Personalized Nutrition Using Microbial Metabolite Phenotype to Stratify Participants and Non-Invasive Host Exfoliomics Reveal the Effects of Flaxseed Lignan Supplementation in a Placebo-Controlled Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Differential Gene Expression Analysis
2.3. Linear Discriminant Analysis (LDA)
2.4. Ingenuity Pathway Analysis
3. Results
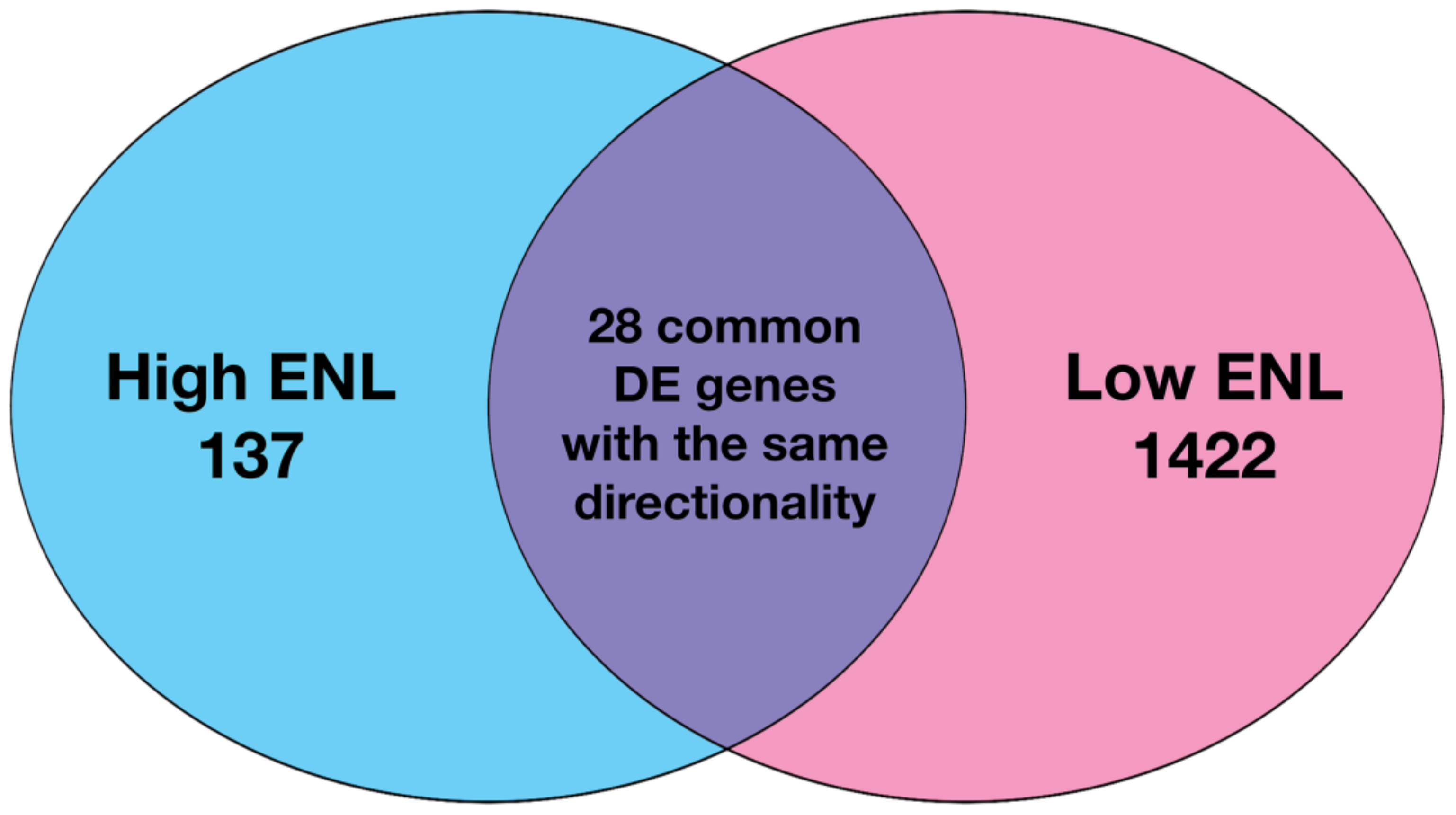
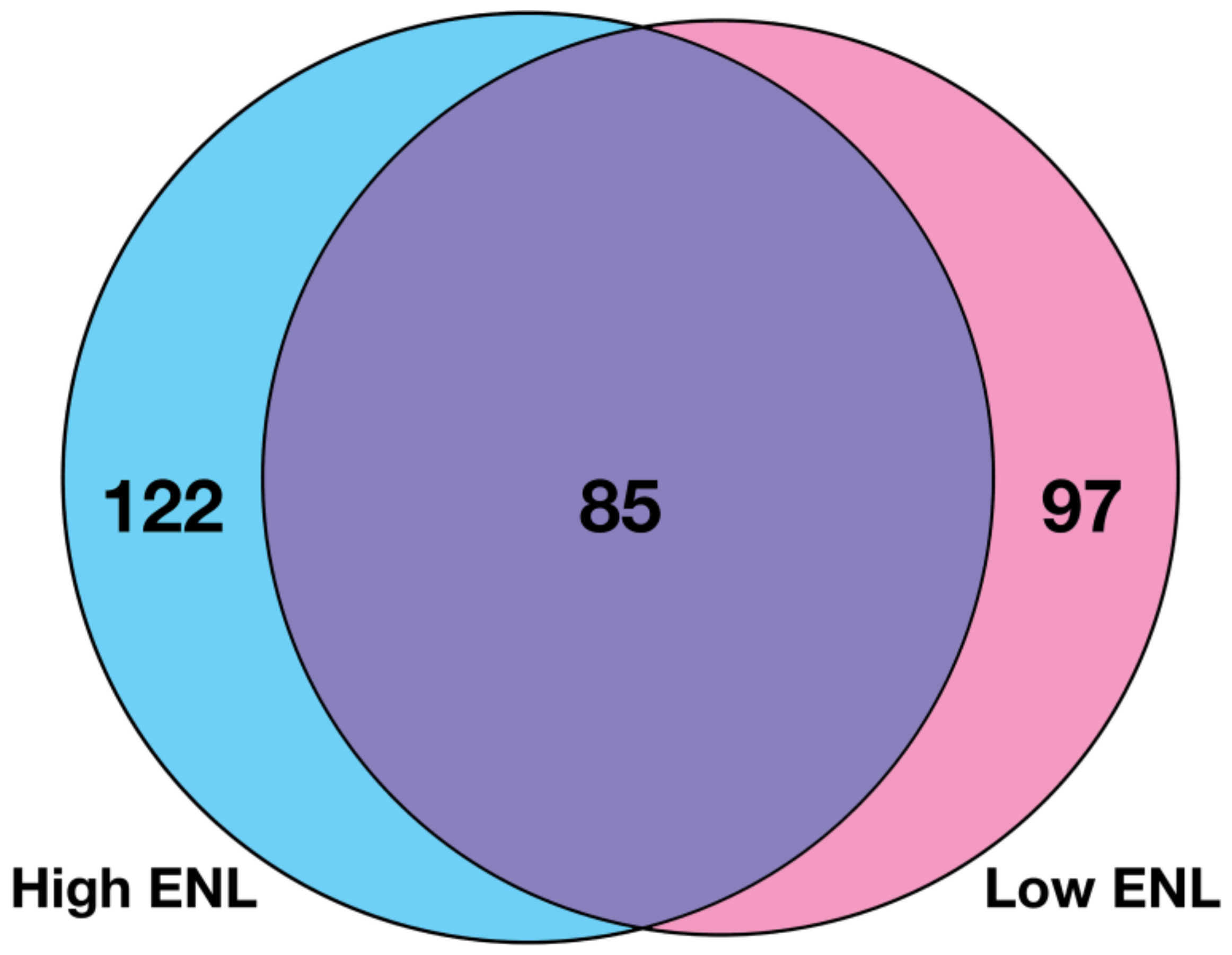
3.1. Gene Expression in Stool Exfoliated Cells
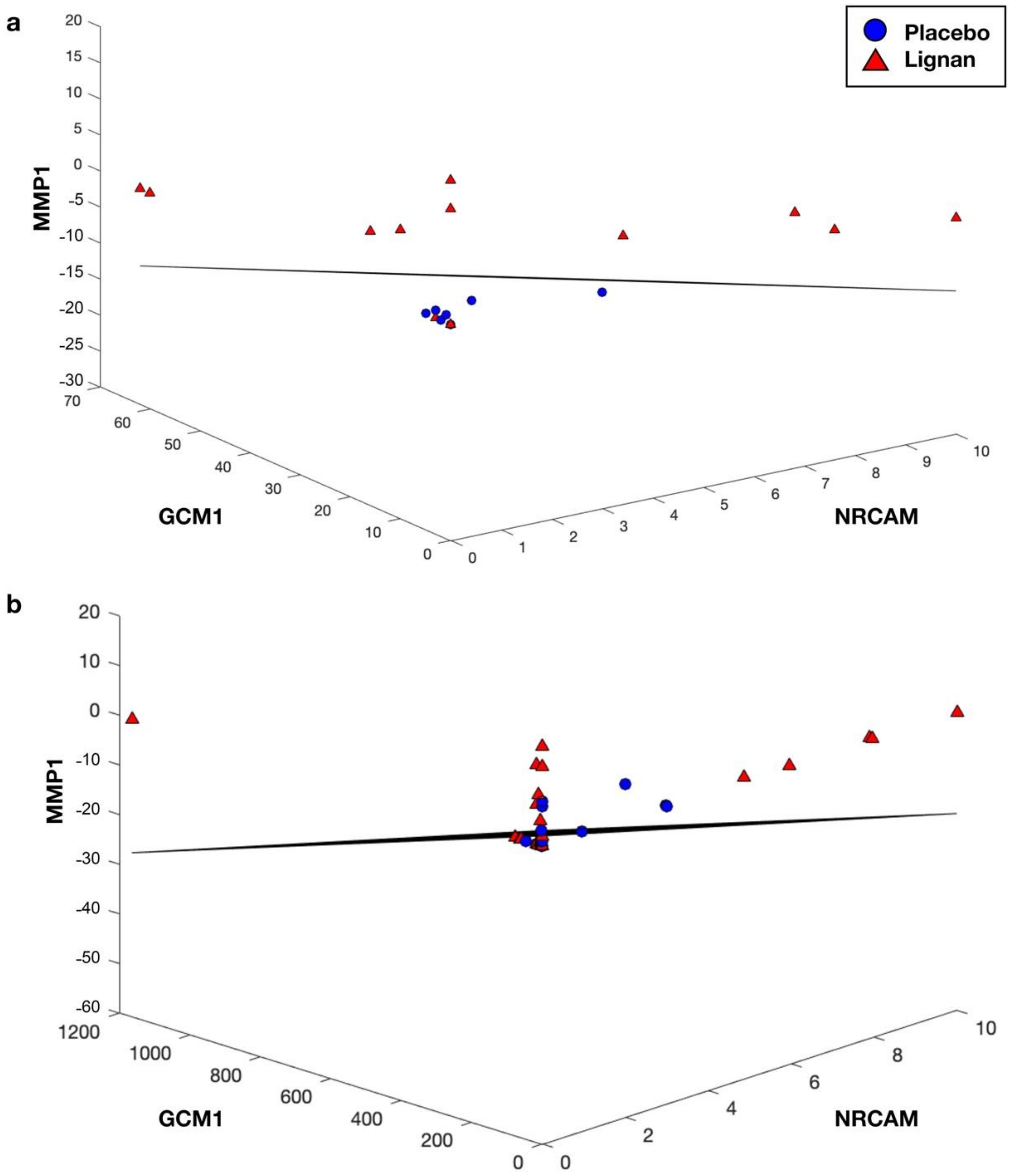
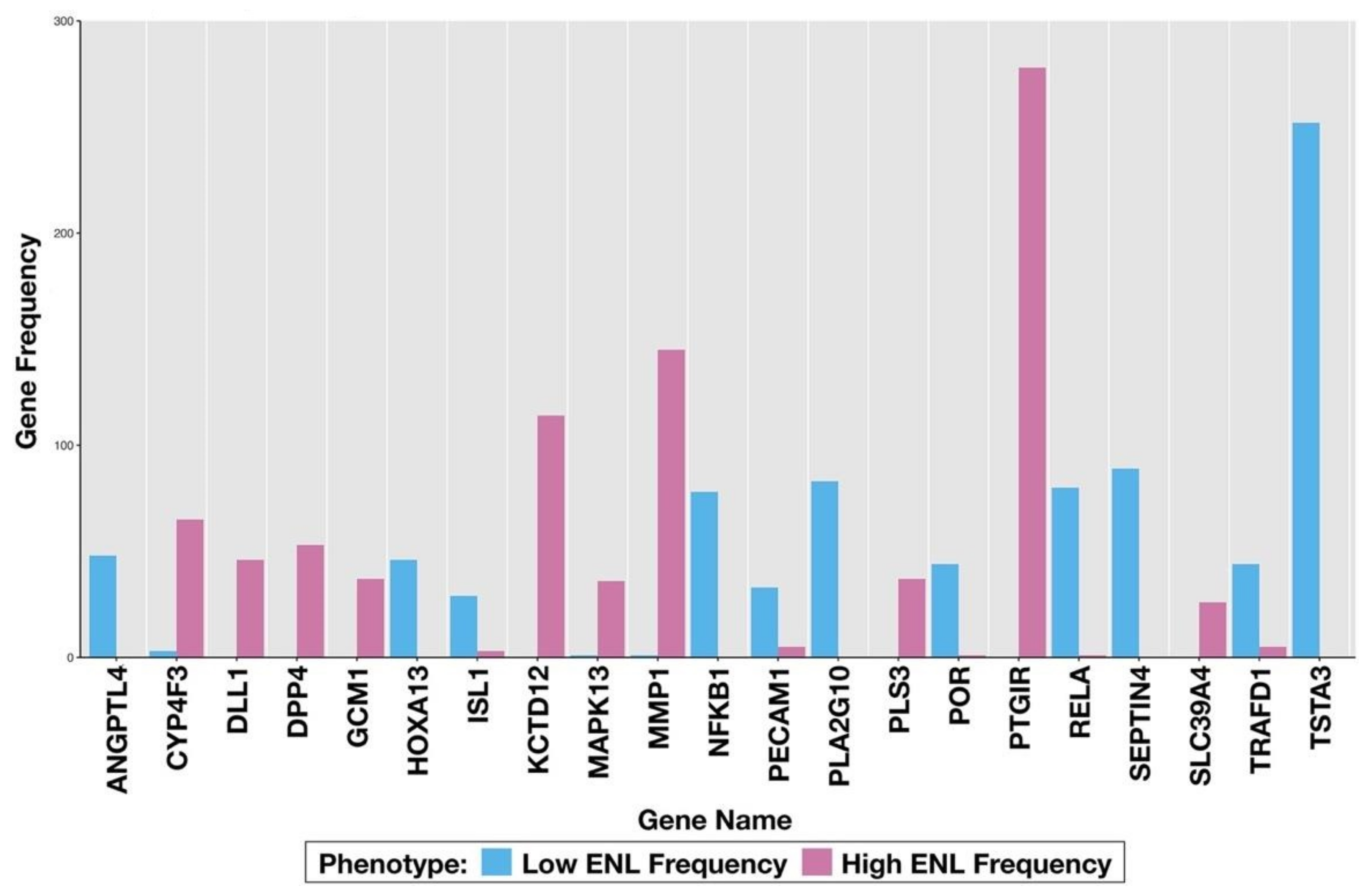
3.2. LDA Classification
3.3. IPA Functional Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, A.; Harrison, T.A.; Cao, Y.; Sakoda, L.C.; Barfield, R.; Giannakis, M.; Song, M.; Phipps, A.I.; Figueiredo, J.C.; Zaidi, S.H.; et al. Intake of Dietary Fruit, Vegetables, and Fiber and Risk of Colorectal Cancer According to Molecular Subtypes: A Pooled Analysis of 9 Studies. Cancer Res. 2020, 80, 4578–4590. [Google Scholar] [CrossRef]
- Hullings, A.G.; Sinha, R.; Liao, L.M.; Freedman, N.D.; Graubard, B.I.; Loftfield, E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 2020, 112, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, S.; Wilson, A.S.; Appolonia, C.N.; Thomas, T.K.; O’Keefe, S.J.D. Fiber, Fat, and Colorectal Cancer: New Insight into Modifiable Dietary Risk Factors. Curr. Gastroenterol. Rep. 2019, 21, 62. [Google Scholar] [CrossRef]
