Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Dietary Assessment
2.4. Quantification of TMAO and Select Amines
2.5. Clinical Chemistry
2.6. Inflammatory Markers
2.7. Microbiota Assessment
2.8. Endothelial Function with EndoPAT
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. TMAO and Recent Food Intake
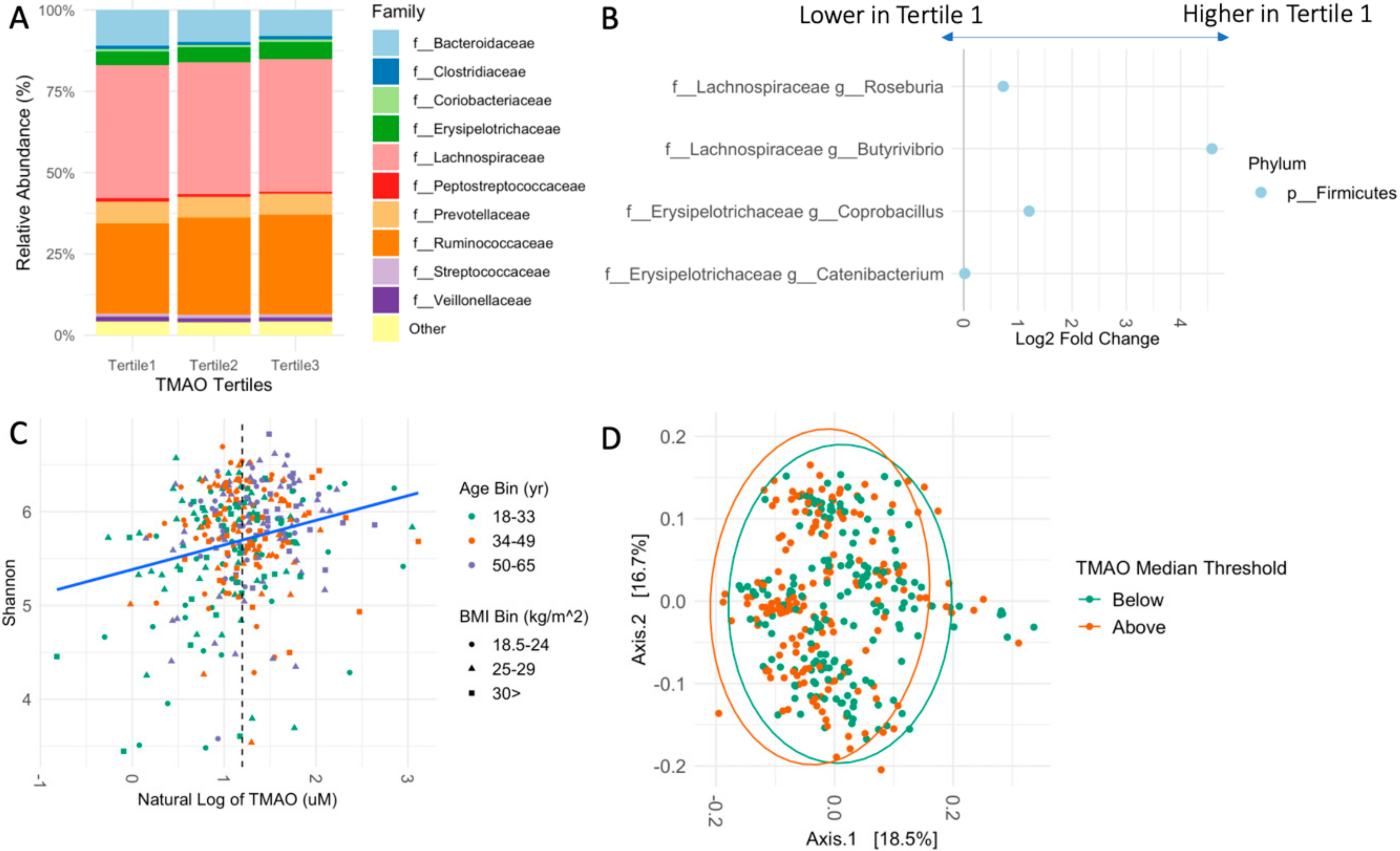
3.3. Fecal Microbiome Relates to TMAO
3.4. Cardiometabolic Markers and TMAO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Center for Disease Control and Prevention. Heart Disease and Stroke. 2020. Available online: https://www.cdc.gov/chronicdisease/pdf/factsheets/Heart-Disease-Stroke-factsheet-H.pdf (accessed on 8 December 2021).
