Role of Zinc in Diabetic Kidney Disease
Abstract
1. Introduction
2. Zinc and Chronic Kidney Disease
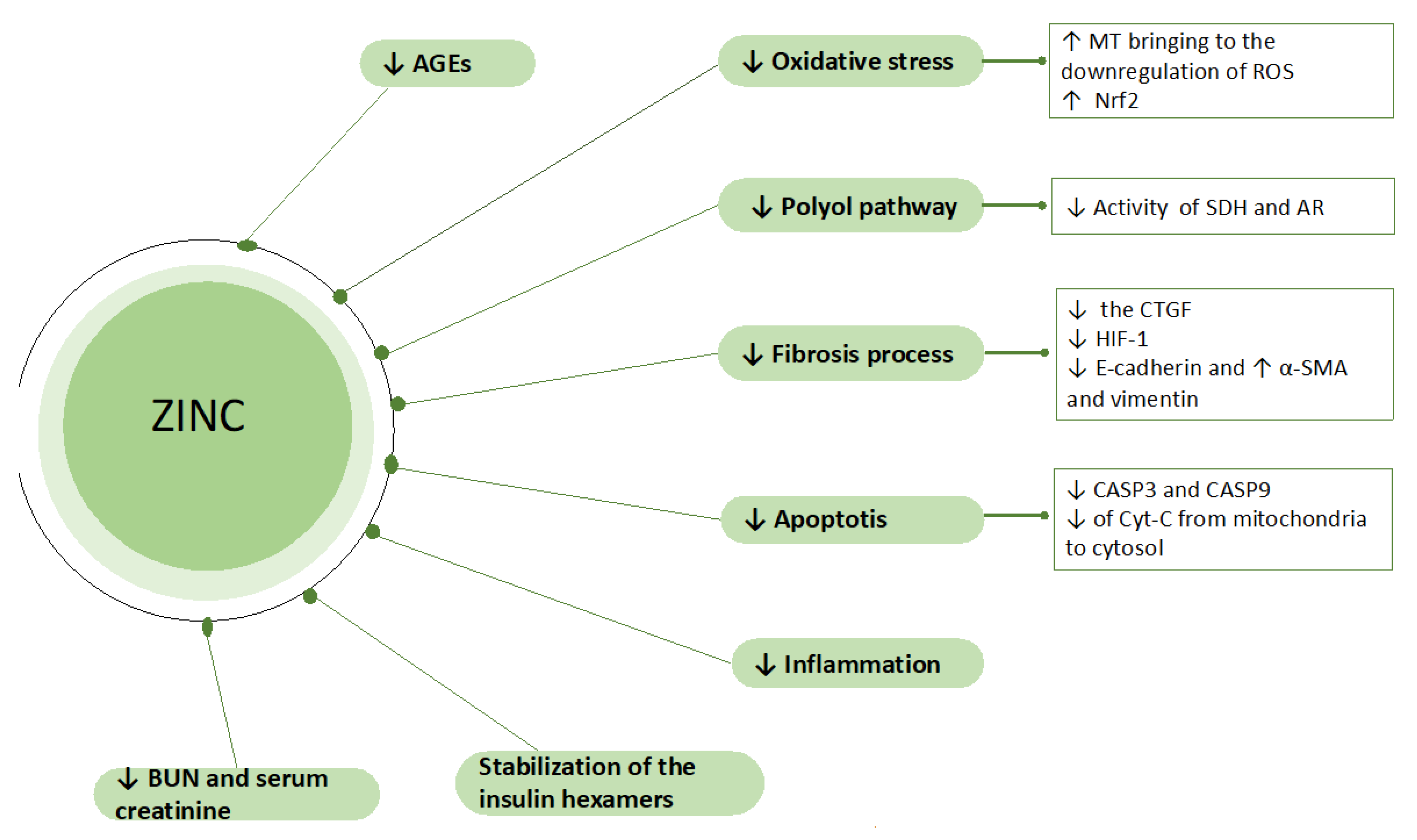
3. Role of the Zinc in DKD: Experimental Studies
4. Role of the Zinc in DKD: Human Studies
5. Zinc Supplementation and Optimal Levels in DKD: In Medio Stat Virtus
6. A Tricky Aspect on the Zinc Supplementation: Mind the Copper
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOPP | advanced oxidation protein products |
| ACR | albumin-to-creatinine ratio |
| AGEs | advanced glycation end products |
| BUN | blood urea nitrogen |
| CKD | Chronic Kidney Disease |
| DM | Diabetes Mellitus |
| DKD | Diabetic Kidney Disease |
| ESRD | end-stage renal disease |
| Fe | iron |
| FE | fractional excretion |
| GFR | glomerular filtration rate |
| HD | hemodialysis |
| HIF | Hypoxia-inducible factor |
| HOMA-IR | homeostasis model assessment—insulin resistance |
| hs-CRP | high-sensitivity c-reactive protein |
| HR | hazard ratio |
| HIF | Hypoxia-inducible factor |
| ICAM-1 | intercellular adhesion molecule 1 |
| LDL | low-density lipoprotein |
| MT | metallothionine |
| mRNA | messenger ribonucleic acid |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 |
| PD | peritoneal dialysis |
| QUICKI | Quantitative Insulin Sensitivity Check Index |
| RCT | randomized controlled trial |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| PKC | protein kinase c |
| TGF-β | Transforming growth factor β |
| TNF-α | tumor necrosis factor-alpha |
| T2DM | type 2 diabetes mellitus |
| WT1 | Will’s tumor gene-1 |
| Zn | Zinc |
| ZnSO4 | Zinc sulfate |
References
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985, 5, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Dvornik, S.; Cuk, M.; Racki, S.; Zaputović, L. Serum zinc concentrations in the maintenance hemodialysis patients. Coll. Antropol. 2006, 30, 125–129. [Google Scholar] [PubMed]
- Nagy, A.; Pethő, D.; Gáll, T.; Zavaczki, E.; Nyitrai, M.; Posta, J.; Zarjou, A.; Agarwal, A.; Balla, G.; Balla, J. Zinc Inhibits HIF-Prolyl Hydroxylase Inhibitor-Aggravated VSMC Calcification Induced by High Phosphate. Front. Physiol. 2020, 10, 1584. [Google Scholar] [CrossRef]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro, O.M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-κB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Pigera, S.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Zinc and diabetes mellitus: Understanding molecular mechanisms and clinical implications. DARU 2015, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Cuppari, L.; Cozzolino, S.M. Iron and zinc status of patients with chronic renal failure who are not on dialysis. J. Ren. Nutr. 2002, 12, 38–41. [Google Scholar] [CrossRef]
- Tavares, A.P.D.S.R.; Mafra, D.; Leal, V.O.; Gama, M.D.S.; Vieira, R.M.M.F.; Brum, I.S.D.C.; Borges, N.A.; Silva, A.A. Zinc Plasma Status and Sensory Perception in Nondialysis Chronic Kidney Disease Patients. J. Ren. Nutr. 2021, 31, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yin, Z.; Lv, Y.; Luo, J.; Shi, W.; Fang, J.; Shi, X. Plasma element levels and risk of chronic kidney disease in elderly populations (≥90 Years old). Chemosphere 2020, 254, 126809. [Google Scholar] [CrossRef]
