Partially Hydrolyzed Guar Gum Suppresses the Development of Sarcopenic Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Analytical Procedures for the Glucose and Insulin Tolerance Tests
2.3. Indirect Calorimetry
2.4. Measurement of Grip Strength
2.5. Muscle Histology
2.6. Liver Histology
2.7. Biochemistry
2.8. Measurement of Free Fatty Acids in the Sera, Liver, Feces, and Plantaris Muscles
2.9. Measurement of SCFA Levels in the Feces and Sera and Amino Acid Concentrations in the Plantaris Muscle, Feces, Sera, and Liver Samples
2.10. Gene Expression Analysis in Murine Liver, Jejunum, White Adipose Tissue, and Soleus Muscle
2.11. Small Intestine Histology
2.12. Protocol for Isolation of Mononuclear Cells from Small Intestines of Mice
2.13. Tissue Preparation and Flow Cytometry
2.14. Statistical Analyses
3. Results
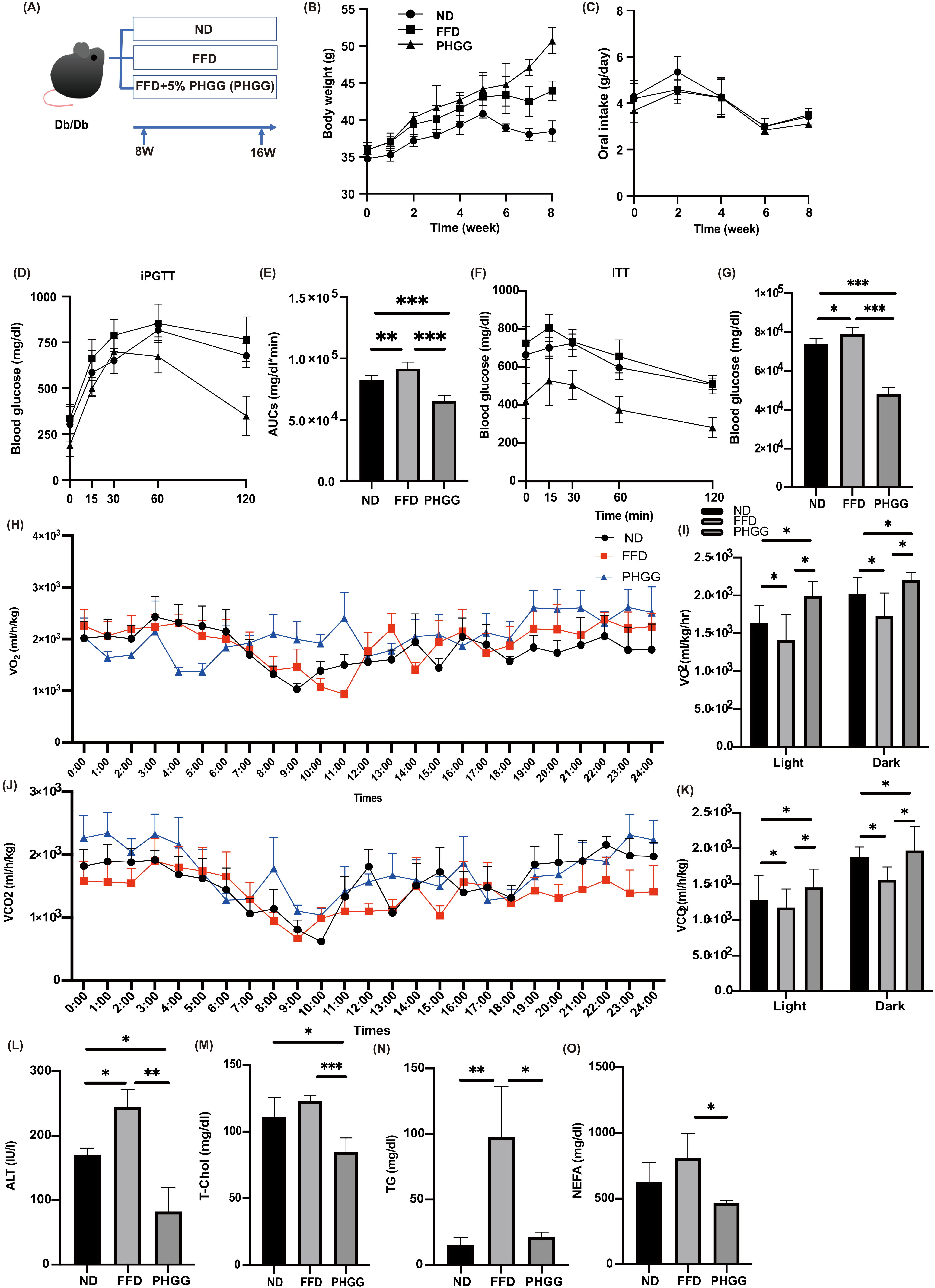
3.1. Glucose Intolerance, Which Was Worsened by FFD, Was Significantly Improved by PHGG Administration
3.2. FFD Decreased the Energy Metabolism, Whereas PHGG Increased
3.3. PHGG Decreased Hepatic Enzyme and Improved Lipid Metabolism
3.4. PHGG Improved Intrahepatic Fat Accumulation and Fibrosis and Decreased the Gene Expression Related to Inflammation and Fibrosis in the Liver and White Adipose Tissue
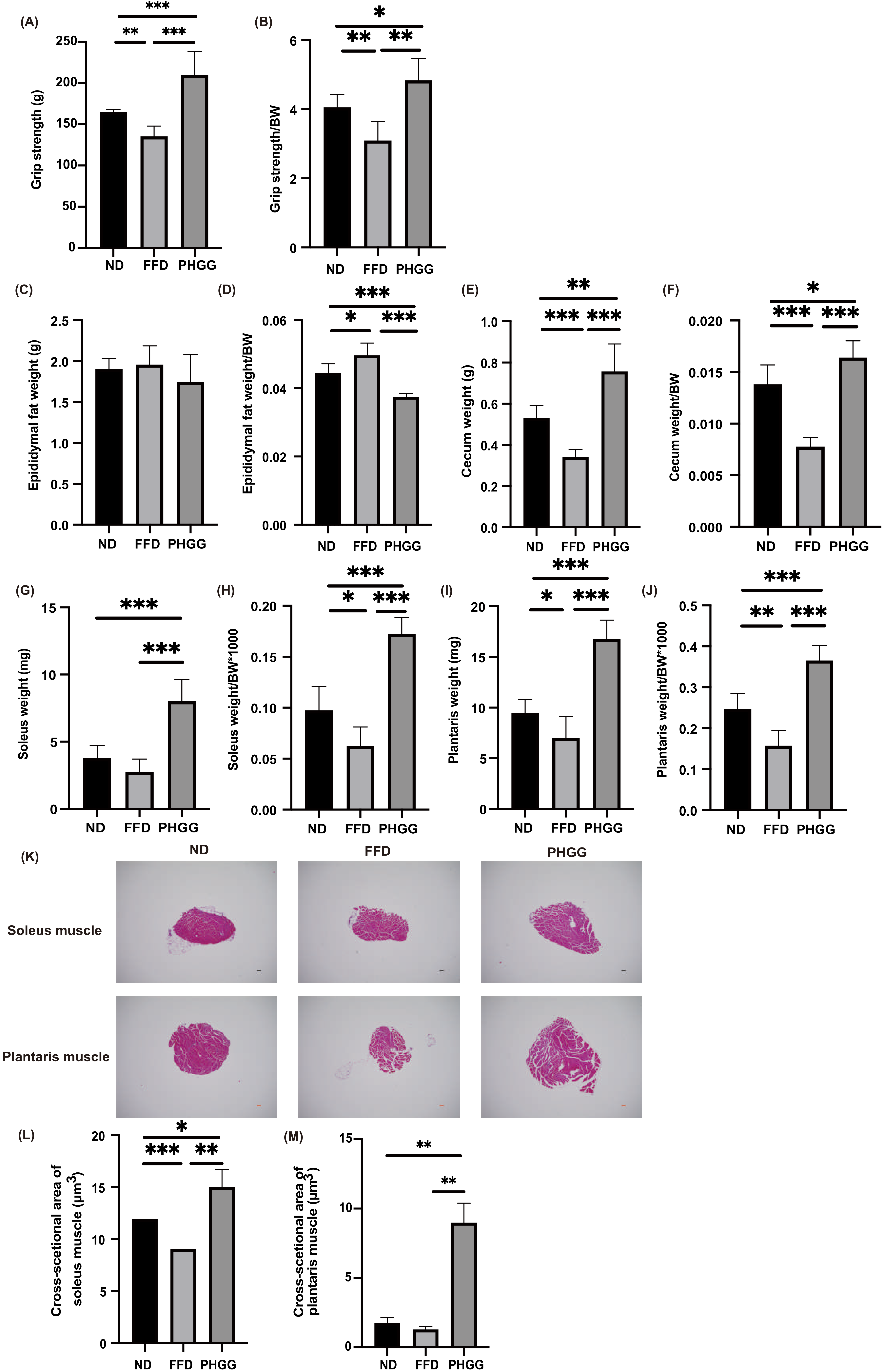
3.5. The Administration of PHGG Improved Sarcopenic Obesity
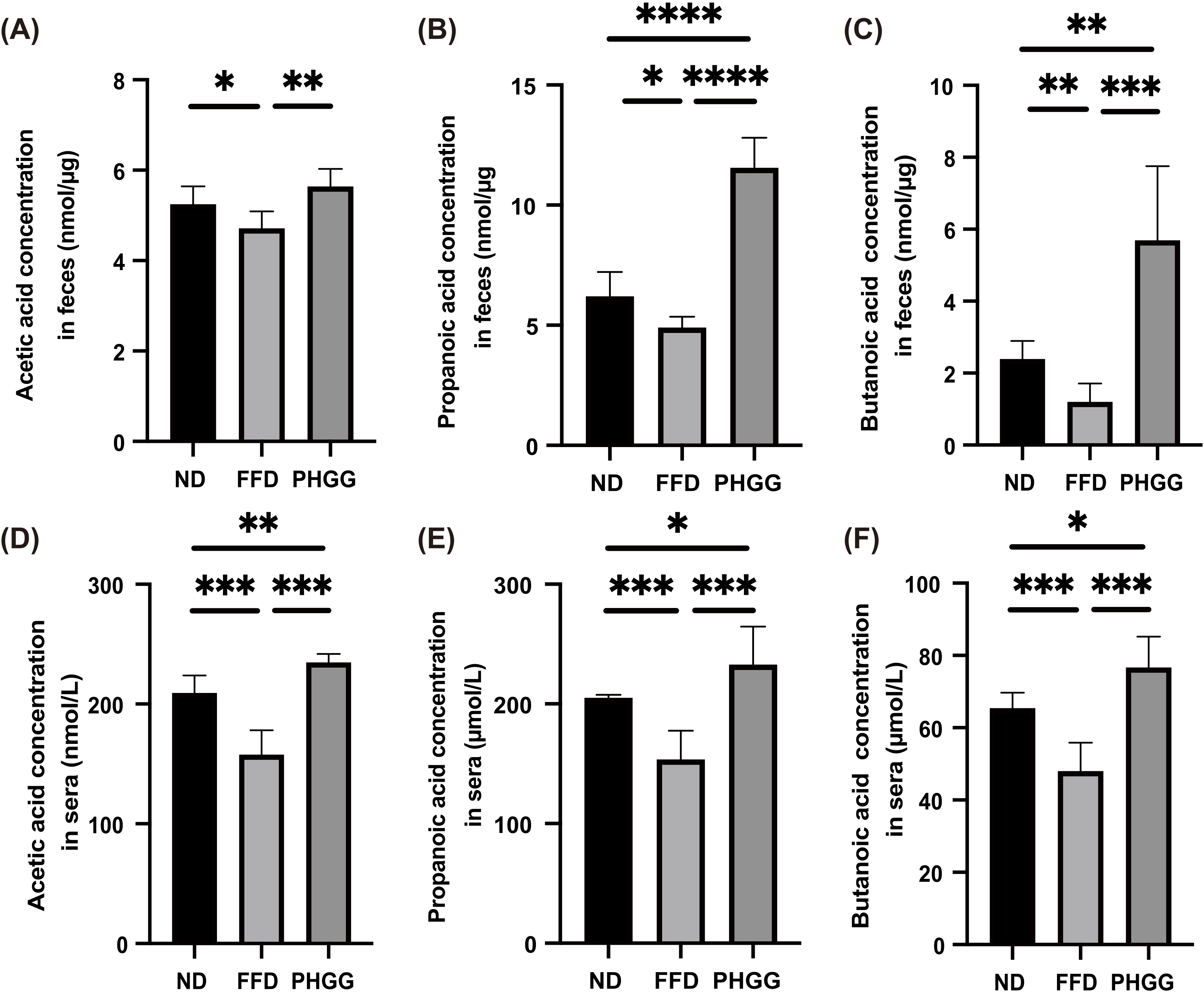
3.6. Short-Chain Fatty Acids in Feces and Sera Increased by the Administration of PHGG
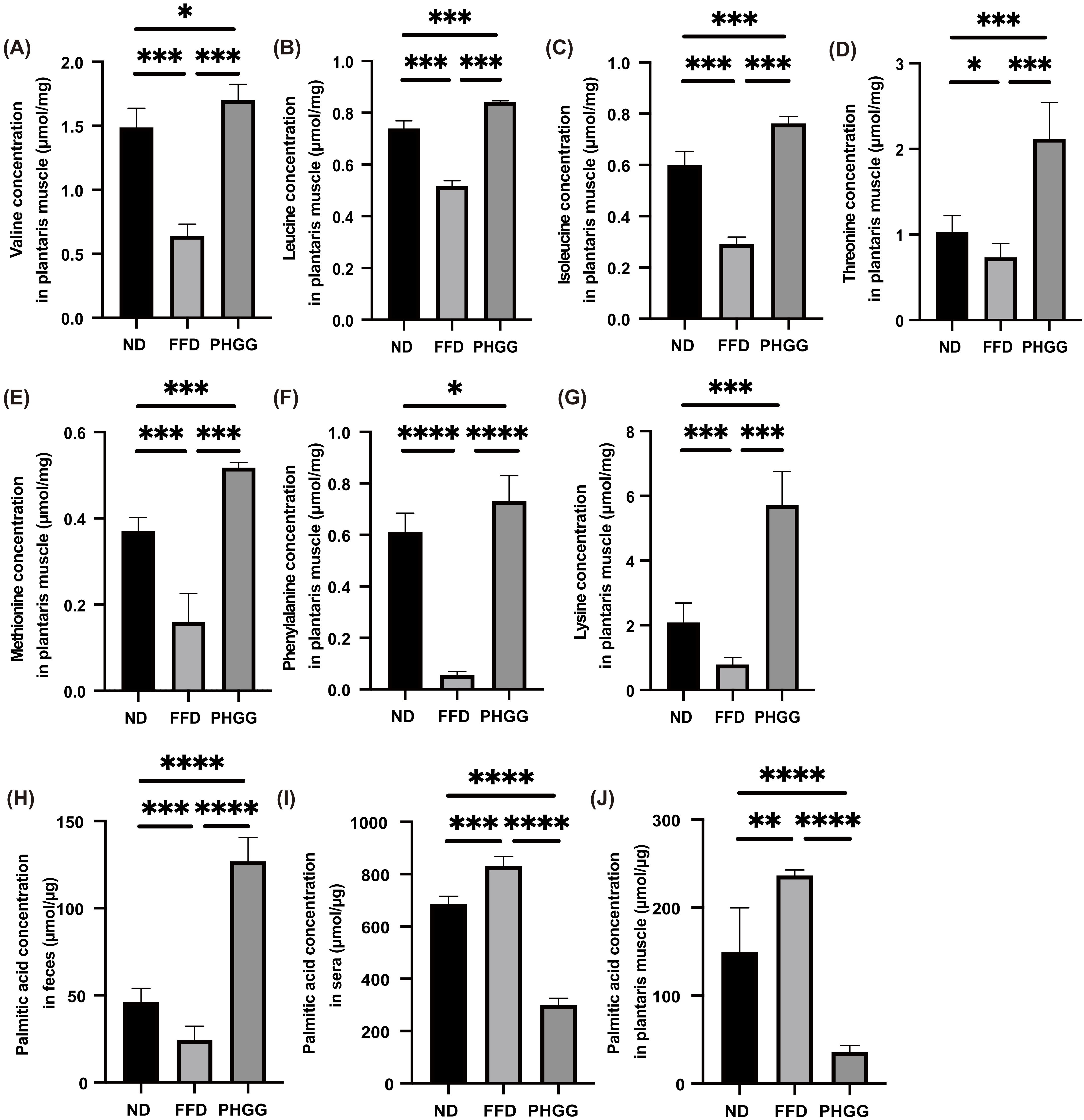
3.7. The Administration of PHGG Significantly Increased Amino Acids Related to Muscle Protein Synthesis in the Plantaris Muscle, Whereas It Decreased Them in the Sera and Liver
3.8. The Administration of PHGG Significantly Decreased Saturated Fatty Acid in the Plantaris Muscle
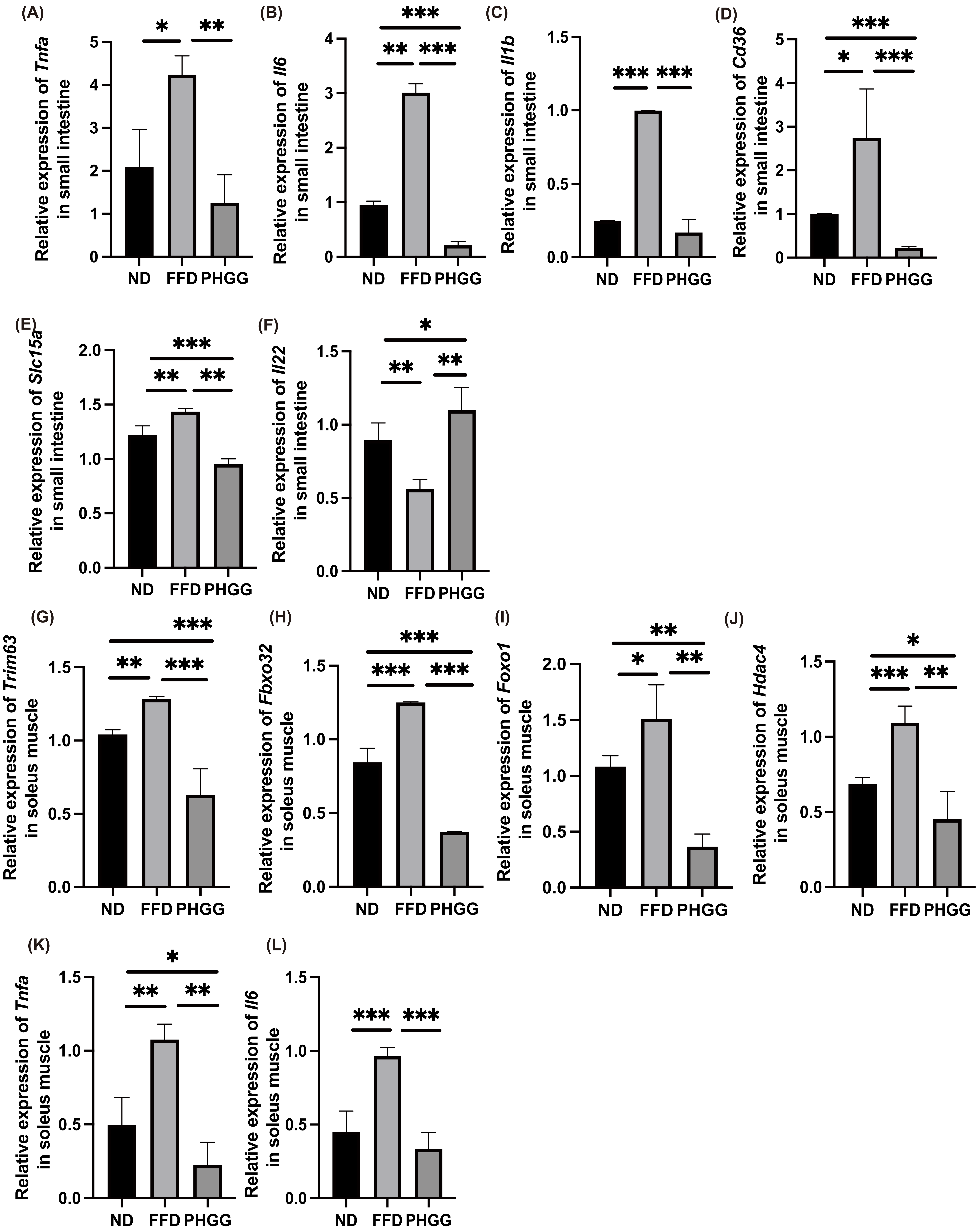
3.9. The Administration of PHGG Decreased the Expression of Genes Related to Inflammation, Amino Acid Transporter, and Fatty Acid Transporter in the Small Intestine
3.10. The Administration of PHGG Decreased the Expression of Genes Related to Muscle Atrophy and Inflammation
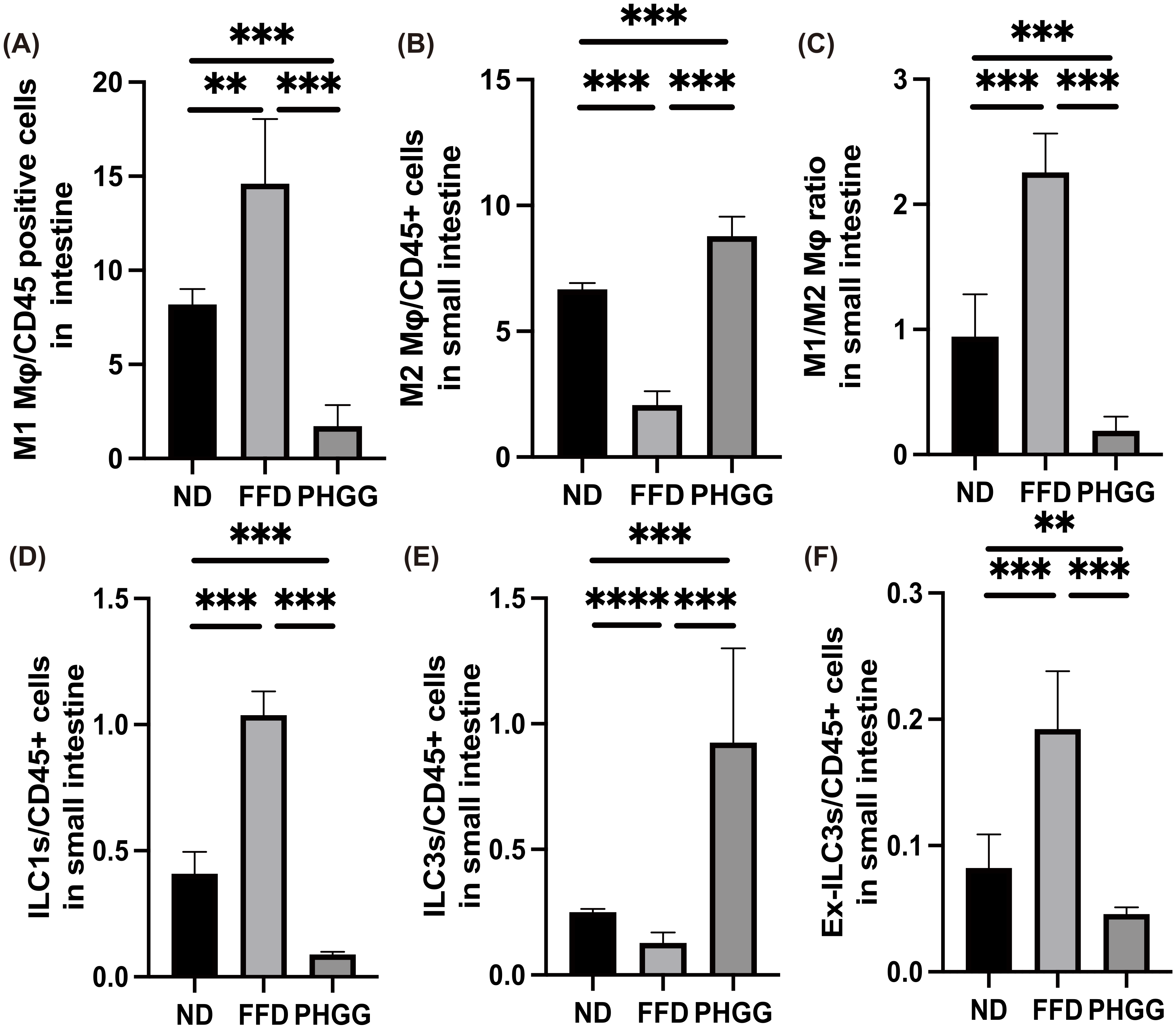
