxo-α-bourbonene and the cisoid isomer for structural determination of the toxic component of Lansium domesticum. Bull. Chem. Soc. Jpn. 1988, 61, 2672–2674. [Google Scholar] [CrossRef]
- Pooasa, S. Limonoids from the timbers of Lansium domestium corr. and the stem barks of Xylocarpus rumphii. J. Marriage Fam. 2019, 56, 181–192. [Google Scholar]
- Yapp, D.T.; Steed, J.W.; Houghton, P.J. 4-Hydroxy-N-methylproline. Crystallogr. Sect. E Struct. Rep. Online 2002, 58, o988–o989. [Google Scholar] [CrossRef]
- Chantrapromma, K.; Saewan, N.; Fun, H.-K.; Chantrapromma, S.; Rahman, A.A. 6-Hydroxymexicanolide. Crystallogr. Sect. E Struct. Rep. Online 2004, 60, o312–o314. [Google Scholar] [CrossRef]
- Ji, K.-L.; Dai, M.-Y.; Xiao, C.-F.; Xu, Y.-K. Two new steroids with NO inhibitory effects from Lansium domesticum. Nat. Prod. Res. 2021, 35, 1147–1152. [Google Scholar] [CrossRef]
- Dong, S.-H.; Zhang, C.-R.; Dong, L.; Wu, Y.; Yue, J.-M. Onoceranoid-type triterpenoids from Lansium domesticum. J. Nat. Prod. 2011, 74, 1042–1048. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kitagawa, T.; Ohta, T.; Yoshida, T.; Imahori, D.; Teo, S.; bin Ahmad, H.S.; Watanabe, T. Structures of triterpenoids from the leaves of Lansium domesticum. J. Nat. Med. 2019, 73, 727–734. [Google Scholar] [CrossRef]
- Putri, N.K.; Fajriah, S.; Yusuf, M.; Maharani, R.; Anshori, J.A.; Supratman, U.; Mayanti, T. 3-Hydroxy-8, 14-secogammacera-7, 14-dien-21-one: A new onoceranoid triterpenes from Lansium domesticum corr. Cv kokossan. Molbank 2020, 2020, M1157. [Google Scholar]
- Tsuchiya, A.; Makita, Y.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M.; Arai, M.A. Isolation and evaluation of cardenolides from Lansium domesticum as notch inhibitors. J. Nat. Med. 2020, 74, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Fadhilah, K.; Wahyuono, S.; Astuti, P. A bioactive compound isolated from Duku (Lansium domesticum Corr) fruit peels exhibits cytotoxicity against T47D cell line. F1000Research 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Marfori, E.C.; Kajiyama, S.I.; Fukusaki, E.-I.; Kobayashi, A. Lansioside D, a new triterpenoid glycoside antibiotic from the fruit peel of Lansium domesticum Correa. J. Pharmacogn. Phytochem. 2015, 3, 140–143. [Google Scholar]
- Alimon, H.; Sani, A.A.; Abdul Azziz, S.; Daud, N.; Arriffin, N.M.; Bakri, Y. Antimicrobial activities of three different seed extracts of Lansium varieties. Pertanika J. Sci. Technol. 2014, 22, 529–540. [Google Scholar]
- Kiang, A.; Tan, E.; Lim, F.; Habaguchi, K.; Nakanishi, K.; Fachan, L.; Ourisson, G. Lansic acid, a bicyclic triterpene. Tetrahedron Lett. 1967, 8, 3571–3574. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018; p. 238.