- Hasanato, R.M. Assessment of trace elements in sera of patients undergoing renal dialysis. Saudi Med. J. 2014, 35, 365–370. [Google Scholar]
- Lobo, J.C.; Stockler-Pinto, M.B.; Farage, N.E.; Faulin Tdo, E.; Abdalla, D.S.; Torres, J.P.; Velarde, L.G.; Mafra, D. Reduced plasma zinc levels, lipid peroxidation, and inflammation biomarkers levels in hemodialysis patients: Implications to cardiovascular mortality. Ren. Fail. 2013, 35, 680–685. [Google Scholar] [CrossRef]
- Dashti-Khavidaki, S.; Khalili, H.; Vahedi, S.M.; Lessan-Pezeshki, M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity. Saudi J. Kidney Dis. Transpl. 2010, 21, 641–645. [Google Scholar]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Masuda, K.; Yamazaki, M.; Kiyohara, C.; Itoh, S.; Wasaki, M.; Inoue, H. Metal ion and vitamin adsorption profiles of phosphate binder ion-exchange resins. Clin. Nephrol. 2010, 73, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Damianaki, K.; Lourenco, J.M.; Braconnier, P.; Ghobril, J.P.; Devuyst, O.; Burnier, M.; Lenglet, S.; Augsburger, M.; Thomas, A.; Pruijm, M. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol. Dial. Transplant. 2020, 35, 1163–1170. [Google Scholar] [CrossRef]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.R.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Dietary zinc intake and incident chronic kidney disease. Clin. Nutr. 2021, 40, 1039–1045. [Google Scholar] [CrossRef]
- Tokuyama, A.; Kanda, E.; Itano, S.; Kondo, M.; Wada, Y.; Kadoya, H.; Kidokoro, K.; Nagasu, H.; Sasaki, T.; Kashihara, N. Effect of zinc deficiency on chronic kidney disease progression and effect modification by hypoalbuminemia. PLoS ONE 2021, 16, e0251554. [Google Scholar] [CrossRef]
- Li, M.S.; Adesina, S.E.; Ellis, C.L.; Gooch, J.L.; Hoover, R.S.; Williams, C.R. NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage. Am. J. Physiol. Cell Physiol. 2017, 312, C47–C55. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, M.Q.; Hu, R.; Yang, Y.; Huang, Y.S.; Xian, S.X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef]
- Pan, H.Z.; Zhang, L.; Guo, M.Y.; Sui, H.; Li, H.; Wu, W.H.; Qu, N.Q.; Liang, M.H.; Chang, D. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol. 2010, 47, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Özcelik, D.; Nazıroglu, M.; Tunçdemir, M.; Çelik, Ö.; Öztürk, M.; Flores-Arce, M.F. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol. Trace Elem. Res. 2012, 150, 342–349. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, X.; Kang, Y.J. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp. Biol. Med. 2002, 227, 214–222. [Google Scholar] [CrossRef]
- Cai, L. Metallothionein as an adaptive protein prevents diabetes and its toxicity. Nonlinearity Biol. Toxicol. Med. 2004, 2, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, Q.; Lu, J.; Zhang, X.; Suen, D.; Tan, Y.; Jin, L.; Xiao, J.; Xie, R.; Rane, M.; et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J. Nutr. Biochem. 2010, 21, 237–246. [Google Scholar] [CrossRef]
