Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection, Quality Assessment and Data Extraction
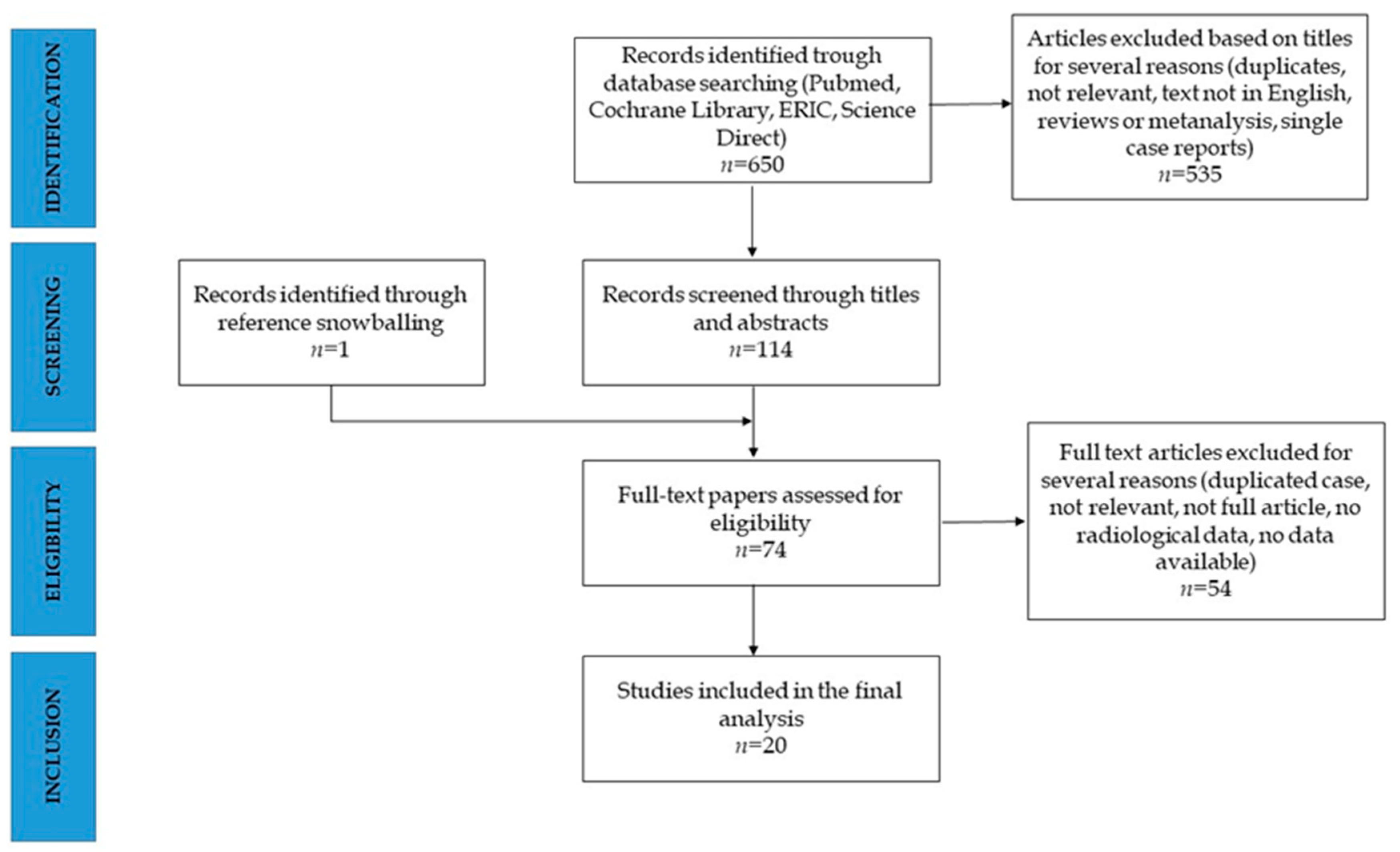
3. Results
3.1. Study Selection
3.2. Study Design and Population
3.3. Bone Health Parameters and Bone Disease Definitions
3.4. Prevalence of MBD and Comparison with Control Population
3.5. Evolution of MBD and Risk Factors for MBD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130 (Suppl 1), S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Abi Nader, E.; Pigneur, B.; Lambe, C. Short Bowel Syndrome as the Leading Cause of Intestinal Failure in Early Life: Some Insights into the Management. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 303–329. [Google Scholar] [CrossRef] [PubMed]
- D’Antiga, L.; Goulet, O. Intestinal failure in children: The European view. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 118–126. [Google Scholar] [CrossRef]
- Allan, P.J.; Lal, S. Metabolic bone diseases in intestinal failure. J. Hum. Nutr. Diet. 2020, 33, 423–430. [Google Scholar] [CrossRef]
- Ward, L.M.; Weber, D.R.; Munns, C.F.; Högler, W.; Zemel, B.S. A Contemporary View of the Definition and Diagnosis of Osteoporosis in Children and Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, e2088–e2097. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.; Fewtrell, M.; Goulet, O.; Molgaard, C.; Picaud, J.C.; Senterre, T.; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Calcium, phosphorus and magnesium. Clin. Nutr. 2018, 37, 2360–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, C.; Virgili, N.; Cuerda, C.; Chicharro, L.; Gómez, P.; Moreno, J.M.; Álvarez, J.; Martí, E.; Matía, P.; Penacho, M.A.; et al. Transversal study on the prevalence of Metabolic Bone Disease (MBD) and Home Parenteral Nutrition (HPN) in Spain: Data from NA-DYA group. Nutr. Hosp. 2010, 25, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Foldes, J.; Rimon, B.; Muggia-Sullam, M.; Gimmon, Z.; Leichter, I.; Steinberg, R.; Menczel, J.; Freund, H.R. Progressive bone loss during long-term home total parenteral nutrition. J. Parenter. Enteral. Nutr. 1990, 14, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Moukarzel, A. Metabolic bone disease associated with total parenteral nutrition. Clin. Nutr. 2000, 19, 217–231. [Google Scholar] [CrossRef]
- Pironi, L.; Morselli Labate, A.M.; Pertkiewicz, M.; Przedlacki, J.; Tjellesen, L.; Staun, M.; De Francesco, A.; Gallenca, P.; Guglielmi, F.W.; Van Gossum, A.; et al. Prevalence of bone disease in patients on home parenteral nutrition. Clin. Nutr. 2002, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, M.; Foulon, V.; De Pourcq, L.; Hiele, M.; Willems, L. Guidelines recommendations on care of adult patients receiving home parenteral nutrition: A systematic review of global practices. Clin. Nutr. 2012, 31, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An up-dated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 372, n71. [Google Scholar] [CrossRef]
- Pollock, A.; Berge, E. How to do a systematic review. Int. J. Stroke 2018, 13, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Research, International and Strategic Business Development Team. Academy of Nutrition and Dietetics, Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2016. [Google Scholar]
- Cannon, R.A.; Byrne, W.J.; Ament, M.E.; Gates, B.; O’Connor, M.; Fonkalsrud, E.W. Home parenteral nutrition in infants. J. Pediatr. 1980, 96, 1098–1104. [Google Scholar] [CrossRef]
- Larchet, M.; Garabédian, M.; Bourdeau, A.; Gorski, A.M.; Goulet, O.; Ricour, C. Calcium metabolism in children during long-term total parenteral nutrition: The influence of calcium, phosphorus, and vitamin D intakes. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 367–375. [Google Scholar] [CrossRef]
- Leonberg, B.L.; Chuang, E.; Eicher, P.; Tershakovec, A.M.; Leonard, L.; Stallings, V.A. Long-term growth and development in children after home parental nutrition. J. Pediatr. 1998, 132, 461–466. [Google Scholar] [CrossRef]
- Dellert, S.F.; Farrell, M.K.; Specker, B.L.; Heubi, J.E. Bone mineral content in children with short bowel syndrome after discontinuation of parental nutrition. J. Pediatr. 1998, 132, 516–519. [Google Scholar] [CrossRef]
- Diamanti, A.; Bizzarri, C.; Basso, M.S.; Gambarara, M.; Cappa, M.; Daniele, A.; Noto, C.; Castro, M. How does long-term parenteral nutrition impact the bone mineral status of children with intestinal failure? J. Bone. Miner. Metab. 2010, 28, 351–358. [Google Scholar] [CrossRef]