3.11. The Administration of PHGG Regulated Inflammatory Responses in Innate Immunity in the LPL of the Small Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of Energy Intake Rather than Protein Intake Is Associated with Sarcopenia in Elderly Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and Determinant Factors of Sarcopenia in Patients with Type 2 Diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Na, W.; Sohn, C. Relationship between Osteosarcopenic Obesity and Dietary Inflammatory Index in Postmenopausal Korean Women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Osaka, T.; Fukuda, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. The Relationship between Hepatic Steatosis and Skeletal Muscle Mass Index in Men with Type 2 Diabetes. Endocr. J. 2016, 63, 877–884. [Google Scholar] [CrossRef]
- Osaka, T.; Hashimoto, Y.; Fukuda, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. Relationship between Skeletal Muscle Mass and Hepatic Fibrosis in Patients with Type 2 Diabetes. Diabetes Metab. 2017, 43, 184–186. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic Obesity: Prevalence and Association with Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Smith, G.I.; Yoshino, J.; Kelly, S.C.; Reeds, D.N.; Okunade, A.; Patterson, B.W.; Klein, S.; Mittendorfer, B. High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell Rep. 2016, 17, 849–861. [Google Scholar] [CrossRef]
- Moyer, V.A. Vitamin D and Calcium Supplementation to Prevent Fractures in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2013, 158, 691–696. [Google Scholar] [CrossRef]
- Buigues, C.; Fernández-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Martínez-Martínez, M.; Verdejo, Y.; Carmen Mascarós, M.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rahman, M.A.; Lee, S.H.; Hwang, H.S.; Kim, H.A.; Yun, J.W. Plasma Proteome Analysis for Anti-Obesity and Anti-Diabetic Potentials of Chitosan Oligosaccharides in Ob/Ob Mice. Proteomics 2009, 9, 2149–2162. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Hashimoto, Y.; Matsumoto, S.; Ushigome, E.; Fukuda, T.; Sennmaru, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. Protein Intake, Especially Vegetable Protein Intake, Is Associated with Higher Skeletal Muscle Mass in Elderly Patients with Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 7985728. [Google Scholar] [CrossRef]
