Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region
Abstract
:1. Introduction
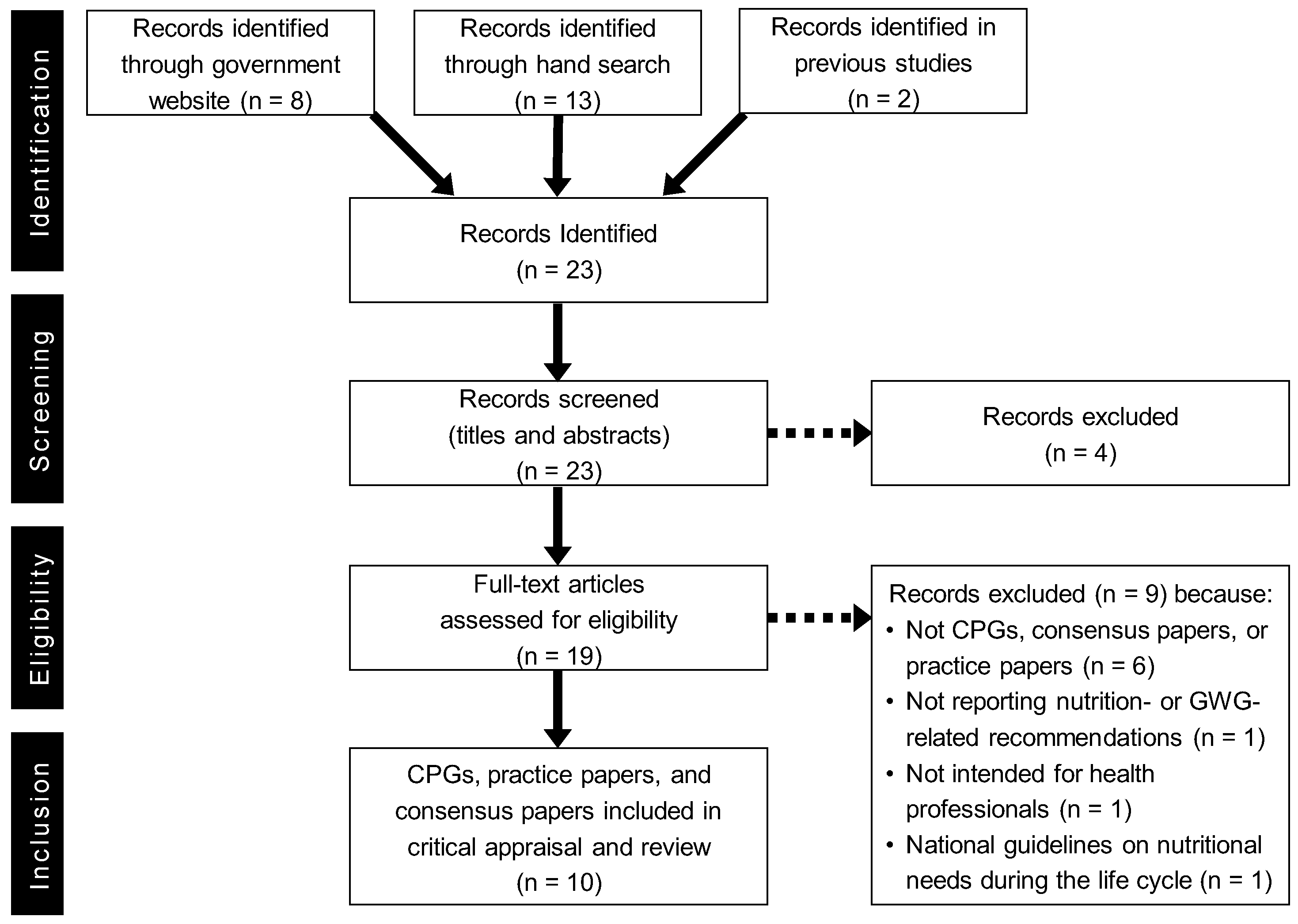
2. Materials and Methods
3. Results
3.1. Characteristics and Quality of Selected CPGs
3.2. Overview of GWG Recommendations
3.3. Overview of Nutritional Recommendations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Regional Office for Europe. Good Maternal Nutrition the Best Start in Life. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/publications/2016/good-maternal-nutrition.-the-best-start-in-life-2016 (accessed on 31 January 2022).
