Effect of Beta 2-Adrenergic Receptor Gly16Arg Polymorphism on Taste Preferences in Healthy Young Japanese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Outline of Study
2.3. Extraction of DNA and Genotyping of the ADRB2 Polymorphism
2.4. Determination of Saliva Flow Rate during Resting and Mastication
2.5. Determination of Taste Preference
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of the Population
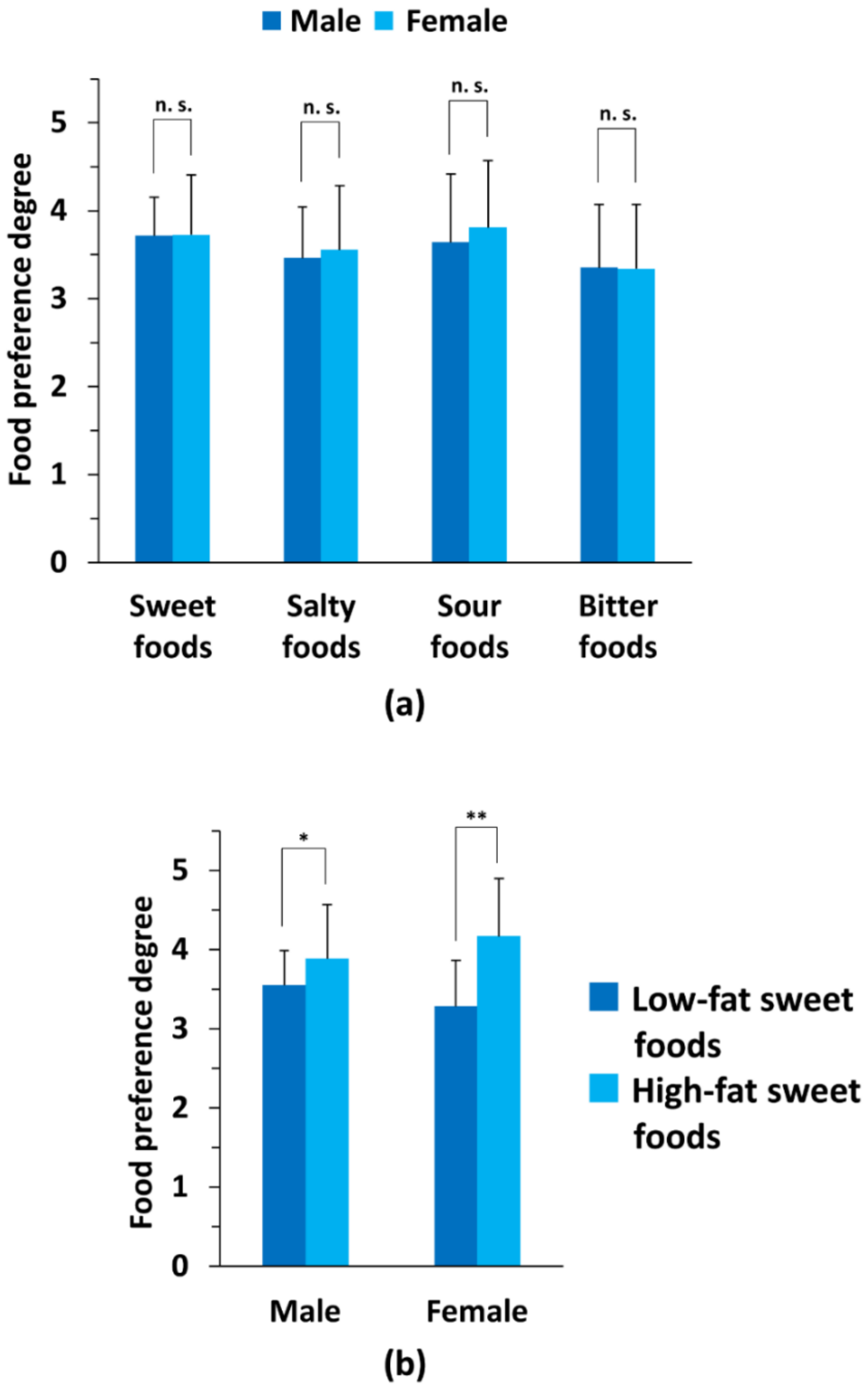
3.2. Comparison of Primary Taste Preference Degrees between the Two Sex Subgroups
3.3. Genotype Frequencies of ADRB2 Gly16Arg (G/C) Polymorphism in Male and Female Subjects
3.4. Comparison of Primary Taste Preference Degrees between Genotype Subgroups in Male and Female Subjects
3.5. Comparison of the Degree of Taste Preference between Low-Fat and High-Fat Sweet Tastes in Each Genotype Subgroup for Male and Female Subjects
3.6. Comparison of Greasy Food Preference Degrees among Genotype Subgroups in Male and Female Subjects
3.7. Multiple Linear Regression Anaysis Using Age and BMI in Male aad Female Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontaine, K.R.; Barofsky, I. Obesity and health-related quality of life. Obes. Rev. 2001, 2, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L. NEDCOM, The Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Obesity and kidney disease: Hidden consequences of the epidemic. Afr. J. Prim. Health Care Fam. Med. 2017, 9, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hong, G.; Tominami, K.; Kudo, T. Oral fat sensitivity is associated with social support for stress coping in young adult men. Tohoku J. Exp. Med. 2018, 244, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D.A. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Chen, Y.; Leiter, L.; Liu, L.; Reeder, B.A. Risk factor correlates of body mass index. CMAJ 1997, 157, S26–S31. [Google Scholar]
- Rexrode, K.M.; Hennekens, C.H.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Rich-Edwards, J.W.; Speizer, F.E.; Manson, J.E. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA 1997, 277, 1539–1545. [Google Scholar] [CrossRef]
- Willett, W.C.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA 1995, 273, 461–465. [Google Scholar] [CrossRef]
- Pang, J.; Xi, C.; Huang, X.; Cui, J.; Gong, H.; Zhang, T. Effects of excess energy intake on glucose and lipid metabolism in C57BL/6 mice. PLoS ONE 2016, 11, e0146675. [Google Scholar] [CrossRef] [PubMed]
- Masih, T.; Dimmock, J.A.; Epel, E.S.; Guelfi, K.J. Stress-induced eating and the relaxation response as a potential antidote: A review and hypothesis. Appetite 2017, 118, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Nakamura, Y.; Nakajima, T.; Sasakawa, Y.; Nakakura-Ohshima, K.; Murakami, T.; Kurosawa, M.; Iwase, Y.; Murakami, D.; Saitoh, I.; et al. Oral feeding behavior during a whole meal. Dent. Oral Craniofac. Res. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Loper, H.B.; La Sala, M.; Dotson, C.; Steinle, N. Taste perception, associated hormonal modulation, and nutrient intake. Nutr. Rev. 2015, 73, 83–91. [Google Scholar] [CrossRef]
