Low Hemoglobin Levels Are Associated with Reduced Psychomotor and Language Abilities in Young Ugandan Children
Abstract
1. Introduction
2. Materials and Methods
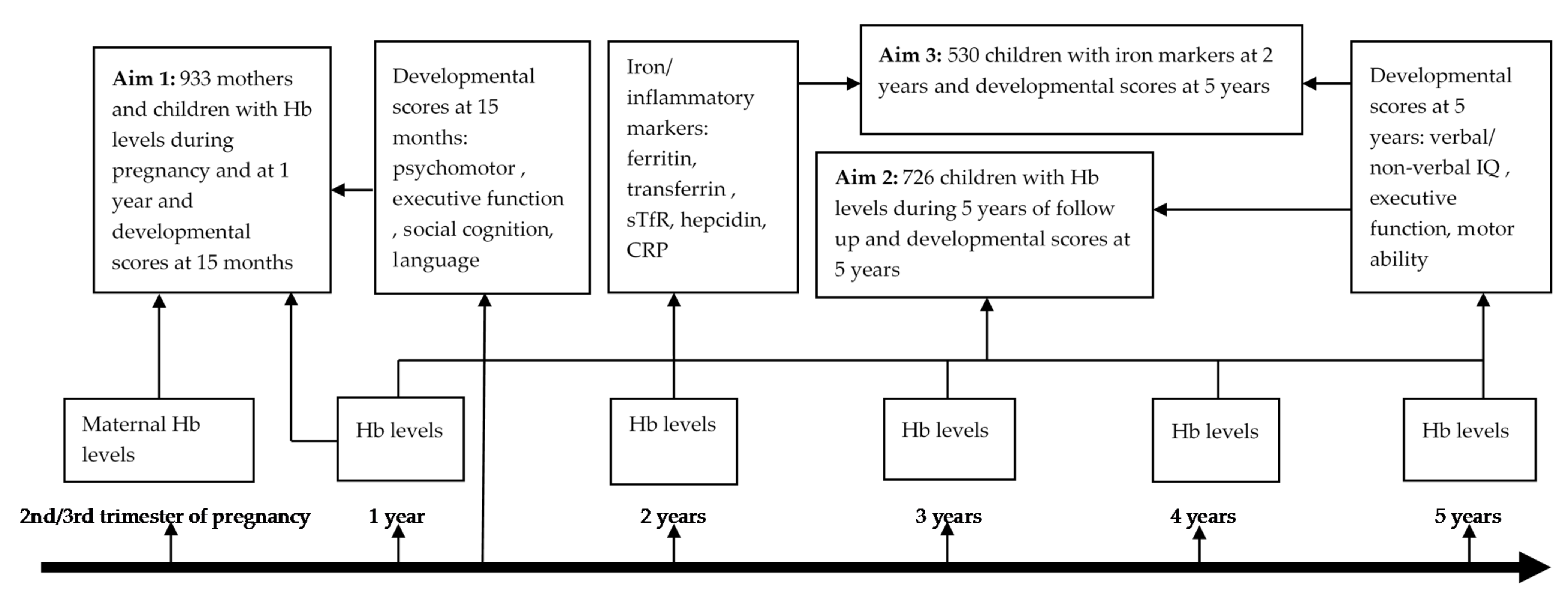
2.1. Study Design and Setting
2.2. Laboratory Procedures and Definitions
2.3. Developmental Assessments
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Study Participants
3.2. Associations between Participant Characteristics and Developmental Scores at 15 Months and Five Years
3.3. Anemia during Pregnancy Is Associated with Reduced Psychomotor Scores in Children at 15 Months
3.4. Lower Hemoglobin Levels at 12 Months of Age Are Associated with Reduced Psychomotor and Language Scores at 15 Months
3.5. Hemoglobin Levels and Anemia in Mothers and Children Are Not Associated with Developmental Scores at Five Years
3.6. Iron Status at Two Years Was Not Associated with Developmental Scores at Five Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCoy, D.C.; Peet, E.D.; Ezzati, M.; Danaei, G.; Black, M.M.; Sudfeld, C.R.; Fawzi, W.; Fink, G. Early childhood developmental status in low-and middle-income countries: National, regional, and global prevalence estimates using predictive modeling. PLoS Med. 2016, 13, e1002034. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Walker, S.P.; Fernald, L.C.H.; Andersen, C.T.; DiGirolamo, A.M.; Lu, C.; McCoy, D.C.; Fink, G.; Shawar, Y.R.; Shiffman, J.; et al. Early childhood development coming of age: Science through the life course. Lancet 2017, 389, 77–90. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Angel, M.D.; Rohner, F. The Proportion of Anemia Associated with Iron Deficiency in Low, Medium, and High Human Development Index Countries: A Systematic Analysis of National Surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef]
- Muriuki, J.M.; Mentzer, A.J.; Webb, E.L.; Morovat, A.; Kimita, W.; Ndungu, F.; Macharia, A.W.; Crane, R.J.; Berkley, J.A.; Lule, S.; et al. Estimating the burden of iron deficiency among African children. BMC Med. 2020, 18, 31. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- WHO. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015; p. 43. [Google Scholar]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Todorich, B.; Pasquini, J.M.; Garcia, C.I.; Paez, P.M.; Connor, J.R. Oligodendrocytes and myelination: The role of iron. Glia 2009, 57, 467–478. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Green, A.R. Iron deficiency and neurotransmitter synthesis and function. Proc. Nutr. Soc. 1978, 37, 173–179. [Google Scholar] [CrossRef]
- Beard, J.L.; Felt, B.; Schallert, T.; Burhans, M.; Connor, J.R.; Georgieff, M.K. Moderate iron deficiency in infancy: Biology and behavior in young rats. Behav. Brain Res. 2006, 170, 224–232. [Google Scholar] [CrossRef]