- Olieman, J.F.; Penning, C.; Spoel, M.; Ijsselstijn, H.; van den Hoonaard, T.L.; Escher, J.C.; Bax, N.M.; Tibboel, D. Long-term impact of infantile short bowel syndrome on nutritional status and growth. Br. J. Nutr. 2012, 107, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Ubesie, A.C.; Heubi, J.E.; Kocoshis, S.A.; Henderson, C.J.; Mezoff, A.G.; Rao, M.B.; Cole, C.R. Vitamin D deficiency and low bone min-eral density in pediatric and young adult intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Mutanen, A.; Mäkitie, O.; Pakarinen, M.P. Risk of metabolic bone disease is increased both during and after weaning off parenteral nutrition in pediatric intestinal failure. Horm. Res. Paediatr. 2013, 79, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Appleman, S.S.; Kalkwarf, H.J.; Dwivedi, A.; Heubi, J.E. Bone deficits in parenteral nutrition-dependent infants and children with intestinal failure are attenuated when accounting for slower growth. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichler, J.; Chomtho, S.; Fewtrell, M.; Macdonald, S.; Hill, S.M. Growth and bone health in pediatric intestinal failure patients receiving long-term parenteral nutrition. Am. J. Clin. Nutr. 2013, 97, 1260–1269. [Google Scholar] [CrossRef] [Green Version]
- Derepas, C.; Kosar, C.; Avitzur, Y.; Wales, P.W.; Courtney-Martin, G. Decreased bone turnover markers in children on long-term parenteral nutrition (PN) for intestinal failure (IF). J. Parenter. Enteral. Nutr. 2015, 39, 85–94. [Google Scholar] [CrossRef]
- Demehri, F.R.; Simha, S.; Stephens, L.; Harris, M.B.; Arnold, M.A.; Brown, P.I.; Teitelbaum, D.H. Pediatric intestinal failure: Predictors of metabolic bone disease. J. Pediatr. Surg. 2015, 50, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Fisher, J.G.; Bairdain, S.; Sparks, E.A.; Zurakowski, D.; Modi, P.B.; Duggan, C.; Jaksic, T. Metabolic bone disease in pediatric intestinal failure patients: Prevalence and risk factors. J. Pediatr. Surg. 2015, 50, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, L.J.; Bechtold, H.M.; Reyen, L.E.; Hall, T.R.; Vargas, J.H. Vitamin D deficiency in children with intestinal failure receiving home parenteral nutrition. J. Parenter. Enteral. Nutr. 2015, 39, 471–475. [Google Scholar] [CrossRef]
- Neelis, E.; Rijnen, N.; Sluimer, J.; Olieman, J.; Rizopoulos, D.; Wijen, E.; Rings, E.; de Koning, B.; Hulst, J. Bone health of children with intestinal failure measured by dual energy X-ray absorptiometry and digital X-ray radiogrammetry. Clin. Nutr. 2018, 37, 687–694. [Google Scholar] [CrossRef]
- Poinsot, P.; Geoffroy, P.; Braillon, P.; Denis, A.; Loras-Duclax, I.; Bacchetta, J.; Touzet, S.; Lachaux, A.; Peretti, N. Longitudinal bone mineralization assessment in children treated with long-term parenteral nutrition for severe intestinal failure. J. Parenter. Enteral. Nutr. 2018, 42, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, K.; Ksiazyk, J.; Kozlowski, D.; Pajdowska, M.; Janusz, M.; Jaworski, M. Nutritional therapy complications in children with ultra-short bowel syndrome include growth deficiency but not cholestasis. Acta Paediatr. 2018, 107, 1088–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvammen, J.A.; Thomassen, R.A.; Kjeserud, C.N.; Saeland, C.; Godang, K.; Bollerslev, J.; Thorsby, P.M.; Juliusson, P.B.; Bentsen, B.S.; Henriksen, C. Bone mineral density and vitamin D in paediatric intestinal failure patients receiving home parenteral nutrition. Clin. Nutr. ESPEN 2020, 39, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.A.; Lambe, C.; Talbotec, C.; Acramel, A.; Pigneur, B.; Goulet, O. Metabolic bone disease in children with in-testinal failure is not associated with the level of parenteral nutrition dependency. Clin. Nutr. 2021, 40, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Pichler, J.; Chomtho, S.; Fewtrell, M.; Macdonald, S.; Hill, S. Body composition in paediatric intestinal failure patients receiving long-term parenteral nutrition. Arch. Dis. Child. 2014, 99, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Louazon, T.; Poinsot, P.; Restier, L.; Belmalih, A.; Loras-Duclaux, I.; Marotte, S.; Heissat, S.; Barnoud, D.; Chambrier, C.; Confavreux, C.B.; et al. A prospective case-control pilot study to evaluate bone microarchitecture in children and teenagers on long-term parenteral nu-trition using HR-pQCT. Sci. Rep. 2021, 11, 9151. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; El-Hajj, F.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M.; International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Aghdassi, E.; Baun, M.; Yeung, M.; Fairholm, L.; Saqui, O.; Allard, J.P. Metabolic Bone Disease in Patients Receiving Home Parenteral Nutrition: A Canadian Study and Review. J. Parenter. Enteral. Nutr. 2006, 30, 492–496. [Google Scholar] [CrossRef]