- Watanabe, K.; Hong, G.; Tominami, K.; Izumi, S.; Hayashi, Y.; Kudo, T.A. Association between beta3-adrenergic receptor Trp64Arg polymorphism and fat preference in healthy young Japanese women. Tohoku J. Exp. Med. 2019, 248, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, sweet, salty, sour and umami taste perception decreases with age: Sex-specific analysis, modulation by genetic variants and taste-preference associations in 18 to 80 year-old subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Dahir, N.S.; Calder, A.N.; McKinley, B.J.; Liu, Y.; Gilbertson, T.A. Sex differences in fat taste responsiveness are modulated by estradiol. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E566–E580. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, N.R.; López-Díaz, J.A.; Wall-Medrano, A.; Jiménez-Castro, J.A.; Angulo, O. Oral fat perception is related with body mass index, preference and consumption of high-fat foods. Physiol. Behav. 2014, 129, 36–42. [Google Scholar] [CrossRef]
- Shin, Y.K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sévigny, J.; Tschöp, M.H.; Maudsley, S.; et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE 2010, 5, e12729. [Google Scholar] [CrossRef]
- Janssen, S.; Depoortere, I. Nutrient sensing in the gut: New roads to therapeutics? Trends Endocrinol. Metab. 2013, 24, 92–100. [Google Scholar] [CrossRef]
- Satoh-Kuriwada, S.; Kawai, M.; Iikubo, M.; Sekine-Hayakawa, Y.; Shoji, N.; Uneyama, H.; Sasano, T. Development of an umami taste sensitivity test and its clinical use. PLoS ONE 2014, 9, e95177. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- True, C.; Arik, A.; Lindsley, S.; Kirigiti, M.; Sullivan, E.; Kievit, P. Early high-fat diet exposure causes dysregulation of the orexin and dopamine neuronal populations in nonhuman primates. Front. Endocrinol. 2018, 9, 508. [Google Scholar] [CrossRef]
- Kozuka, C.; Yabiku, K.; Sunagawa, S.; Ueda, R.; Taira, S.; Ohshiro, H.; Ikema, T.; Yamakawa, K.; Higa, M.; Tanaka, H.; et al. Brown rice and its component, γ-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes 2012, 61, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, C.; Kaname, T.; Shimizu-Okabe, C.; Takayama, C.; Tsutsui, M.; Matsushita, M.; Abe, K.; Masuzaki, H. Impact of brown rice-specific γ-oryzanol on epigenetic modulation of dopamine D2 receptors in brain striatum in high-fat-diet-induced obesity in mice. Diabetologia 2017, 60, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, D.; Passilly-Degrace, P.; Besnard, P. Molecular mechanisms of fat preference and overeating. Ann. N. Y. Acad. Sci. 2008, 1141, 163–175. [Google Scholar] [CrossRef]
- Adachi, S.; Endo, Y.; Mizushige, T.; Tsuzuki, S.; Matsumura, S.; Inoue, K.; Fushiki, T. Increased levels of extracellular dopamine in the nucleus accumbens and amygdala of rats by ingesting a low concentration of a long-chain Fatty Acid. Biosci. Biotechnol. Biochem. 2013, 77, 2175–2180. [Google Scholar] [CrossRef][Green Version]
