Neuroprotective Effect of 1,3-dipalmitoyl-2-oleoylglycerol Derived from Rice Bran Oil against Cerebral Ischemia-Reperfusion Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
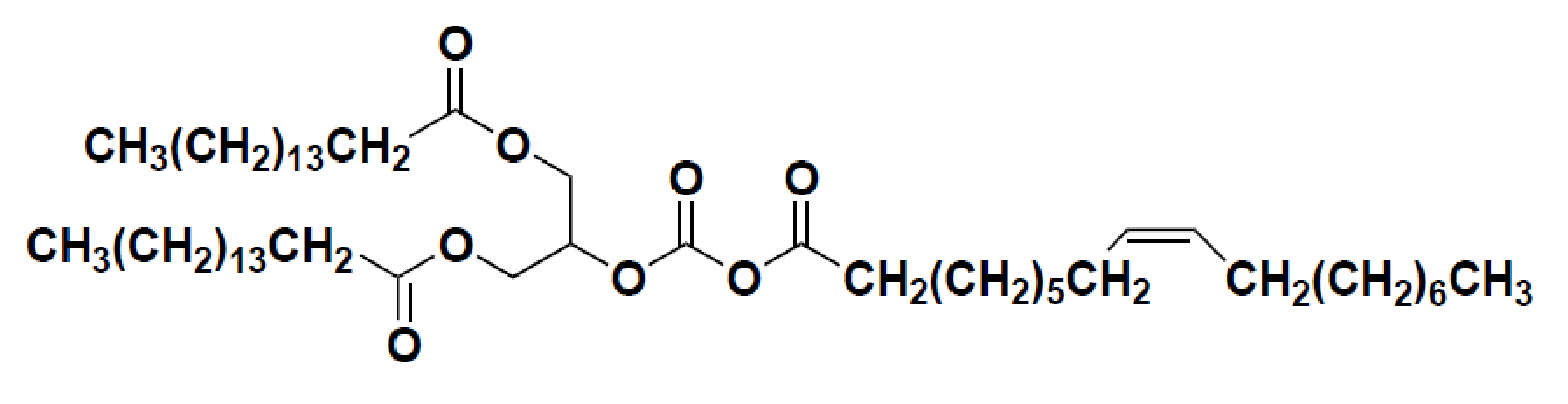
2.1. Preparation of POP
2.2. Chemicals and Reagents
2.3. Experimental Animals
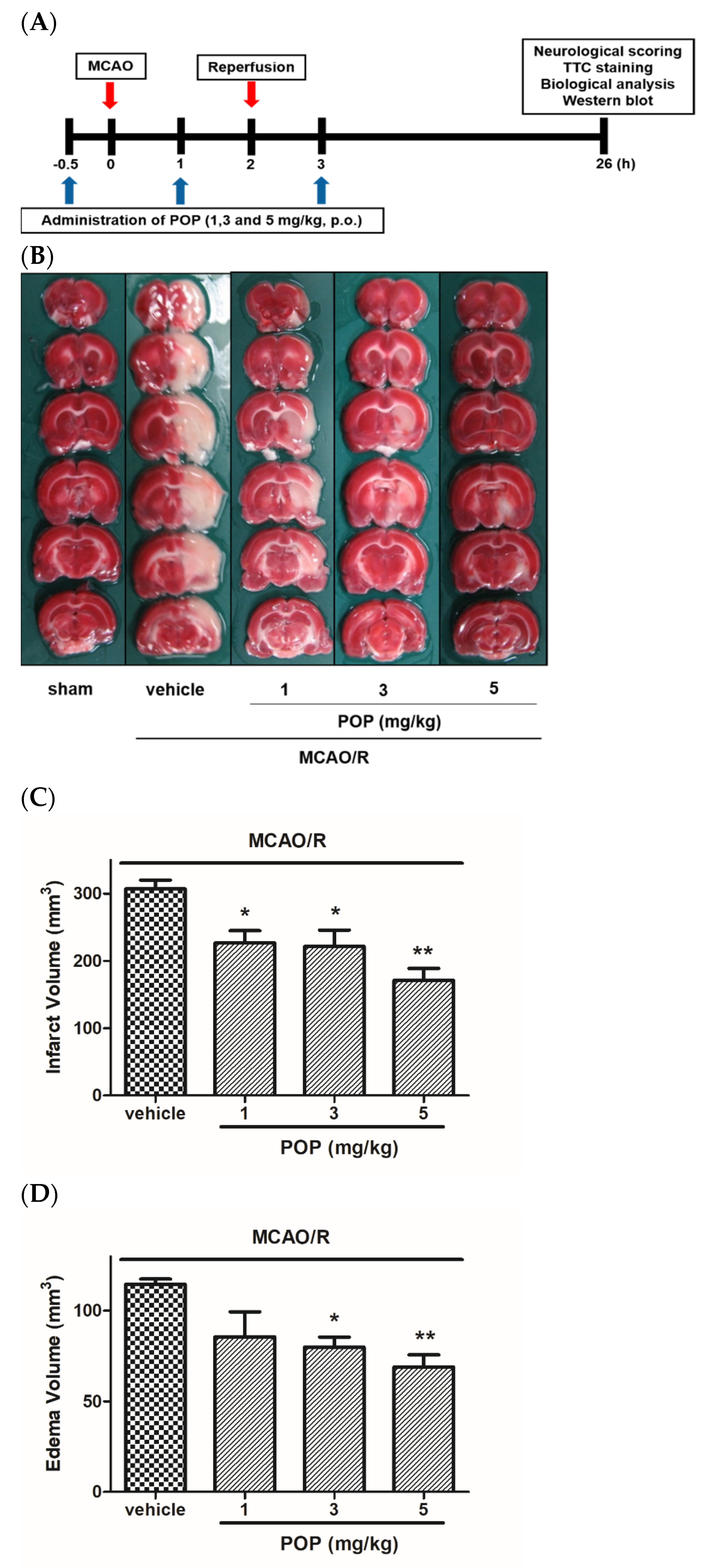
2.4. MCAO/R-Induced Transient Cerebral Ischemia in Rats and POP Treatment
2.5. Neurological Deficit Score and Cerebral Infarct/Edema
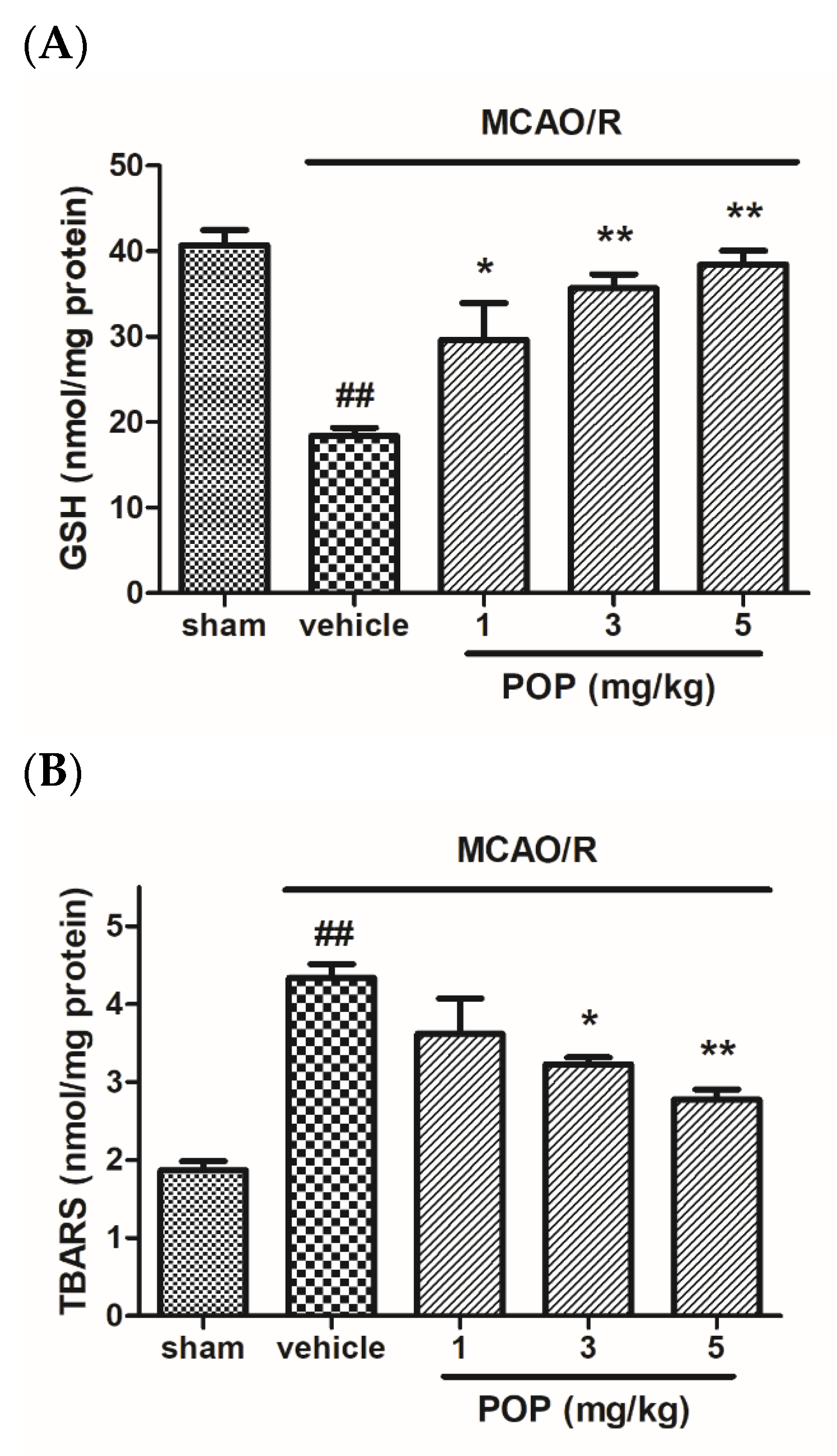
2.6. Biochemical Analysis
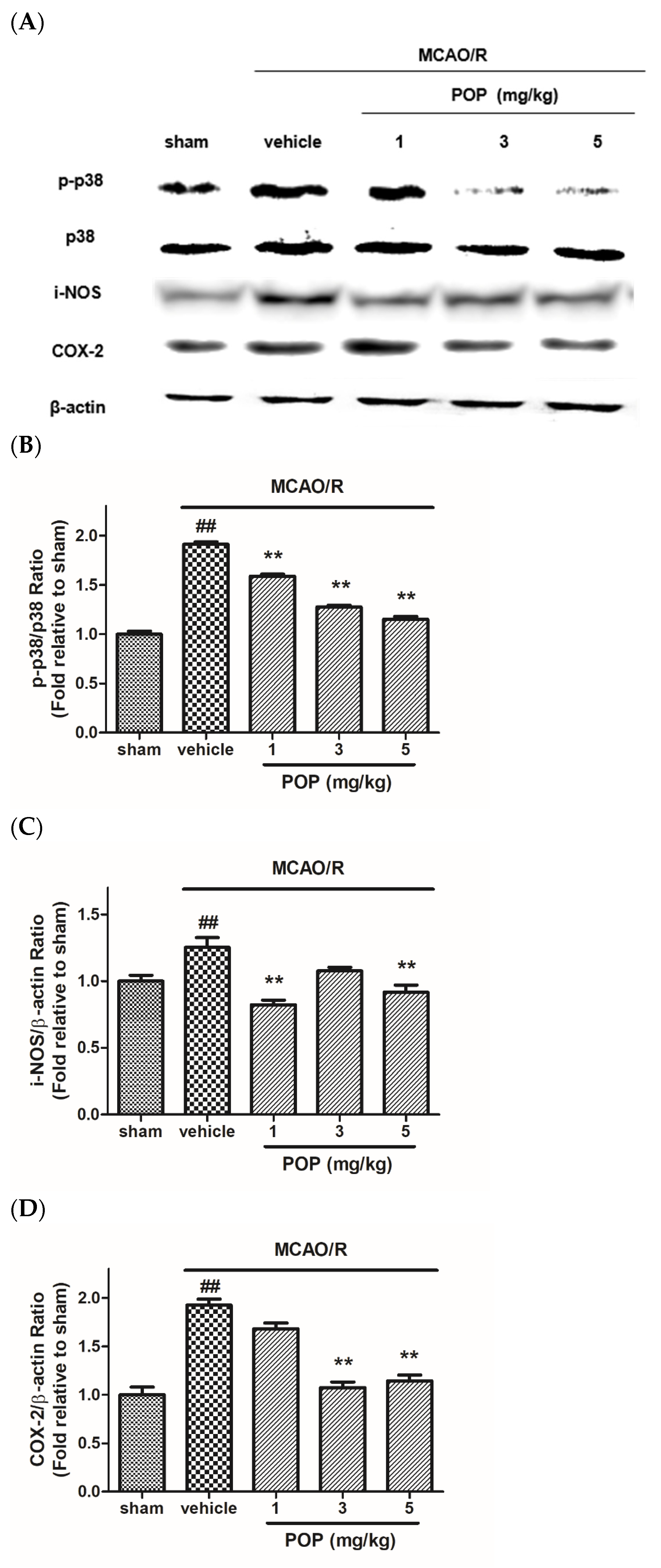
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. POP Attenuated Infarct/Edema Formation and Neurological Functional Deficits Induced by MCAO/R in Rats
3.2. POP Alleviated GSH Depletion and Lipid Peroxidation after MCAO/R in Rat Brains
3.3. POP Reversed Changes in the Expression of Apoptosis-Associated Proteins after MCAO/R in Ipsilateral Rat Brains
3.4. POP Reduced the Upregulation of p38 MAPK and Inflammation-Related Proteins after MCAO/R in Ipsilateral Rat Brains
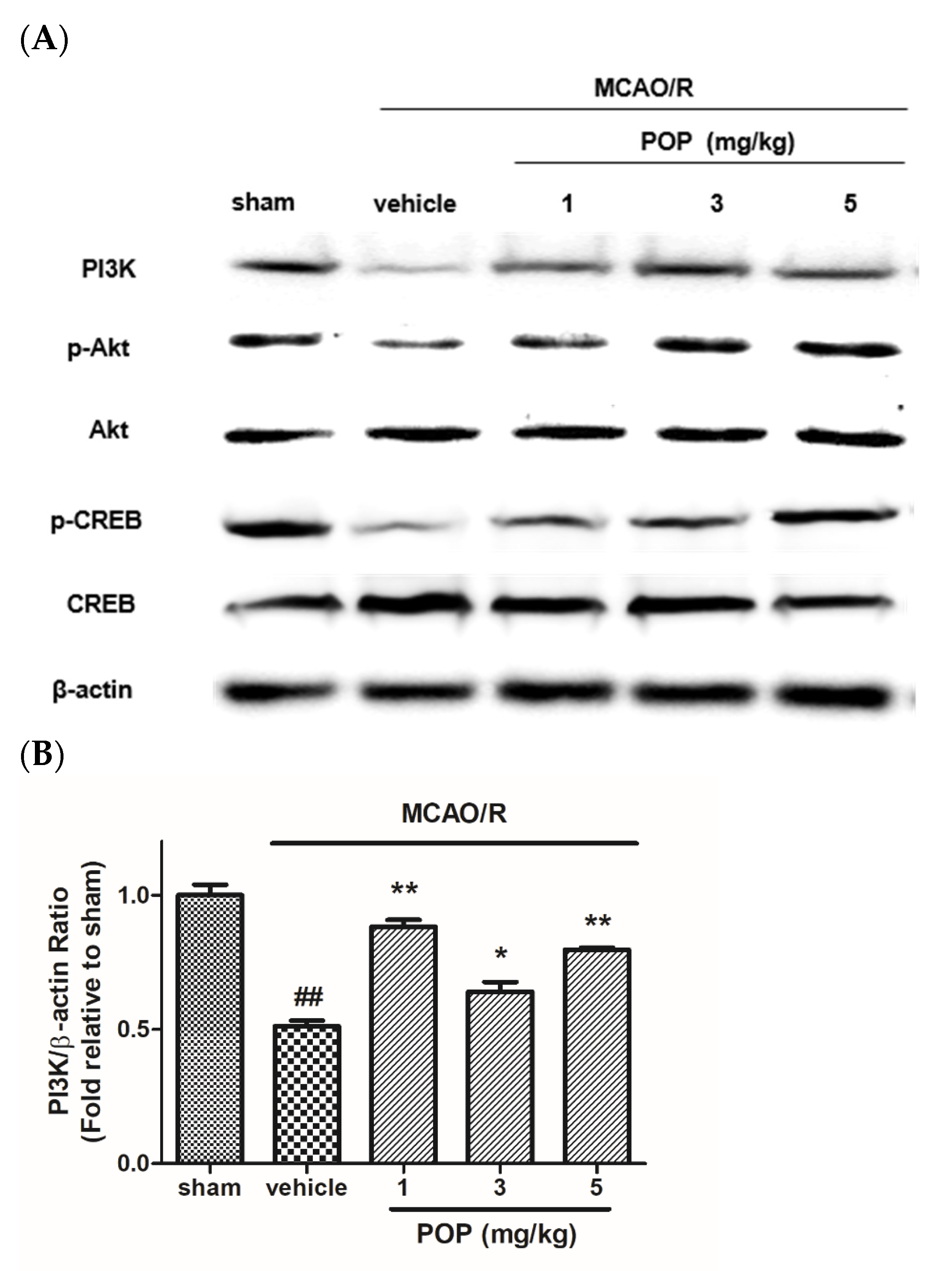
3.5. POP Attenuated the Suppression of PI3K/Akt/CREB Signaling after MCAO/R in Ipsilateral Rat Brains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, P.M.; Steinberg, G.K. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology and Its Impact on Clinical Treatments. Neuron 2015, 87, 297–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.M.; Wissman, S.; Albers, G.W.; Jhamandas, J.H.; Madden, K.P.; Hamilton, S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 