- The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 8 December 2021).
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose–Response Meta-analysis. Adv. Nutr. 2020, 11, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. CCLM 2017, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEntyre, C.J.; Lever, M.; Chambers, S.T.; George, P.M.; Slow, S.; Elmslie, J.L.; Florkowski, C.M.; Lunt, H.; Krebs, J.D. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann. Clin. Biochem. 2015, 52, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Corbin, K.D.; da Costa, K.-A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study1234. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Missimer, A.; Fernandez, M.L.; DiMarco, D.M.; Norris, G.H.; Blesso, C.N.; Murillo, A.G.; Vergara-Jimenez, M.; Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; et al. Compared to an Oatmeal Breakfast, Two Eggs/Day Increased Plasma Carotenoids and Choline without Increasing Trimethyl Amine N -Oxide Concentrations. J. Am. Coll. Nutr. 2018, 37, 140–148. [Google Scholar] [CrossRef]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; et al. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; O’Connor, L.E.; Wang, Y.; Gertz, E.R.; Campbell, W.W.; Bennett, B.J. Adopting a Mediterranean-style eating pattern with low, but not moderate, unprocessed, lean red meat intake reduces fasting serum trimethylamine N-oxide (TMAO) in adults who are overweight or obese. Br. J. Nutr. 2021, 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Jameson, E.; Crosatti, M.; Schafer, H.; Rajakumar, K.; Bugg, T.D.H.; Chen, Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 4268–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ–Butyrobetaine is a pro-atherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrell, M.; Bazeley, P.; Wang, Z.; Levison, B.S.; Li, X.S.; Jia, X.; Krauss, R.M.; Knight, R.; Lusis, A.J.; Garcia-Garcia, J.C.; et al. Fecal Microbiome Composition Does Not Predict Diet-Induced TMAO Production in Healthy Adults. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2021, 10, e021934. [Google Scholar] [CrossRef]
- Baldiviez, L.M.; Keim, N.L.; Laugero, K.D.; Hwang, D.H.; Huang, L.; Woodhouse, L.R.; Burnett, D.J.; Zerofsky, M.S.; Bonnel, E.L.; Allen, L.H.; et al. Design and implementation of a cross-sectional nutritional phenotyping study in healthy US adults. BMC Nutr. 2017, 3, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute. Reviewing & Cleaning ASA24 Data. 2021. Available online: https://epi.grants.cancer.gov/asa24/resources/cleaning.html (accessed on 8 February 2022).
- Bouzid, Y.Y.; Arsenault, J.E.; Bonnel, E.L.; Cervantes, E.; Kan, A.; Keim, N.L.; Lemay, D.G.; Stephensen, C.B. Effect of Manual Data Cleaning on Nutrient Intakes Using the Automated Self-Administered 24-Hour Dietary Assessment Tool (ASA24). Curr. Dev. Nutr. 2021, 5, nzab005. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Basic Steps in Calculating HEI Scores. 2021. Available online: https://epi.grants.cancer.gov/hei/calculating-hei-scores.html (accessed on 8 February 2022).