- Walton, G.E.; van den Heuvel, E.G.H.M.; Kosters, M.H.W.; Rastall, R.A.; Tuohy, K.M.; Gibson, G.R. A Randomised Crossover Study Investigating the Effects of Galacto-Oligosaccharides on the Faecal Microbiota in Men and Women over 50 Years of Age. Br. J. Nutr. 2012, 107, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Niu, A.-J.; Wu, J.-M.; Yu, D.-H.; Wang, R. Protective Effect of Lycium Barbarum Polysaccharides on Oxidative Damage in Skeletal Muscle of Exhaustive Exercise Rats. Int. J. Biol. Macromol. 2008, 42, 447–449. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Mao, X.; Wu, Y.; Ouyang, J. Astragalus Polysaccharide Improves Insulin Sensitivity in KKAy Mice: Regulation of PKB/GLUT4 Signaling in Skeletal Muscle. J. Ethnopharmacol. 2010, 127, 32–37. [Google Scholar] [CrossRef]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of Partially Hydrolyzed Guar Gum in the Treatment of Irritable Bowel Syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef]
- Takayama, S.; Katada, K.; Takagi, T.; Iida, T.; Ueda, T.; Mizushima, K.; Higashimura, Y.; Morita, M.; Okayama, T.; Kamada, K.; et al. Partially Hydrolyzed Guar Gum Attenuates Non-Alcoholic Fatty Liver Disease in Mice through the Gut-Liver Axis. World J. Gastroenterol. 2021, 27, 2160–2176. [Google Scholar] [CrossRef]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the Metabolic Syndrome Profile by Soluble Fibre—Guar Gum—In Patients with Type 2 Diabetes: A Randomised Clinical Trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef]
- Yasukawa, Z.; Naito, Y.; Takagi, T.; Mizushima, K.; Tokunaga, M.; Ishihara, N.; Juneja, L.R.; Yoshikawa, T. Partially Hydrolyzed Guar Gum Affects the Expression of Genes Involved in Host Defense Functions and Cholesterol Absorption in Colonic Mucosa of Db/Db Male Mice. J. Clin. Biochem. Nutr. 2012, 51, 33–38. [Google Scholar] [CrossRef]