h after symptom onset. The ATLANTIS Study: A randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999, 282, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M. Antithrombotic and thrombolytic therapy for ischemic stroke. J. Thromb. Thrombolysis 1999, 7, 165–169. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, Q.; Hu, Z.; Tang, X. Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front. Neurosci. 2019, 13, 1036. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Nan, G. The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke. J. Mol. Neurosci. 2016, 59, 90–98. [Google Scholar] [CrossRef]
- Asih, P.R.; Prikas, E.; Stefanoska, K.; Tan, A.R.P.; Ahel, H.I.; Ittner, A. Functions of p38 MAP Kinases in the Central Nervous System. Front. Mol. Neurosci. 2020, 13, 570586. [Google Scholar] [CrossRef]
- Jie, P.; Hong, Z.; Tian, Y.; Li, Y.; Lin, L.; Zhou, L.; Du, Y.; Chen, L.; Chen, L. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015, 6, e1775. [Google Scholar] [CrossRef] [Green Version]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.A.; Kury, L.A.; Li, T.; Zeb, A.; Koh, P.O.; Liu, F.; Zhou, Q.; Hussain, I.; Khan, A.U.; Jiang, Y.; et al. Polydatin Attenuates Neuronal Loss via Reducing Neuroinflammation and Oxidative Stress in Rat MCAO Models. Front. Pharm. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Park, H.; Lee, J.E.; Kang, T.C. Blockade of 67-kDa Laminin Receptor Facilitates AQP4 Down-Regulation and BBB Disruption via ERK1/2-and p38 MAPK-Mediated PI3K/AKT Activations. Cells 2020, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsieh, H.L.; Chi, P.L.; Yang, C.C.; Hsiao, L.D.; Yang, C.M. Upregulation of COX-2/PGE2 by ET-1 mediated through Ca2+-dependent signals in mouse brain microvascular endothelial cells. Mol. Neurobiol. 2014, 49, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Kim, H.; Yoon, K.S.; Choe, W.; Kang, I. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: Possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-kappaB signaling pathways. Neurosci. Lett. 2009, 464, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, J.; Hu, L.; Kong, L.; Wang, T.; Di, M.; Li, C.; Gui, S. miR-130a alleviates neuronal apoptosis and changes in expression of Bcl-2/Bax and caspase-3 in cerebral infarction rats through PTEN/PI3K/Akt signaling pathway. Exp. Ther. Med. 2020, 19, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Gibon, V.; Danthine, S. Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation. Foods 2020, 9, 1891. [Google Scholar] [CrossRef]
- Shekarchizadeh, H.; Tikani, R.; Kadivar, M. Optimization of cocoa butter analog synthesis variables using neural networks and genetic algorithm. J. Food Sci. Technol. 2014, 51, 2099–2105. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, T.; Fukui, K.; Taniguchi, K.; Nagaoka, S.; Yamaoto, T.; Hashimoto, Y. Absorption and metabolism of lipids in rats depend on fatty acid isomeric position. J. Nutr. 1996, 126, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Lee, H.K.; Yoo, H.-s.; Seong, Y.H. Protective Effect of Rice Bran Oil against β-Amyloid Protein-Induced Memory Impairment and Neuronal Death in Mice. Nat. Prod. Sci. 2020, 26, 221–229. [Google Scholar]