- Kable, M.E.; Chin, E.L.; Storms, D.; Lemay, D.G.; Stephensen, C.B. Tree-based Analysis of Dietary Diversity Captures Associations between Fiber Intake and Gut Microbiota Composition in a Healthy U.S. Adult Cohort. J. Nutr. 2021, 152, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Comeau, A.M.; Douglas, G.M.; Langille, M.G.I. Microbiome Helper: A Custom and Streamlined Workflow for Microbiome Research. mSystems 2017, 2, e00127-16. [Google Scholar] [CrossRef] [Green Version]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Quince, C.; Lanzen, A.; Davenport, R.J.; Turnbaugh, P.J. Removing Noise from Pyrosequenced Amplicons. BMC Bioinform. 2011, 12, 38. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet 2011, 17, 3. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e003970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andraos, S.; Jones, B.; Lange, K.; Clifford, S.A.; Thorstensen, E.B.; Kerr, J.A.; Wake, M.; Saffery, R.; Burgner, D.P.; O’Sullivan, J.M. Trimethylamine N-oxide (TMAO) Is not Associated with Cardiometabolic Phenotypes and Inflammatory Markers in Children and Adults. Curr. Dev. Nutr. 2021, 5, nzaa179. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Hullar, M.A.J.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Agriculture. Food Patterns Equivalents Intakes from Food: Mean Amounts Consumed per Individual by Gender and Age, What We Eat in America, NHANES, 2009–2010. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fped/table_1_fped_gen_0910.pdf (accessed on 4 January 2022).
- U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025; U.S. Department of Agriculture: Washington, DC, USA, 2020. [Google Scholar]
- Wu, W.-K.; Chen, C.-C.; Liu, P.-Y.; Panyod, S.; Liao, B.-Y.; Chen, P.-C.; Kao, H.-L.; Kuo, H.-C.; Kuo, C.-H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host–diet–gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Pidcock, S.E.; Skvortsov, T.; Santos, F.G.; Courtney, S.J.; Sui-Ting, K.; Creevey, C.J.; Huws, S.A. Phylogenetic systematics of Butyrivibrio and Pseudobutyrivibrio genomes illustrate vast taxonomic diversity, open genomes and an abundance of carbohydrate-active enzyme family isoforms. Microb. Genom. 2021, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 (FMO3) Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.T.; Schartum-Hansen, H.; Pedersen, E.R.; Ueland, P.M.; Tell, G.S.; Mellgren, G.; Njølstad, P.R.; Seifert, R.; Strand, E.; Karlsson, T.; et al. Prospective Associations of Systemic and Urinary Choline Metabolites with Incident Type 2 Diabetes. Clin. Chem. 2016, 62, 755–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Li, X.S.; Wu, Y.; Wang, Z.; Khaw, K.-T.; Wareham, N.J.; Nieuwdorp, M.; Boekholdt, S.M.; Hazen, S.L. Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the EPIC-Norfolk prospective population study. Am. Heart J. 2021, 236, 80–86. [Google Scholar] [CrossRef]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakhtangadze, T.; Tak, R.S.; Singh, U.; Baig, M.S.; Bezsonov, E. Gender Differences in Atherosclerotic Vascular Disease: From Lipids to Clinical Outcomes. Front. Cardiovasc. Med. 2021, 8, 707889. [Google Scholar] [CrossRef] [PubMed]
