Guava Fruit and Acacia pennata Vegetable Intake Association with Frailty of Older Adults in Northern Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Measurement
2.2.1. Assessment of Frailty
- (a)
- Unintended weight loss was indicated when older adults lost at least 4.5 kg over the past 12 months by self-reporting.
- (b)
- Exhaustion was determined whether they felt exhausted using the following two statements: (a) I felt that everything I did was an effort; (b) I could not get going. The question asked was “How often in the last week did you feel this way?” 0 = rarely or none of the time (<1 day), 1 = some or a little of the time (1–2 days), 2 = a moderate amount of the time (3–4 days), or 3 = most of the time. The degree of exhaustion was further rated, and the score from 2 to 4 suggested a positive finding.
- (c)
- Slowness was determined by walking along a 15-foot path and considered in conjunction with height and sex. A positive finding was suggested when they met one of the following criteria. Men with height ≤173 cm showed a walk time ≥7 s or with height >173 cm showed a walk time ≥6 s. Women with height ≤159 cm showed a walk time ≥7 s or with height >159 cm showed a walk time ≥6 s.
- (d)
- Weakness was determined by grip strength of the non-dominant hand in relation to body mass index (BMI) and sex. Grip strength was measured using a handheld dynamometer (Takei TKK5001®). We used the handgrip criteria recommended by the consensus report of the Asian working group for sarcopenia [36]. A positive finding was suggested when the handgrip was less than 26 kg for men or less than 16 kg for women.
- (e)
- Low physical activity was determined by self-reported frequency of engagement in activities requiring low to moderate levels of energy but was indicated when they performed the physical activities three times or fewer a month [37].
2.2.2. Determination of Total Phenolic Compounds, Total Flavonoids, and Antioxidant Capacity of Fruits and Vegetables
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Prevalence of Physical Frailty in Older Adults
3.3. Prevalence of Dietary Intake in Older Adults
3.4. The Association between Fruit and Vegetable Intakes and Frailty in Older Adults
3.5. Total Phenolic Compounds, Total Flavonoids, and Antioxidant Capacity of Certain Fruit and Vegetable Intakes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallis, S.; Wall, J.; Biram, R.; Romero-Ortuno, R. Association of the clinical frailty scale with hospital outcomes. QJM Int. J. Med. 2015, 108, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.E.; Al Snih, S.; Berges, I.M.; Ray, L.A.; Markides, K.S.; Ottenbacher, K.J. Frailty and 10-Year Mortality in Community-Living Mexican American Older Adults. Gerontology 2009, 55, 644–651. [Google Scholar] [CrossRef]
- Siriwardhana, D.D.; Hardoon, S.; Rait, G.; Weerasinghe, M.C.; Walters, K.R. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 2018, 8, e018195. [Google Scholar] [CrossRef] [Green Version]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Taffett, G.E. Physiology of Aging. In Geriatric Medicine: An Evidence-Based Approach; Springer: New York, NY, USA, 2003; pp. 27–35. [Google Scholar]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Setiati, S.; Laksmi, P.W.; Aryana, I.S.; Sunarti, S.; Widajanti, N.; Dwipa, L.; Seto, E.; Istanti, R.; Ardian, L.J.; Chotimah, S.C. Frailty state among Indonesian elderly: Prevalence, associated factors, and frailty state transition. BMC Geriatr. 2019, 19, 182. [Google Scholar] [CrossRef]
- Badrasawi, M.; Shahar, S.; Singh, D.K.A. Risk Factors of Frailty Among Multi-Ethnic Malaysian Older Adults. Int. J. Gerontol. 2017, 11, 154–160. [Google Scholar] [CrossRef]
- Siriwardhana, D.D.; Weerasinghe, M.C.; Rait, G.; Falcaro, M.; Scholes, S.; Walters, K.R. Prevalence of frailty in rural community-dwelling older adults in Kegalle district of Sri Lanka: A population-based cross-sectional study. BMJ Open 2019, 9, e026314. [Google Scholar] [CrossRef]
- Thinuan, P.; Siviroj, P.; Lerttrakarnnon, P.; Lorga, T. Prevalence and Potential Predictors of Frailty among Community-Dwelling Older Persons in Northern Thailand: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 4077. [Google Scholar] [CrossRef]