- Chu, X.; Qi, C.; Zou, L.; Fu, X. Intraluminal suture occlusion and ligation of the distal branch of internal carotid artery: An improved rat model of focal cerebral ischemia-reperfusion. J. Neurosci. Methods 2008, 168, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Menzies, S.A.; Hoff, J.T.; Betz, A.L. Middle cerebral artery occlusion in rats: A neurological and pathological evaluation of a reproducible model. Neurosurgery 1992, 31, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Burkhalter, A.; Ladou, J. A fluorometric method for the determination of hippuric acid. J. Lab. Clin. Med. 1961, 57, 813–818. [Google Scholar] [PubMed]
- Dawn-Linsley, M.; Ekinci, F.J.; Ortiz, D.; Rogers, E.; Shea, T.B. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J. Neurosci. Methods 2005, 141, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Ferrer, I.; Planas, A.M. Signaling of cell death and cell survival following focal cerebral ischemia: Life and death struggle in the penumbra. J. Neuropathol. Exp. Neurol. 2003, 62, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Jang, J.Y.; Yoo, H.S.; Seong, Y.H. Neuroprotective effect of phytoceramide against transient focal ischemia-induced brain damage in rats. Arch. Pharm. Res. 2015, 38, 2241–2250. [Google Scholar] [CrossRef]