- Joakimsen, O.; Bønaa, K.H.; Stensland-Bugge, E.; Jacobsen, B.K. Age and Sex Differences in the Distribution and Ultrasound Morphology of Carotid Atherosclerosis: The Tromsø Study. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 3007–3013. [Google Scholar] [CrossRef] [Green Version]
- Esposito, T.; Varriale, B.; D’Angelo, R.; Amato, A.; Sidoti, A. Regulation of flavin-containing mono-oxygenase (Fmo3) gene expression by steroids in mice and humans. Horm. Mol. Biol. Clin. Investig. 2014, 20. [Google Scholar] [CrossRef]
| Tertile 1 | Tertile 2 | Tertile 3 | p | Padj | ||
|---|---|---|---|---|---|---|
| TMAO range (μM) | 0.44–2.77 | 2.78–3.9 | 3.91–22.50 | |||
| Descriptive | n | 121 | 120 | 120 | ||
| Age (yr) | 36.96 (13.43) | 39.89 (13.28) | 44.04 (13.93) | <0.001 | 0.002 | |
| Sex (% Female) | 66 (54.5) | 58 (48.3) | 65 (54.2) | 0.557 | 1.0 | |
| Ethnicity (%) | 0.001 | 0.016 | ||||
| Caucasian | 67 (55.8) | 71 (59.7) | 82 (68.9) | |||
| Hispanic | 9 (7.5) | 21 (17.6) | 19 (16.0) | |||
| African American | 5 (4.2) | 6 (5.0) | 5 (4.2) | |||
| Asian | 29 (24.2) | 8 (6.7) | 7 (5.9) | |||
| Multiple Ethnicities | 7 (5.8) | 10 (8.4) | 4 (3.4) | |||
| Other | 3 (2.5) | 3 (2.5) | 2 (1.7) | |||
| Anthropometrics | BMI (kg/m2) | 26.63 (4.71) | 27.02 (4.70) | 28.12 (5.19) | 0.05 | 0.135 |
| Waist Circumference (cm) | 83.10 (13.12) | 84.67 (12.00) | 87.58 (12.74) | 0.023 | 0.074 | |
| Endothelial | Systolic BP (mmHg) | 118.26 (10.92) | 120.94 (10.39) | 119.60 (11.78) | 0.177 | 0.337 |
| Diastolic BP (mmHg) | 68.07 (9.69) | 69.54 (8.61) | 67.40 (8.47) | 0.169 | 0.337 | |
| Reactive Hyperemia Index | 2.21 (0.55) | 2.26 (0.56) | 2.21 (0.55) | 0.768 | 0.768 | |
| Augmentation Index | 0.55 (21.09) | 5.08 (23.92) | 3.45 (21.80) | 0.302 | 0.419 | |
| Plasma Chemistry | HDL (mg/dL) | 56.72 (16.72) | 55.36 (16.81) | 53.48 (14.81) | 0.295 | 0.419 |
| LDL (mg/dL) | 106.08 (29.42) | 109.56 (31.22) | 114.69 (33.20) | 0.101 | 0.2131 | |
| Cholesterol (mg/dL) | 172.11 (32.89) | 174.84 (35.18) | 178.10 (36.26) | 0.41 | 0.487 | |
| Triglycerides (mg/dL) | 91.99 (47.41) | 99.76 (54.33) | 99.33 (40.12) | 0.364 | 0.461 | |
| Glucose (mg/dL) | 92.33 (7.61) | 95.27 (22.36) | 96.52 (10.76) | 0.084 | 0.199 | |
| Insulin (ρM) | 56.92 (36.91) | 59.34 (38.17) | 73.08 (75.17) | 0.04 | 0.1210 | |
| Inflammatory | TNF-α (ρg/mL) | 1.97 (0.74) | 2.06 (0.82) | 2.46 (0.89) | <0.001 | 0.000 |
| IL-6 (ρg/mL) | 0.90 (1.94) | 0.66 (0.60) | 0.82 (0.57) | 0.309 | 0.419 | |
| CRP (mg/dL) | 0.35 (0.54) | 0.38 (0.84) | 0.44 (0.71) | 0.583 | 0.651 | |
| TMAO | Cystatin C (μM) | 0.83 (0.12) | 0.85 (0.16) | 0.89 (0.13) | 0.001 | 0.005 |
| TMAO (μM) | 2.05 (0.55) | 3.35 (0.32) | 6.18 (3.11) | <0.001 | 0.000 | |
| Choline (μM) | 8.82 (2.05) | 9.15 (2.08) | 9.65 (1.93) | 0.006 | 0.024 | |
| Betaine (μM) | 44.70 (16.69) | 44.02 (15.73) | 45.81 (18.64) | 0.715 | 0.755 | |
| Carnitine (μM) | 34.80 (8.91) | 35.85 (8.44) | 36.77 (8.20) | 0.198 | 0.343 |
| β | p | Padj | ||