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Potential anti-malarial agents from endophytic fungi: A review. Mini Rev. Med. Chem. 2018, 18, 1110–1132. [Google Scholar] [CrossRef]
- Arnason, J.T.; Guillet, G.; Durst, T. Phytochemical diversity of insect defenses in tropical and temperate plant families. In Advances in Insect Chemical Ecology; Carde, R.T., Carde, J.G., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 1–20. [Google Scholar]
- Purwani, S.; Nahar, J.; Zulfikar, Z.; Nurlelasari, N.; Mayanti, T. Molecular docking on kokosanolide A and C for anticancer activity against human breast cancer cell MCF-7. J. Kim. Valensi 2021, 1, 52–57. [Google Scholar] [CrossRef]
- Koul, O. Phytochemicals and insect control: An antifeedant approach. Crit. Rev. Plant Sci. 2008, 27, 1–24. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A.; Saritha, V.; Duraipandiyan, V.; Muthukumar, C.; Kim, S.-J. Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol. 2013, 13, 105. [Google Scholar] [CrossRef]
- Purrington, C. Secondary Products. Antifeedant Substances in Plants; Brian Thomas, E.O.A.P.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Leatemia, J.A.; Isman, M.B. Insecticidal activity of crude seed extracts of annona spp., Lansium domesticum and Sandoricum koetjape against lepidopteran larvae. Phytoparasitica 2004, 32, 30–37. [Google Scholar] [CrossRef]
- Adam, D. How far will global population rise? Researchers can’t agree. Nature 2021, 597, 462–465. [Google Scholar] [CrossRef]
- Verma, S.; Yadav, A. Rising trends towards the development of oral herbal male contraceptive: An insight review. Futur. J. Pharm. Sci. 2021, 7, 23. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Telikepalli, H.; McGhee, E.; Shankel, D.M. Natural antimutagenic agents. Mutat. Res. Fundam. Mol. Mech. Mutagenes. 1996, 350, 143–152. [Google Scholar] [CrossRef]
- Akram, M.; Riaz, M.; Wadood, A.W.C.; Hazrat, A.; Mukhtiar, M.; Ahmad Zakki, S.; Daniyal, M.; Shariati, M.A.; Said Khan, F.; Zainab, R. Medicinal plants with anti-mutagenic potential. Biotechnol. Biotechnol. Equip. 2020, 34, 309–318. [Google Scholar] [CrossRef]
- Bhattacharya, S. Natural antimutagens: A review. Res. J. Med. Plant 2011, 5, 116–126. [Google Scholar] [CrossRef]
- Klungsupya, P.; Suthepakul, N.; Laovitthayanggoon, S.; Thongdon, A.; Trangwacharakul, S.; Phornchirasilp, S. Investigation on antioxidant, antimutagenic and cytotoxic properties of active fractions of Thai longkong (Lansium domesticum corr.) fruits. J. Ethnobiol. Ethanopharmacol. 2012, 1, 1–9. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting drug chemo-resistance in cancer using natural products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Khalili, R.M.A.; Noratiqah, J.M.; Norhaslinda, R.; Amin, B.A.; Roslan, A.; Zubaidi, A.L.A. Cytotoxicity effect and morphological study of different Duku (Lansium domesticum corr.) extract towards human colorectal adenocarcinoma cells line (HT-29). Pharmacog. J. 2017, 9, 757–761. [Google Scholar] [CrossRef]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The canonical notch signaling pathway: Structural and biochemical insights into shape, sugar, and force. Dev. Cell 2017, 41, 228–241. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G.S.; Wu, K. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett. 2015, 369, 20–27. [Google Scholar] [CrossRef]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Subandrate, S.; Sinulingga, S.; Wahyuni, S.; Altiyan, M.F.; Fatmawati, F. Antioxidant potential of Lansium domesticum corr. Seed extract in white male rat (Rattus novergicus) induced by alcohol. Molekul 2016, 11, 1–8. [Google Scholar] [CrossRef][Green Version]
- Yamin, Y.; Ruslin, R.; Sabarudin, S.; Sida, N.A.; Kasmawati, H. Determination of antiradical activity, total phenolic, and total flavonoid contents of extracts and fractions of langsat (Lansium domesticum coor.) Seeds. Borneo J. Pharm. 2020, 3, 249–256. [Google Scholar] [CrossRef]