- Lampe, J.W.; Kim, E.; Levy, L.; Davidson, L.A.; Goldsby, J.S.; Miles, F.L.; Navarro, S.L.; Randolph, T.W.; Zhao, N.; Ivanov, I.; et al. Colonic mucosal and exfoliome transcriptomic profiling and fecal microbiome response to a flaxseed lignan extract intervention in humans. Am. J. Clin. Nutr. 2019, 110, 377–390. [Google Scholar] [CrossRef]
- Halldin, E.; Eriksen, A.K.; Brunius, C.; da Silva, A.B.; Bronze, M.; Hanhineva, K.; Aura, A.M.; Landberg, R. Factors Explaining Interpersonal Variation in Plasma Enterolactone Concentrations in Humans. Mol. Nutr. Food Res. 2019, 63, e1801159. [Google Scholar] [CrossRef]
- Hullar, M.A.; Lancaster, S.M.; Li, F.; Tseng, E.; Beer, K.; Atkinson, C.; Wahala, K.; Copeland, W.K.; Randolph, T.W.; Newton, K.M.; et al. Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol. Biomark. Prev. 2015, 24, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Navarro, S.L.; Schwarz, Y.; Gu, H.; Djukovic, D.; Randolph, T.W.; Shojaie, A.; Kratz, M.; Hullar, M.A.J.; Lampe, P.D.; et al. Plasma metabolite abundances are associated with urinary enterolactone excretion in healthy participants on controlled diets. Food Funct. 2017, 8, 3209–3218. [Google Scholar] [CrossRef]
- George, N.I.; Bowyer, J.F.; Crabtree, N.M.; Chang, C.W. An Iterative Leave-One-Out Approach to Outlier Detection in RNA-Seq Data. PLoS ONE 2015, 10, e0125224. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Zhou, X.; Lindsay, H.; Robinson, M.D. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 2014, 42, e91. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B-Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Zhao, C.; Ivanov, I.; Davidson, L.A.; Goldsby, J.S.; Lupton, J.R.; Mathai, R.A.; Monaco, M.H.; Rai, D.; Russell, W.M.; et al. Noninvasive stool-based detection of infant gastrointestinal development using gene expression profiles from exfoliated epithelial cells. Am. J. Physiol. -Gastrointest. Liver Physiol. 2010, 298, G582–G589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ivanov, I.; Dougherty, E.R.; Hartman, T.J.; Lanza, E.; Bobe, G.; Colburn, N.H.; Lupton, J.R.; Davidson, L.A.; Chapkin, R.S. Noninvasive detection of candidate molecular biomarkers in subjects with a history of insulin resistance and colorectal adenomas. Cancer Prev. Res. (Phila) 2009, 2, 590–597. [Google Scholar] [CrossRef]
- Braga-Neto, U.; Dougherty, E. Bolstered error estimation. Pattern Recognit. 2004, 37, 1267–1281. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, L.; Yin, R.; Hudlikar, R.; Li, S.; Kuo, H.D.; Peter, R.; Sargsyan, D.; Guo, Y.; Liu, X.; et al. Epigenetics/epigenomics and prevention by curcumin of early stages of inflammatory-driven colon cancer. Mol. Carcinog 2020, 59, 227–236. [Google Scholar] [CrossRef]
- Masoudkabir, F.; Sarrafzadegan, N. The interplay of endothelial dysfunction, cardiovascular disease, and cancer: What we should know beyond inflammation and oxidative stress. Eur. J. Prev. Cardiol. 