- Imaizumi, M.; Takeda, M.; Fushiki, T. Effects of oil intake in the conditioned place preference test in mice. Brain Res. 2000, 870, 150–156. [Google Scholar] [CrossRef]
- Yoneda, T.; Taka, Y.; Okamura, M.; Mizushige, T.; Matsumura, S.; Manabe, Y.; Tsuzuki, S.; Inoue, K.; Fushiki, T. Reinforcing effect for corn oil stimulus was concentration dependent in an operant task in mice. Life Sci. 2007, 81, 1585–1592. [Google Scholar] [CrossRef]
- Takeda, M.; Imaizumi, M.; Sawano, S.; Manabe, Y.; Fushiki, T. Long-term optional ingestion of corn oil induces excessive caloric intake and obesity in mice. Nutrition 2001, 17, 117–120. [Google Scholar] [CrossRef]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Newman, L.P.; Keast, R.S. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A. The role of dietary fat in obesity. Semin. Vasc. Med. 2005, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Paeratakul, S.; Popkin, B.M. Dietary fat and obesity: A review of animal, clinical and epidemiological studies. Physiol. Behav. 2004, 83, 549–555. [Google Scholar] [CrossRef]
- Haryono, R.Y.; Sprajcer, M.A.; Keast, R.S. Measuring oral fatty acid thresholds, fat perception, fatty food liking, and papillae density in humans. J. Vis. Exp. 2014, 88, e51236. [Google Scholar] [CrossRef]
- Asano, M.; Hong, G.; Matsuyama, Y.; Wang, W.; Izumi, S.; Izumi, M.; Toda, T.; Kudo, T. Association of oral fat sensitivity with body mass index, taste preference, and eating habits in healthy Japanese young adults. Tohoku J. Exp. Med. 2016, 238, 93–103. [Google Scholar] [CrossRef]
- Keast, R.S.; Costanzo, A. Is fat the sixth taste primary? Evidence and implications. Flavour 2015, 4, 5. [Google Scholar] [CrossRef]
- Mattes, R.D. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol. Behav. 2011, 104, 624–631. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K.; Abumrad, N.A. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1823–R1832. [Google Scholar] [CrossRef]
- Bouchard, C. Long-term programming of body size. Nutr. Rev. 1996, 54, S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Large, V.; Hellström, L.; Reynisdottir, S.; Lönnqvist, F.; Eriksson, P.; Lannfelt, L.; Arner, P. Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J. Clin. Investig. 1997, 100, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Naka, I.; Hikami, K.; Nakayama, K.; Koga, M.; Nishida, N.; Kimura, R.; Furusawa, T.; Natsuhara, K.; Yamauchi, T.; Nakazawa, M.; et al. A functional SNP upstream of the beta-2 adrenergic receptor gene (ADRB2) is associated with obesity in Oceanic populations. Int. J. Obes. 2013, 37, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R. Pharmacogenetics of the human beta-adrenergic receptors. Pharm. J. 2007, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Masuo, K.; Katsuya, T.; Fu, Y.; Rakugi, H.; Ogihara, T.; Tuck, M.L. Beta2- and beta3-adrenergic receptor polymorphisms are related to the onset of weight gain and blood pressure elevation over 5 years. Circulation 2005, 111, 3429–3434. [Google Scholar] [CrossRef]