- Breton, A.B.; Fox, J.A.; Brownson, M.P.; McEchron, M.D. Postnatal nutritional iron deficiency impairs dopaminergic-mediated synaptic plasticity in the CA1 area of the hippocampus. Nutr. Neurosci. 2015, 18, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef] [PubMed]
- Bourque, S.L.; Iqbal, U.; Reynolds, J.N.; Adams, M.A.; Nakatsu, K. Perinatal Iron Deficiency Affects Locomotor Behavior and Water Maze Performance in Adult Male and Female Rats. J. Nutr. 2008, 138, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Youdim, M.E.; Mostofsky, D.I. Brain iron-deficiency causes reduced learning capacity in rats. Pharmacol. Biochem. Behav. 1986, 25, 141–144. [Google Scholar] [CrossRef]
- Prado, E.L.; Abbeddou, S.; Adu-Afarwuah, S.; Arimond, M.; Ashorn, P.; Ashorn, U.; Bendabenda, J.; Brown, K.H.; Hess, S.Y.; Kortekangas, E.; et al. Predictors and pathways of language and motor development in four prospective cohorts of young children in Ghana, Malawi, and Burkina Faso. J. Child Psychol. Psychiatry 2017, 58, 1264–1275. [Google Scholar] [CrossRef]
- Mireku, M.O.; Davidson, L.L.; Koura, G.K.; Ouédraogo, S.; Boivin, M.J.; Xiong, X.; Accrombessi, M.M.K.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. Prenatal Hemoglobin Levels and Early Cognitive and Motor Functions of One-Year-Old Children. Pediatrics 2015, 136, e76–e83. [Google Scholar] [CrossRef]
- Mireku, M.O.; Davidson, L.L.; Boivin, M.J.; Zoumenou, R.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. Prenatal Iron Deficiency, Neonatal Ferritin, and Infant Cognitive Function. Pediatrics 2016, 138, e20161319. [Google Scholar] [CrossRef]
- Webb, E.L.; Mawa, P.A.; Ndibazza, J.; Kizito, D.; Namatovu, A.; Kyosiimire-Lugemwa, J.; Nanteza, B.; Nampijja, M.; Muhangi, L.; Woodburn, P.W. Effect of single-dose anthelmintic treatment during pregnancy on an infant’s response to immunisation and on susceptibility to infectious diseases in infancy: A randomised, double-blind, placebo-controlled trial. Lancet 2011, 377, 52–62. [Google Scholar] [CrossRef]
- WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Guideline on Use of Ferritin Concentrations to Assess Iron Status in Populations; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- WHO Multicentre Growth Reference Study Group; de Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar]
- Abubakar, A.; Holding, P.; Van De Vijver, F.J.R.; Bomu, G.; van Baar, A. Developmental monitoring using caregiver reports in a resource-limited setting: The case of Kilifi, Kenya. Acta Paediatr. 2010, 99, 291–297. [Google Scholar] [CrossRef]