- Nygaard, A.; Skallerup, A.; Olesen, S.S.; Køhler, M.; Vinter-Jensen, L.; Kruse, C.; Vestergaard, P.; Højgaard Rasmussen, H. Osteoporosis in patients with intestinal insufficiency and intestinal failure: Prevalence and clinical risk factors. Clin. Nutr. 2018, 37, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Colomb, V.; Dabbas, M.; Goulet, O.; Talbotec, C.; Corriol, O.; Ricour, C. Prepubertal growth in children with long-term parenteral nutrition. Horm. Res. 2002, 58, 2–6. [Google Scholar] [CrossRef]
- Ferrone, M.; Geraci, M.A. Review of the Relationship Between Parenteral Nutrition and Metabolic Bone Disease. Nutr. Clin. Pract. 2007, 22, 329. [Google Scholar] [CrossRef]
- Shike, M.; Sturtridge, W.C.; Tam, C.S.; Harrison, J.E.; Jones, G.; Murray, T.M.; Husdan, H.; Whitwell, J.; Wilson, D.R.; Jeejeebhoy, K.N. A possible role of vitamin D in the genesis of parenteral-nutrition-induced metabolic bone disease. Ann. Intern. Med. 1981, 95, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Verhage, A.H.; Cheong, W.K.; Allard, J.P.; Jeejeebhoy, K.N. Harry M. Vars Research Award: Increase in lumbar spine bone mineral content in patients on long-term parenteral nutrition without vitamin D supplementation. J. Parenter. Enteral. Nutr. 1995, 19, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Hill., S.; Ksiazyk, J.; Prell, C.; Tabbers, M.; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Home parenteral nutrition. Clin. Nutr. 2018, 37, 2401–2408. [Google Scholar] [CrossRef] [Green Version]
- Pastore, S.; Londero, M.; Barbieri, F.; Di Leo, G.; Paparazzo, R.; Ventura, A. Treatment with pamidronate for osteoporosis complicating long-term intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 615–618. [Google Scholar] [CrossRef] [PubMed]
| Cannon RA, 1980 [16] | Larchet M, 1991 [17] | Leonberg BL, 1998 [18] | Dellert SF, 1998 [19] | Diamanti A, 2010 [20] | Olieman JF, 2012 [21] | Ubesie AC, 2013 [22] | Mutanen A, 2013 [23] | Appleman SS, 2013 [24] | Pichler J, 2014 [35] | Derepas C, 2015 [26] | Demehri FR, 2015 [27] | Khan FA, 2015 [28] | Wozniak LJ, 2015 [29] | Neelis E, 2018 [30] | Poinsot P, 2018 [31] | Olszweska K, 2018 [32] | Kvammen JA, 2020 [33] | Nader EA, 2021 [34] | Louazon T, 2021 [36] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relevance questions | ||||||||||||||||||||
| 1. Would implementing the studied intervention or procedure (if found successful) result in improved outcomes for the patients/clients/population group? (NA for some Epi studies) | N/A | N/A | N/A | N/A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Did the authors study an outcome (dependent variable) or topic that the patients/clients/population group would care about? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Is the focus of the intervention or procedure (independent variable) or topic of study a common issue of concern to dietetics practice? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is the intervention or procedure feasible? (NA for some epidemiological studies) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Validity questions | ||||||||||||||||||||
| 1. Was the research question clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the selection of study subjects/patients free from bias? | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Were study groups comparable? | N/A | N/A | N/A | Yes | Yes | N/A | N/A | Yes | Yes | Yes | Yes | N/A | N/A | Yes | Yes | Yes | N/A | Yes | Yes | Yes |
| 4. Was method of handling withdrawals described? | Yes | Yes | N/A | N/A | Yes | Yes | Yes | Yes | N/A | Yes | N/A | Yes | No | Yes | Yes | Yes | Yes | N/A | Yes | Yes |
| 5. Was blinding used to prevent introduction of bias? | No | No | No | Unclear | No | No | no | No | Yes | No | Yes | No | No | Yes | No | No | no | no | no | no |
| 6. Were intervention/therapeutic regimens/exposure factor or procedure and any comparison(s) described in detail? Were intervening factors described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Were outcomes clearly defined and the measurements valid and reliable? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the statistical analysis appropriate for the study design and type of outcome indicators? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Are conclusions supported by results with biases and limitations taken into consideration? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Is bias due to study’s funding or sponsorship unlikely? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| TOTAL SCORE | Neutral | Neutral | Neutral | Positive | Positive | Neutral | Neutral | Positive | Positive | Positive | Positive | Neutral | Neutral | Positive | Positive | Positive | Neutral | Positive | Positive | Positive |