- Apridamayanti, P.; Fajriaty, I.; Hatita, E. Antioxidant activity and analgesic assessment of Lansium domesticum stem bark infusion. Nusant. Biosci. 2018, 10, 71–75. [Google Scholar] [CrossRef]
- Nur, S.; Rumiyati, R.; Lukitaningsih, E. Screening of antioxidants, anti-aging and tyrosinase inhibitory activities of ethanolic and ethyl acetate extracts of fruit flesh and fruit peel langsat (Lansium domesticum corr) in vitro. Maj. Obat. Tradis. 2017, 22, 63–72. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, S.R.; Mohamed, G.A.; Khayat, M.T.; Ahmed, S.; Abo-Haded, H.; Alshali, K.Z. Mangostanaxanthone VIIII, a new xanthone from Garcinia mangostana pericarps, α-amylase inhibitory activity, and molecular docking studies. Rev. Bras. Farmacogn. 2019, 29, 206–212. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Zayed, M.F.; Ross, S.A. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017, 70, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.-J.; Zheng, S.-Q.; Huang, X.-B.; Xing, T.-K.; Wu, G.-S.; Sun, H.-Y.; Qi, S.-H.; Luo, H.-R. Current perspective in the discovery of anti-aging agents from natural products. Nat. Prod. Bioprospect. 2017, 7, 335–404. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Mills, S.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Emanuela, R.; Garret, A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar]
- Wang, T.-X.; Wu, G.-J.; Jiang, J.-G. Natural products with analgesic effect from herbs and nutraceuticals used in traditional chinese medicines. Curr. Mol. Med. 2020, 20, 461–483. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Abouelhassan, Y.; Garrison, A.T.; Yang, H.; Chávez-Riveros, A.; Burch, G.M.; Huigens, R.W., III. Recent progress in natural-product-inspired programs aimed to address antibiotic resistance and tolerance. J. Med. Chem. 2019, 62, 7618–7642. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells. Ann. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef]
- Sun, J.; Xiang, H.; Yang, L.-L.; Chen, J.-B. A review on steroidal 5α-reductase inhibitors for treatment of benign prostatic hyperplasia. Curr. Med. Chem. 2011, 18, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Srivilai, J.; Minale, G.; Scholfield, C.N.; Ingkaninan, K. Discovery of natural steroid 5 alpha-reductase inhibitors. Assay Drug Dev. Technol. 2019, 17, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; de Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.J.; García-Martínez, O. Potential effects of phenolic compounds that can be found in olive oil on wound healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Cucurbitacin E glucoside alleviates concanavalin A-induced hepatitis through enhancing SIRT1/Nrf2/HO-1 and inhibiting NF-ĸB/NLRP3 signaling pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Jaiswal, L.; Aparna, R.S.L.; Prasad, R.G.S.V.; Kumar, G.P.; Manohara, C.M. Wound healing potential of green synthesized silver nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Express 2015, 5, 159–164. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.M.; Siddiqui, M.; AlSalhi, M.S.; Alrokayan, S.A. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2011, 43, 1266–1271. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Aparna, R.; Prasad, R. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 2014, 137, 75–78. [Google Scholar] [CrossRef]
- Rahma, K.; Widodo, A.D.W.; Setiabudi, R.J.; Roestamadji, R.I.; Rochmanti, M.; Lestari, P. Antibacterial activity of synthesized silver nanoparticle using langsat leaf extract (Lansium domesticum var. pubescen kooders et valeton) as bioreductor against Escherichia coli and Staphylococcus aureus. In Proceedings of the 1st Jenderal Soedirman International Medical Conference (JIM) in Conjunction with the Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia (KIBI), SCITEPRESS—Science and Technology, Online Meeting, 28–29 November 2021; Volume 1, pp. 298–304. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Landau, M. Exogenous factors in skin aging. Environ. Factors Ski. Dis. 2007, 35, 1–13. [Google Scholar]