- Abubakar, A.; Holding, P.; van Baar, A.; Newton, C.; Van De Vijver, F.J.R. Monitoring psychomotor development in a resource-limited setting: An evaluation of the Kilifi Developmental Inventory. Ann. Trop. Paediatr. 2008, 28, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Nampijja, M.; Apule, B.; Lule, S.; Akurut, H.; Muhangi, L.; Webb, E.L.; Lewis, C.; Elliott, A.M.; Alcock, K.J. Effects of Maternal Worm Infections and Anthelminthic Treatment during Pregnancy on Infant Motor and Neurocognitive Functioning. J. Int. Neuropsychol. Soc. 2012, 18, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Nampijja, M.; Apule, B.; Lule, S.; Akurut, H.; Muhangi, L.; Elliott, A.M.; Alcock, K.J. Adaptation of Western measures of cognition for assessing 5-year-old semi-urban Ugandan children. Br. J. Educ. Psychol. 2010, 80, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 359S–371S. [Google Scholar] [PubMed]
- Nampijja, M.; Kizindo, R.; Apule, B.; Lule, S.; Muhangi, L.; Titman, A.; Elliott, A.; Alcock, K.; Lewis, C. The role of the home environment in neurocognitive development of children living in extreme poverty and with frequent illnesses: A cross-sectional study. Wellcome Open Res. 2018, 3, 152. [Google Scholar] [CrossRef] [PubMed]
- WHO. Nutritional Anaemias: Tools for Effective Prevention and Control; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Geng, F.; Mai, X.; Zhan, J.; Xu, L.; Zhao, Z.; Georgieff, M.; Shao, J.; Lozoff, B. Impact of Fetal-Neonatal Iron Deficiency on Recognition Memory at 2 Months of Age. J. Pediatr. 2015, 167, 1226–1232. [Google Scholar] [CrossRef]
- Blackburn, S. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 216–218. [Google Scholar]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Bremner, J.G.; Wachs, T.D. The Wiley-Blackwell Handbook of Infant Development, Volume 2: Applied and Policy Issues; Blackwell Publishers Limited: West Sussex, UK, 2010; pp. 14–27. [Google Scholar]
- Kariger, P.K.; Stoltzfus, R.J.; Olney, D.; Sazawal, S.; Black, R.; Tielsch, J.; Frongillo, E.A.; Khalfan, S.S.; Pollitt, E. Iron Deficiency and Physical Growth Predict Attainment of Walking but Not Crawling in Poorly Nourished Zanzibari Infants. J. Nutr. 2005, 135, 814–819. [Google Scholar] [CrossRef]
- Olney, D.K.; Pollitt, E.; Kariger, P.K.; Khalfan, S.S.; Ali, N.S.; Tielsch, J.; Sazawal, S.; Black, R.; Mast, D.; Allen, L.H.; et al. Young Zanzibari Children with Iron Deficiency, Iron Deficiency Anemia, Stunting, or Malaria Have Lower Motor Activity Scores and Spend Less Time in Locomotion. J. Nutr. 2007, 137, 2756–2762. [Google Scholar] [CrossRef][Green Version]
- Madanijah, S.; Sukandar, D.; Fitri, Y.; Ahmad, A. Assessment of Anemia Status on Physical Development Skills of Children Under Two Years Old in Aceh, Indonesia. J. Nutr. Sci. Vitaminol. 2020, 66, S463–S467. [Google Scholar] [CrossRef]
- Rothman, M.; Faber, M.; Covic, N.; Matsungo, T.M.; Cockeran, M.; Kvalsvig, J.D.; Smuts, C.M. Infant Development at the Age of 6 Months in Relation to Feeding Practices, Iron Status, and Growth in a Peri-Urban Community of South Africa. Nutrients 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.A.; Hassan, E.S.; Yasien, D.G. Effect of iron deficiency anemia on language development in preschool Egyptian children. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110114. [Google Scholar] [CrossRef] [PubMed]