- Hale, M.; Shah, S.; Clegg, A. Frailty, inequality and resilience. Clin. Med. 2019, 19, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Gill, T.M.; Gahbauer, E.A.; Allore, H.G.; Han, L. Transitions Between Frailty States Among Community-Living Older Persons. Arch. Intern. Med. 2006, 166, 418–423. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Graham, J.E.; Al Snih, S.; Raji, M.; Samper-Ternent, R.; Ostir, G.V.; Markides, K.S. Mexican Americans and Frailty: Findings from the Hispanic Established Populations Epidemiologic Studies of the Elderly. Am. J. Public Health 2009, 99, 673–679. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low Nutrient Intake Is an Essential Component of Frailty in Older Persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Inzitari, M.; For the International Association of Gerontology and Geriatrics (IAGG) Task Force for Nutrition in the Elderly; Doets, E.; Bartali, B.; Benetou, V.; Di Bari, M.; Visser, M.; Volpato, S.; Gambassi, G.; Topinkova, E.; et al. Nutrition in the age-related disablement process. J. Nutr. Health Aging 2011, 15, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, C.; Alix, E.; Sallé, A.; Berrut, G.; Ritz, P. Energy requirements in frail elderly people: A review of the literature. Clin. Nutr. 2007, 26, 16–24. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Thalacker-Mercer, A.E.; Fleet, J.C.; Craig, B.A.; Carnell, N.S.; Campbell, W.W. Inadequate protein intake affects skeletal muscle transcript profiles in older humans2. Am. J. Clin. Nutr. 2007, 85, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Beasley, J.M.; Lacroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.F.; Woods, N.F.A.; Michael, Y.L.; Curb, J.D.; Prentice, R.L. Protein Intake and Incident Frailty in the Women’s Health Initiative Observational Study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012, 51, 173–179. [Google Scholar] [CrossRef]
- Gao, J.; Jia, Y.; Dai, J.; Fu, H.; Wang, Y.; Yan, H.; Zhu, Y.; Nie, X. Association of Fruit and Vegetable Intake and Frailty among Chinese Elders: A Cross-Sectional Study in Three Cities. J. Nutr. Health Aging 2019, 23, 890–895. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Rahi, B.; Peres, K.; Colpo, M.; Dartigues, J.-F.; Bandinelli, S.; Feart, C.; Rodríguez-Artalejo, F. Consumption of fruit and vegetables and risk of frailty: A dose-response analysis of 3 prospective cohorts of community-dwelling older adults. Am. J. Clin. Nutr. 2016, 104, 132–142. [Google Scholar] [CrossRef]
- Fung, T.T.; Struijk, E.A.; Rodriguez-Artalejo, F.; Willett, W.C.; Lopez-Garcia, E. Fruit and vegetable intake and risk of frailty in women 60 years old or older. Am. J. Clin. Nutr. 2020, 112, 1540–1546. [Google Scholar] [CrossRef]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Jivraj, S.; Sekiguchi, K.; Walters, K. Fruit and Vegetable Consumption and Frailty: A Systematic Review. J. Nutr. Health Aging 2018, 22, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Kojima, G.; Iliffe, S.; Jivraj, S.; Walters, K. Fruit and Vegetable Consumption and Incident Prefrailty and Frailty in Community-Dwelling Older People: The English Longitudinal Study of Ageing. Nutrients 2020, 12, 3882. [Google Scholar] [CrossRef]
- Bonnefoy, M.; Berrut, G.; LeSourd, B.; Ferry, M.; Gilbert, T.; Guerin, O.; Hanon, O.; Jeandel, C.; Paillaud, E.; Raynaud-Simon, A.; et al. Frailty and nutrition: Searching for evidence. J. Nutr. Health Aging 2015, 19, 250–257. [Google Scholar] [CrossRef]
- León-Muñoz, L.M.; Guallar-Castillón, P.; López-García, E.; Rodríguez-Artalejo, F. Mediterranean Diet and Risk of Frailty in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2014, 15, 899–903. [Google Scholar] [CrossRef]
- Ntanasi, E.; Yannakoulia, M.-H.; Kosmidis, M.; Anastasiou, C.A.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Scarmeas, N. Adherence to Mediterranean Diet and Frailty. J. Am. Med. Dir. Assoc. 2018, 19, 315–322.e2. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef]
- Center of Disease Control and Prevention. Epi Info™ Downloads. Available online: https://www.cdc.gov/epiinfo/support/downloads.html (accessed on 15 September 2021).