| First Author, Year | Study Details (Study Type, Participants, Years, Country) | Intestinal Failure/pn- Dependence Definitions | Bone Disease Measurement and Definitions | Study Population (Number, Age, Sex, Length of pn) | Control Group or Reference Population | Prevalence of Bone Disease and/or Comparison with Control Group | Vitamin d Status | Follow-Up Results | Factors Associated with Bone Density or Metabolic Bone Disease |
|---|---|---|---|---|---|---|---|---|---|
| Cannon RA, 1980 [16] | Observational, single-center study (OSCS). Children on HTPN at August 1979 with a follow-up of 2 years. USA. | TPN begun within 2 months of age and still ongoing. | Development of pathologic fractures or loose teeth plus radiologic evidence of rickets were assessed. | 8 children, age 7–24 months, 5 males. Duration of TPN: 7–24 months. | No | 37.5% had bone disease. | Supplementation with vitamin D resulted in healing of bone lesions in one patient. | Not evaluated | Not reported |
| Larchet M, 1991 [17] | OSCS. Children on cyclic HPN at November 1983, with a follow-up of 4 years. France. | HPN for SBS. | Osteopenia assessed on wrist and tibia X-ray. Sserum Ca, P, ALP, PTH, and urinary Ca and P were annually measured. Bone biopsy. | 7 children, age 4–14 years, 4 males. Duration of PN at study start: 19–79 months. | No | 85.7% had signs of moderate osteopenia. The cortico-diaphyseal indices of the tibia were normal (>0.5). | Mean 25-(OH)D levels dropped from 23 ± 6 ng/mL (1983) to 5.5 ± 2 (1987), (p < 0.03). | No change on radiologic signs of osteopenia during the 4 years of the study. | Not reported |
| Leonberg BL, 1998 [18] | OSCS. Children with IF, weaned off PN in the previous 7 years (at least since 6 months) evaluated for a nutritional and bone disease screening. USA. | PN for more than 4 months, beginning in the 1st postnatal week, including 1 month of HPN. | BMC of the radius measured by single-photon absorptiometry, compared with age appropriate norms. | 9 children, age 2.8–6 years (mean, SD: 4.9 ± 1), 5 males. Duration of PN: 5–39 months (mean, SD: 14.6 ± 11.4). Duration of HPN: 8.5 ± 5 months. Mean time of PN cessation: 3.4 ±1.4 years | 18 healthy controls matched for age, gender, and race. | Total BMC of SBS children was reduced compared to controls but not different after correction for weight and height (p = 0.8). 44.4% had BMC below normal. | Median 25-(OH)D levels: 26 ng/mL. | Not evaluated | 75% subjects with low BMC were consuming diets deficient in calcium. No correlation of BMC with PN duration or time since discontinuation. |
| Dellert SF, 1998 [19] | OSCS, case-control. Children with SBS, totally weaned from PN and with a bone disease screening (1984–1994). USA. | SBS (intestinal resection, omphalocele, gastroschisis) receiving at least 1 month of PN. | BMC measured by DXA in subjects and controls. Serum Ca and P levels were measured. | 18 children, age 2–8 years, 9 males. Duration of PN: 1–67 months (median 7 months). Discontinuation of PN: 1–8 years (median 3 years). | 36 healthy controls matched for age and gender. | BMC of cases was reduced compared to controls. After adjustment for weight and height, there was no difference in BMC between SBS and controls. Serum Ca e P were comparable. | Mean serum 25-(OH)D were lower in children with SBS respect to controls (26.0 ± 9.8 ng/mL vs. 41.3 ± 8.4 ng/mL, p = 0.002); mean serum 1,25-(OH)2D were similar. | Not evaluated | BMC was not correlated with PN duration or time since PN discontinuation. |