- Bastian, T.W.; von Hohenberg, W.C.; Mickelson, D.J.; Lanier, L.M.; Georgieff, M.K. Iron Deficiency Impairs Developing Hippocampal Neuron Gene Expression, Energy Metabolism, and Dendrite Complexity. Dev. Neurosci. 2016, 38, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.S.; Tkac, I.; Magid, R.; O’Connor, M.B.; Andrews, N.C.; Schallert, T.; Gunshin, H.; Georgieff, M.K.; Petryk, A. Iron Is Essential for Neuron Development and Memory Function in Mouse Hippocampus. J. Nutr. 2009, 139, 672–679. [Google Scholar] [CrossRef]
- Krebs, N.F.; Lozoff, B.; Georgieff, M.K. Neurodevelopment: The Impact of Nutrition and Inflammation during Infancy in Low-Resource Settings. Pediatrics 2017, 139, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, C.; Liu, X.; Wang, Z.; Luo, R.; Li, S.; Yu, Y.; Alderman, H. Nutrition, Cognition, and Social Emotion among Preschoolers in Poor, Rural Areas of South Central China: Status and Correlates. Nutrients 2021, 13, 1322. [Google Scholar] [CrossRef]
- Paxson, C.; Schady, N. Cognitive development among young children in Ecuador the roles of wealth, health, and parenting. J. Hum. Resour. 2007, 42, 49–84. [Google Scholar] [CrossRef]
- Gashu, D.; Stoecker, B.J.; Bougma, K.; Adish, A.; Haki, G.D.; Marquis, G.S. Stunting, selenium deficiency and anemia are associated with poor cognitive performance in preschool children from rural Ethiopia. Nutr. J. 2015, 15, 38. [Google Scholar] [CrossRef]
- Thorisdottir, A.V.; Gunnarsdottir, I.; Palsson, G.I.; Gretarsson, S.J.; Thorsdottir, I. Iron status and developmental scores in 6-year-olds highlights ongoing need to tackle iron deficiency in infants. Acta Paediatr. 2013, 102, 914–919. [Google Scholar] [CrossRef]
- Lozoff, B.; Jimenez, E.; Wolf, A.W. Long-Term Developmental Outcome of Infants with Iron Deficiency. N. Engl. J. Med. 1991, 325, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Pala, E.; Erguven, M.; Guven, S.; Erdogan, M.; Balta, T. Psychomotor Development in Children with Iron Deficiency and Iron-Deficiency Anemia. Food Nutr. Bull. 2010, 31, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Smuts, C.M.; Malan, L.; Kvalsvig, J.; van Stuijvenberg, M.E.; Hurrell, R.F.; Zimmermann, M.B. Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: A randomized, double-blind, placebo-controlled intervention in South Africa. Am. J. Clin. Nutr. 2012, 96, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.J.; Kvalsvig, J.D.; Chwaya, H.M.; Montresor, A.; Albonico, M.; Tielsch, J.; Savioli, L.; Pollitt, E. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: Double blind, placebo controlled study. BMJ 2001, 323, 1389. [Google Scholar] [CrossRef]