- Apostolova, M.D.; Choo, K.H.; Michalska, A.E.; Tohyama, C. Analysis of the possible protective role of metallothionein in streptozotocin-induced diabetes using metallothionein-null mice. J. Trace Elem. Med. Biol. 1997, 11, 1–7. [Google Scholar] [CrossRef]
- He, X.; Kan, H.; Cai, L.; Ma, Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J. Mol. Cell Cardiol. 2009, 46, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Renal. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.J.; Joshi, P.C.; Fan, X.; Brown, L.A.; Ritzenthaler, J.D.; Roman, J.; Guidot, D.M. Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol. Clin. Exp. Res. 2011, 35, 1519–1528. [Google Scholar] [CrossRef]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef]
- Yang, F.; Li, B.; Dong, X.; Cui, W.; Luo, P. The beneficial effects of zinc on diabetes-induced kidney damage in murine rodent model of type 1 diabetes mellitus. J. Trace Elem. Med. Biol. 2017, 42, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, D.; Chi, Z.H.; Chu, Q.; Zhao, C.; Ma, R.Z.; Zhao, Y.; Li, H. Effect of zinc on high glucose-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells. Int. J. Mol. Med. 2015, 35, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, D.; Fan, J.; Lian, X.; Zhao, Y.; Wang, X.; Chi, Z.H.; Zhang, P. Zinc Attenuates Tubulointerstitial Fibrosis in Diabetic Nephropathy Via Inhibition of HIF Through PI-3K Signaling. Biol. Trace Elem. Res. 2016, 173, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ning, X.; Zhang, Y.; Lu, Y.; Nie, Y.; Han, S.; Liu, L.; Du, R.; Xia, L.; He, L.; et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009, 75, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, D.; Lian, X.; Chi, Z.H.; Wang, X.; Zhao, Y.; Ping, Z. Effect of zinc deficiency on mouse renal interstitial fibrosis in diabetic nephropathy. Mol. Med. Rep. 2016, 14, 5245–5252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Chu, Q.; Wang, Z.Y.; Li, H.; Chi, Z.H. Zinc modulates high glucose-induced apoptosis by suppressing oxidative stress in renal tubular epithelial cells. Biol. Trace Elem. Res. 2014, 158, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, P.; Lu, X.; Li, C.; Dong, X.; Yang, F.; Luo, P.; Li, B. Nrf2 participates in the anti-apoptotic role of zinc in Type 2 diabetic nephropathy through Wnt/β-catenin signaling pathway. J. Nutr. Biochem. 2020, 84, 108451. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Pradeep, S.R.; Srinivasan, K. Zinc supplementation alleviates the progression of diabetic nephropathy by inhibiting the overexpression of oxidative-stress-mediated molecular markers in streptozotocin-induced experimental rats. J. Nutr. Biochem. 2018, 54, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tan, Y.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol. Mech. Methods 2013, 23, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Karatug, A.; Kaptan, E.; Bolkent, S.; Mutlu, O.; Yanardag, R. Alterations in kidney tissue following zinc supplementation to STZ-induced diabetic rats. J. Trace Elem. Med. Biol. 2013, 27, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bonner, R.; Albajrami, O.; Hudspeth, J.; Upadhyay, A. Diabetic Kidney Disease. Prim Care 2020, 47, 645–659. [Google Scholar] [CrossRef]