- Fall, C.H. Evidence for the Intra-Uterine Programming of Adiposity in Later Life. Ann. Hum. Biol. 2011, 38, 410–428. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Lutsiv, O.; Mulla, S.; Rosen, A.; Beyene, J.; McDonald, S.D.; Knowledge Synthesis Group. Low Gestational Weight Gain and the Risk of Preterm Birth and Low Birthweight: A Systematic Review and Meta-Analyses. Acta Obstet. Gynecol. Scand. 2011, 90, 935–954. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational Weight Gain Across Continents and Ethnicity: Systematic Review and Meta-Analysis of Maternal and Infant Outcomes in More Than One Million Women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R.; Athens, M.A.; Thompson, G.H.; Bradshaw, B.S.; Stern, M.P. Birthweight and Adult Health Outcomes in a Biethnic Population in the USA. Diabetologia 1994, 37, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Durmuş, B.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Jaddoe, V.W. Risk Factors and Outcomes of Maternal Obesity and Excessive Weight Gain During Pregnancy. Obesity 2013, 21, 1046–1055. [Google Scholar] [CrossRef]
- Baran, J.; Weres, A.; Czenczek-Lewandowska, E.; Leszczak, J.; Kalandyk-Osinko, K.; Łuszczki, E.; Sobek, G.; Mazur, A. Excessive Gestational Weight Gain: Long-Term Consequences for the Child. J. Clin. Med. 2020, 9, 3795. [Google Scholar] [CrossRef]
- Institute of Medicine (US), Committee on Clinical Practice Guidelines. Guidelines for Clinical Practice: From Development to Use; Field, M.J., Lohr, K.N., Eds.; National Academies Press: Washington, DC, USA, 1992.
- Alavi, N.; Haley, S.; Chow, K.; McDonald, S.D. Comparison of National Gestational Weight Gain Guidelines and Energy Intake Recommendations. Obes. Rev. 2013, 14, 68–85. [Google Scholar] [CrossRef]
- Scott, C.; Andersen, C.T.; Valdez, N.; Mardones, F.; Nohr, E.A.; Poston, L.; Loetscher, K.C.; Abrams, B. No Global Consensus: A Cross-Sectional Survey of Maternal Weight Policies. BMC Pregnancy Childbirth 2014, 14, 167. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Theodoridis, X.; Gkiouras, K.; Lampropoulou, M.; Petalidou, A.; Patelida, M.; Tsirou, E.; Papoutsakis, C.; Goulis, D.G. Methodological Quality of Clinical Practice Guidelines for Nutrition and Weight Gain During Pregnancy: A Systematic Review. Nutr. Rev. 2020, 78, 546–562. [Google Scholar] [CrossRef]
- Paez, A. Gray Literature: An Important Resource in Systematic Reviews. J. Evid. Based Med. 2017, 10, 233–240. [Google Scholar] [CrossRef]
- Mahood, Q.; Van Eerd, D.; Irvin, E. Searching for Grey Literature for Systematic Reviews: Challenges and Benefits. Res. Synth. Methods 2014, 5, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Kelly, S.E.; Hsieh, S.C.; Skidmore, B.; Wells, G.A. Systematic Reviews of Clinical Practice Guidelines: A Methodological Guide. J. Clin. Epidemiol. 2019, 108, 64–76. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Countries. 2021. Available online: https://www.who.int/countries (accessed on 31 January 2022).