| Diamanti A, 2010 [20] | OSCS, case-control. Children with IF on a BMD evaluation program (September 2005-September 2007). Italy. | IF: condition needing PN providing at least 75% of total calories for >4 weeks or at least 50% calories for >3 months. | BMD (BMC, areal BMD, BMAD, BMD z-score) measured by DXA at baseline in patients and controls and after 1 year (in 9 patients). BMC reduction: BMD z-score <−1. Development of fractures was assessed. | 24 children, age 3.5–17.5 years (6.7 ± 5.2 years), 14 males. 7 off PN. Duration of HPN: 3–181 months (median 38). | 24 healthy controls matched for age and gender. Mean age: 6.5 ± 3.9 years, 18 males. | 83% had BMD z-score ≤ −1. BMD z-score, BMD and BMAD were significantly lower in patients compared to controls (p < 0.05 and p < 0.01). Subjects with BMD z-score >−1 were lower in patients (17%) compared to controls (50%). 17% developed fractures. | Not evaluated. | All DXA variables were increased at the 2nd DXA in 7/9 patients at 1 year follow-up. | No correlation between bone mineral status and PN duration and nutrient intake. Significant correlation between BMC and BMD and weight and height. BMD z-scores were higher in SBS-IF compared to medical causes of IF. |
| Olieman JF, 2012 [21] | OSCS, cross-sectional. Children with infantile SBS (within 1 year) and off PN invited for a nutritional/bone disease screening (January 1975–January 2003). Netherland. | IF: 70% of resection of SB or PN needed for >42 days after resection or residual SB length <50 cm for preterm and <75 cm for term neonates | BMD LS, BMD TB, BMC, LBM, and %BF were measured by DXA and compared to Dutch reference data. | 40 subjects, 16 males, mean age 14.8 ± 6.8 years, including 31 children, mean age 11.8 ± 4.2 years. Duration of PN: median 110 days (43–2345) | Dutch reference population. | In children, mean BMC, LBM*, and BMD LS were lower than the reference values (p < 0.05). | Not evaluated. | Not evaluated | Not reported |
| Ubesie AC, 2013 [22] | OSCS, retrospective. IF patients aged ≥3 years under nutritional follow-up in a 5-year period (2007–2012). USA. | IF: need for PN support for more than 30 days. | Lumbar spine BMD (L1–L4) was measured by DXA (within 6 months from serum evaluation) and compared to reference values. BMD z-score were adjusted for age and height for “short” patients (height <5th percentile on CDC charts). Reduced BMD: BMD ≤ −2 z-score. | 123 IF patients, median age: 4 years (3–22 years), 71 (57.7) % males. PN duration not indicated. 80 had a DEXA scan. | Reference population (not specified). | 12.5% had a low BMD z-score (adjusted for height). | 40% had vitamin D deficiency (25-(OH)D < 20 ng/mL). | Not evaluated | PN dependence at bone health evaluation was associated with reduced BMD. Increased age was significantly associated with vitamin D deficiency (p = 0.04) and reduced BMD z-score (p = 0.01) at a binary logistic regression analysis. At a logistic regression model, age >10 years was associated only to reduced BMD (p = 0.02). |
| Mutanen A, 2013 [23] | OSCS, cross sectional. Children with IF invited to a bone disease screening (January 1984–August 2010). Finland. | IF: 50% resection of SB or duration of PN >30 days. | BMD in the left proximal femur and lumbar spine was measured by DXA, and compared with reference values. BMD z-score ≤ −1 was considered low. BMD z-scores were corrected for bone age. Vertebral compression >20% was considered abnormal. PTH levels were measured. Occurrence of fractures was assessed. | 41 IF children, 27 males, mean age 9.9 years (0.2–27). PN duration 41 months (0.7–250). 30 off PN, time after weaning off: 9 years (0.3–27). 30 had a DXA study. | Reference population (not specified). | BMD z-score was ≤−1 in 70% and ≤−2 in 43%. 3.8% had sustained fractures. 3.8% had vertebral compression. | 41% had vitamin D deficiency (25-(OH)D < 15 ng/mL), and 44% had secondary hyperparathyroidism. Mean 25-(OH)D levels: 21.6 ng/mL on PN; 23.6 ng/mL in patients off PN (p = 0.642). | Not evaluated | At the multiple regression analysis duration of EN after weaning PN, duration of PN and calcium supplementation were the significant predictors for a lower lumbar spine BMD z-score. |
| Appleman SS, 2013 [24] | Observational, case-control, double-center study. PN-dependent IF children invited to a bone disease screening. USA. | PN-dependent IF: inability to sustain growth without PN | BMC and BMD of the lumbar spine were measured by DXA. PTH was measured. | 20 IF children, median age 26 months (6–127), 9 males (45%). Median time on PN: 18.5 months (4–103) | 49 healthy controls median age 25 months (7–127), 22 males (45%). | Lumbar spine BMC was 15% lower (p = 0.0009), and BMD was 12% lower (p = 0.0035) in IF subjects compared to controls. No differences after adjusting for weight and height. | IF participants had higher serum 25(OH)D than controls (mean levels: 40 vs. 30 ng/mL, p = 0.0005). IF patients had lower prevalence (5% vs. 14%) of vitamin D insufficiency (25(OH)D < 20 ng/mL). | Not evaluated | No association between aluminum concentration and BMC or BMD. |