- Ohashi, Y.; Sumitani, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Fujisawa, T. Consumption of Partially Hydrolysed Guar Gum Stimulates Bifidobacteria and Butyrate-Producing Bacteria in the Human Large Intestine. Benef. Microbes 2015, 6, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Harada, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Ogasawara, Y.; Juneja, L.R.; Fujisawa, T. Faecal Fermentation of Partially Hydrolyzed Guar Gum. J. Funct. Foods 2012, 4, 398–402. [Google Scholar] [CrossRef]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota—Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Dávila, I.; Gullón, B.; Alonso, J.L.; Labidi, J.; Gullón, P. Vine Shoots as New Source for the Manufacture of Prebiotic Oligosaccharides. Carbohydr. Polym. 2019, 207, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pylkas, A.M.; Juneja, L.R.; Slavin, J.L. Comparison of Different Fibers for In Vitro Production of Short Chain Fatty Acids by Intestinal Microflora. J. Med. Food 2005, 8, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wu, M.; Hao, Y.; Deng, H. Exploring the Preventive Effect and Mechanism of Senile Sarcopenia Based on “Gut-Muscle Axis”. Front. Bioeng. Biotechnol. 2020, 8, 590869. [Google Scholar] [CrossRef]
- Aoki, T.; Oyanagi, E.; Watanabe, C.; Kobiki, N.; Miura, S.; Yokogawa, Y.; Kitamura, H.; Teramoto, F.; Kremenik, M.J.; Yano, H. The Effect of Voluntary Exercise on Gut Microbiota in Partially Hydrolyzed Guar Gum Intake Mice under High-Fat Diet Feeding. Nutrients 2020, 12, 2508. [Google Scholar] [CrossRef]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of Three Types of Mixed Anesthetic Agents Alternate to Ketamine in Mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef]
- Kawano, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Sasano, R.; et al. Erythritol Ameliorates Small Intestinal Inflammation Induced by High-Fat Diets and Improves Glucose Tolerance. Int. J. Mol. Sci. 2021, 22, 5558. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Osaka, T.; Senmaru, T.; Fukuda, T.; Hamaguchi, M.; Fukui, M. MiR-23b-3p Acts as a Counter-Response against Skeletal Muscle Atrophy. J. Endocrinol. 2020, 244, 535–547. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Maekawa, M.; Kanno, T. Reestimation of aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio based on JSCC consensus method—Changes of criteria for a differential diagnosis of hepatic disorders following the alteration from Karmen method to JSCC method. Nihon Shokakibyo Gakkai Zasshi 1994, 91, 154–161. (In Japanese) [Google Scholar] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Christmass, M.A.; Mitoulas, L.R.; Hartmann, P.E.; Arthur, P.G. A Semiautomated Enzymatic Method for Determination of Nonesterified Fatty Acid Concentration in Milk and Plasma. Lipids 1998, 33, 1043–1049. [Google Scholar] [CrossRef]
- Okamura, T.; Nakajima, H.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Hamaguchi, M.; Asano, M.; Yamazaki, M.; et al. Low Circulating Dihomo-Gamma-Linolenic Acid Is Associated with Diabetic Retinopathy: A Cross Sectional Study of Kamogawa-Dm Cohort Study. Endocr. J. 2021, 68, 421–428. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.-E.; van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate Lymphoid Type 2 Cells Sustain Visceral Adipose Tissue Eosinophils and Alternatively Activated Macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wu, S.; Cheng, L.; Shen, Y.; Ma, W.; She, W.; Yang, C.; Wang, J.; Jiang, W. Type 3 Innate Lymphoid Cell: A New Player in Liver Fibrosis Progression. Clin. Sci. 2018, 132, 2565–2582. [Google Scholar] [CrossRef]
- Ono, Y.; Nagai, M.; Yoshino, O.; Koga, K.; Nawaz, A.; Hatta, H.; Nishizono, H.; Izumi, G.; Nakashima, A.; Imura, J.; et al. CD11c+ M1-like Macrophages (MΦs) but Not CD206+ M2-like MΦ Are Involved in Folliculogenesis in Mice Ovary. Sci. Rep. 2018, 8, 8171. [Google Scholar] [CrossRef]
- Noack, J.; Timm, D.; Hospattankar, A.; Slavin, J. Fermentation Profiles of Wheat Dextrin, Inulin and Partially Hydrolyzed Guar Gum Using an In Vitro Digestion Pretreatment and In Vitro Batch Fermentation System Model. Nutrients 2013, 5, 1500–1510. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Higashimura, Y.; Ushiroda, C.; Mizushima, K.; Ohashi, Y.; Yasukawa, Z.; Ozeki, M.; Tokunaga, M.; Okubo, T.; et al. Partially Hydrolysed Guar Gum Ameliorates Murine Intestinal Inflammation in Association with Modulating Luminal Microbiota and SCFA. Br. J. Nutr. 2016, 116, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Wako, N.; Okubo, T.; Ishihara, N. Influence of Partially Hydrolyzed Guar Gum on Constipation in Women. J. Nutr. Sci. Vitaminol. 1994, 40, 251–259. [Google Scholar] [CrossRef]