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing Guideline Development, Reporting and Evaluation in Health Care. CMAJ 2010, 182, E839–E842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann-Eßer, W.; Siering, U.; Neugebauer, E.A.; Lampert, U.; Eikermann, M. Systematic review of current guideline appraisals performed with the Appraisal of Guidelines for Research & Evaluation II instrument-a third of AGREE II users apply a cut-off for guideline quality. J. Clin. Epidemiol. 2018, 95, 120–127. [Google Scholar] [CrossRef]
- Bargeri, S.; Iannicelli, V.; Castellini, G.; Cinquini, M.; Gianola, S. AGREE II appraisals of clinical practice guidelines in rehabilitation showed poor reporting and moderate variability in quality ratings when users apply different cuff-offs: A methodological study. J. Clin. Epidemiol. 2021, 139, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.T.; Hofer, O.J.; Harding, J.E.; Wall, C.R.; Crowther, C.A. Dietary recommendations for women with gestational diabetes mellitus: A systematic review of clinical practice guidelines. Nutr. Rev. 2021, 79, 988–1021. [Google Scholar] [CrossRef]
- Family Health Bureau; Ministry of Health. Maternal Care Package. A Guide to Field Healthcare Workers; Family Health Bureau, Ministry of Health: Colombo, Sri Lanka, 2011. Available online: http://maternalnutritionsouthasia.com/wp-content/uploads/maternal_care_package_a_guide_to_field_healthcare_workers_english-1.pdf (accessed on 31 January 2022).
- Maternal and Reproductive Health Division; The Republic of the Union of Myanmar Ministry of Health and Sports. National Guidelines for Antenatal Care for Service Providers; Maternal and Reproductive Health Division, Ministry of Health and Sports: Nay Pyi Taw, Myanmar, 2018. Available online: http://mohs.gov.mm/su/oysLCg (accessed on 31 January 2022).
- Department of Health; Australian Government. Clinical Practice Guidelines: Pregnancy Care 2020 Edition; National Medical Health and Research Council: Canberra, Australia, 2021. Available online: https://www.health.gov.au/resources/pregnancy-care-guidelines (accessed on 31 January 2022).
- Obstetrics Subgroup; Chinese Society of Obstetrics and Gynecology; Chinese Medical Association. Guideline of Preconception and Prenatal Care (2018). Zhonghua Fu Chan Ke Za Zhi 2018, 53, 7–13. (In Chinese) [Google Scholar] [CrossRef]
- Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists. Obstetrics and Gynecology Practice Guidelines/Obstetrics 2020; Japan Society of Obstetrics and Gynecology: Tokyo, Japan, 2020; Available online: https://www.jsog.or.jp/activity/pdf/gl_sanka_2020.pdf (accessed on 31 January 2022). (In Japanese)
- Ministry of Health, New Zealand. Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women: A Background Paper; Ministry of Health: Wellington, New Zealand, 2006. Available online: https://www.health.govt.nz/publication/food-and-nutrition-guidelines-healthy-pregnant-and-breastfeeding-women-background-paper (accessed on 31 January 2022).
- Ministry of Health, New Zealand. Guidance for Healthy Weight Gain in Pregnancy; Ministry of Health: Wellington, New Zealand, 2014. Available online: https://www.health.govt.nz/publication/guidance-healthy-weight-gain-pregnancy (accessed on 31 January 2022).
- Philippine Obstetrical and Gynecological Society. Clinical Practice Guidelines on Maternal Nutrition and Supplementation; Philippine Obstetrical and Gynecological Society: Manila, Philippine, 2013; Available online: http://docshare03.docshare.tips/files/28353/283537702.pdf (accessed on 31 January 2022).
- Ministry of Health, Vietnam. National Guidelines on Nutrition for Pregnant Women and Breatfeeding Mothers; Ministry of Health: Ha Noi, Vietnam, 2017. Available online: https://cvdvn.files.wordpress.com/2017/04/hdqg_dinh-dc6b0e1bba1ng.pdf (accessed on 31 January 2022). (In Vietnamese)
- The Public Health Division of the Pacific Community. Pacific Guidelines for Healthy Eating during Pregnancy: A Handbook for Health Professionals and Educators; Pacific Community: Noumea, New Caredonia, 2019; Available online: https://www.spc.int/updates/blog/2020/07/now-available-pacific-guidelines-for-healthy-eating-during-pregnancy (accessed on 31 January 2022).