- Schürks, M.; Kurth, T.; Ridker, P.M.; Buring, J.E.; Zee, R.Y. Association between polymorphisms in the beta 2-adrenoceptor gene and migraine in women. Headache 2009, 49, 235–244. [Google Scholar] [CrossRef]
- Petrone, A.; Zavarella, S.; Iacobellis, G.; Zampetti, S.; Vania, A.; Di Pietro, S.; Galgani, A.; Leonetti, F.; Di Mario, U.; Buzzetti, R. Association of beta2 adrenergic receptor polymorphisms and related haplotypes with triglyceride and LDL-cholesterol levels. Eur. J. Hum. Genet. 2006, 14, 94–100. [Google Scholar] [CrossRef]
- Green, S.A.; Liggett, S.B. A proline-rich region of the third intracellular loop imparts phenotypic beta 1-versus beta 2-adrenergic receptor coupling and sequestration. J. Biol. Chem. 1994, 269, 26215–26219. [Google Scholar] [CrossRef]
- Green, S.A.; Turki, J.; Bejarano, P.; Hall, I.P.; Liggett, S.B. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 1995, 13, 25–33. [Google Scholar] [CrossRef]
- Moore, P.E.; Laporte, J.D.; Abraham, J.H.; Schwartzman, I.N.; Yandava, C.N.; Silverman, E.S.; Drazen, J.M.; Wand, M.P.; Panettieri, R.A., Jr.; Shore, S.A. Polymorphism of the beta(2)-adrenergic receptor gene and desensitization in human airway smooth muscle. Am. J. Respir. Crit. Care Med. 2000, 162, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Koryakina, Y.; Jones, S.M.; Cornett, L.E.; Seely, K.; Brents, L.; Prather, P.L.; Kofman, A.; Kurten, R.C. Effects of the β-agonist, isoprenaline, on the down-regulation, functional responsiveness and trafficking of β2-adrenergic receptors with N-terminal polymorphisms. Cell Biol. Int. 2012, 36, 1171–1183. [Google Scholar] [CrossRef]
- Evans, D.A.; McLeod, H.L.; Pritchard, S.; Tariq, M.; Mobarek, A. Interethnic variability in human drug responses. Drug Metab. Dispos. 2001, 29, 606–610. [Google Scholar]
- Munakata, M.; Harada, Y.; Ishida, T.; Saito, J.; Nagabukuro, A.; Matsushita, H.; Koga, N.; Ohsaki, M.; Imagawa, K.; Shiratsuchi, T. Molecular-based haplotype analysis of the beta 2-adrenergic receptor gene (ADRB2) in Japanese asthmatic and non-asthmatic subjects. Allergol. Int. 2006, 55, 191–198. [Google Scholar] [CrossRef]
- Konno, S.; Hizawa, N.; Makita, H.; Shimizu, K.; Sakamoto, T.; Kokubu, F.; Saito, T.; Endo, T.; Ninomiya, H.; Iijima, H.; et al. The effects of a Gly16Arg ADRB2 polymorphism on responses to salmeterol or montelukast in Japanese patients with mild persistent asthma. Pharm. Genom. 2014, 24, 246–255. [Google Scholar] [CrossRef]
- Kaneko, Y.; Masuko, H.; Sakamoto, T.; Iijima, H.; Naito, T.; Yatagai, Y.; Yamada, H.; Konno, S.; Nishimura, M.; Noguchi, E.; et al. Asthma phenotypes in Japanese adults-their associations with the CCL5 and ADRB2 genotypes. Allergol. Int. 2013, 62, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Izumi, S.; Hong, G.; Iwasaki, K.; Izumi, M.; Matsuyama, Y.; Chiba, M.; Toda, T.; Kudo, T.A. Gustatory salivation is associated with body mass index, daytime sleepiness, and snoring in healthy young adults. Tohoku J. Exp. Med. 2016, 240, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Omori, R. Comparisons of the taste sensitivity between three generations. Bull. Fac. Educ. Utsunomiya Univ. 2013, 63, 201–210. [Google Scholar]