2020, 27, 2075–2076. [Google Scholar] [CrossRef]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Aksentijevich, I.; McDermott, M.F.; O’Shea, J.J.; Kastner, D.L. TNFRSF1A mutations and autoinflammatory syndromes. Curr. Opin. Immunol. 2000, 12, 479–486. [Google Scholar] [CrossRef]
- Zhang, J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J. Clin. Investig. 2007, 117, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Levy, L.; Curtis, K.R.; Elkon, I.; Kahsai, O.J.; Ammar, H.S.; Randolph, T.W.; Hong, N.N.; Carnevale Neto, F.; Raftery, D.; et al. Effect of a Flaxseed Lignan Intervention on Circulating Bile Acids in a Placebo-Controlled Randomized, Crossover Trial. Nutrients 2020, 12, 1837. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Precision (Personalized) Nutrition: Understanding Metabolic Heterogeneity. Ann. Rev. Food Sci. Technol. 2020, 11, 71–92. [Google Scholar] [CrossRef] [PubMed]

| Low ENL Placebo | High ENL Placebo | p Value * | Low ENL Flax | High ENL Flax | p Value * | |
|---|---|---|---|---|---|---|
| SECO (µmol/24 h) | 0.48 (0.63) | 0.30 (0.36) | 0.28 | 4.37 (4.45) | 7.16 (5.71) | 0.007 |
| END (µmol/24 h) | 1.27 (3.12) | 1.12 (2.46) | 0.12 | 10.83 (15.97) | 17.77 (14.15) | 0.002 |
| ENL (µmol/24 h) | 3.29 (4.05) | 11.83 (19.94) | 0.002 | 7.46 (6.97) | 61.75 (36.42) | <0.001 |
| Gene 1 | Gene 2 | Gene 3 | Bresub Error | ∆ε Bolstered |
|---|---|---|---|---|
| ANXA3 | 0.2454 | |||
| NR6A1 | 0.2498 | |||
| KCTD12 | 0.2521 | |||
| EPHB1 | 0.2557 | |||
| PRKCH | 0.2573 | |||