- Nobaek, S.; Johansson, M.-L.; Molin, G.; Ahrné, S.; Jeppsson, B. Alteration of Intestinal Microflora Is Associated with Reduction in Abdominal Bloating and Pain in Patients with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, A. Probiotics in the Treatment of Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2004, 38, S104–S106. [Google Scholar] [CrossRef] [PubMed]
- Shimada, R.; Yoshimura, M.; Murakami, K.; Ebihara, K. Plasma Concentrations of GLP-1 and PYY in Rats Fed Dietary Fiber Depend on the Fermentability of Dietary Fiber and Respond to an Altered Diet. Int. J. Clin. Nutr. Diet. 2015, 1, 103. [Google Scholar] [CrossRef][Green Version]
- MacDonald, P.E.; El-kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51 (Suppl. 3), S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. Gut Hormones as Pharmaceuticals. From Enteroglucagon to GLP-1 and GLP-2. Regul. Pept. 2000, 93, 45–51. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The Biology of Innate Lymphoid Cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Vosshenrich, C.A.J.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial Flora Drives Interleukin 22 Production in Intestinal NKp46+ Cells That Provide Innate Mucosal Immune Defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border Patrol: Regulation of Immunity, Inflammation and Tissue Homeostasis at Barrier Surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; de Sauvage, F.J.; et al. Interleukin-22 Mediates Early Host Defense against Attaching and Effacing Bacterial Pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Figliuolo, V.R.; dos Santos, L.M.; Abalo, A.; Nanini, H.; Santos, A.; Brittes, N.M.; Bernardazzi, C.; de Souza, H.S.P.; Vieira, L.Q.; Coutinho-Silva, R.; et al. Sulfate-Reducing Bacteria Stimulate Gut Immune Responses and Contribute to Inflammation in Experimental Colitis. Life Sci. 2017, 189, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Fachi, J.L.; Sécca, C.; Rodrigues, P.B.; de Mato, F.C.P.; di Luccia, B.; de Souza Felipe, J.; Pral, L.P.; Rungue, M.; de Melo Rocha, V.; Sato, F.T.; et al. Acetate Coordinates Neutrophil and ILC3 Responses against C. Difficile through FFAR2. J. Exp. Med. 2020, 217, e20190489. [Google Scholar] [CrossRef]