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22 (Suppl. 1), 3–15. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Campo, G.D.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef] [PubMed]
- Gerardo Yanowsky-Escatell, F.; Andrade-Sierra, J.; Pazarín-Villaseñor, L.; Santana-Arciniega, C.; De Jesús Torres-Vázquez, E.; Samuel Chávez-Iñiguez, J.; Ángel Zambrano-Velarde, M.; Martín Preciado-Figueroa, F. The Role of Dietary Antioxidants on Oxidative Stress in Diabetic Nephropathy. Iran J. Kidney Dis. 2020, 14, 81–94. [Google Scholar] [PubMed]
- Radcliffe, N.J.; Seah, J.M.; Clarke, M.; MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Clinical predictive factors in diabetic kidney disease progression. J. Diabetes Investig. 2017, 8, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Torreggiani, M.; Pellicanò, V.; Cernaro, V.; Messina, R.M.; Longhitano, E.; Siligato, R.; Gembillo, G.; Esposito, C.; Piccoli, G.B. Kidney Biopsy in Type 2 Diabetic Patients: Critical Reflections on Present Indications and Diagnostic Alternatives. Int. J. Mol. Sci. 2021, 22, 5425. [Google Scholar] [CrossRef] [PubMed]
- Amatruda, M.; Gembillo, G.; Giuffrida, A.E.; Santoro, D.; Conti, G. The Aggressive Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes: Pathogenetic Mechanisms and Potential Therapies. Medicina 2021, 57, 868. [Google Scholar] [CrossRef]
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef] [PubMed]
- Bilous, R.W.; Gonzalez-Campoy, J.M.; Fradkin, J.E.; Mauer, M.; Molitch, M.E.; Narva, A.S.; Nelson, R.G.; Sharma, K.; Tuttle, K.R.; Rocco, M.V.; et al. KDOQI Clinical Practice Guideline for Diabetes CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [Google Scholar] [CrossRef]
- Gembillo, G.; Cernaro, V.; Salvo, A.; Siligato, R.; Laudani, A.; Buemi, M.; Santoro, D. Role of Vitamin D Status in Diabetic Patients with Renal Disease. Medicina 2019, 55, 273. [Google Scholar] [CrossRef] [PubMed]
- Cernaro, V.; Loddo, S.; Macaione, V.; Ferlazzo, V.T.; Cigala, R.M.; Crea, F.; De Stefano, C.; Genovese, A.R.R.; Gembillo, G.; Bolignano, D.; et al. RAS inhibition modulates kynurenine levels in a CKD population with and without type 2 diabetes mellitus. Int. Urol. Nephrol. 2020, 52, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Bolignano, D.; Cernaro, V.; Gembillo, G.; Baggetta, R.; Buemi, M.; D’Arrigo, G. Antioxidant agents for delaying diabetic kidney disease progression: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0178699. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Chimienti, F.; Wheeler, M.B. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes. Metab. 2009, 11, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Kucuk, O.; Sarkar, F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Pompano, L.M.; Boy, E. Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, Q.; Liu, G.; Tan, Y.; Cai, L. Analysis of serum and urinal copper and zinc in Chinese northeast population with the prediabetes or diabetes with and without complications. Oxidative Med. Cell. Longev. 2013, 2013, 635214. [Google Scholar] [CrossRef] [PubMed]