- Institute of Medicine (US), Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Institute of Medicine (US), Subcommittee on Nutritional Status and Weight Gain during Pregnancy. Nutrition During Pregnancy. Part I: Weight Gain. Part II: Nutrient Supplements; National Academies Press: Washington, DC, USA, 1990. [Google Scholar]
- Huang, X.; Tan, H.; Cai, M.; Shi, T.; Mi, C.; Lei, J. Gestational Weight Gain in Chinese Women—Results From a Retrospective Cohort in Changsha, China. BMC Pregnancy Childbirth 2018, 18, 185. [Google Scholar] [CrossRef]
- Morisaki, N.; Nagata, C.; Jwa, S.C.; Sago, H.; Saito, S.; Oken, E.; Fujiwara, T. Pre-Pregnancy BMI-Specific Optimal Gestational Weight Gain for Women in Japan. J. Epidemiol. 2017, 27, 492–498. [Google Scholar] [CrossRef]
- Choi, S.K.; Lee, G.; Kim, Y.H.; Park, I.Y.; Ko, H.S.; Shin, J.C. Determining Optimal Gestational Weight Gain in the Korean Population: A Retrospective Cohort Study. Reprod. Biol. Endocrinol. 2017, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Ee, T.X.; Allen, J.C., Jr.; Malhotra, R.; Koh, H.; Østbye, T.; Tan, T.C. Determining Optimal Gestational Weight Gain in a Multiethnic Asian Population. J. Obstet. Gynaecol. Res. 2014, 40, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B. Food taboos: Their origins and purposes. J. Ethnobiol. Ethnomed. 2009, 29, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iradukunda, F. Food taboos during pregnancy. Health Care Women Int. 2020, 41, 159–168. [Google Scholar] [CrossRef]
- Withers, M.; Kharazmi, N.; Lim, E. Traditional beliefs and practices in pregnancy, childbirth and postpartum: A review of the evidence from Asian countries. Midwifery 2018, 56, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Sae-tan, S.; Lambert, C.; Biesalski, H.K. Plant-based food taboos in pregnancy and the postpartum period in Southeast Asia—A systematic review of literature. Food Sci. Nutr. 2018, 48, 949–961. [Google Scholar] [CrossRef]
- Köhler, R.; Lambert, C.; Biesalski, H.K. Animal-based food taboos during pregnancy and the postpartum period of Southeast Asian women—A review of literature. Food Res. Int. 2019, 115, 480–486. [Google Scholar] [CrossRef] [PubMed]
| Criterion | Description |
|---|---|
| (P) Population | Pregnant women |
| (I) Interventions | Any nutritional/dietary intervention for achieving a healthy pregnancy outcome |
| (C) Comparators | Any comparator or comparison. No key CPG content is of interest |
| (A) Attributes of eligible CPGs | (1) National and international CPGs, including consensus papers or practice papers (2) In a full-text format that is publicly available (3) Published since the year 2000 in any language (4) Issued from professional or governmental organisations in the Asia-Pacific region (5) Reporting nutrition- or/and GWG-related recommendations (6) Intended for health professionals (7) Latest version (8) With no restrictions on their quality, as assessed by the AGREE II instrument |
| (R) Recommendation characteristics and other considerations | Not applicable |
| Country/Region (Year of Publication) | CPG Title | Language | Name and Location of Publishing Organisation | Organisation Level of Body | Range of Topics Addressed | Intended Audience | |||
|---|---|---|---|---|---|---|---|---|---|
| Governmental | Professional or Scientific | Maternal Care | GWG | Diet/Nutrition in Pregnancy | |||||
| Sri Lanka (2011) [20] | Maternal Care PackageA Guide to Field Healthcare Workers | English | Family Health Bureau, Ministry of Health, Colombo, Sri Lanka | ✓ | ✓ | ✓ | ✓ | Health workers who provide maternal and newborn care | |