- Tominaga, T.; Ohnishi, S.T. Interrelationship of brain edema, motor deficits, and memory impairment in rats exposed to focal ischemia. Stroke 1989, 20, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Li, W.; Yang, S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Mukda, S.; Chen, S.D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Serteser, M.; Ozben, T.; Gumuslu, S.; Balkan, S.; Balkan, E. Lipid peroxidation in rat brain during focal cerebral ischemia: Prevention of malondialdehyde and lipid conjugated diene production by a novel antiepileptic, lamotrigine. Neurotoxicology 2002, 23, 111–119. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 89–95. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Jia, L.; Chen, Y.; Tian, Y.H.; Zhang, G. MAPK pathway mediates the anti-oxidative effect of chicoric acid against cerebral ischemia-reperfusion injury in vivo. Exp. Ther. Med. 2018, 15, 1640–1646. [Google Scholar] [CrossRef] [Green Version]
- Hirabayashi, H.; Takizawa, S.; Fukuyama, N.; Nakazawa, H.; Shinohara, Y. Nitrotyrosine generation via inducible nitric oxide synthase in vascular wall in focal ischemia-reperfusion. Brain Res. 2000, 852, 319–325. [Google Scholar] [CrossRef]
- Iadecola, C.; Gorelick, P.B. The Janus face of cyclooxygenase-2 in ischemic stroke: Shifting toward downstream targets. Stroke 2005, 36, 182–185. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C.; Niwa, K.; Nogawa, S.; Zhao, X.; Nagayama, M.; Araki, E.; Morham, S.; Ross, M.E. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1294–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol. Res. 2019, 68, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Lo, E.H. The Role of the PI3K Pathway in the Regeneration of the Damaged Brain by Neural Stem Cells after Cerebral Infarction. J. Clin. Neurol. 2015, 11, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijilafu; Hur, E.M.; Liu, C.M.; Jiao, Z.; Xu, W.L.; Zhou, F.Q. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat. Commun. 2013, 4, 2690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, T.; Li, N.; Wu, L.; Gu, J.; Li, C.; Zhao, C.; Liu, W.; Shan, L.; Yu, P.; et al. Tetramethylpyrazine nitrone activates the BDNF/Akt/CREB pathway to promote post-ischaemic neuroregeneration and recovery of neurological functions in rats. Br. J. Pharmacol. 2018, 175, 517–531. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Xi, G.M.; Li, W.C.; Ling, H.Y.; Qu, P.; Fang, X.B. Cyclic-AMP response element binding protein and tau are involved in the neuroprotective mechanisms of nerve growth factor during focal cerebral ischemia/reperfusion in rats. J. Clin. Neurosci. 2010, 17, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Hoy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Antonios, N.; Angiolillo, D.J.; Silliman, S. Hypertriglyceridemia and ischemic stroke. Eur. Neurol. 2008, 60, 269–278. [Google Scholar] [CrossRef]