- Lephart, E.D. Equol’s anti-aging effects protect against environmental assaults by increasing skin antioxidant defense and ECM proteins while decreasing oxidative stress and inflammation. Cosmetics 2018, 5, 16. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Tulipani, S.; Gonzàles-Paramàs, A.M.; Santos-Buelga, C.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Photoprotective potential of strawberry (Fragaria ananassa) extract against UV-A irradiation damage on human fibroblasts. J. Agric. Food Chem. 2012, 60, 2322–2327. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Lukitaningsih, E.; Saputro, A.H.; Widiasri, M.; Khairunnisa, N.; Prabaswari, N.; Kuswahyuningsih, R. In vitro antiaging analysis of topical pharmaceutical preparation containing mixture of strawberry fruit, pomelo peel, and langsat fruit extracts. Indones. J. Chemom. Pharm. Anal. 2020, 1, 53–61. [Google Scholar] [CrossRef]
- Hiola, R.; Rizky, N.; Hasan, H.; Thomas, N. Formulation and evaluation of langsat (Lansium domesticum corr.) peel ethanol extracts lotion as anti-mosquito repellent. J. Rep. Pharm. Sci. 2018, 7, 250–259. [Google Scholar]
- Wih, W.L.; Ranti, A.S.; Wasitaatmadja, S.; Suryaningsih, S.; Junardy, F.; Maily, M. Penelitian bahan pencerah dan pelembab kulit dari tanaman Indonesia. Maj. Ilmu Kefarmasian 2009, 6, 1. [Google Scholar]
- Luepke, N. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Curry, A.; Gettings, S.; McEwen, G. The Cosmetic, Toiletry, and Fragrance Association Safety Testing Guidelines: Evaluation of Primary Skin Irritation Potential; CTFA: Washington, DC, USA, 1991; Volume 6, pp. 1–5. [Google Scholar]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of olive mill wastewater by membrane processes to recover natural antioxidant compounds for cosmeceutical and nutraceutical applications or functional foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]

| Forms/Variety | Botanical Characteristics | Ref. |
|---|---|---|
| Langsat | Fruits are bunched together ≈ 20 on one brown thick spike up to 20 cm length. Its fruit is oval or round ≈ 2–3 cm long and has a yellowish skin, which when peeled release a latex, showing up a translucent white flesh that is divided into segments and has 1–3 seeds. On ripping, the flesh is fairly aromatic and juicy with a sweet-acidic taste. | [11] |
| Duku | Fruits are bunched together ≈ 8–12, on one brown thick spike up to 20 cm length. Duku fruit is featured from langsat fruit by its larger size (3–5 cm in diameter), round shape, and much thicker skin that is comparatively free from latex. Also, it is generally more aromatic and sweeter than langsat. | [11] |
| Dokong (Longkong) | Fruits are occurred in bunches (25–30 fruits/bunch). Its fruit is globular with leathery, thick, and yellow skin, free of latex. The edible portion is juicy and fleshy is thin-skinned, nearly seedless, and free of latex, with uneven five-fragmented translucent white adhering aril. It has a nice aroma with a slightly sour and sweet taste. | [12,13] |
| Duku-langsat | It is round, brownish-yellow, and intermediate in size. It has a sweet flesh and thinner skin than that of duku. | [5] |
| L. domesticum var. typica | Inflorescence: rachises, young branchlets, under the surface of leaves, and calyx sparsely pubescent or sub-glabrous. Fruit: oblong-obovoid or ellipsoid, pericarp thin with little milky juice, seeds small, aril thick and smooth. | [14] |
| L. domesticum var. pubescens Koorders et Valeton | Inflorescence: young branchlets, rachises, calyx densely pubescent, under the surface of leaves. Fruit: sub-globose, pericarp thick with milky copious juice, thin and sour aril, large seeds. | [14] |
| Nationality | Name |
|---|---|
| English | Langsat, Duku |
| Burmese | Duku, Langsak |
| Filipino | Lanzone, Buahan, Lansones, Lanzon, Lansone |
| Indonesian | Langsat, Kokosan, Lanset Duku, Langsa, Lansot, Lasa, Lansat |