- Al-Timimi, D.J.; Sulieman, D.M.; Hussen, K.R. Zinc status in type 2 diabetic patients: Relation to the progression of diabetic nephropathy. J. Clin. Diagn. Res. 2014, 8, CC04. [Google Scholar] [CrossRef]
- Lin, C.C.; Shih, C.T.; Lee, C.H.; Huang, Y.L. Changes in Trace Elements During Early Stages of Chronic Kidney Disease in Type 2 Diabetic Patients. Biol. Trace Elem. Res. 2018, 186, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Yanagisawa, H.; Suka, M.; Yoshida, Y.; Onishi, Y.; Tahara, T.; Kikuchi, T.; Kushiyama, A.; Anai, M.; Takahashi, K.; et al. Synergistic association of the copper/zinc ratio under inflammatory conditions with diabetic kidney disease in patients with type 2 diabetes: The Asahi Diabetes Complications Study. J. Diabetes Investig. 2022, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Jalali, M.; Siassi, F.; Hosseini, M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care 2005, 28, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.M.; Ismail, S.H.; Hussein, K.I.; Bakir, I.H.; Sahib, A.S.; Khalaf, B.H.; Hussain, S.A. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J. Pineal Res. 2006, 41, 189–193. [Google Scholar] [CrossRef]
- Parham, M.; Amini, M.; Aminorroaya, A.; Heidarian, E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: A double blind, randomized, placebo-controlled, cross-over trial. Rev. Diabet. Stud. 2008, 5, 102–109. [Google Scholar] [CrossRef]
- El-Ashmony, S.M.A.; Morsi, H.K.; Abdelhafez, A.M. Effect of zinc supplementation on glycemic control, lipid profile, and renal functions in patients with type II diabetes: A single blinded, randomized, placebo-controlled, trial. J Biol Agric Health 2012, 2, 33. [Google Scholar]
- Narváez-Caicedo, C.; Moreano, G.; Sandoval, B.A.; Jara-Palacios, M.Á. Zinc Deficiency among Lactating Mothers from a Peri-Urban Community of the Ecuadorian Andean Region: An Initial Approach to the Need of Zinc Supplementation. Nutrients 2018, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Fischer Walker, C.; Ezzati, M.; Black, R. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur. J. Clin. Nutr. 2009, 63, 591–597. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar] [PubMed]
- Mayo Clinic Laboratories Website. Available online: https://www.mayocliniclabs.com/test-catalog/overview/8620#Clinical-and-Interpretive (accessed on 16 March 2022).
- Wessells, K.R.; King, J.C.; Brown, K.H. Development of a Plasma Zinc Concentration Cutoff to Identify Individuals with Severe Zinc Deficiency Based on Results from Adults Undergoing Experimental Severe Dietary Zinc Restriction and Individuals with Acrodermatitis Enteropathica. J. Nutr. 2014, 144, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S. Coeliac disease, insulin-like growth factor, bone mineral density, and zinc. Scand. J. Gastroenterol. 2000, 35, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Ojuawo, A.; Keith, L. The serum concentrations of zinc, copper and selenium in children with inflammatory bowel disease. Cent. Afr. J. Med. 2002, 48, 116–119. [Google Scholar] [PubMed]
- Maxfield, L.; Shukla, S.; Crane, J.S. Zinc Deficiency. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493231/ (accessed on 17 March 2022).