- Freiberg, J.J.; Tybjaerg-Hansen, A.; Jensen, J.S.; Nordestgaard, B.G. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 2008, 300, 2142–2152. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, T.; Slowik, A.; Gryz, E.A.; Szczudlik, A. Lower serum triglyceride level is associated with increased stroke severity. Stroke 2004, 35, e151–e152. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Kim, Y.S.; Lee, D.H.; Lee, S.H.; Park, H.J.; Lee, D.; Kim, H. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci. Rep. 2019, 9, 10732. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.K.; Jang, J.Y.; Yoo, H.-S.; Seong, Y.H. Neuroprotective Effect of 1,3-dipalmitoyl-2-oleoylglycerol Derived from Rice Bran Oil against Cerebral Ischemia-Reperfusion Injury in Rats. Nutrients 2022, 14, 1380. https://doi.org/10.3390/nu14071380
Lee HK, Jang JY, Yoo H-S, Seong YH. Neuroprotective Effect of 1,3-dipalmitoyl-2-oleoylglycerol Derived from Rice Bran Oil against Cerebral Ischemia-Reperfusion Injury in Rats. Nutrients. 2022; 14(7):1380. https://doi.org/10.3390/nu14071380
Chicago/Turabian StyleLee, Hong Kyu, Ji Yeon Jang, Hwan-Su Yoo, and Yeon Hee Seong. 2022. "Neuroprotective Effect of 1,3-dipalmitoyl-2-oleoylglycerol Derived from Rice Bran Oil against Cerebral Ischemia-Reperfusion Injury in Rats" Nutrients 14, no. 7: 1380. https://doi.org/10.3390/nu14071380
APA StyleLee, H. K., Jang, J. Y., Yoo, H.-S., & Seong, Y. H. (2022). Neuroprotective Effect of 1,3-dipalmitoyl-2-oleoylglycerol Derived from Rice Bran Oil against Cerebral Ischemia-Reperfusion Injury in Rats. Nutrients, 14(7), 1380. https://doi.org/10.3390/nu14071380