- Prior, S.J.; Goldberg, A.P.; Ryan, A.S. ADRB2 haplotype is associated with glucose tolerance and insulin sensitivity in obese postmenopausal women. Obesity 2011, 19, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, M.L.; Muzzio, D.O.; Iglesias Molli, A.E.; Belli, S.H.; Graffigna, M.N.; Levalle, O.A.; Frechtel, G.D.; Cerrone, G.E. Association between β2-adrenoceptor (ADRB2) haplotypes and insulin resistance in PCOS. Clin. Endocrinol. 2013, 78, 600–606. [Google Scholar] [CrossRef]
- Drewnowski, A.; Greenwood, M.R. Cream and sugar: Human preferences for high-fat foods. Physiol. Behav. 1983, 30, 629–633. [Google Scholar] [CrossRef]
- Tenk, C.M.; Felfeli, T. Sucrose and fat content significantly affects palatable food consumption in adolescent male and female rats. Appetite 2017, 118, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sugino, T.; Aoyagi, S.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. Effects of citric acid and l-carnitine on physical fatigue. J. Clin. Biochem. Nutr. 2007, 41, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Lillioja, S.; Knowler, W.C.; Christin, L.; Freymond, D.; Abbott, W.G.; Boyce, V.; Howard, B.V.; Bogardus, C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N. Engl. J. Med. 1988, 318, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Kovacs, E.M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005, 13, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Azadpour, N.; Tartibian, B.; Koşar, Ş.N. Effects of aerobic exercise training on ACE and ADRB2 gene expression, plasma angiotensin II level, and flow-mediated dilation: A study on obese postmenopausal women with prehypertension. Menopause 2017, 24, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Sollars, S.I. Contributory role of sex differences in the variations of gustatory function. J. Neurosci. Res. 2017, 95, 594–603. [Google Scholar] [CrossRef]
- Wright, P.; Crow, R.A. Menstrual cycle: Effect on sweetness preferences in women. Horm. Behav. 1973, 4, 387–391. [Google Scholar] [CrossRef]
- Verma, P.; Mahajan, K.K.; Mittal, S.; Ghildiyal, A. Salt preference across different phases of menstrual cycle. Indian J. Physiol. Pharmacol. 2005, 49, 99–102. [Google Scholar]




| All (Japanese, n = 52) | Male (n = 26) | Female (n = 26) | Male vs. Female | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | p-Value # | |
| Age (year) | 23.4 | 3.1 | 20–35 | 23.7 | 4.2 | 20–35 | 23.1 | 2.9 | 20–33 | n.s. |
| Height (cm) | 166.6 | 8.6 | 151.1–182.5 | 173.6 | 5.9 | 165.6–182.5 | 159.7 | 5.0 | 151.1–168.4 | <0.0001 ** |
| Weight (kg) | 59.5 | 11.3 | 44.2–105.0 | 66.3 | 9.4 | 49.6–105.0 | 52.6 | 5.1 | 44.2–65.2 | <0.0001 ** |
| BMI (kg/m2) | 21.3 | 2.9 | 17.1–34.4 | 22.0 | 2.7 | 17.1–34.4 | 20.6 | 2.0 | 17.8–26.6 | n.s. |