| Pichler J, 2014 [35] | OSCS. Children aged >5 years with IF and being on HPN (2002–2010). UK. | IF: HPN for at least 6 months. | BMD and BMAD were calculated by DXA + bone age assessed by X-ray. Low BMD or BMAD: ≤−2 SDS. Biochemical markers (also ALP) were measured. Occurrence of fractures was recorded. | 45 subjects, 24 males, age 7.7 years (5–18). Time of HPN: 5 years (3.2–12). 18 (40%) on TPN, 26 (58%) on PPN, 1 (2%) off PN. | UK reference data. | 42% had BMD -≤−2 SDS and 31% had BMAD ≤−2 SDS. Mean BMD SDS was −1.7 ± 1.6; mean BMAD SDS was −1.4 ± 1.5. 37% had a history of fractures (median 1.3), all non-pathological. | Mean 25(OH)D levels: 57.6 ± 6.44 ng/mL. 7% patients had vitamin D insufficiency (25(OH)D < 25 ng/mL). | 35/42 had repeated DXA at 1 and 2 years. BMD declined significantly at 1 and 2 years, while BMAD only at 1 year. In a multivariate model, the only significant predictor of a change in the BMD SDS was a change in the weight SDS. | No difference of BMD and BMAD SDS according to diagnosis, degree of dependence on PN, and presence of 25-(OH)D deficiency. In a multivariate model, age at the time of DXA was the only predictor of BMD SDS. |
| Derepas C, 2015 [26] | OSCS, case-control. IF children on HPN (June 2012–August 2012). Canada. | IF: need of PN providing at least 25% calories for more than 6 weeks. | BMD measured annually by DXA on the lumbar spine (L1–L4) and compared to reference values. Reduced BMD: BMD z-score ≤ −1. Serum OC, CTx, BSAP, and PTH levels were assessed. | 13 IF children, age 1.2–10.7 years, 12 males. Duration of PN: 0.5–12.5 years. 9 subjects had DXA (7 results available) | 20 healthy controls matched for age and gender. | Mean serum OC and CTx concentration were lower in IF compared to controls (p < 0.01 and p < 0.05). No differences in BSAP and PTH levels. BMD measured in 9/13 IF subjects ranged from 0.401 to 0.838 g/cm2. BMD z-score ranged from −3.526 to +1.5. 57% subjects had BMD z-score ≤ −1. 28.5% had BMD z-score ≤ −2. | Not evaluated | Not evaluated | A significant inverse relation was found between BMD and serum OC but no with length of PN, age, weight, or height z-score. BMD z scores was inversely related to CTx also after controling for weight z-scores. |
| Demehri FR, 2015 [27] | OSCS, retrospective. Patients with IF (2006–2012) who underwent DXA screening (at 6 years). USA | IF: PN dependence of at least 60 days. | BMD measured by DXA at the lumbar spine (L1–L4). BMD z-scores were derived from reference values. MBD was defined as BMD z-score ≤ −1. Serum Ca, P, and PTH measured within 4 weeks of DXA scan. History of pathologic fractures and bone pain. | 36 IF subjects, 21 males 25 off PN (69.4%). Mean PN duration 5.1 ± 5.4 years. 17 subjects had a repeated DXA. | Reference U.S. population. | Mean BMD z-score was −1.16 ± 1.32. 50% had MBD. 11.1% had pathologic fractures, and 16.6% had history of bone pain. | Mean 25(OH)D levels: at 1st DXA, 25.11 ± 13.05 ng/mL; at 2nd DXA, 23.67 ± 12.73 ng/mL (p = 0.821). 63.8% had vitamin D deficiency (25(OH)D ≤30 ng/mL), and 25% had hyperparathyroidism (>55 pg/mL). No change in serum 25-(OH)D (p = 0.821), despite an increase in % of subjects on vitamin D supplementation. | In 17/36 with repeated DXA, no change at the 2nd DXA (after 2 ± 1.1 years) occurred (p = 0.199). | Duration of PN and serum 25-(OH)D were predictors of BMD z-score at univariate analysis. In a multivariate analysis, the only significant predictor of a reduced BMD z-score was the length of PN (B = −0.132, p = 0.006). |
| Khan FA, 2015 [28] | OSCS, retrospective. Patients with IF who underwent DXA screening at 5 years of age (2004–2013). USA. | Not reported. | Whole-body DXA was performed, and BMD z-scores were determined using normative data. For patients with repeated DXA scans, the lowest BMD z-scores were recorded. MBD: BMD z-score ≤ −2. Serum levels Ca, P, and PTH were measured. History of fractures was recorded. | 65 IF subjects, 34 males. 39 off PN (60%). Mean PN duration 44.2 ± 43.2 months. | Reference U.S. population. | BMD z-score ≤ −2 was reported in 34%. 29% experienced at least 1 fracture. | Mean 25(OH)D levels: 27 ± 42 ng/mL. 42% had vitamin D deficiency (25(OH)D ≤30 ng/mL). | Not evaluated. | At univariate analysis, patients with BMD z-score ≤ −2 had lower WAZ (p = 0.01), lower Ca (p = 0.04), and higher PTH levels (p = 0.006). At the multivariable logistic regression analysis, WAZ (R = 1.8, p = 0.03) and serum Ca (R = 3.8, p = 0.02) were independent factors of a low BMD z-score. |