| GCM1 | 0.2597 | |||
| PTGIR | 0.2626 | |||
| PROX1 | 0.2662 | |||
| DLL1 | 0.2681 | |||
| PAX6 | 0.2687 | |||
| MMP1 | PTGIR | 0.1568 | 0.1058 | |
| CYP4F3 | PTGIR | 0.1721 | 0.0905 | |
| DLL1 | PTGIR | 0.1729 | 0.0897 | |
| PLS3 | PTGIR | 0.1741 | 0.0885 | |
| FOXA1 | KCTD12 | 0.1770 | 0.0751 | |
| COX4I1 | SLC39A4 | 0.1785 | 0.1179 | |
| KCTD12 | MAPK13 | 0.1788 | 0.0733 | |
| KCTD12 | RBL2 | 0.1846 | 0.0675 | |
| NR6A1 | PTGIR | 0.1847 | 0.0651 | |
| VDR | KCTD12 | 0.1848 | 0.0674 | |
| NRCAM | GCM1 | MMP1 | 0.1157 | 0.1066 |
| NRCAM | MMP1 | PLD3 | 0.1282 | 0.0920 |
| CA14 | GCM1 | MMP1 | 0.1291 | 0.0932 |
| OTUB1 | CYP4F3 | PTGIR | 0.1296 | 0.0425 |
| GCM1 | MMP1 | PECAM1 | 0.1298 | 0.0924 |
| BMP4 | GCM1 | MMP1 | 0.1331 | 0.0740 |
| COX4I1 | FOXO3 | SLC39A4 | 0.1340 | 0.0445 |
| DLL1 | MMP1 | PTGIR | 0.1342 | 0.0226 |
| MMP1 | PTGIR | WNT5A | 0.1346 | 0.0222 |
| CYP4F3 | MMP1 | PTGIR | 0.1375 | 0.0193 |
| Gene 1 | Gene 2 | Gene 3 | Bresub Error | ∆ε Bolstered |
|---|---|---|---|---|
| ANXA3 | 0.2454 | |||
| NR6A1 | 0.2498 | |||
| KCTD12 | 0.2521 | |||
| EPHB1 | 0.2557 | |||
| PRKCH | 0.2573 | |||
| GCM1 | 0.2597 | |||
| PTGIR | 0.2626 | |||
| PROX1 | 0.2662 | |||
| DLL1 | 0.2681 | |||
| PAX6 | 0.2687 | |||
| ANGPTL4 | RELA | 0.1702 | 0.0164 | |
| SEPTIN4 | TSTA3 | 0.1773 | 0.1020 | |
| ISL1 | TSTA3 | 0.1799 | 0.1111 | |
| ANGPTL4 | GJB1 | 0.1803 | 0.0063 | |
| NFKB1 | TRAFD1 | 0.1824 | 0.1428 | |
| NFKB1 | TSTA3 | 0.1835 | 0.1076 | |