| Italian | Lansio, Lanzone |
| Malay | Langseh, Lansa, Langsep, Kokosan, Pijitan |
| Thai | Longkong, Duku, Langsat |
| Vietnamese | Bo‘N-Bon |
| Chinese | Lan Sa, Lan Sa Guo |
| Japanese | Ransa |
| Spanish | Arbol De Lanza, Lanzón |
| Portuguese | Arbol-Do-Lanza |
| Surinam | Duki |
| Malaysia | Dokong, Duku Hutan, Duku, Duku-Langsat, Langsat-Hutan, Longkong, Langsat |
| Korean | Lang Sat |
| Danish | Langsat, Langsep |
| French | Lansium, Langsep |
| Dutch | Doekoe, Langsep |
| Costa Rica | Duki |
| Cuba | Duku, Kokosan |
| German | Doko, Echter-Lanzebaum, Duku, Lansabaum, Langsta, Lansibaum |
| Honduras | Duki |
| Taiwan | Lan sa guo |
| Kenya | lengeset |
| Sundanese | Kokosan, Pisitan |
| Javanese | Langsep, langsat, celoring |
| Madurese | Langsep |
| Forms/Variety | Botanical Characteristics | Ref. |
|---|---|---|
| Fruit peels | In Java, it is dried and burned as incense in the sick people’s rooms and to repel mosquitoes. It is utilized to cure diarrhea and intestinal parasites. Fruit peels are used as an arrow poison. | [25,26] |
| It is applied to the skin as a moisturizer and skin whitening cream. Borneo, it is utilized as talc powder by indigenous females of Dayak for skin protection from the sun. | [10,27] | |
| Seeds | Pulverized seeds mixed with water are utilized as a vermifuge for children. Also, they are utilized as a febrifuge. In Peninsular Malaysia, among the Sakai the bitter seeds were crushed and utilized for curing fevers. In the Philippines, pounded seeds mixed with water are used for deworming and ulcers. | [22,23,28] |
| Bark | A poultice of bark used against scorpion stings. A decoction is taken for malaria and dysentery treatment in Java, Borneo, and Malaya. A tincture is useful as an anti-colic or anti-diarrhetic. In Kenya, the bark is used for spleen and fever. In Borneo, bark stew water decoction is taken by rural communities as an antifertility medicine. | [8,17,26,29,30,31] |
| Resin | It halts diarrhea and intestinal spasms. The resin from the bark is given for swellings, flatulence, and spasm. | [8] |
| Leaf | Its juice is utilized as eye drops to eleminate inflammation. A decoction of leaves and bark has been taken for curing dysentery. The Philippines used leaves for the control of mosquitoes. In Ibans in Sarawak, Malaysia leaves are used to treat fever. | [26,32,33] |
| Peel and flesh | It is used as facial masks, wash gels, and toners. Peel is known to be toxic to domestic animals. | [10,34] |
| Wood tar | It is used for blackening teeth. | [5] |
| Wood | It is used for tool handles, house posts, and rafters | [16] |
| Bark and fruit | The fruit skin‘s juice and bark are utilized as a Dyak arrow poison. | [5] |
| Seed and bark | A decoction of seed and bark is used for the enlargement of spleen and fever in Kenya. | [30] |
| Stem | The decoction of the langsat stems and bark of Pterocarpus indica assists treating dysentery. | [35] |
| Compound Name | Biological Activity | Assay, Organism, or Cell Line | Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| Germacrene D (1) | Antimicrobial | Agar well/Escherichia coli | 12.0 mm (CZ) * | Chloramphenicol 23.0 mm (CZ) | [58] |
| Agar well/Pseudomonas aeruginosa | 11.0 mm (CZ) | Chloramphenicol 8.0 (CZ) | [58] | ||
| Agar well/Candida albicans | 14.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Aspergillus niger | 13.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Trichophyton mentagrophytes | 13.0 mm (CZ) | Chloramphenicol 50.0 (mm (CZ) | [58] | ||
| Lansioside A (35) | Anti-leukotriene D4 | leukotriene D4/guinea pig ileum | 2.4 ppm (IC50) | - | [19] |
| Lansioside B (36) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrugresistant strain) | >20.0 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Lansioside C (37) | Antimicrobial | Agar well/Staphylococcus aureus | 19.0 mm (CZ) | Chloramphenicol 25.0 mm (CZ) | [58] |
| Agar well/Escherichia coli | 12.0 mm (CZ) | Chloramphenicol 23.0 mm (CZ) | [58] | ||