| Wozniak LJ, 2015 [29] | OSCS, retrospective. IF children under follow-up (January 2012–June 2012) who underwent at least 1 determination of serum 25-(OH)D between July 2010 and June 2012. USA. | IF: HPN required for at least 6 months. | Osteopenia evaluated on subjective analysis of standard radiology studies (bone mineralization of axial skeleton, long bones, and blurring of the cortical white line). History of fractures was assessed. | 27 IF children, median age 5.5 (IQR: 2.7–8.2 years), 12 (44%) males. Median PN duration: 3.5 years (IQR: 2.6–6.9). | No | 59% had osteopenia. 11% had fractures. | 41% had vitamin D insufficiency (25(OH)D = 20–29 ng/mL); 1 child had vitamin D deficiency (25(OH)D < 20 ng/mL). Supplementation with vitamin D resulted in improvement of 25-(OH)D levels (2/27) and in improvement at blood drawn following the study period (4/27). | Not evaluated. | At univariate and multivariate logistic regression analysis, the only variable associated with 25-(OH)D levels was diagnosis of SBS. Duration of PN was not correlated. |
| Neelis E, 2018 [30] | OSCS, retrospective. IF children (followed between 2000 and 2015) who underwent at least one DXA or DXR. The Netherlands. | IF: PN needed for >6 weeks. | Total-body and lumbar spine (L2, L2–4) BMD was measured by DXA after 4–5 years of age. BMD z-scores were determined by national reference values. BMD was adjusted to the bone size calculating the BMAD. Low BMD or BMAD: z-score ≤ −2. DXR was used to calculate the BHI that was compared to a reference population. History of fractures was recorded. | 46 IF subjects, 20 (44%) males. Age at 1st DXA: 6 years (IQR: 5.5–9.9). 28 off PN (76%). Median PN duration: 9.4 months (IQR: 4.6–14.3). 37 had 1st DXA. 13 children had multiple DXA scans after a median time of 2.1 years (1.09–2.44). | Reference Dutch population. | 24.3% had a low BMD (either BMD TB, LS or BMAD z-score ≤ −2) at 1st DXA. Median BMD TB, BMD LS, and BMAD z-scores were significantly lower compared to the reference population (p = 0.006; p < 0.001 and p = 0.004). 50% had low BHI (z score ≤ −2) at 1st radiography. 4/46 children developed multiple fractures (100% were PN dependent at time of 1st fracture). | 33% had vitamin D insufficiency (25(OH)D < 20 ng/mL); no differences between children on PN or weaned off. | Median change in z-scores per year was +0.16 SD for BMD TB and +0.09 for BMD LS. | Patients still on PN at 1st DXA had lower median BMD TB z-score (p = 0.048), and the proportion of children with BMD TB z-scores ≤ −2 was higher (p = 0.008). At univariate analysis, an older age, a lower HFA z-score, a HFA z-score ≤ −2, a higher WFH z-score, and a longer PN duration were related to lower BMD LS z-scores. In a multivariate model older age, longer duration of PN and surgical IF were related to lower z-scores. |
| Poinsot P, 2018 [31] | OSCS, retrospective. IF children (January 2004–January 2014) on HPN with at least 2 DXA (on HPN since at least 2 years from last DXA). France. | IF: children on HPN for at least 6 months. | TBMC, LTM, and FM were measured by DXA and adjusted for ideal WFH. LBM** was defined as a TBMC z-score ≤ −2, following the reference values. Serum Ca, P, and ALP were measured within 3 months of DXA assessment. | 31 children, 14 males (45%). Median age at 1st DXA: median age: 2.9 years (0.4–13.3). Median PN duration: 2.7 years (0.1–12.7). Median age at last DXA 11.4 years (3.7–19.7), and median PN duration: 9.2 years (2.9–19.6). Median time between 1st and last DXA: 6.2 years (0.7–16.6). | Reference population composed of 68 healthy children (2–24.9 years, 31 males) and 55 newborns (gestational age 33–40 weeks). | 45% had LBM** at 1st DXA. Median TBMC z-score at 1st DXA was −1.9 SD (−5.3–2.6). 71% had TBMC z-score for ideal WFH ≤−1. | Median 25(OH)D levels: at 1st DXA, 15.4 ng/mL; at last DXA, 14.0 ng/mL. | TBMC z-score adjusted for ideal WFH increased significantly at last DXA (+0.1 ± 0.04 per year, p = 0.012). At last DXA, 29% had LBM (p = 0.197 with 1st DXA). | IF etiology (CIPO, HD, and CE) and a lower plasmatic creatinine level were related to LBM** prevalence at baseline. At last DXA, LBM** was associated with a shorter PN duration (p = 0.045) and a younger age (p = 0.023). The risk of the LBM** decreased significantly with the duration of HPN (OR 0.9 per year of PN, p = 0.018). |