| Body fat (%) | 21.9 | 8.0 | 6.9–36.0 | 15.6 | 5.4 | 6.9–33.4 | 28.1 | 3.7 | 21.6–36.0 | <0.0001 ** |
| All (Japanese, n = 52) | Male (n = 26) | Female (n = 26) | Male vs. Female | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value # | ||
| Sweet food | Azuki-bean jelly (Yokan) | 3.40 | 1.12 | 3.58 | 0.81 | 3.23 | 1.37 | n.s. |
| Steamed bean-jam bun (Manju) | 3.63 | 0.97 | 3.69 | 0.88 | 3.58 | 1.06 | n.s. | |
| Candy | 3.21 | 1.00 | 3.38 | 0.85 | 3.04 | 1.11 | n.s. | |
| Ice cream | 4.27 | 0.91 | 4.23 | 0.76 | 4.31 | 1.05 | n.s. | |
| Chocolate | 4.02 | 1.00 | 3.77 | 0.95 | 4.27 | 1.00 | n.s. | |
| Strawberry sponge cake (Japanese-style shortcake) | 3.79 | 1.07 | 3.65 | 0.98 | 3.92 | 1.16 | n.s. | |
| Salty food | Potato chips | 3.88 | 1.08 | 4.04 | 0.82 | 3.73 | 1.28 | n.s. |
| Salted squids (Ika shiokawa) | 2.90 | 1.35 | 2.96 | 1.31 | 2.85 | 1.41 | n.s. | |
| Salted kelp (Shio Kombu) | 3.06 | 1.23 | 3.00 | 1.02 | 3.12 | 1.42 | n.s. | |
| Pickled vegetables (Tsukemono) | 3.35 | 1.08 | 3.15 | 0.92 | 3.54 | 1.21 | n.s. | |
| Salted cod roe (Tarako) | 3.48 | 1.26 | 3.31 | 1.12 | 3.65 | 1.38 | n.s. | |
| Salted salmon | 3.73 | 0.93 | 3.77 | 0.82 | 3.69 | 1.05 | n.s. | |
| Miso soup (Misoshiru) | 4.15 | 0.83 | 4.00 | 0.80 | 4.31 | 0.84 | n.s. | |
| Sour food | Japanese orange (Mikan) | 4.35 | 0.95 | 4.38 | 0.80 | 4.31 | 1.09 | n.s. |
| Hassaku orange | 3.62 | 1.03 | 3.46 | 0.95 | 3.77 | 1.11 | n.s. | |
| Pickled Japanese plum (Umeboshi) | 3.44 | 1.27 | 3.38 | 1.13 | 3.50 | 1.42 | n.s. | |
| Yogurt | 4.19 | 0.86 | 4.08 | 0.84 | 4.31 | 0.88 | n.s. | |
| Lemon | 3.19 | 1.09 | 3.12 | 1.07 | 3.27 | 1.12 | n.s. | |
| Grapefruit | 3.56 | 1.19 | 3.42 | 1.17 | 3.69 | 1.23 | n.s. | |
| Bitter food | Celery | 2.54 | 1.24 | 2.42 | 1.06 | 2.65 | 1.41 | n.s. |
| Tea | 4.37 | 0.69 | 4.35 | 0.63 | 4.38 | 0.75 | n.s. | |
| Green pepper | 3.38 | 1.07 | 3.58 | 1.03 | 3.19 | 1.10 | n.s. | |
| Parsley | 2.81 | 1.24 | 2.85 | 1.19 | 2.77 | 1.31 | n.s. | |
| Coffee | 3.63 | 1.19 | 3.58 | 1.24 | 3.69 | 1.16 | n.s. | |
| SNP | Male (n = 26) | Female (n = 26) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratio of Genotypes | Chi-Square Test | Ratio of Genotypes | Chi-Square Test | |||||||
| ADRB2 | GG | GC | CC | χ2 | p-Value # | GG | GC | CC | χ2 | p-Value |
| Gly16Arg | (Gly16Gly) | (Gly16Arg) | (Arg16Arg) | (Gly16Gly) | (Gly16Arg) | (Arg16Arg) | ||||
| (refSNP #: rs1042713) | n = 7 (26.9) | n = 15 (57.7) | n = 4 (15.4) | 0.746 | n.s. | n = 6 (23.1) | n = 13 (50.0) | n = 7 (26.9) | <0.001 | n.s. |
| GG (Gly16Gly) (n = 7) | GC (Gly16Arg) (n = 15) | CC (Arg16Arg) (n = 4) | GG vs. GC | GG vs. CC | GC vs. CC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value # | p-Value # | p-Value # | ||
| Sweet food | Azuki-bean jelly (Yokan) | 3.43 | 0.98 | 3.67 | 0.72 | 3.50 | 1.00 | n.s. | n.s. | n.s. |
| Steamed bean-jam bun (Manju) | 3.29 | 1.11 | 3.93 | 0.70 | 3.50 | 1.00 | n.s. | n.s. | n.s. | |
| Candy | 3.57 | 1.13 | 3.47 | 0.52 | 2.75 | 1.26 | n.s. | n.s. | n.s. | |