| ANGPTL4 | TSTA3 | 0.1861 | 0.0005 | |
| PECAM1 | TSTA3 | 0.1862 | 0.0990 | |
| BCL2 | ANGPTL4 | 0.1871 | 0.2701 | |
| NFKB1 | PROX1 | 0.1906 | 0.1360 | |
| CDH3 | SEPTIN4 | TSTA3 | 0.1427 | 0.0346 |
| PLA2G10 | HOXA13 | ULK1 | 0.1429 | 0.0779 |
| PLA2G10 | HOXA13 | MAML1 | 0.1429 | 0.0779 |
| FAM129A | SEPTIN4 | TSTA3 | 0.1450 | 0.0323 |
| CACNB4 | RELA | TSTA3 | 0.1465 | 0.0708 |
| NANOG | RELA | TSTA3 | 0.1468 | 0.0639 |
| CYP4F3 | HOXA13 | KCTD17 | 0.1523 | 0.0685 |
| POR | NFKB1 | TRAFD1 | 0.1537 | 0.0288 |
| RELA | SEPTIN4 | TSTA3 | 0.1537 | 0.0237 |
| WNT4 | RELA | TSTA3 | 0.1540 | 0.0565 |
| Upstream Regulator | Molecule Type | Phenotype | Predicted Activation State | Activation z-Score | Target Molecules in Dataset |
|---|---|---|---|---|---|
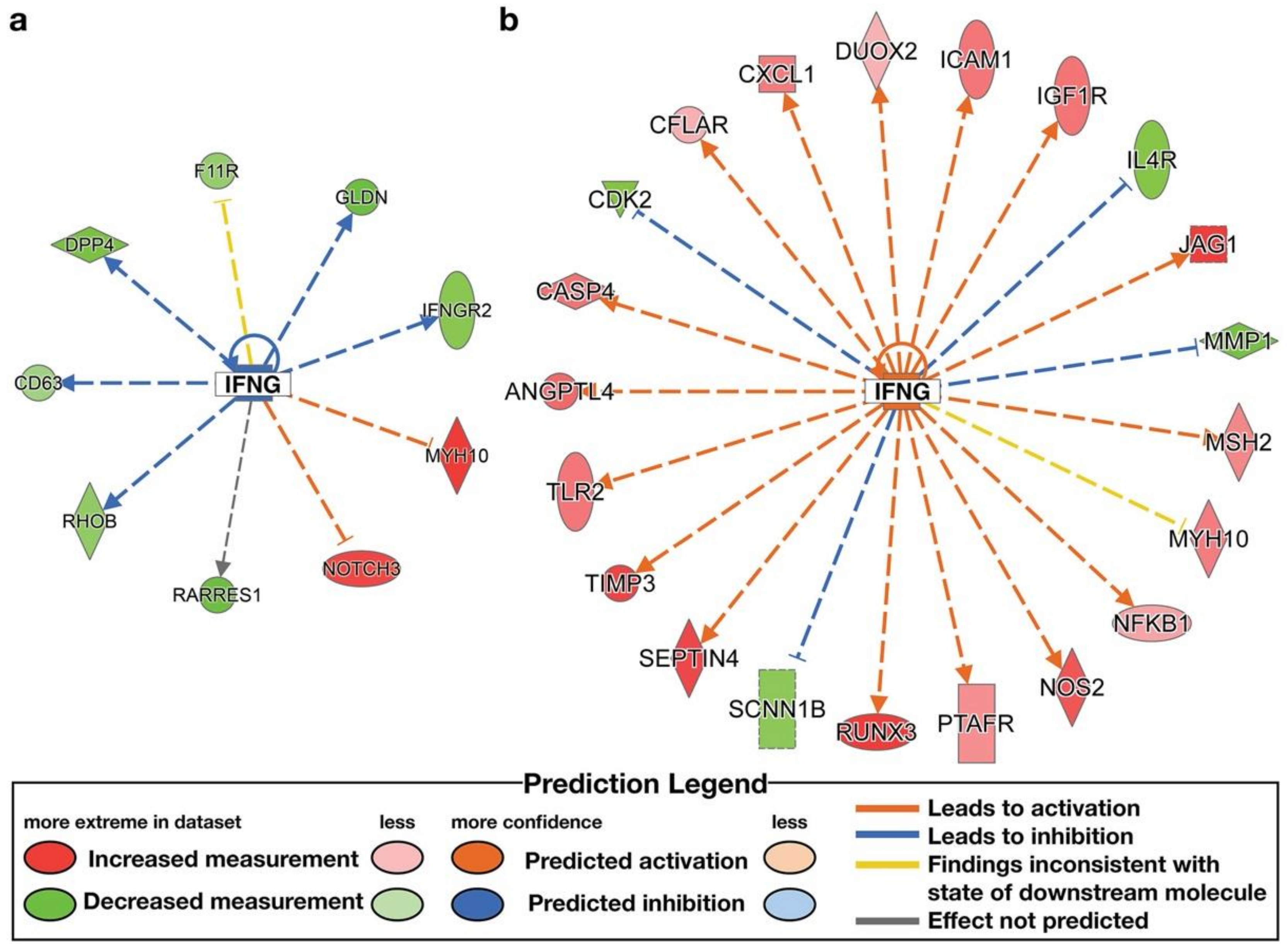
| IFNG | cytokine | High ENL | Inhibited | −2.094 | CD63, DPP4, F11R, GLDN, IFNGR2, MYH10, NOTCH3, RARRES1, RHOB |
| IFNG | cytokine | Low ENL | Activated | 3.343 | ACE, ADCY5, ADGRG2, ADRA2A, AGER, ANGPTL4, APOBEC3G, APOL1, ATM, ATP2A2, BLNK, CASP4, CCL3, CDH5, CDK2, CFB, CFLAR, CHRNG, CIITA, COL1A1, COL1A2, CRIM1, CSF1, CSF2RB, CTSC, CXCL1, CXCL11, CYRIA, DEPP1, DUOX2, EDN1, EGR3, ELK1, ETV7, FANCF, FCGR3A/FCGR3B, FKBP5, FTX, GAL3ST1, GBP2, GNAO1, GNG7, HDAC9, HERC6, HLADMA, ICAM1, IFI16, IGF1, IGF1R, IL1R1, IL1RN, IL3RA, IL4R, IREB2, ISL1, ITPR1, JAG1, JAK3, KYNU, LCP2, LY75, LYN, MAP2, MITF, MMP1, MSH2, MX2, MYH10, NEURL3, NFE2L3, NFKB1, NLRP3, NOS2, P2RY14, PAPPA, PECAM1, PHF11, PIM2, PLA2G7, PLAAT3, PSME2, PTAFR, QPRT, RAE1, RUNX3, SCIMP, SCLY, SCNN1B, SCUBE1, SEPTIN4, SLC1A2, SNAP25, SOCS3, TIMP3, TLR2, TNFAIP2, TNFRSF12A, TSC22D3, UBA2, VRK2 |