- Silpakit, O.; Silpakit, C.; Pukdeenaul, P. A Comparison Study of Cognitive Impairment Screening Tools: CDT, IQCODE VS MMSE. Siriraj Med. J. 2007, 59, 361. [Google Scholar]
- Dementia Association of Thailand, MSET10. The Dementia Association of Thailand Newsletter. (In Thai). Available online: https://thaidementia.com/news/assets/files/DAT_news_letter_10.pdf (accessed on 15 September 2021).
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Danilovich, M.K.; Diaz, L.; Corcos, D.M.; Ciolino, J.D. Relationship between SHARE-FI Frailty Scores and Physical Performance Measures in Older Adult Medicaid Recipients. Geriatrics 2018, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Punvittayagul, C.; Chariyakornkul, A.; Sankam, P.; Wongpoomchai, R. Inhibitory Effect of Thai Purple Rice Husk Extract on Chemically Induced Carcinogenesis in Rats. Molecules 2021, 26, 360. [Google Scholar] [CrossRef]
- Lengkidworraphiphat, P.; Wongpoomchai, R.; Taya, S.; Jaturasitha, S. Effect of genotypes on macronutrients and antioxidant capacity of chicken breast meat. Asian-Australas. J. Anim. Sci. 2020, 33, 1817–1823. [Google Scholar] [CrossRef]
- Ghoreishy, S.M.; Asoudeh, F.; Jayedi, A.; Mohammadi, H. Fruit and vegetable intake and risk of frailty: A systematic review and dose response meta-analysis. Ageing Res. Rev. 2021, 71, 101460. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Rincón, M.; Pulido, R.; Saura-Calixto, F. Guava Fruit (Psidium guajava L.) as a New Source of Antioxidant Dietary Fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Gull, J.; Sultana, B.; Anwar, F.; Naseer, R.; Ashraf, M.; Ashrafuzzaman, M. Variation in Antioxidant Attributes at Three Ripening Stages of Guava (Psidium guajava L.) Fruit from Different Geographical Regions of Pakistan. Molecules 2012, 17, 3165–3180. [Google Scholar] [CrossRef] [Green Version]
- Kamala, M.; Banu, M.S.; Senthil, R.; Anand, A.V. Anti Hyperglycemic and Ant‰ Hyperlipidemic Potentials of Psidium guajava Fruit Extract—A Review. Res. J. Pharm. Technol. 2011, 4, 1033–1036. [Google Scholar]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [Green Version]
- Wongsa, P.; Chaiwarit, J.; Zamaludien, A. In vitro screening of phenolic compounds, potential inhibition against α-amylase and α-glucosidase of culinary herbs in Thailand. Food Chem. 2012, 131, 964–971. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Joseph, J.M.; Manian, S. Antioxidant and Free Radical Scavenging Activities of Indian Acacias: Acacia leucophloea (Roxb.) Willd., Acacia ferruginea Dc., Acacia dealbata Link. and Acacia pennata (L.) Willd. Int. J. Food Prop. 2013, 16, 1717–1729. [Google Scholar] [CrossRef]
- Dongmo, A.; Nguelefack, T.; Lacaille-Dubois, M. Antinociceptive and anti-inflammatory activities of Acacia pennata wild (Mimosaceae). J. Ethnopharmacol. 2005, 98, 201–206. [Google Scholar] [CrossRef]
- Dongmo, A.B.; Miyamoto, T.; Yoshikawa, K.; Arihara, S.; Lacaille-Dubois, M.-A. Flavonoids from Acacia pennata and their Cyclooxygenase (COX-1 and COX-2) Inhibitory Activities. Planta Med. 2007, 73, 1202–1207. [Google Scholar] [CrossRef]
- Rifai, Y.; Arai, M.A.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Terpenoids and a Flavonoid Glycoside from Acacia pennata Leaves as Hedgehog/GLI-Mediated Transcriptional Inhibitors. J. Nat. Prod. 2010, 73, 995–997. [Google Scholar] [CrossRef]
- El-Taher, E.M.M.; El-Sherei, M.M.; El Dine, R.S.; Elnaggar, D.M.; Khalil, W.K.B.; Kassem, S.M.; Elkhateeb, A.; Kassem, M.E.S. Acacia pennata L. leaves: Chemical profiling and impact on DNA damage, alteration of genotoxicity—Related genes expression and ROS generation in hepatic tissues of acetaminophen treated male rats. Adv. Tradit. Med. 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Lomarat, P.; Chancharunee, S.; Anantachoke, N.; Kitphati, W.; Sripha, K.; Bunyapraphatsara, N. Bioactivity-guided Separation of the Active Compounds in Acacia pennata Responsible for the Prevention of Alzheimer’s Disease. Nat. Prod. Commun. 2015, 10, 1431–1434. [Google Scholar] [CrossRef] [Green Version]
- Thongwat, D.; Ganranoo, L.; Chokchaisiri, R. Larvicidal and Pupicidal Activities of Crude and Fractionated Extracts of Acacia pennata (L.) Willd. Subsp Insuavis Shoot Tips against Aedes aegypti (L.) (Diptera: Culicidae). Southeast Asian J. Trop. Med. Public Health 2017, 48, 27–36. [Google Scholar]
- Department of Disease Control of Thailand Thai National Health Survey 2019–2020. Available online: https://ddc.moph.go.th/dncd/ncdstrategy/public/home/media/142 (accessed on 20 February 2022).