| Ice cream | 4.57 | 0.79 | 4.13 | 0.74 | 4.00 | 0.82 | n.s. | n.s. | n.s. | |
| Chocolate | 4.00 | 0.82 | 3.73 | 0.96 | 3.50 | 1.29 | n.s. | n.s. | n.s. | |
| Strawberry sponge cake (Japanese style shortcake) | 4.14 | 0.90 | 3.47 | 0.92 | 3.50 | 1.29 | n.s. | n.s. | n.s. | |
| Salty food | Potato chips | 4.29 | 0.76 | 3.93 | 0.88 | 4.00 | 0.82 | n.s. | n.s. | n.s. |
| Salted squids (Ika shiokawa) | 3.14 | 1.35 | 3.20 | 1.26 | 1.75 | 0.96 | n.s. | n.s. | n.s. | |
| Salted kelp (Shio Kombu) | 2.86 | 1.07 | 3.13 | 0.99 | 2.75 | 1.26 | n.s. | n.s. | n.s. | |
| Pickled vegetables (Tsukemono) | 2.86 | 0.90 | 3.27 | 0.80 | 3.25 | 1.50 | n.s. | n.s. | n.s. | |
| Salted cod roe (Tarako) | 3.43 | 1.13 | 3.27 | 1.22 | 3.25 | 0.96 | n.s. | n.s. | n.s. | |
| Salted salmon | 3.43 | 0.53 | 3.87 | 0.92 | 4.00 | 0.82 | n.s. | n.s. | n.s. | |
| Miso soup (Misoshiru) | 3.57 | 0.79 | 4.27 | 0.80 | 3.75 | 0.50 | n.s. | n.s. | n.s. | |
| Sour food | Japanese orange (Mikan) | 4.29 | 0.76 | 4.47 | 0.83 | 4.25 | 0.96 | n.s. | n.s. | n.s. |
| Hassaku orange | 3.14 | 0.90 | 3.67 | 0.98 | 3.25 | 0.96 | n.s. | n.s. | n.s. | |
| Pickled Japanese plum (Umeboshi) | 2.43 | 0.98 | 3.73 | 0.96 | 3.75 | 1.26 | 0.028 * | n.s. | n.s. | |
| Yogurt | 3.86 | 0.90 | 4.20 | 0.86 | 4.00 | 0.82 | n.s. | n.s. | n.s. | |
| Lemon | 2.71 | 0.76 | 3.27 | 1.03 | 3.25 | 1.71 | n.s. | n.s. | n.s. | |
| Grapefruit | 2.43 | 0.98 | 3.73 | 1.10 | 4.00 | 0.82 | 0.034 * | 0.047 * | n.s. | |
| Bitter food | Celery | 2.00 | 1.15 | 2.60 | 1.12 | 2.50 | 0.58 | n.s. | n.s. | n.s. |
| Tea | 3.86 | 0.38 | 4.53 | 0.64 | 4.50 | 0.58 | n.s. | n.s. | n.s. | |
| Green pepper | 2.86 | 1.07 | 3.93 | 0.96 | 3.50 | 0.58 | n.s. | n.s. | n.s. | |
| Parsley | 2.43 | 1.13 | 3.07 | 1.33 | 2.75 | 0.50 | n.s. | n.s. | n.s. | |
| Coffee | 3.14 | 0.69 | 4.00 | 1.36 | 2.75 | 0.96 | n.s. | n.s. | n.s. | |
| GG (Gly16Gly) (n = 6) | GC (Gly16Arg) (n = 13) | CC (Arg16Arg) (n = 7) | GG vs. GC | GG vs. CC | GC vs. CC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value # | p-Value # | p-Value # | ||
| Sweet food | Azuki-bean jelly (Yokan) | 3.00 | 1.41 | 3.46 | 1.36 | 3.00 | 1.00 | n.s. | n.s. | n.s. |
| Steamed bean-jam bun (Manju) | 3.83 | 0.75 | 3.62 | 1.39 | 3.29 | 0.49 | n.s. | n.s. | n.s. | |
| Candy | 3.50 | 1.52 | 2.77 | 0.93 | 3.14 | 1.07 | n.s. | n.s. | n.s. | |
| Ice cream | 4.67 | 0.52 | 4.23 | 1.30 | 4.14 | 0.90 | n.s. | n.s. | n.s. | |
| Chocolate | 4.50 | 0.84 | 4.46 | 0.97 | 3.71 | 1.11 | n.s. | n.s. | n.s. | |
| Strawberry sponge cake (Japanese style shortcake) | 4.00 | 1.55 | 4.08 | 1.04 | 3.57 | 1.13 | n.s. | n.s. | n.s. | |
| Salty food | Potato chips | 4.00 | 1.67 | 3.23 | 1.17 | 4.43 | 0.79 | n.s. | n.s. | n.s. |
| Salted squids (Ika shiokawa) | 3.50 | 1.22 | 2.85 | 1.63 | 2.29 | 0.95 | n.s. | n.s. | n.s. | |
| Salted kelp (Shio Kombu) | 3.33 | 1.51 | 3.38 | 1.50 | 2.43 | 1.13 | n.s. | n.s. | n.s. | |
| Pickled vegetables (Tsukemono) | 4.50 | 0.55 | 3.46 | 1.27 | 2.86 | 1.07 | n.s. | 0.038 * | n.s. | |