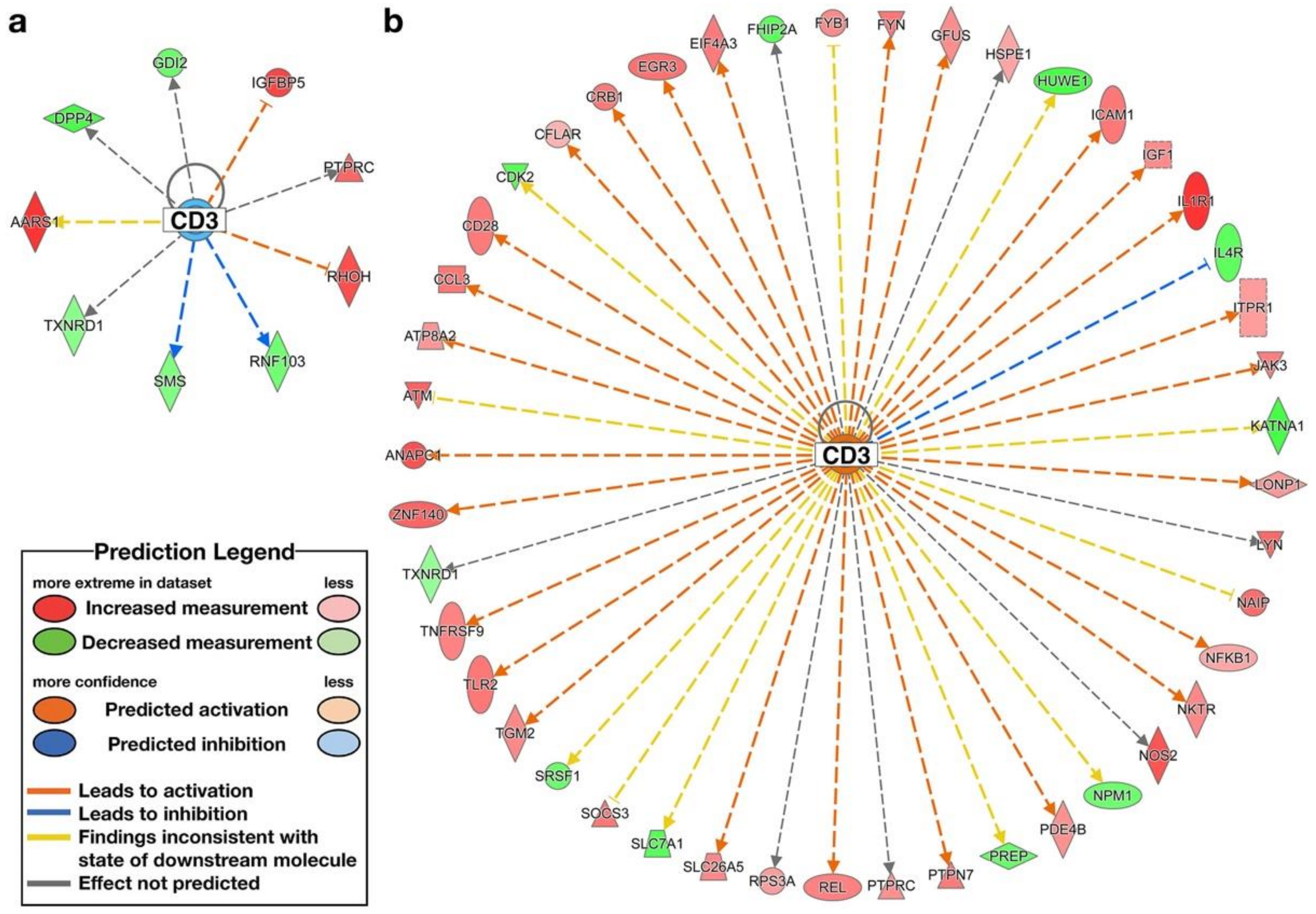
| CD3 | complex | High ENL | −1.342 | AARS1, DPP4, GDI2, IGFBP5, PTPRC, RHOH, RNF103, SMS, TXNRD1 | |
| CD3 | complex | Low ENL | Activated | 2.802 | ANAPC1, ATM, ATP8A2, CCL3, CD28, CDK2, CFLAR, CRB1, EGR3, EIF4A3, FHIP2A, FYB1, FYN, GFUS, HSPE1, HUWE1, ICAM1, IGF1, IL1R1, IL4R, ITPR1, JAK3, KATNA1, LONP1, LYN, NAIP, NFKB1, NKTR, NOS2, NPM1, PDE4B, PREP, PTPN7, PTPRC, REL, RPS3A, SLC26A5, SLC7A1, SOCS3, SRSF1, TGM2, TLR2, TNFRSF9, TXNRD1, ZNF140 |
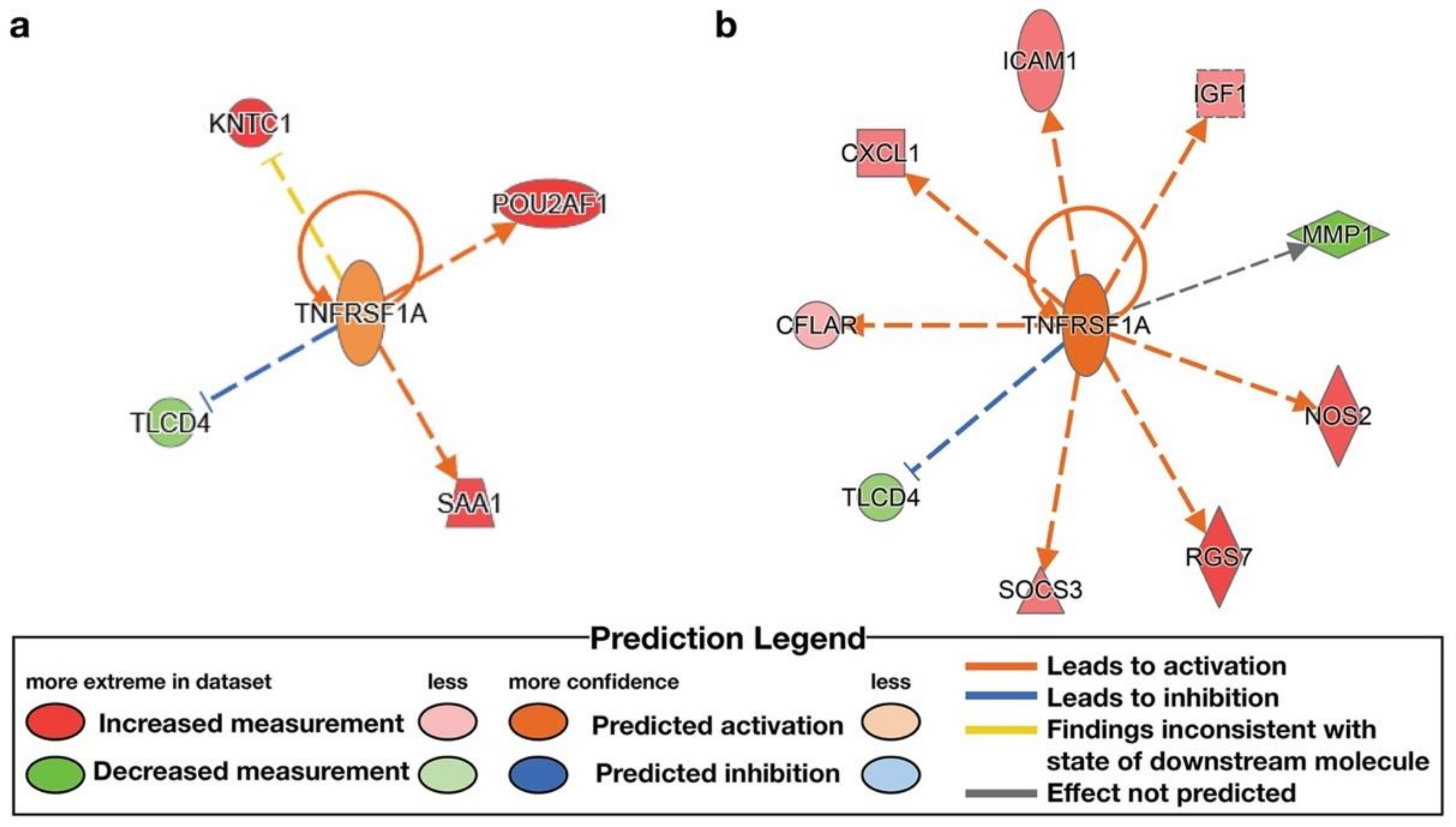
| TNFRSF1A | trans- membrane receptor | High ENL | 1.091 | KNTC1, POU2AF1, SAA1, TLCD4 | |
| TNFRSF1A | trans- membrane receptor | Low ENL | Activated | 2.758 | CFLAR, CXCL1, ICAM1, IGF1, MMP1, NOS2, RGS7, SOCS3, TLCD4 |
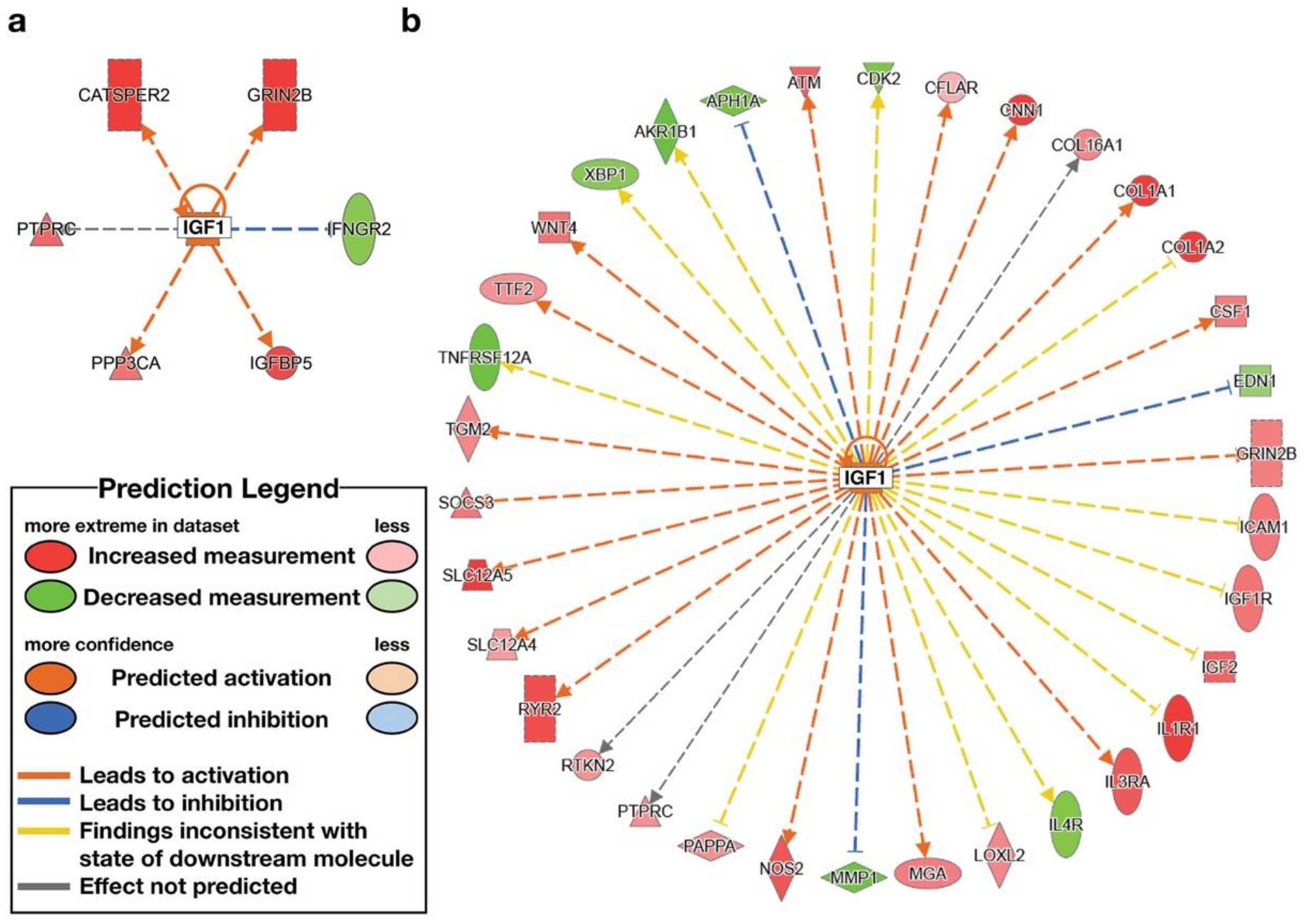
| IGF1 | growth factor | High ENL | Activated | 2.172 | CATSPER2, GRIN2B, IFNGR2, IGFBP5, PPP3CA, PTPRC |
| IGF1 | growth factor | Low ENL | 1.828 | AKR1B1, APH1A, ATM, CDK2, CFLAR, CNN1, COL16A1, COL1A1, COL1A2, CSF1, EDN1, GRIN2B, ICAM1, IGF1, IGF1R, IGF2, IL1R1, IL3RA, IL4R, LOXL2, MGA, MMP1, NOS2, PAPPA, PTPRC, RTKN2, RYR2, SLC12A4, SLC12A5, SOCS3, TGM2, TNFRSF12A, TTF2, WNT4, XBP1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullens, D.A.; Ivanov, I.; Hullar, M.A.J.; Randolph, T.W.; Lampe, J.W.; Chapkin, R.S. Personalized