- Bosma, M.; Kersten, S.; Hesselink, M.K.C.; Schrauwen, P. Re-Evaluating Lipotoxic Triggers in Skeletal Muscle: Relating Intramyocellular Lipid Metabolism to Insulin Sensitivity. Prog. Lipid Res. 2012, 51, 36–49. [Google Scholar] [CrossRef]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered Lipid Metabolism and the Pathogenesis of Insulin Resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef]
- Tsintzas, K.; Chokkalingam, K.; Jewell, K.; Norton, L.; Macdonald, I.A.; Constantin-Teodosiu, D. Elevated Free Fatty Acids Attenuate the Insulin-Induced Suppression of PDK4 Gene Expression in Human Skeletal Muscle: Potential Role of Intramuscular Long-Chain Acyl-Coenzyme A. J. Clin. Endocrinol. Metab. 2007, 92, 3967–3972. [Google Scholar] [CrossRef]
- Bryner, R.W.; Woodworth-Hobbs, M.E.; Williamson, D.L.; Alway, S.E. Docosahexaenoic Acid Protects Muscle Cells from Palmitate-Induced Atrophy. ISRN Obes. 2012, 2012, 647348. [Google Scholar] [CrossRef]
- Woodworth-Hobbs, M.E.; Hudson, M.B.; Rahnert, J.A.; Zheng, B.; Franch, H.A.; Price, S.R. Docosahexaenoic Acid Prevents Palmitate-Induced Activation of Proteolytic Systems in C2C12 Myotubes. J. Nutr. Biochem. 2014, 25, 868–874. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Mori, J.; Yamaguchi, M.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. ILC2s Improve Glucose Metabolism through the Control of Saturated Fatty Acid Absorption within Visceral Fat. Front. Immunol. 2021, 12, 669629. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Delcourt, I.; Rance, M.; Giraudet, C.; Walrand, S.; Bedu, M.; Duche, P.; Boirie, Y. Changes in Basal and Insulin and Amino Acid Response of Whole Body and Skeletal Muscle Proteins in Obese Men. J. Clin. Endocrinol. Metab. 2009, 94, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Liu, Y.; Jacobs, R.; Rider, M.H. A Novel PKB/Akt Inhibitor, MK-2206,Effectivelyinhibits Insulin-Stimulated Glucose Metabolism and Protein Synthesis in Isolated Rat Skeletal Muscle. Biochem. J. 2012, 447, 137–147. [Google Scholar] [CrossRef]