- Boivin, M.J.; Giordani, B. Improvements in Cognitive Performance for Schoolchildren in Zaire, Africa, Following an Iron Supplement and Treatment for Intestinal Parasites. J. Pediatr. Psychol. 1993, 18, 249–264. [Google Scholar] [CrossRef]
- Bouhouch, R.R.; El-Fadeli, S.; Andersson, M.; Aboussad, A.; Chabaa, L.; Zeder, C.; Kippler, M.; Baumgartner, J.; Sedki, A.; Zimmermann, M.B. Effects of wheat-flour biscuits fortified with iron and EDTA, alone and in combination, on blood lead concentration, iron status, and cognition in children: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1318–1326. [Google Scholar] [CrossRef]
- Beard, J.L.; Wiesinger, J.A.; Connor, J.R. Pre- and Postweaning Iron Deficiency Alters Myelination in Sprague-Dawley Rats. Dev. Neurosci. 2003, 25, 308–315. [Google Scholar] [CrossRef]
- Ortiz, E.; Pasquini, J.M.; Thompson, K.; Felt, B.; Butkus, G.; Beard, J.; Connor, J.R. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J. Neurosci. Res. 2004, 77, 681–689. [Google Scholar] [CrossRef]
- Yehuda, S.; Youdim, M.B. Brain iron: A lesson from animal models. Am. J. Clin. Nutr. 1989, 50, 618–629. [Google Scholar] [CrossRef]
- Beard, J.L. Why Iron Deficiency Is Important in Infant Development. J. Nutr. 2008, 138, 2534–2536. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.R. Iron Status and Neural Functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Dallman, P.R. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. 1986, 6, 13–40. [Google Scholar] [CrossRef] [PubMed]
| Variable | Analysis for Aim 1; Maternal Hb during Pregnancy, Child Hb 12 Months and Development at 15 Months (n = 933) | Analysis for Aim 2; Annual Hb and Development at 5 Years (n = 726) | Analysis for Aim 3; Iron Status at 2 Years and Development at 5 Years (n = 530) |
|---|---|---|---|
| Age at Hb/iron assessment, mean (SD) | 12 months | Annually (1–5 years) | 2.4 (0.8) years |
| Age at developmental assessment, mean (SD) | 15.4 (0.5) months | 5.0 (0.8) years | 5.0 (0.8) years |
| Sex, male, n/total (%) | 470/932 (50.4) | 355/724 (49.0) | 260/530 (49.1) |
| * Any malaria parasitemia, n (%) | 46/929 (5.0) | 102/726 (14.1) | 72/530 (13.7) |
| ** Clinical malaria episodes, n (%) | |||
| None | 674/932 (72.3) | 297/722 (41.2) | 216/530 (40.8) |
| ≥1 | 258/932 (27.7) | 425/722 (58.9) | 314/530 (59.3) |
| *** Helminthic infections | |||
| Schistosomiasis, n/total (%) | 0/877 (0) | 28/726 (3.9) | 15/530 (2.8) |
| Hookworm, n/total (%) | 2/877(0.2) | 19/726 (2.6) | 13/530 (2.5) |
| Any worm infection, n/total (%) | 21/877 (2.4) | 140/726 (19.3) | 101/530 (19.1) |
| Treatment with albendazole, n/total (%) | 462/930 (49.7) | 354/724 (48.9) | 254/530 (47.9) |
| Nutritional status at developmental assessment | |||
| Stunting, n/total (%) | 127/921 (13.8) | 187/710 (26.3) | 136/520 (26.2) |
| Underweight, n/total (%) | 79/932 (8.9) | 70/714 (9.8) | 59/523 (11.3) |