| Agar well/Pseudomonas aeruginosa | 12.0 mm (CZ) | Chloramphenicol 8.0 mm (CZ) | [58] | ||
| Agar well/Bacillus subtitis | 26.0 mm (CZ) | Chloramphenicol 20.0 mm (CZ) | [58] | ||
| Agar well/Candida albicans | 13.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Aspergillus niger | 14.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Trichophyton mentagrophytes | 20.0 mm (CZ) | Chloramphenicol 50.0 mm (CZ) | [58] | ||
| Methyl lansiolate (38) | Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine sensitive strain) | 0.65 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] |
| Microculture radioisotope/P. falciparum (W2, chloroquine resistant strain) | 0.76 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 128.0 cells % survival | - | [68] | |
| Lansiolic acid (39) | Antimicrobial | Agar well/Staphylococcus aureus | 12.0 mm (CZ) | Chloramphenicol 25.0 mm (CZ) | [58] |
| Agar well/Escherichia coli | 11.0 mm (CZ) | Chloramphenicol 23.0 mm (CZ) | [58] | ||
| Agar well/Pseudomonas aeruginosa | 12.0 mm (CZ) | Chloramphenicol 8.0 mm (CZ) | [58] | ||
| Agar well/Bacillus subtitis | 13.0 mm (CZ) | Chloramphenicol 20.0 mm (CZ) | [58] | ||
| Agar well/Candida albicans | 14.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Aspergillus niger | 14.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Trichophyton mentagrophytes | 14.0 mm (CZ) | Chloramphenicol 50.0 mm (CZ) | [58] | ||
| Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine-sensitive strain) | >10 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] | |
| Microculture radioisotope/P. falciparum (W2, chloroquine- resistant strain) | >10 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Microculture radioisotope/P. falciparum (K1, multidrug-resistant strain) | >20.0 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] | ||
| Cytotoxicity | SRB/KB | 116.1 cells % survival | - | [68] | |
| Dukunolide C (42) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug-resistant strain) | 5.2 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Kokosanolide A (47) | Cytotoxicity | MTT/MCF-7 | 8.62 μg/mL (IC50) | - | [98] |
| Kokosanolide B (48) | Antibacterial | Disc diffusion/E. coli | 8.0 mm (IZD) | Vancomycin 17.5 mm (IZD) Chloramphenicol 18.5 mm (IZD) Sulphonamide 9 mm (IZD) | [77] |
| 8,14-Secogammacera-7,14-diene-3,21-dione (51) | Antibacterial | Disc diffusion/E. coli | 7.5 mm (IZD) | Vancomycin 17.5 mm (IZD) Chloramphenicol 18.5 mm (IZD) Sulphonamide 9 mm (IZD) | [77] |
| α,γ-Onoceradienedione = 8,14-Secogammacera-7,14(27)-diene-3,21-dione (52) | Antimicrobial | Agar well/Pseudomonas aeruginosa | 13.0 mm (CZ) | Chloramphenicol 8.0 mm (CZ) | [58] |
| Agar well/Candida albicans | 13.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Aspergillus niger | 12.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Trichophyton mentagrophytes | 13.0 mm (CZ) | Chloramphenicol 50.0 mm (CZ) | [58] | ||
| Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine sensitive strain) | 1.66 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] | |
| Microculture radioisotope/P. falciparum (W2, chloroquine resistant strain) | 1.83 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 131.5 cells % survival | - | [68] | |
| MTT/HeLa | 32.39 μg/mL (IC50) | Doxorubicin 2.83 μg/mL (IC50) | [80] | ||
| MTT/T-47D | 30.69 μg/mL (IC50) | Doxorubicin 0.04 μg/mL (IC50) | [80] | ||
| MTT/A549 | 13.71 μg/mL (IC50) | - | [80] | ||