| Myanmar (2018) [21] | National Guidelines for Antenatal Care For Service Providers | English | Maternal and Reproductive Health Division, Ministry of Health and Sports, Nay Pyi Taw, Myanmar | ✓ | ✓ | ✓ | Service providers at all levels of the health system | ||
| Australia (2020) [22] | Clinical Practice Guidelines: Pregnancy Care 2020 Edition | English | Australian Government Department of Health, Canberra, Australia | ✓ | ✓ | ✓ | ✓ | All health professionals who contribute to pregnancy care, including midwives, obstetricians, general practitioners, Aboriginal and Torres Strait Islander health workers and allied health professionals | |
| China (2018) [23] | Guidelines on preconception care and prenatal care (Translated) | Chinese | Obstetricians Group-Obstetrics and Gynecology Branch-Chinese Medical Association, Beijing, China | ✓ | ✓ | ✓ | ✓ | Clinicians | |
| Japan (2020) [24] | Guideline for Gynecological Practice 2020 edition | Japanese | Japan Society of Obstetrics and Gynecology, Tokyo, JapanJapan Association of Obstetricians and Gynecologists, Tokyo, Japan | ✓ | ✓ | ✓ | ✓ | Physicians engaged in obstetric care | |
| New Zealand (2006, revised 2008) [25] | Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women: A background paper (Food and Nutrition) | English | Ministry of Health, Wellington, New Zealand | ✓ | ✓ | ✓ | Health practitioners – including dietitians, nutritionists, midwives, doctors, nurses, primary health care providers, health promoters, and teachers | ||
| New Zealand (2014) [26] | Guidance for Healthy Weight Gain in Pregnancy (Weight Gain) | English | Ministry of Health, Wellington, New Zealand | ✓ | ✓ | Health practitioners | |||
| Philippines (2013) [27] | Clinical Practice Guidelines on Maternal Nutrition and Supplementation First Edition | English | Philippine Obstetrical and Gynecological Society, (Foundation), Inc. Metro Manila, Philippine | ✓ | ✓ | ✓ | The obstetrican-gynecologist, the general practitioner, the patient, the student, and the allied medical practitioner | ||
| Vietnam (2017) [28] | National Guidelines on Nutrition for Pregnant Women and Breastfeeding Mothers (Translated) | Vietnamese | Ministry of Health, Ha Noi, Vietnam | ✓ | ✓ | ✓ | Health professionals | ||
| The Pacific Community (New Caledonia) (2019) [29] | Pacific Guidelines for Healthy Eating During PregnancyA Handbook for Health Professionals and Educators | English | Public Health Division of the Pacific CommunityNoumea, New Caledonia | ✓ | ✓ | ✓ | Health professionals in the Pacific who provide advice related to family planning or pregnancies | ||
| Country/Region | AGREE II Domain (%) | Overall Quality | Recommendation (% of Reviewers) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scope and Purpose | Stakeholder Involvement | Rigour of Development | Clarity of Presentation | Applicability | Editorial Independence | Yes | Yes, Needs Modification | No | ||
| Sri Lanka [20] | 53 | 53 | 6 | 42 | 25 | 13 | 25 | 100 | ||
| Myanmar [21] | 69 | 53 | 17 | 53 | 40 | 13 | 42 | 50 | 50 | |
| Australia [22] | 100 | 97 | 91 | 94 | 96 | 100 | 100 | 100 | ||
| China [23] | 72 | 50 | 16 | 67 | 31 | 4 | 25 | 100 | ||
| Japan [24] | 81 | 81 | 61 | 86 | 13 | 92 | 67 | 100 | ||
| New Zealand (Food and Nutrition) [25] | 81 | 61 | 24 | 75 | 44 | 0 | 58 | 100 | ||
| New Zealand (Weight Gain) [26] | 97 | 53 | 25 | 72 | 40 | 0 | 50 | 100 | ||
| Philippines [27] | 44 | 33 | 25 | 53 | 13 | 0 | 33 | 100 | ||
| Vietnam [28] | 28 | 28 | 4 | 61 | 10 | 0 | 25 | 100 | ||
| The Pacific Community (New Caledonia) [29] | 81 | 44 | 18 | 67 | 2 | 0 | 33 | 100 | ||