- Kobayashi, S.; Suga, H.; Sasaki, S.; Three-generation Study of Women on Diets and Health Study Group. Diet with a combination of high protein and high total antioxidant capacity is strongly associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutr. J. 2017, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Ribeiro, R.V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Simpson, S.J.; Hirani, V. Prospective Associations Between Dietary Antioxidant Intake and Frailty in Older Australian Men: The Concord Health and Ageing in Men Project. J. Gerontol. Ser. A 2019, 75, 348–356. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Woodhouse, K.W. Frailty, inflammation and the elderly. Biogerontology 2010, 11, 635–641. [Google Scholar] [CrossRef]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Rockwood, K. Changes with age in the distribution of a frailty index. Mech. Ageing Dev. 2004, 125, 517–519. [Google Scholar] [CrossRef]
- Crews, D.E.; Zavotka, S. Aging, Disability, and Frailty: Implications for Universal Design. J. Physiol. Anthr. 2006, 25, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Kane, A.E.; Howlett, S.E. Sex differences in frailty: Comparisons between humans and preclinical models. Mech. Ageing Dev. 2021, 198, 111546. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Veronese, N.; Maggi, S.; Baggio, G.; De Rui, M.; Bolzetta, F.; Zambon, S.; Sartori, L.; Perissinotto, E.; Crepaldi, G.; et al. Marital Status and Frailty in Older People: Gender Differences in the Progetto Veneto Anziani Longitudinal Study. J. Women’s Health 2016, 25, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Grande, G.; Vetrano, D.L.; Maggi, S.; Sergi, G.; Welmer, A.-K.; Rizzuto, D. Gender Differences in the Relationship Between Marital Status and the Development of Frailty: A Swedish Longitudinal Population-Based Study. J. Women’s Health 2020, 29, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Walters, K.; Iliffe, S.; Taniguchi, Y.; Tamiya, N. Marital Status and Risk of Physical Frailty: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 322–330. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Palmer, K.M.; Galluzzo, L.; Giampaoli, S.; Marengoni, A.; Bernabei, R.; Onder, G. Hypertension and frailty: A systematic review and meta-analysis. BMJ Open 2018, 8, e024406. [Google Scholar] [CrossRef]