| Domesticulide A (58) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | >20.0 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Domesticulide B (59) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 3.2 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Domesticulide C (60) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 2.4 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Domesticulide D (61) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 6.9 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Domesticulide E (62) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | >20.0 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| 6-Hydroxymexicanolide (63) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | >20.0 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| 6-Acetoxymexicanolide (64) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 9.7 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Methyl angolensate (65) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 5.9 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Methyl 6-hydroxyangolensate (66) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | >20.0 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Methyl 6-acetoxyangolensate (67) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 3.8 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Azadiradione (68) | Antimalarial | Microculture radioisotope/P. falciparum (K1, multidrug resistant strain) | 2.9 µg/mL (IC50) | Artemisinin 0.001–0.003 µg/mL (IC50) | [65] |
| Onoceratriene (69) | Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine sensitive strain) | >10 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] |
| Microculture radioisotope/P. falciparum (W2, chloroquine resistant strain) | >10 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 108.3 cells % survival | - | [68] | |
| Lansionic acid = 3-ketolansiolic acid (70) | Antimicrobial | Agar well/Escherichia coli | 12.0 mm (CZ) | Chloramphenicol 23.0 mm (CZ) | [58] |
| Agar well/Pseudomonas aeruginosa | 12.0 mm (CZ) | Chloramphenicol 8.0 mm (CZ) | [58] | ||
| Agar well/Bacillus subtitis | 13.0 mm (CZ) | Chloramphenicol 20.0 mm (CZ) | [58] | ||
| Agar well/Candida albicans | 12.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Aspergillus niger | 14.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Trichophyton mentagrophytes | 15.0 mm (CZ) | Chloramphenicol 50.0 mm (CZ) | [58] | ||
| Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine-sensitive strain) | >10 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] | |
| Microculture radioisotope/P. falciparum (W2, chloroquine-resistant strain) | >10 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 129.1 cells % survival | - | [68] | |
| Lansionic acid A = Lansiolic acid A (71) | Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine-sensitive strain) | >10 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] |
| Microculture radioisotope/P. falciparum (W2, chloroquine resistant strain) | >10 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 134.5 cells % survival | - | [68] | |
| 21α-Hydroxyonocera-8(26),14-dien-3-one = 3-keto-22-hydroxyonoceradiene (72) | Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine-sensitive strain) | 2.41 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] |
| Microculture radioisotope/P. falciparum (W2, chloroquine-resistant strain) | >10 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 113.9 cells % survival | - | [68] | |
| Methyl lansiolate A (73) | Antimalarial | Microculture radioisotope/P. falciparum (D6, chloroquine-sensitive strain) | 0.69 µg/mL (IC50) | Artemisinin 0.0015 µg/mL (IC50) Chloroquine 0.0045 µg/mL (IC50) | [68] |
| Microculture radioisotope/P. falciparum (W2, chloroquine-resistant strain) | 1.02 µg/mL (IC50) | Artemisinin 0.0035 µg/mL (IC50) Chloroquine 0.0065 µg/mL (IC50) | [68] | ||
| Cytotoxicity | SRB/KB | 66.7 cells % survival | - | [68] | |
| Lamesticumin A (85) | Antibacterial | Microdilution/S. aureus | 6.25 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] |
| Microdilution/S. epidermidis | 12.5 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/M. luteus | 6.25 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/B. subtilis | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/M. pyogenes | 3.12 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] | ||