| Country/Region | Recommended as: | BMI (kg/m2) | Weight Gain (kg) | Evidence Based on |
|---|---|---|---|---|
| Sri Lanka [20] | Expected weight gain in kg | <18.5 | 12.5–18 | Not mentioned |
| 18.5–24.9 | 11.5–16 | |||
| 25–29.9 | 7–11.5 | |||
| ≥30 | ≤6.8 | |||
| Australia [22] | IOM recommendations for weight gain in pregnancy | <18.5 | 12.5–18 | NHMRC 2013 based on IOM 2009 |
| 18.5–24.9 | 11.5–16 | |||
| 25–29.9 | 7–11.5 | |||
| ≥30 | 5–9 | |||
| Recommendations for weight gain in pregnancy among women from Asian backgrounds | <18.5 | 12.5–18 | NHMRC 2013 based on IOM 2009 and matched with Asian BMI cut-offs | |
| 18.5–22.9 | 11.5–16 | |||
| 23–27.5 | 7–11.5 | |||
| >27.5 | ≤7 | |||
| China [23] | Recommendations on the range of weight gain during pregnancy (Translated) | <18.5 | 12.5–18 | American College of Obstetricians and Gynecologists. Committee Opinion No. 548 and No. 549 |
| 18.5–24.9 | 11.5–16 | |||
| 25–29.9 | 7–11.5 | |||
| ≥30 | 5–9 | |||
| Japan [24] | Recommended values for weight gain during pregnancy (Translated) | <18.5 | 9–12 | Japan Society for the Study of Obesity, Diagnostic criteria for obesity 2011; Ministry of Health, Labor and Welfare, Healthy Parents and Children 21 |
| 18.5–25 | 7–12 | |||
| >25 | Individualised (standard: up to 5 kg) | |||
| New Zealand (Food and Nutrition) [25] | Recommended total weight gain in pregnant women, by pre-pregnancy BMI (kg/m2) | <19.8 | 12.5–18 | IOM 1990 |
| 19.8–26 | 11.5–16 | |||
| 26–29 | 7–11 | |||
| >29 | 6 | |||
| New Zealand (Weight Gain) [26] | Recommendations for total and average rate of weight gain during pregnancy, by pre-pregnancy BMI | <18.5 | 12.5–18 | IOM and NRC 2009 |
| 18.5–24.9 | 11.5–16 | |||
| 25–29.9 | 7–11.5 | |||
| ≥30 | 5–9 | |||
| Philippines [27] | Recommended total weight gain by pre-pregnancy BMI Classification of Pregnancy BMI | <18.5 | 12.7–18.1 | IOM 2009 |
| 18.5–24.9 | 11.3–15.9 | |||
| 25–29.9 | 6.8–11.3 | |||
| ≥30 | 5–9.1 | |||
| Vietnam [28] | Recommended weight gain | <18.5 | At least 25% of pre-pregnancy weight | Not mentioned |
| 18.5–24.9 | 10–12 | |||
| >25 | At least 15% of pre-pregnancy weight | |||
| The Pacific Community (New Caledonia) [29] | How much weight gain to recommend | <18.5 | 12.5–18 | Not mentioned |
| 18.5–24.9 | 11.5–16 | |||
| 25–29.9 | 7–11.5 | |||
| >30 | 5–9 |
| Country | Dietary Advice | Additional Energy Intake (kcal/day) | Nutritional Supplementation | ||
|---|---|---|---|---|---|
| Foods to Choose | Foods to Avoid/Limit | Supplement to Take for All Pregnant Women | Supplement to Take for Specific Conditions | ||
| Sri Lanka [20] | ✓ | Not mentioned | +360 | Iron (60 mg) and folic acid (400 μg) with vitamin C (50 mg) per day after a period of amenorrhoea of 12 weeks for 6 months during pregnancy and 6 months after delivery Folic acid (5 mg) during first trimester Calcium (No mention of dosage) | Iron (double dose) for 3 months and monitor the progress for women with both moderate and severe anaemia Folic acid should be taken until the next pregnancy for women who have a history of having children with neural tube defects |
| Myanmar [21] | ✓ | ✓ | +300 | Iron (60 mg) daily after the first trimester Folic acid (400 μg) daily starting in the first trimester and up to 37 completed weeks, and then twice daily up to delivery Vitamin B1 (10 mg) daily 1 month before pregnancy, during pregnancy, and 3 months after delivery | Vitamin B12 supplementation may be needed if a woman has a vegetarian or vegan diet Multivitamin and mineral supplements may be needed for women who are vegetarian, drink alcohol, use cigarettes or drugs, have been on a weight-loss program, and adolescents with poor nutrition Iron (double dose daily) for 3 months for women with moderate anaemia |