- Huong Do, T.T.; Hindlet, P.; Dupriet-Waligora, A.J.; Kapel, N.; Neveux, N.; Mignon, V.; Deloménie, C.; Farinotti, R.; Fève, B.; Buyse, M. Disturbed Intestinal Nitrogen Homeostasis in a Mouse Model of High-Fat Diet-Induced Obesity and Glucose Intolerance. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E668–E680. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Adegoke, O.A.J.; Bates, H.E.; Kiraly, M.A.; Vranic, M.; Riddell, M.C.; Marliss, E.B. Exercise in ZDF Rats Does Not Attenuate Weight Gain, but Prevents Hyperglycemia Concurrent with Modulation of Amino Acid Metabolism and AKT/MTOR Activation in Skeletal Muscle. Eur. J. Nutr. 2015, 54, 751–759. [Google Scholar] [CrossRef]
| Ingredients (g/kg) | ND | FFD | PHGG |
|---|---|---|---|
| PHGG | 0 | 0 | 50 |
| Cellulose | 50 | 0 | 0 |
| Corn starch | 397.486 | 447.486 | 397.486 |
| Casein | 200 | ||
| L-cystine | 3 | ||
| Sucrose | 100 | ||
| Soybean oil | 70 | ||
| AIN-93 mineral mixture | 35 | ||
| AIN-93G vitamin mixture | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamura, T.; Hamaguchi, M.; Mori, J.; Yamaguchi, M.; Mizushima, K.; Abe, A.; Ozeki, M.; Sasano, R.; Naito, Y.; Fukui, M. Partially Hydrolyzed Guar Gum Suppresses the Development of Sarcopenic Obesity. Nutrients 2022, 14, 1157. https://doi.org/10.3390/nu14061157
Okamura T, Hamaguchi M, Mori J, Yamaguchi M, Mizushima K, Abe A, Ozeki M, Sasano R, Naito Y, Fukui M. Partially Hydrolyzed Guar Gum Suppresses the Development of Sarcopenic Obesity. Nutrients. 2022; 14(6):1157. https://doi.org/10.3390/nu14061157
Chicago/Turabian StyleOkamura, Takuro, Masahide Hamaguchi, Jun Mori, Mihoko Yamaguchi, Katsura Mizushima, Aya Abe, Makoto Ozeki, Ryoichi Sasano, Yuji Naito, and Michiaki Fukui. 2022. "Partially Hydrolyzed Guar Gum Suppresses the Development of Sarcopenic Obesity" Nutrients 14, no. 6: 1157. https://doi.org/10.3390/nu14061157
APA StyleOkamura, T., Hamaguchi, M., Mori, J., Yamaguchi, M., Mizushima, K., Abe, A., Ozeki, M., Sasano, R., Naito, Y., & Fukui, M. (2022). Partially Hydrolyzed Guar Gum Suppresses the Development of Sarcopenic Obesity. Nutrients, 14(6), 1157. https://doi.org/10.3390/nu14061157