| Wasting, n/total (%) | 39/921 (4.2) | 53/713 (7.4) | 40/523 (7.7) |
| Household socioeconomic status, n/total (%) | |||
| 1 (lowest) | 51/916 (5.6) | 46/713 (6.5) | 30/521 (5.8) |
| 2 | 68/916 (7.4) | 45/713 (6.3) | 38/521 (13.1) |
| 3 | 287/916 (31.3) | 219/713 (30.7) | 163/521 (31.3) |
| 4 | 268/916 (29.3) | 210/713 (29.5) | 143/521 (27.5) |
| 5 | 189/916 (20.6) | 142/713 (19.9) | 108/521 (20.7) |
| 6 (highest) | 53/916 (5.8) | 51/713 (7.2) | 39/521 (7.5) |
| Child Anemia/Iron Status | |
|---|---|
| Anemia | |
| Moderate anemia at age one, n/total (%) | 436/933 (46.7) |
| Moderate anemia at age two, n/total (%) | 127/615 (20.7) |
| Moderate anemia at age three, n/total (%) | 26/641 (4.1) |
| Moderate anemia at age four, n/total (%) | 13/399 (3.3) |
| Moderate anemia at age five, n/total (%) | 31/713 (4.4) |
| Any moderate anemia event from one to five years of age, n/total (%) | 342/726 (47.1) |
| Any mild anemia event from one to five years of age, n/total (%) | 550/726 (75.8) |
| Microcytic anemia at 12 months of age, n/total (%) | 427/933 (45.8) |
| Anemia associated with malaria at 12 months of age, n/total (%) | 40/929 (4.3) |
| Anemia associated with stunting at 12 months of age, n/total (%) | 67/921 (7.3) |
| Annual hemoglobin (Hb) levels | |
| Hb at age 1 (g/dL), mean (95% CI) (n = 933) | 9.9 (9.8, 10.0) |
| Hb at age 2 (g/dL), mean (95% CI) (n = 615) | 10.9 (10.8, 11.0) |
| Hb at age 3 (g/dL), mean (95% CI) (n = 641) | 11.7 (11.6, 11.8) |
| Hb at age 4 (g/dL), mean (95% CI) (n = 399) | 12.1 (11.7, 12.6) |
| Hb at age 5 (g/dL), mean (95% CI) (n = 713) | 12.0 (11.9, 12.1) |
| Iron status | |
| Iron deficiency at 2 years of age, n/total (%) | 152/490 (31.0) |
| Iron deficiency anemia at 2 years of age, n/total (%) | 82/441 (18.6) |
| Iron and inflammation biomarkers at two years of age | |
| Ferritin (μg/L), mean (95% CI) (n = 495) | 21.7 (19.8, 23.7) |
| Transferrin (g/L), mean (95% CI) (n = 517) | 2.7 (2.7, 2.8) |
| sTfR (mg/L) mean (95% CI) (n = 518) | 6.3 (5.9, 6.7) |
| Hepcidin (μg/L) mean (95% CI) (n = 516) | 7.8 (7.0, 8.6) |
| C-reactive protein (mg/L) mean (95% CI) (n = 519) | 1.3 (1.2, 1.5) |
| Maternal Hb/ anemia status at enrolment in pregnancy | |
| Hb levels (g/dL), mean (95% CI) (n = 933) | 11.4 (11.3, 11.4) |
| Mild anemia, n/total (%) | 336/933 (36.0) |
| Moderate anemia, n/total (%) | 147/933 (15.8) |
| Developmental Domain | n | Univariable Model β (95% CI) | p Value | n | Multivariable Model * β (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Maternal Hemoglobin Levels and Developmental Scores at 15 Months | ||||||
| Executive function (A not B + self-control) | 686 | −0.01 (−0.06, 0.04) | 0.71 | 619 | −0.002 (−0.06, 0.05) | 0.93 |
| Psychomotor (fine motor + gross motor) | 915 | 0.06 (0.02, 0.11) | 0.01 | 828 | 0.05 (0.0002, 0.09) | 0.05 |