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Matthew, A.W.; Kevin, P.K. Chapter 36—Nutritional Toxicologic Pathology. In Wallig, Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Wanda, M.H., Colin, G.R., Matthew, A., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1077–1121. ISBN 9780124157590. [Google Scholar] [CrossRef]
- Al-Maroof, R.A.; Al-Sharbatti, S.S. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med. J. 2006, 27, 344–350. [Google Scholar] [PubMed]
- Gunasekara, P.; Hettiarachchi, M.; Liyanage, C.; Lekamwasam, S. Effects of zinc and multimineral vitamin supplementation on glycemic and lipid control in adult diabetes. Diabetes, Metabolic Syndrome and Obesity: Targets Ther. 2011, 4, 53–60. [Google Scholar] [CrossRef][Green Version]
- Fernández-Cao, J.C.; Warthon-Medina, M.; HMoran, V.; Arija, V.; Doepking, C.; Serra-Majem, L.; Lowe, N.M. Zinc Intake and Status and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Zhao, J.; Han, X.Y.; Zhou, X.H.; Wu, J.; Ji, L.N. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin. Med. J. 2015, 128, 3276–3282. [Google Scholar] [CrossRef]
- Jayawardena, R.; Ranasinghe, P.; Galappatthy, P.; Malkanthi, R.; Constantine, G.; Katulanda, P. Effects of zinc supplementation on diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2012, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.W.; Giroux, A.; L’Abbé, M.R. Effect of zinc supplementation on copper status in adult man. Am. J. Clin. Nutr. 1984, 40, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Koi, S.; Arai, K.; Nogami, A.; Toma, H.; Yamamoto, M.; Miura, O.; Nagao, T. Impaired hematopoiesis due to copper deficiency in a hemodialysis patient supplemented with zinc. Rinsho Ketsueki. 2020, 61, 1487–1491. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Yacoubian, C.; Watson, N.; Morrison, I. The risk of copper deficiency in patients prescribed zinc supplements. J. Clin. Pathol. 2015, 68, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Laouali, N.; MacDonald, C.J.; Shah, S.; El Fatouhi, D.; Mancini, F.R.; Fagherazzi, G.; Boutron-Ruault, M.C. Dietary Copper/Zinc Ratio and Type 2 Diabetes Risk in Women: The E3N Cohort Study. Nutrients 2021, 13, 2502. [Google Scholar] [CrossRef]
| Study, year | Patients (n.) | Controls (n.) | Results |
|---|---|---|---|
| Jiancheng Xu et al. [64] 2013 | 189 patients with DM or prediabetes Age 20–65, mean age 55 IFG: (n = 12) IGT: (n = 15) T1D: (n = 25) T2D: (n = 137) DKD: (n = 24) DR: (n = 34) DPN: (n = 50) | 50 healthy patients | Plasma Zn (mg/L): Control group 0.81 (0.67–0.93) vs. IFG group 0.75 (0.70–0.84) (NS) vs. IGT 0.77 (0.67–0.87) (NS) vs. T1D 0.59 (0.53–0.75) (p = 0.056) vs. T2D 0.61 (0.51–0.75) (p < 0.001) Urinary Zn (mg/L): Control 0.20 (0.14–0.32) vs. IFG 0.32 (0.26–0.37) (NS) vs. IGT 0.27 (0.19–0.41) (NS) vs. T1D 0.86 (0.67–0.91) p < 0.001 vs. T2D 0.48 (0.38–0.57) p < 0.001 Plasma Zn (mg/L): T2D 0.73 (0.55–0.79) vs. DKD 0.59 (0.48–0.76) (NS) vs. DR 0.58 (0.46–0.63) p = 0.002 vs. DPN 0.63 (0.59–0.75) p = 0.08 Urinary Zn (mg/L): T2D 0.47 (0.28–0.53) vs. DKD 0.44 (0.30–0. 52) (NS) vs. DR 0.45 (0.25–0.52) (NS) vs. DPN 0.52 (0.44–0.63) (p < 0.001) |