- Aprahamian, I.; Sassaki, E.; Dos Santos, M.F.; Izbicki, R.; Pulgrossi, R.C.; Biella, M.M.; Borges, A.C.N.; Sassaki, M.M.; Torres, L.M.; Ícaro, S.F.; et al. Hypertension and frailty in older adults. J. Clin. Hypertens. 2017, 20, 186–192. [Google Scholar] [CrossRef] [Green Version]
- El Assar, M.; Laosa, O.; Mañas, L.R. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Graciani, A.; Guallar-Castillón, P.; López-García, E.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Diabetes and Risk of Frailty and Its Potential Mechanisms: A Prospective Cohort Study of Older Adults. J. Am. Med. Dir. Assoc. 2015, 16, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, S.; Levin, R.; Dkaidek, S.; Edelman, S.; Resnick, T.; Menczel, J. Frailty and osteoporosis in older women—A prospective study. Osteoporos. Int. 2014, 25, 763–768. [Google Scholar] [CrossRef]
- Gunawardene, P.; Bermeo, S.; Vidal, C.; Al Saedi, A.; Chung, P.; Boersma, D.; Phu, S.; Pokorski, I.; Suriyaarachchi, P.; Demontiero, O.; et al. Association Between Circulating Osteogenic Progenitor Cells and Disability and Frailty in Older Persons: The Nepean Osteoporosis and Frailty Study. J. Gerontol. Ser. A 2015, 71, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Maeda, K.; Satake, S.; Matsui, Y.; Arai, H. Osteosarcopenia, the co-existence of osteoporosis and sarcopenia, is associated with social frailty in older adults. Aging 2021, 34, 535–543. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, Y.; Zhan, J.-K.; Tang, Z.-Y.; He, J.-Y.; Tan, P.; Deng, H.-Q.; Huang, W.; Liu, Y.-S. Sarco-Osteoporosis: Prevalence and Association with Frailty in Chinese Community-Dwelling Older Adults. Int. J. Endocrinol. 2015, 2015, 482940. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Total, n (%) (n = 350) | Physical Frailty Status, n (%) | ||
|---|---|---|---|---|
| Non-Frail (n = 154, 44.0%) | Frail (n = 196, 56%) | p-Value | ||
| Sex a | ||||
| Male | 90 (26.7) | 48 (53.3) | 42 (46.7) | 0.013 * |
| Female | 260 (74.3) | 106 (40.8) | 154 (59.2) | |
| Age, years a, Mean ± SD | 69.31 ± 6.96 | 67.47 ± 5.70 | 70.76 ± 7.51 | |
| 60–64 | 106 (30.3) | 57 (53.8) | 49 (46.2) | <0.001 ** |
| 65–74 | 158 (45.1) | 69 (43.7) | 89 (56.3) | |
| ≥75 | 86 (24.6) | 28 (32.6) | 58 (67.4) | |
| Education a | ||||
| No school | 46 (13.1) | 16 (34.8) | 30 (65.2) | 0.077 |