|---|---|---|---|---|
| Protein | Non-processed Meat | 0.035 | 0.300 | 0.703 |
| Processed Meat | 0.041 | 0.331 | 0.703 | |
| Poultry | 0.025 | 0.458 | 0.734 | |
| Seafood High in ω-3 | −0.011 | 0.851 | 0.928 | |
| Seafood Low in ω-3 | 0.090 | 0.116 | 0.629 | |
| Eggs | 0.035 | 0.582 | 0.764 | |
| Nuts | 0.002 | 0.967 | 0.969 | |
| Legumes | 0.021 | 0.631 | 0.764 | |
| Total Protein Foods | 0.076 | 0.168 | 0.629 | |
| Vegetables | Dark Green Vegetables | −0.126 | 0.060 | 0.629 |
| Red and Orange Vegetables | −0.088 | 0.360 | 0.703 | |
| Starchy Vegetables | 0.047 | 0.526 | 0.764 | |
| Other Vegetables | −0.066 | 0.369 | 0.703 | |
| Total Vegetables | −0.080 | 0.210 | 0.629 | |
| Grains | Whole Grains | −0.011 | 0.147 | 0.922 |
| Refined Grains | −0.063 | 0.180 | 0.629 | |
| Total Grains | −0.016 | 0.807 | 0.629 | |
| Dairy | Milk | 0.033 | 0.637 | 0.764 |
| Cheese | −0.051 | 0.410 | 0.703 | |
| Yogurt | 0.047 | 0.386 | 0.703 | |
| Total Dairy | −0.038 | 0.575 | 0.764 | |
| Miscellaneous | Total Choline | 0.111 | 0.208 | 0.629 |
| Total Fiber | −0.087 | 0.185 | 0.629 | |
| HEI Total Score | <0.001 | 0.969 | 0.969 |
| β | p | Padj | ||
|---|---|---|---|---|
| TMAO | Betaine (μM) | −1.376 | 0.422 | 0.630 |
| Carnitine (μM) | 1.444 | 0.081 | 0.331 | |
| Choline (μM) | 0.234 | 0.249 | 0.543 | |
| Anthropometrics | Waist circumference (cm) | 1.965 | 0.117 | 0.331 |
| BMI (kg/m2) | 0.829 | 0.100 | 0.331 | |
| Endothelial | Systolic BP (mmHg) | −0.233 | 0.838 | 0.950 |
| Diastolic BP (mmHg) | −1.756 | 0.054 | 0.305 | |
| Reactive Hyperemia Index | −0.007 | 0.906 | 0.963 | |
| Augmentation Index 75 | −1.200 | 0.481 | 0.630 | |
| Clinical Chemistry | HDL (mg/dL) | −1.683 | 0.287 | 0.543 |
| LDL (mg/dL) | 2.225 | 0.467 | 0.630 | |
| Cholesterol (mg/dL) | −0.039 | 0.991 | 0.991 | |
| Glucose (mg/dL) | 0.022 | 0.052 | 0.305 | |
| Insulin (mg/dL) | 0.069 | 0.272 | 0.517 | |
| Triglycerides (mg/dL) | 0.010 | 0.825 | 0.950 | |
| Inflammatory | CRP (mg/dL) | 0.129 | 0.326 | 0.554 |
| TNF-α (ρg/mL) | 0.112 | 0.001 | 0.024 | |
| IL-6 (ρg/mL) | 0.072 | 0.283 | 0.543 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, K.L.; Gertz, E.R.; Cervantes, E.; Bonnel, E.L.; Stephensen, C.B.; Kable, M.E.; Bennett, B.J. Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults. Nutrients 2022, 14, 1376. https://doi.org/10.3390/nu14071376
James KL, Gertz ER, Cervantes E, Bonnel EL, Stephensen CB, Kable ME, Bennett BJ. Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults. Nutrients. 2022; 14(7):1376. https://doi.org/10.3390/nu14071376
Chicago/Turabian StyleJames, Kristen L., Erik R. Gertz, Eduardo Cervantes, Ellen L. Bonnel, Charles B. Stephensen, Mary E. Kable, and Brian J. Bennett. 2022. "Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults" Nutrients 14, no. 7: 1376. https://doi.org/10.3390/nu14071376
APA StyleJames, K. L., Gertz, E. R., Cervantes, E., Bonnel, E. L., Stephensen, C. B., Kable, M. E., & Bennett, B. J. (2022). Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults. Nutrients, 14(7), 1376. https://doi.org/10.3390/nu14071376