| Australia [22] | ✓ | ✓ | Not mentioned | Folic acid (400 μg/day) ideally from 1 month before conception and throughout the first 3 months Iodine (150 μg/day) Women with pre-existing thyroid conditions should seek advice from their medical practitioner before taking a supplement | Iron (80–300 mg weekly or 30–60 mg daily) supplementation to pregnant women based on their haemoglobin concentration at 28 weeks Calcium supplement for women at risk of hypertension (pre-eclampsia) Omega-3 long-chain polyunsaturated fatty acids (800 mg DHA and 100 mg EPA/day), if they are low in omega-3 |
| China [23] | Not mentioned | Not mentioned | Not mentioned | Folate (400–800 μg/day) or folate-contained multivitamins from 3 months before pregnancy to 3 months of pregnancy Iron (60 mg/day) for women without anaemia Calcium (0.6–1.5 g/day) | Iron (100–200 mg/day) for women diagnosed with anaemia Folate (4 mg) every day for a woman who has previously given birth to a baby with neural tube defects |
| Japan [24] | Not mentioned | Not mentioned | +50 (1st tri) +250 (2nd tri) +450 (3rd tri) | Folic acid (400 μg) daily from before conception | Folic acid (4–5 mg/day) from preconception to the 11th week of pregnancy for women with a history of pregnancy with neural tube defects |
| New Zealand (Food and Nutrition) [25] | ✓ | ✓ | +0 (1st tri) +340 (2nd tri) +452 (3rd tri) | Folic acid (800 μg) daily for at least 4 weeks before and 12 weeks after conception | Calcium for women who consume little or no milk and milk products Iron if indicated by monitoring of iron status Vitamin B12 for pregnant and breastfeeding vegan women Folic acid (5 mg) for women at increased risk of having a pregnancy affected by an neural tube defect for at least 4 weeks before and 12 weeks after conception Vitamin D for covered women |
| Philippines [27] | Not mentioned | Not mentioned | +300 (2nd and 3rd tri) | Iron (30–60 mg) and folic acid (400 μg) daily throughout pregnancy | Iron is doubled (120 mg) if she is large, has twin foetuses, or begins supplementation late in pregnancy Folic acid (5 mg) prior to conception for women at high risk of having a child with neural tube defect |
| Vietnam [28] | ✓ | ✓ | +50 (1st tri) +250 (2nd tri) +450 (3rd tri) | Iron (60 mg) and folic acid (400 μg) every day through pregnancy until 1 month after childbirth, or multi-micronutrients, as required. | Not mentioned |
| The Pacific Community (New Caledonia) [29] | ✓ | ✓ | +300 (2nd tri) +400 or 450 (3rd tri) | Iron (30–60 mg) and folic acid (400 μg) * every day | Iron (120 mg) and folic acid (400 μg) * every day if diagnosed with anaemia Calcium (1.5–2 g) per day for women with low calcium intake |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoyama, T.; Li, D.; Bay, J.L. Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region. Nutrients 2022, 14, 1288. https://doi.org/10.3390/nu14061288
Aoyama T, Li D, Bay JL. Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region. Nutrients. 2022; 14(6):1288. https://doi.org/10.3390/nu14061288
Chicago/Turabian StyleAoyama, Tomoko, Donglai Li, and Jacquie Lindsay Bay. 2022. "Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region" Nutrients 14, no. 6: 1288. https://doi.org/10.3390/nu14061288
APA StyleAoyama, T., Li, D., & Bay, J. L. (2022). Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region. Nutrients, 14(6), 1288. https://doi.org/10.3390/nu14061288