| Primary school | 212 (60.6) | 89 (42.0) | 123 (58.0) | |
| Secondary school | 92 (26.3) | 49 (53.3) | 43 (46.7) | |
| Marital status a | ||||
| Single | 50 (14.3) | 15 (30.0) | 35 (70.0) | 0.028 * |
| Married | 171 (48.9) | 86 (50.3) | 85 (49.7) | |
| Widow/divorced/separated | 129 (36.9) | 53 (41.1) | 76 (58.9) | |
| Occupation in the past a | ||||
| Farmers | 181 (52.9) | 81 (44.8) | 100 (55.2) | 0.338 |
| Merchants | 85 (24.9) | 40 (47.1) | 45 (52.9) | |
| Official workers | 33 (9.6) | 17 (51.5) | 16 (48.5) | |
| Housekeeper/unemployed | 43 (12.6) | 14 (32.6) | 29 (67.4) | |
| Incomes, USD per month a | ||||
| ≤30 | 118 (33.7) | 50 (42.4) | 68 (57.6) | 0.103 |
| 31–90 | 137 (39.1) | 55 (40.1) | 82 (59.9) | |
| 91–180 | 52 (14.9) | 31 (59.6) | 21 (40.4) | |
| >180 | 43 (12.3) | 18 (41.9) | 25 (58.1) | |
| No. of underlying diseases a | ||||
| None | 128 (36.6) | 67 (52.3) | 61 (47.7) | 0.035 * |
| 1 | 131 (37.4) | 55 (42.0) | 76 (58.0) | |
| ≥2 | 91 (26.0) | 32 (35.2) | 59 (64.8) | |
| Underlying diseases | ||||
| Hypertension a | 177 (49.4) | 65 (36.7) | 112 (63.3) | 0.006 * |
| Diabetes mellitus a | 44 (12.6) | 13 (29.5) | 31 (70.5) | 0.039 * |
| Cardiovascular diseases a | 17 (4.9) | 8 (47.1) | 9 (52.9) | 0.794 |
| Stroke b | 5 (1.4) | 2 (40.0) | 3 (60.0) | 0.612 |
| Arthritis a | 72 (20.6) | 29 (40.3) | 43 (59.7) | 0.475 |
| Osteoporosis a | 17 (4.9) | 3 (17.6) | 14 (82.4) | 0.025 * |
| COPD b | 8 (2.3) | 4 (50.0) | 4 (50.0) | 0.500 |
| Parameters | Total (n = 350) | Non-Frail (n = 154) | Frail (n = 196) | p-Value |
|---|---|---|---|---|
| a Weight (kg), Mean ± SD | 54.11 ± 10.10 | 55.74 ± 9.41 | 52.83 ± 10.46 | 0.007 * |
| b Unintended weight loss, n (%) | 32 (9.1) | 29 (90.6) | 3 (9.4) | <0.001 ** |
| b Self-reported exhaustion, n (%) | 7 (2.0) | 0 (0.0) | 7 (100.0) | 0.016 * |
| b Low activity, n (%) | 63 (18.0) | 62 (98.4) | 1 (1.6) | <0.001 ** |
| c Walking time (sec), Mean ± SD | 6.27 ± 2.29 | 5.17 ± 0.83 | 7.14 ± 2.67 | <0.001 ** |
| b Slow walking speed, n (%) | 112 (32.0) | 1 (0.9) | 111 (99.1) | <0.001 ** |
| c Grip strength (kg), Mean ± SD | 22.59 ± 6.51 | 25.59 ± 5.74 | 20.23 ± 6.11 | <0.001 ** |
| b Low grip strength, n (%) | 102 (29.1) | 0 (0.0) | 102 (100.0) | <0.001 ** |
| Types of Fruits and Vegetables ≠ | Total (n = 350), n (%) | Non-Frail (n = 154) n (%) | Frail (n = 196) n (%) | p-Value |