| Al Timini et al. [65] 2014 | 300 T2D patients Age 43.5–71.6 Group II: Diabetic, normotensive (n = 145) Group III: Diabetic, hypertensive (n = 41) Group IV: Diabetic, normotensive with microalbuminuria (n = 62) Group V: Diabetic, hypertensive with microalbuminuria (n = 52) | 100 apparently healthy subjects Age 45.7–69.2 Group I: Nondiabetic, normotensive (n = 100) | Urinary zinc/creatine (ug/g): 2.33 + 1.18 in patient groups vs. 1.01 + 0.57 in control (p value < 0.001) Serum zinc (ug/dL): 70.0 + 19.2 in patient groups vs. 86.2 + 15.2 in control (p value < 0.001) Group I: 86.2 + 15.2 Group II: 79.2 + 15.0 Group III: 77.9 + 17.2 Group IV: 56.8 + 13.8 Group V: 55.0 + 14.2 eGFR > 90 mL/min/1.73 m2: 107 (45.7%) had serum zinc levels < 70 ug/dL and 127 (54.3%) > 70 ug/dL (total 234) eGFR 60–89 mL/min/1.73 m2: 38 (76.0%) had serum zinc levels < 70 ug/dL and 12 (24.0%) > 70 ug/dL (total 50) eGFR 30–59 mL/min/1.73 m2: 14 (93.3%) had serum zinc levels < 70 ug/dL and 1 (6.7%) > 70 ug/dL (total 15) eGFR 15–29 mL/min/1.73 m2: 1 (100%) had serum zinc levels < 70 μg/dL and none > 70 μg/dL (total 1) |
| Lin et al. [66] 2018 | 148 T2D patients with CKD Age 62.4 ± 9.8 CKD Stage 1 eGFR > 90 mL/min/1.73 m2 (n = 25) CKD Stage 2 eGFR 60–89 mL/min/1.73 m2 (n = 49) CKD Stage 3a eGFR 45–69 mL/min/1.73 m2 (n = 40) CKD Stage 3b eGFR 30–44 mL/min/1.73 m2 (n = 34 | No control group | Zinc (ppm) Stage 1: 1.0 ± 0.2 Stage 2: 0.9 ± 0.2 Stage 3a: 0.9 ± 0.2 Stage 3b: 0.8 ± 0.2 p = <0.001 |
| Takao et al. [67] 2021 | 651 patients with T2D Age 65 ± 9.7 DKD group (n = 220) No DKD group (n = 431) | No control group | Cu (microg/dL): 97.0 ± 15.6 in no DKD group vs. 100.5 ± 15.5 in DKD group (p = 0.007) Zn (microg/dL): 85.4 ± 11.3 in no DKD group vs. 82.1 ± 11.6 in DKD group (p = 0.0005) Cu/Zn ratio: 1.155 ± 0.242 in no DKD group vs. 1.247 ± 0.265 in DKD group (p < 0.0001) The optimal Cu\Zn cut-off value for detecting DKD was 1.1648 |
| Study, Year | Population | Intervention | Control | Follow-Up | Renal Outcomes |
|---|---|---|---|---|---|
| Farvid et al. [68] 2005 | 76 T2D patients with microalbuminuria Divided into 4 groups: M, V, MV, P Age: 50 ± 9 for groups P, V, MV 52 ± 8 for group M | Group M (n = 18): Zinc sulfate 15 mg and magnesium oxide 100 mg twice a day Group V (n = 20): 100 mg vitamin C and 50 UI vitamin E twice a day Group MV (n = 19): Both supplementation of group M and V | Group P (n = 19): Placebo | 3 months | Microalbuminuria (mg/g creatinine): No significative reduction after zinc supplementation in group P and M Significative reduction in group V (35.6 (6.2–64.9) vs. 22.1 (5.2–39.0) (p = 0.034) and in group MV (29.3 (3.2 to 61.9) vs. 10.8 (4.2–17.3) (p = 0.005) after zinc supplementation NAG (units/g Cr): No significative reduction after zinc supplementation in group M, V, MV, and P Urine protein (g/g creatinine): No significative reduction after zinc supplementation in group M, V, MV, and P |
| Kadhim et al. [69] 2006 | 46 patients with T2D Divided into 3 groups: A, B, C, and control group Aged 49.1 ± 6.0 | Group B (n = 18): 10 mg of melatonin + 50 mg of zinc acetate + metformin 2550 mg/die + dietary control program Group C (n = 13): 10 mg of melatonin + 50 mg of zinc acetate + dietary control program | Group A (n = 15): Placebo + metformin 2550 mg/die Control (n = 17): healthy subjects in the same age range of patients | 3 months | Microalbuminuria (mg/g creatinine): No significative difference in group A after Zinc supplementation Significative reduction in group B after 30 days and after 90 days Baseline 249.05 ± 24.74. After 30 days 188.61 ± 13.88 (p < 0.01). After 90 days 156.22 ± 14.26 (p < 0.01). Significative difference between group B and group A after 90 days. Significative reduction in group C after 30 days and after 90 days. Baseline 254.69 ± 32.30. After 30 days 215.07 ± 30.96 (p < 0.01). After 90 days 621.07 ± 18.59 (p < 0.05). Significative difference between group C and group A after 90 days Plasma creatinine (mg/dL): No significative difference in group A, group B, and group C after Zinc supplementation Plasma urea (mg/dL): No significative difference in group A, group B, and group C after Zinc supplementation |