| Social cognition (recognition of self and others + self-care) | 933 | 0.03 (−0.1, 0.07) | 0.19 | 843 | 0.02 (−0.03, 0.06) | 0.50 |
| Language | 933 | 0.02 (−0.02, 0.06) | 0.08 | 843 | −0.01 (−0.05, 0.04) | 0.81 |
| Child hemoglobin levels at 12 months and developmental scores at 15 months | ||||||
| Executive function (A not B + self-control) | 686 | 0.01 (−0.04, 0.07) | 0.60 | 619 | 0.03 (−0.03, 0.09) | 0.28 |
| Psychomotor (fine motor + gross motor) | 915 | 0.07 (0.03, 0.12) | 0.001 | 828 | 0.07 (0.02, 0.12) | 0.003 |
| Social cognition (recognition of self and others + self-care) | 933 | 0.03 (−0.4, 0.10) | 0.40 | 843 | 0.13 (−0.10, 0.36) | 0.28 |
| Language | 933 | 0.04 (−0.01, 0.08) | 0.09 | 843 | 0.05 (0.002, 0.10) | 0.04 |
| Developmental Domain | n | Univariable Model β (95% CI) | p Value | n | Multivariable Model * β (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Mild Maternal Anemia during Pregnancy and Developmental Scores at 15 Months (n = 336/933) | ||||||
| Executive function (A not B + self-control) | 686 | −0.03 (−0.19, 0.12) | 0.68 | 619 | 0.03 (−0.20, 0.13) | 0.68 |
| Psychomotor (fine motor + gross motor) | 915 | −0.19 (−0.32, −0.05) | 0.01 | 828 | −0.14 (−0.27, −0.002) | 0.05 |
| Social cognition (recognition of self and others +self-care) | 933 | −0.04 (−0.17, 0.09) | 0.55 | 843 | 0.02 (−0.12, 0.16) | 0.77 |
| Language | 933 | −0.01 (−0.14, 0.12) | 0.88 | 843 | 0.05 (−0.09, 0.19) | 0.48 |
| Moderate child anemia at 12 months and developmental scores at 15 months (436/933) a | ||||||
| Executive function (A not B + self-control) | 686 | −0.002 (−0.15, 0.15) | 0.97 | 619 | −0.03 (−0.20, 0.13) | 0.70 |
| Psychomotor (fine motor + gross motor) | 915 | −0.18 (−0.31, −0.06) | 0.01 | 828 | −0.21 (−0.35, −0.08) | 0.002 |
| Social cognition (recognition of self and others +self-care) | 933 | −0.16 (−0.29, −0.03) | 0.01 | 843 | −0.59 (−1.24, 0.05) | 0.07 |
| Language | 933 | −0.05 (−0.18, 0.08) | 0.45 | 846 | −0.09 (−0.23, 0.05) | 0.19 |
| b Combined maternal anemia during pregnancy and child anemia at 12 months and developmental scores at 15 months (n = 175/933) | ||||||
| Executive function (A not B + self-control) | 686 | −0.08 (−0.27, 0.11) | 0.41 | 619 | −0.10 (−0.30, 0.11) | 0.36 |
| Psychomotor (fine motor + gross motor) | 915 | −0.36 (−0.52, −0.19) | 0.0002 | 828 | −0.35 (−0.52, −0.19) | 0.00004 |
| Social cognition (recognition of self and others +self-care) | 933 | −0.12 (−0.29, 0.04) | 0.14 | 843 | −0.08 (−0.25, 0.10) | 0.39 |
| Language | 933 | 0.02 (−0.14, 0.19) | 0.78 | 843 | 0.03 (−0.14, 0.21) | 0.71 |
| Developmental Domain | n | Univariable Model β (95% CI) | p Value | n | Multivariable Model * β (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Hemoglobin Levels at 5 Years and Developmental Scores at 5 Years | ||||||
| Verbal and non-verbal IQ | 713 | 0.0002 (−0.06, 0.06) | 0.99 | 681 | −0.002 (−0.06, 0.06) | 0.96 |