| Microdilution/B. cereus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Cytotoxicity | MTT/T-47D | 15.68 µg/mL (IC50) | Doxorubicin 0.18 µg/mL (IC50) | [35] | |
| 3β-Hydroxyonocera-8(26),14-dien-21-one (90) | Antimicrobial | Agar well/Escherichia coli | 11.0 mm (CZ) | Chloramphenicol 23.0 mm (CZ) | [58] |
| Agar well/Pseudomonas aeruginosa | 12.0 mm (CZ) | Chloramphenicol 8.0 mm (CZ) | [58] | ||
| Agar well/Candida albicans | 14.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Aspergillus niger | 15.0 mm (CZ) | Chloramphenicol 10.0 mm (CZ) | [58] | ||
| Agar well/Trichophyton mentagrophytes | 13.0 mm (CZ) | Chloramphenicol 50.0 mm (CZ) | [58] | ||
| Obebioside A (93) | Notch inhibitor | Luciferase/LS174T cells | 1.65 μM (IC50) | DAPT 20 nM (IC50) | [90] |
| Honghelin (95) | Notch inhibitor | Luciferase/LS174T cells | 0.62 μM (IC50) | DAPT 20 nM (IC50) | [90] |
| Obeside B (96) | Notch inhibitor | Luciferase/LS174T cells | 0.51 μM (IC50) | DAPT 20 nM (IC50) | [90] |
| 2-Ethyl,l,3-(2‘-menthene)propenal (99) | Cytotoxicity | MTT/T-47D | 48.58 µg/mL (IC50) | Doxorubicin 0.43 µg/mL (IC50) | [35] |
| MTT/HepG2 | 127.45 µg/mL (IC50) | Doxorubicin 1.18 µg/mL (IC50) | [35] | ||
| Lamesticumin G (100) | α-Glucosidase inhibitory | Colorimetric/Maltase | 2.27 mM (IC50) | Acarbose 0.0021 mM (IC50) | [66] |
| 17(20)E-dyscusin B (101) | NO inhibition | MTS/RAW264.7 | 9.13 μM (IC50) | L-NMMA 0.18 µM (IC50) | [86] |
| 17(20)Z-dyscusin B (102) | NO inhibition | MTS/RAW264.7 | 14.03 μM (IC50) | L-NMMA 0.18 µM (IC50) | [86] |
| Lamesticumin B (106) | Antibacterial | Microdilution/S. aureus | 6.25 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] |
| Microdilution/S. epidermidis | 12.5 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/M. luteus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/B. subtilis | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/M. pyogenes | 3.12 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] | ||
| Microdilution/B. cereus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Lamesticumin C (107) | Antibacterial | Microdilution/S. aureus | 6.25 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] |
| Microdilution/S. epidermidis | 12.5 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/M. luteus | 6.25 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/B. subtilis | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Microdilution/M. pyogenes | 3.12 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] | ||
| Microdilution/B. cereus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Lamesticumin D (108) | Antibacterial | Microdilution/B. subtilis | 6.25 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] |
| Microdilution/B. cereus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Lamesticumin E (109) | Antibacterial | Microdilution/B. subtilis | 12.5 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] |
| Microdilution/M. pyogenes | 6.25 µg/mL (MIC) | Magnolol 25.0 µg/mL (MIC) | [87] | ||
| Microdilution/B. cereus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
| Lamesticumin F (110) | Antibacterial | Microdilution/B. subtilis | 12.5 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] |
| Microdilution/B. cereus | 3.12 µg/mL (MIC) | Magnolol 12.5 µg/mL (MIC) | [87] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M. Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities. Nutrients 2022, 14, 1531. https://doi.org/10.3390/nu14071531
Abdallah HM, Mohamed GA, Ibrahim SRM. Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities. Nutrients. 2022; 14(7):1531. https://doi.org/10.3390/nu14071531
Chicago/Turabian StyleAbdallah, Hossam M., Gamal A. Mohamed, and Sabrin R. M. Ibrahim. 2022. "Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities" Nutrients 14, no. 7: 1531. https://doi.org/10.3390/nu14071531
APA StyleAbdallah, H. M., Mohamed, G. A., & Ibrahim, S. R. M. (2022). Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities. Nutrients, 14(7), 1531. https://doi.org/10.3390/nu14071531