Nutrition Using Microbial Metabolite Phenotype to Stratify Participants and Non-Invasive Host Exfoliomics Reveal the Effects of Flaxseed Lignan Supplementation in a Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 2377. https://doi.org/10.3390/nu14122377
Mullens DA, Ivanov I, Hullar MAJ, Randolph TW, Lampe JW, Chapkin RS. Personalized Nutrition Using Microbial Metabolite Phenotype to Stratify Participants and Non-Invasive Host Exfoliomics Reveal the Effects of Flaxseed Lignan Supplementation in a Placebo-Controlled Crossover Trial. Nutrients. 2022; 14(12):2377. https://doi.org/10.3390/nu14122377
Chicago/Turabian StyleMullens, Destiny A., Ivan Ivanov, Meredith A. J. Hullar, Timothy W. Randolph, Johanna W. Lampe, and Robert S. Chapkin. 2022. "Personalized Nutrition Using Microbial Metabolite Phenotype to Stratify Participants and Non-Invasive Host Exfoliomics Reveal the Effects of Flaxseed Lignan Supplementation in a Placebo-Controlled Crossover Trial" Nutrients 14, no. 12: 2377. https://doi.org/10.3390/nu14122377
APA StyleMullens, D. A., Ivanov, I., Hullar, M. A. J., Randolph, T. W., Lampe, J. W., & Chapkin, R. S. (2022). Personalized Nutrition Using Microbial Metabolite Phenotype to Stratify Participants and Non-Invasive Host Exfoliomics Reveal the Effects of Flaxseed Lignan Supplementation in a Placebo-Controlled Crossover Trial. Nutrients, 14(12), 2377. https://doi.org/10.3390/nu14122377