| Salted cod roe (Tarako) | 4.50 | 0.84 | 3.62 | 1.50 | 3.00 | 1.29 | n.s. | n.s. | n.s. | |
| Salted salmon | 3.17 | 0.98 | 3.85 | 1.21 | 3.86 | 0.69 | n.s. | n.s. | n.s. | |
| Miso soup (Misoshiru) | 4.50 | 0.84 | 4.31 | 0.85 | 4.14 | 0.90 | n.s. | n.s. | n.s. | |
| Sour food | Japanese orange (Mikan) | 4.83 | 0.41 | 4.31 | 1.03 | 3.86 | 1.46 | n.s. | n.s. | n.s. |
| Hassaku orange | 3.67 | 0.82 | 4.08 | 1.04 | 3.29 | 1.38 | n.s. | n.s. | n.s. | |
| Pickled Japanese plum (Umeboshi) | 4.00 | 1.67 | 3.69 | 1.32 | 2.71 | 1.25 | n.s. | n.s. | n.s. | |
| Yogurt | 4.67 | 0.82 | 4.38 | 0.87 | 3.86 | 0.90 | n.s. | n.s. | n.s. | |
| Lemon | 3.83 | 0.98 | 3.38 | 1.12 | 2.57 | 0.98 | n.s. | n.s. | n.s. | |
| Grapefruit | 4.00 | 1.26 | 4.08 | 1.12 | 2.71 | 0.95 | n.s. | n.s. | 0.045 * | |
| Bitter food | Celery | 3.17 | 1.60 | 2.85 | 1.46 | 1.86 | 0.90 | n.s. | n.s. | n.s. |
| Tea | 4.67 | 0.52 | 4.46 | 0.66 | 4.00 | 1.00 | n.s. | n.s. | n.s. | |
| Green pepper | 3.17 | 0.75 | 3.38 | 1.39 | 2.86 | 0.69 | n.s. | n.s. | n.s. | |
| Parsley | 2.67 | 1.51 | 2.92 | 1.44 | 2.57 | 0.98 | n.s. | n.s. | n.s. | |
| Coffee | 2.83 | 1.33 | 4.38 | 0.96 | 3.14 | 0.38 | 0.009 ** | n.s. | 0.021 * | |
| Question: Do You Like Greasy Foods? | Genotype: GG (Gly16Gly) | Genotype: GC (Gly16Arg) | Genotype: CC (Arg16Arg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 7) | Female (n = 6) | Male (n = 15) | Female (n = 13) | Male (n = 4) | Female (n = 7) | ||||||||
| Scale | Score | n | % | n | % | n | % | n | % | n | % | n | % |
| 1. I like them very much | 4 | 4 | 13.8 | 0 | 0.0 | 1 | 8.3 | 4 | 13.8 | 0 | 0.0 | 0 | 0.0 |
| 2. I like them moderately | 3 | 3 | 10.3 | 3 | 25.0 | 10 | 83.3 | 4 | 13.8 | 2 | 16.7 | 3 | 25.0 |
| 3. I dislike them moderately | 2 | 0 | 0.0 | 3 | 25.0 | 4 | 33.3 | 5 | 17.2 | 2 | 16.7 | 4 | 33.3 |
| 4. I dislike them very much | 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narita, K.; Kudo, T.-a.; Hong, G.; Tominami, K.; Izumi, S.; Hayashi, Y.; Nakai, J. Effect of Beta 2-Adrenergic Receptor Gly16Arg Polymorphism on Taste Preferences in Healthy Young Japanese Adults. Nutrients 2022, 14, 1430. https://doi.org/10.3390/nu14071430
Narita K, Kudo T-a, Hong G, Tominami K, Izumi S, Hayashi Y, Nakai J. Effect of Beta 2-Adrenergic Receptor Gly16Arg Polymorphism on Taste Preferences in Healthy Young Japanese Adults. Nutrients. 2022; 14(7):1430. https://doi.org/10.3390/nu14071430
Chicago/Turabian StyleNarita, Kohei, Tada-aki Kudo, Guang Hong, Kanako Tominami, Satoshi Izumi, Yohei Hayashi, and Junichi Nakai. 2022. "Effect of Beta 2-Adrenergic Receptor Gly16Arg Polymorphism on Taste Preferences in Healthy Young Japanese Adults" Nutrients 14, no. 7: 1430. https://doi.org/10.3390/nu14071430
APA StyleNarita, K., Kudo, T.-a., Hong, G., Tominami, K., Izumi, S., Hayashi, Y., & Nakai, J. (2022). Effect of Beta 2-Adrenergic Receptor Gly16Arg Polymorphism on Taste Preferences in Healthy Young Japanese Adults. Nutrients, 14(7), 1430. https://doi.org/10.3390/nu14071430