| Olszewska K, 2018 [32] | OSCS. Patients with ultra-short bowel syndrome on HPN under nutritional follow-up. Poland. | IF: residual small bowel <10 cm at the time of resection (first 2 months of life) and receiving PN for at least 6 months. | Antero-posterior spinal and total bone mass density measured by DXA, and BMD expressed as z- scores. Decreased BMD TB: ≤−1 z-score. Abnormal bone mineralization: BMDs ≤−1 z-score. | 17 children, age 0.8–14.2 years (median 6.6), 9 males on HPN. Median PN duration: 6.6 years (0.8–14.2 years). 8 subjects had DXA. | Reference population (not specified). | 50% had BMD ts ≤ −1 z-score, and 87.5% had BMD s ≤ −1 z-score. | Mean 25(OH)D levels: 20.1 ng/mL. 53% had vitamin D deficiency (25(OH)D < 20 ng/mL). | Not evaluated. | BMD z-scores were not correlated with body mass or body height SDS, age or PN length. |
| Kvammen JA, 2020 [33] | OSCS, cross sectional, controlled. Children with IF on HPN (March 2017–September 2017) who underwent a bone disease screening. Norway. | IF: children dependent on HPN for >6 months. | BMD was measured by DXA total body (TB) and spinal (LS at L2–L4). BMD z-scores were calculated according to reference values and corrected for bone age (if height was <−2 z-scores). Suboptimal BMD: BMD ≤−1 to −1.99 z-score. Low BMD: ≤−2 z-score. | 19 IF children, mean age: 10.1 years, SD: 3.5, 13 males (68%). 100% on HPN, 4 on TPN. 12 IF children had DXA. | Reference population composed of 50 healthy controls, mean age: 10 years, SD: 3.6, 18 males (36%). 46 controls had DXA. | IF group had significantly lower median BMD z-score for total body (p < 0.001) and lumbar spine (p = 0.01). 25% IF subjects had suboptimal BMD TB, 17% had suboptimal BMD LS (vs. 5% of healthy controls), and 25% of IF children had low BMD. | IF group had lower 1,25-(OH)2D levels compared to controls (29.6 vs. 57.5 pg/mL, p < 0.001). IF patients and healthy had similar level of 25(OH)D (28.4 vs. 32.4 ng/mL, p = 0.29). | Not evaluated | Not reported |
| Nader EA, 2021 [34] | OSCS, retrospective. IF children >5 years on HPN, who underwent a DXA screening (January 2016–December 2018). France. | IF: HPN for at least 2 years | BMD of the lumbar spine (L1–L4), left femur, and total body were measured using DXA and expressed as z-scores (compared to reference values). BMD values were adjusted to height or statural age. Low BMD: ≤−2 z-score. | 40 IF children, 24 males, median age 12.4 ± 4.5 years. HPN duration 12.4 years ± 4.4. | Reference population (not specified). | BMD LS ≤−2 z-score: 30% BMD LS adjusted ≤−2 z-score: 18% LF BMD ≤−2 z-score: 15% LF BMD adjusted ≤−2 z-score: 15% BMD TB ≤−2 z-score: 23% BMD TB adjusted ≤−2 z-score: 18% | Mean 25(OH)D levels: 26.5 ± 9.1 ng/mL; Median 25(OH)D levels: 25 ng/mL. | Not evaluated | No correlation between indication of PN, duration, and degree of PN dependency and level of 25-(OH)D3. |
| Louazon T, 2021 [36] | Cross-sectional, case-control, single-center study. IF children, aged >9 years, on HPN who underwent a bone assessment (March 2014–June 2015). France | IF: HPN for at least 2 years | vBMD was evaluated by HR-pQCT at the non dominant limb. BMC and BMD were measured by TB and spine (L1–L4) DXA. Serum NMID osteocalcin and PTH were measured. | 11 IF children, 8 males, median age: 16 years (9–19). Median PN duration: 10.3 years (6.4–18.3). 2 off PN. | 20 healthy controls, 16 males, median age: 16 years (10–17). | In IF children, increased PTH (p = 0.003) and reduced OC-diasorin (p = 0.005). At the tibia, IF children had low trabecular area compared to controls (p = 0.003). BMD TB was lower in IF subjects (p = 0.039). 18% of IF patients had BMC TB ≤−2 DS. | No differences in mean 25-(OH)D3 levels between patients and controls (17.6 vs. 22.8 ng/mL, p = NS). | Not evaluated | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, S.; Quattrini, S.; Palpacelli, A.; Catassi, G.N.; Lionetti, M.E.; Catassi, C. Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review. Nutrients 2022, 14, 995. https://doi.org/10.3390/nu14050995
Gatti S, Quattrini S, Palpacelli A, Catassi GN, Lionetti ME, Catassi C. Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review. Nutrients. 2022; 14(5):995. https://doi.org/10.3390/nu14050995
Chicago/Turabian StyleGatti, Simona, Sara Quattrini, Alessandra Palpacelli, Giulia N. Catassi, Maria Elena Lionetti, and Carlo Catassi. 2022. "Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review" Nutrients 14, no. 5: 995. https://doi.org/10.3390/nu14050995
APA StyleGatti, S., Quattrini, S., Palpacelli, A., Catassi, G. N., Lionetti, M. E., & Catassi, C. (2022). Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review. Nutrients, 14(5), 995. https://doi.org/10.3390/nu14050995