| Parham et al. [70] 2008 | 50 patients with T2D and microalbuminuria Divided into group 1 (n = 21) and group 2 (n = 18) Age 52 ± 9.3 (Group 1) and 54.5 ± 9.2 (Group 2) | Zinc Sulfate 132 mg 1 capsule per day (30 mg elemental Zn) | Placebo (30 mg of lactose) | Group 1: 3 months of zinc, 4 weeks of wash out, and 3 months of placebo Group 2: 3 months of placebo, 4 weeks of wash out, and 3 months of zinc | Urinary albumin excretion (mg/g): Significant reduction after 3 months of Zn supplementation in both Group 1 (86.5 ± 57 at baseline vs. 75 ± 71 after supplementation) and Group 2 (90 ± 60 at baseline vs. UAE = 78 ± 57 after supplementation) (p < 0.05) No significant reduction in both group after placebo Creatinin clearance (mL/min/1.73 m2): No significant difference in both groups concerning Creatinine Clearance GFR (mL/min/1.73 m2) No significant difference in both groups concerning GFR |
| El-Ashmony et al. [71] 2012 | Patients with T2D Age: 48.46 ± 4.61 in Zn Group and 48.20 ± 4.09 in placebo group. Patients on regular use of antidiabetic drugs (no insuline), with HB1Ac concentration of 8% or greater | Zn group (n = 26) 40 mg zinc sulfate once daily | Control group (n = 30) Placebo | 8 weeks | BUN (mg/dL): Zn group: before treatment 24.15 ± 6.28 and 21.15 ± 6.04 after treatment (p < 0.001) No significative difference in control group Serum creatinine (mg/dL): Zn group: before treatment 0.90 ± 0.42 and 0.82 ± 0.42 after treatment (p < 0.001) No significative difference in control group |
| Khan et al. [72] 2013 | 54 T2D patients with microalbuminuria divided into 2 groups: OHA alone or OHA + zinc. Age 56.1 ± 8.5 | Zn group (n = 27) Zinc Sulfate 50 mg 1 capsule per day + OHA | Control group (n = 27) OHA alone | 12 weeks | Urine microalbumin (mg/day): Significant reduction after 12 weeks in Zinc group Pretrial: 146.87 ± 30.83 before treatment vs. 80.70 ± 33.99 after treatment p value = <0.0001 No reduction in control group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gembillo, G.; Visconti, L.; Giuffrida, A.E.; Labbozzetta, V.; Peritore, L.; Lipari, A.; Calabrese, V.; Piccoli, G.B.; Torreggiani, M.; Siligato, R.; et al. Role of Zinc in Diabetic Kidney Disease. Nutrients 2022, 14, 1353. https://doi.org/10.3390/nu14071353
Gembillo G, Visconti L, Giuffrida AE, Labbozzetta V, Peritore L, Lipari A, Calabrese V, Piccoli GB, Torreggiani M, Siligato R, et al. Role of Zinc in Diabetic Kidney Disease. Nutrients. 2022; 14(7):1353. https://doi.org/10.3390/nu14071353
Chicago/Turabian StyleGembillo, Guido, Luca Visconti, Alfio Edoardo Giuffrida, Vincenzo Labbozzetta, Luigi Peritore, Antonella Lipari, Vincenzo Calabrese, Giorgina Barbara Piccoli, Massimo Torreggiani, Rossella Siligato, and et al. 2022. "Role of Zinc in Diabetic Kidney Disease" Nutrients 14, no. 7: 1353. https://doi.org/10.3390/nu14071353
APA StyleGembillo, G., Visconti, L., Giuffrida, A. E., Labbozzetta, V., Peritore, L., Lipari, A., Calabrese, V., Piccoli, G. B., Torreggiani, M., Siligato, R., & Santoro, D. (2022). Role of Zinc in Diabetic Kidney Disease. Nutrients, 14(7), 1353. https://doi.org/10.3390/nu14071353