| Executive function | 713 | −0.04 (−0.10, 0.02) | 0.15 | 681 | −0.06 (−0.11, 0.002) | 0.06 |
| Motor ability | 713 | −0.004 (−0.05, 0.05) | 0.88 | 681 | −0.001 (−0.05, 0.05) | 0.99 |
| Any moderate anemia event below five years and developmental scores at five years | ||||||
| Verbal and non-verbal IQ | 726 | −0.18 (−0.31, 0.08) | 0.25 | 693 | 0.02 (−0.19, 0.22) | 0.22 |
| Executive function | 726 | 0.01 (−0.18, 0.20) | 0.91 | 693 | 0.11 (−0.08, 0.30) | 0.25 |
| Motor ability | 726 | −0.04 (−0.19, 0.12) | 0.66 | 693 | 0.04 (−0.13, 0.20) | 0.67 |
| Iron Parameter | n | Multivariable Model, β (95% CI) | p Value |
|---|---|---|---|
| Verbal/ Non-Verbal IQ | |||
| ID | 471 | 0.09 (−0.16, 0.35) | 0.45 |
| IDA | 454 | −0.02 (−0.37, 0.32) | 0.90 |
| Ferritin (μg/L) | 471 | −0.12 (−0.25, 0.02) | 0.09 |
| Transferrin (g/L) | 495 | 0.14 (0.06, 0.33) | 0.18 |
| sTfR (mg/L) | 490 | −0.15 (−0.32, 0.02) | 0.09 |
| Hepcidin (μg/L) | 489 | −0.03 (−0.14, 0.07) | 0.54 |
| Executive function | |||
| ID | 471 | 0.21 (−0.04, 0.45) | 0.09 |
| IDA | 454 | −0.11(−0.44, 0.22) | 0.53 |
| Ferritin (μg/L) | 471 | −0.11 (−0.24, 0.02) | 0.11 |
| Transferrin (g/L) | 495 | 0.13 (−0.06, 0.32) | 0.18 |
| sTfR (mg/L) | 490 | −0.05 (−0.21, 0.11) | 0.54 |
| Hepcidin (μg/L) | 489 | −0.05 (−0.14, 0.05) | 0.32 |
| Motor ability | |||
| ID | 471 | 0.12 (−0.09, 0.34) | 0.25 |
| IDA | 454 | 0.09 (−0.19, 0.38) | 0.52 |
| Ferritin (μg/L) | 471 | −0.07 (−0.19, 0.04) | 0.20 |
| Transferrin (g/L) | 495 | 0.01 (−0.18, 0.15) | 0.87 |
| sTfR(mg/L) | 490 | −0.07 (−0.21, 0.07) | 0.32 |
| Hepcidin (μg/L) | 489 | −0.04 (−0.12, 0.05) | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nampijja, M.; Mutua, A.M.; Elliott, A.M.; Muriuki, J.M.; Abubakar, A.; Webb, E.L.; Atkinson, S.H. Low Hemoglobin Levels Are Associated with Reduced Psychomotor and Language Abilities in Young Ugandan Children. Nutrients 2022, 14, 1452. https://doi.org/10.3390/nu14071452
Nampijja M, Mutua AM, Elliott AM, Muriuki JM, Abubakar A, Webb EL, Atkinson SH. Low Hemoglobin Levels Are Associated with Reduced Psychomotor and Language Abilities in Young Ugandan Children. Nutrients. 2022; 14(7):1452. https://doi.org/10.3390/nu14071452
Chicago/Turabian StyleNampijja, Margaret, Agnes M. Mutua, Alison M. Elliott, John Muthii Muriuki, Amina Abubakar, Emily L. Webb, and Sarah H. Atkinson. 2022. "Low Hemoglobin Levels Are Associated with Reduced Psychomotor and Language Abilities in Young Ugandan Children" Nutrients 14, no. 7: 1452. https://doi.org/10.3390/nu14071452
APA StyleNampijja, M., Mutua, A. M., Elliott, A. M., Muriuki, J. M., Abubakar, A., Webb, E. L., & Atkinson, S. H. (2022). Low Hemoglobin Levels Are Associated with Reduced Psychomotor and Language Abilities in Young Ugandan Children. Nutrients, 14(7), 1452. https://doi.org/10.3390/nu14071452