|---|---|---|---|---|
| Fruits | ||||
| Banana | 265 (75.7) | 120 (45.3) | 145 (54.7) | 0.393 |
| Papaya | 218 (62.3) | 92 (42.2) | 126 (57.8) | 0.384 |
| Mango | 173 (49.4) | 76 (43.9) | 97 (56.1) | 0.979 |
| Orange | 153 (43.7) | 67 (43.8) | 86 (56.2) | 0.945 |
| Watermelon | 128 (36.6) | 58 (45.3) | 70 (54.7) | 0.707 |
| Pineapple | 122 (34.9) | 61 (50.0) | 61 (50.0) | 0.098 |
| Rambutan | 122 (34.9) | 46 (37.7) | 76 (62.3) | 0.083 |
| Mangosteen | 113 (32.3) | 47 (41.6) | 66 (58.4) | 0.531 |
| Durian | 75 (21.5) | 36 (48.0) | 39 (52.0) | 0.446 |
| Guava | 60 (17.1) | 36 (60.0) | 24 (40.0) | 0.005 * |
| Vegetables | ||||
| Lettuce | 306 (87.4) | 134 (43.8) | 172 (56.2) | 0.835 |
| Long bean | 174 (49.7) | 81 (46.6) | 93 (53.4) | 0.339 |
| Ivy gourd | 156 (44.6) | 71 (45.5) | 85 (54.5) | 0.609 |
| Eggplant | 127 (36.3) | 52 (40.9) | 75 (59.1) | 0.385 |
| Morning glory | 120 (34.3) | 53 (44.2) | 67 (55.8) | 0.964 |
| Gurmar (Local vegetable) | 106 (30.3) | 48 (45.3) | 58 (54.7) | 0.750 |
| Cauliflower | 94 (26.9) | 34 (36.2) | 60 (63.8) | 0.074 |
| Cucumber | 67 (19.1) | 31 (46.3) | 36 (53.7) | 0.677 |
| Cabbage | 67 (19.1) | 34 (50.7) | 33 (49.3) | 0.216 |
| Malabar spinach | 58 (16.6) | 20 (34.5) | 38 (65.5) | 0.110 |
| Melientha suavis | 58 (16.6) | 19 (32.8) | 39 (67.2) | 0.059 |
| Collard greens | 52 (14.9) | 18 (34.6) | 34 (65.4) | 0.140 |
| Acacia pennata | 44 (12.6) | 27 (61.4) | 17 (38.6) | 0.013 * |
| Frailty Status (Non-Frail/Frail) | ||||||
|---|---|---|---|---|---|---|
| cOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Sex | ||||||
| Male | Ref. | Ref. | ||||
| Female | 1.66 | 1.03, 2.69 | 0.039 * | 1.59 | 0.92, 2.79 | 0.099 |
| Age, years | ||||||
| 60–64 | Ref. | Ref. | ||||
| 65–74 | 1.50 | 0.91, 2.46 | 0.108 | 1.26 | 0.74, 2.14 | 0.401 |
| ≥75 | 2.41 | 1.33, 4.35 | 0.004 * | 2.01 | 1.02, 3.94 | 0.042 * |
| Marital status | ||||||
| Single | Ref. | Ref. | ||||
| Married | 0.42 | 0.22, 0.83 | 0.13 * | 0.46 | 0.22, 0.96 | 0.038 * |
| Widow/divorced/separated | 0.61 | 0.31, 1.24 | 0.172 | 0.56 | 0.27, 1.19 | 0.131 |
| Incomes (USD per month) | ||||||
| ≤30 | Ref. | Ref. | ||||
| 30–90 | 1.10 | 0.66, 1.81 | 0.719 | 1.16 | 0.67, 2.01 | 0.600 |
| 91–180 | 0.50 | 0.26, 0.97 | 0.040 * | 0.62 | 0.30, 1.27 | 0.189 |
| >180 | 1.02 | 0.50, 2.07 | 0.954 | 1.44 | 0.65, 3.16 | 0.369 |
| No. of underlying diseases | ||||||
| None | Ref. | Ref. | ||||
| 1 | 1.52 | 0.93, 2.48 | 0.096 | 1.43 | 0.84, 2.43 | 0.185 |
| ≥2 | 2.03 | 1.17, 3.52 | 0.012 * | 2.07 | 1.15, 3.73 | 0.016 * |
| Regularly consumed | ||||||
| Guava fruit | 0.46 | 0.26, 0.81 | 0.007 * | 0.52 | 0.28, 0.96 | 0.037 * |
| Acacia pennata vegetable | 0.45 | 0.23, 0.85 | 0.015 * | 0.42 | 0.21, 0.83 | 0.012 * |
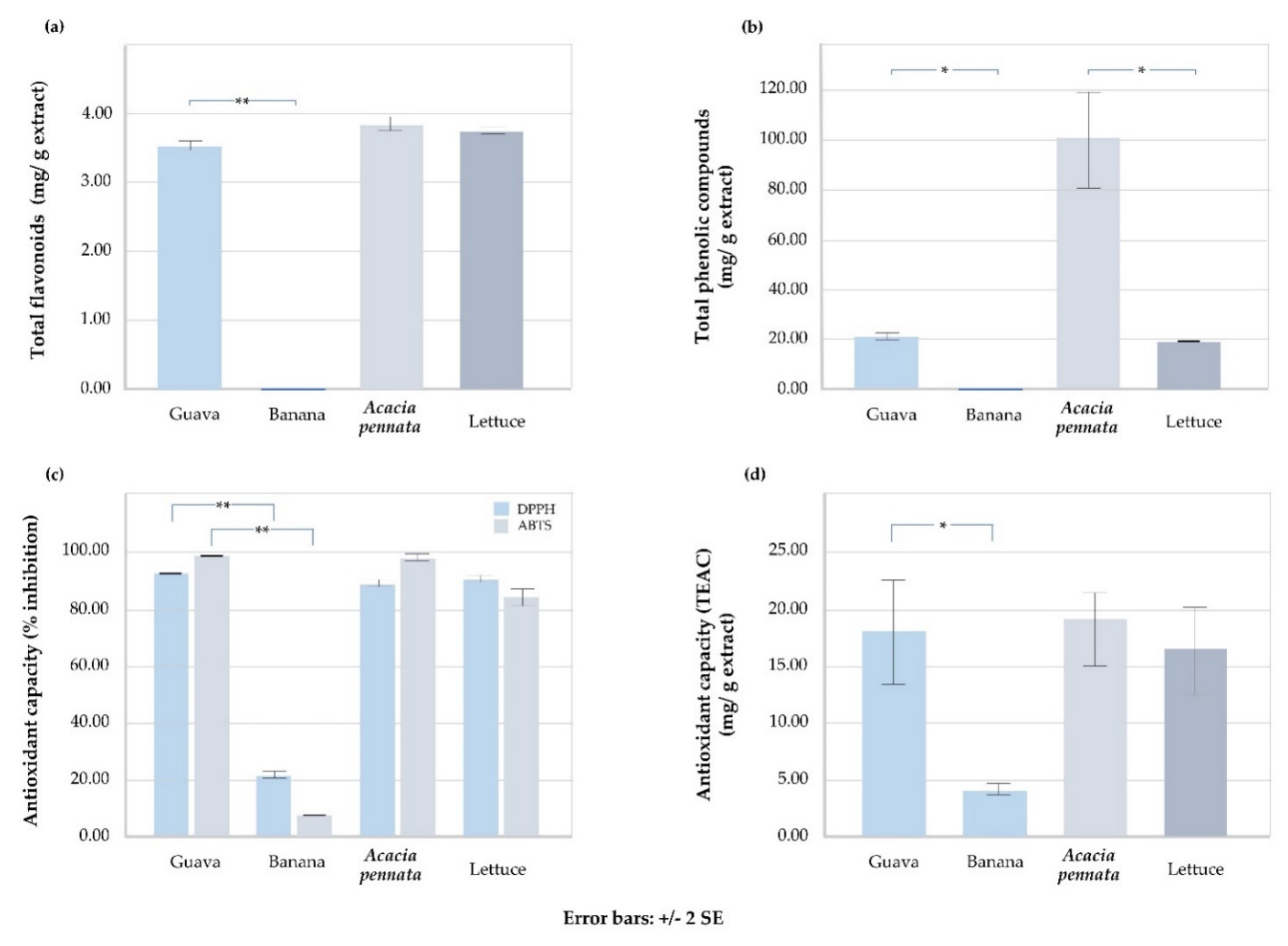
| Total Flavonoids (mg/g) | Total Phenolic Compounds (mg/g) | Antioxidant Capacity | |||
|---|---|---|---|---|---|
| % Radical Scavenging | TEAC (mg/g) | ||||
| DPPH | ABTS | FRAP | |||
| Guava | 3.530 ± 0.007 | 20.517 ± 1.542 | 91.893 ± 0.278 | 97.745 ± 0.247 | 17.850 ± 3.802 |
| Banana | 0.000 ± 0.000 | 0.000 ± 0.000 | 21.700 ± 1.380 | 6.845 ± 0.205 | 3.607 ± 0.692 |
| p-value | <0.001 ** | 0.002 * | <0.001 ** | <0.001 | 0.002 * |
| Acacia pennata | 3.783 ± 0.146 | 100.603 ± 15.623 | 89.023 ± 1.589 | 96.480 ± 1.329 | 18.537 ± 2.879 |
| Lettuce | 3.650 ± 0.052 | 18.683 ± 0.032 | 91.437 ± 1.712 | 85.490 ± 3.663 | 16.53 ± 3.309 |
| p-value | 0.211 | 0.012 * | 0.148 | 0.116 | 0.477 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruangsuriya, J.; Wongpoomchai, R.; Srichairatanakool, S.; Sirikul, W.; Buawangpong, N.; Siviroj, P. Guava Fruit and Acacia pennata Vegetable Intake Association with Frailty of Older Adults in Northern Thailand. Nutrients 2022, 14, 1192. https://doi.org/10.3390/nu14061192
Ruangsuriya J, Wongpoomchai R, Srichairatanakool S, Sirikul W, Buawangpong N, Siviroj P. Guava Fruit and Acacia pennata Vegetable Intake Association with Frailty of Older Adults in Northern Thailand. Nutrients. 2022; 14(6):1192. https://doi.org/10.3390/nu14061192
Chicago/Turabian StyleRuangsuriya, Jetsada, Rawiwan Wongpoomchai, Somdet Srichairatanakool, Wachiranun Sirikul, Nida Buawangpong, and Penprapa Siviroj. 2022. "Guava Fruit and Acacia pennata Vegetable Intake Association with Frailty of Older Adults in Northern Thailand" Nutrients 14, no. 6: 1192. https://doi.org/10.3390/nu14061192
APA StyleRuangsuriya, J., Wongpoomchai, R., Srichairatanakool, S., Sirikul, W., Buawangpong, N., & Siviroj, P. (2022). Guava Fruit and Acacia pennata Vegetable Intake Association with Frailty of Older Adults in Northern Thailand. Nutrients, 14(6), 1192. https://doi.org/10.3390/nu14061192









