An Evaluation of Probability of Adequate Nutrient Intake (PANDiet) Scores as a Diet Quality Metric in Irish National Food Consumption Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Food Consumption Data
2.3. Anthropometric Measurements and Socio-Demographic Factors
2.4. Total Estimated Energy Calculations
2.5. Biofluid Data Collection and Analyses
2.6. PANDiet Score Calculation
2.7. PANDiet Score Validation
2.8. Statistical Analyses
3. Results
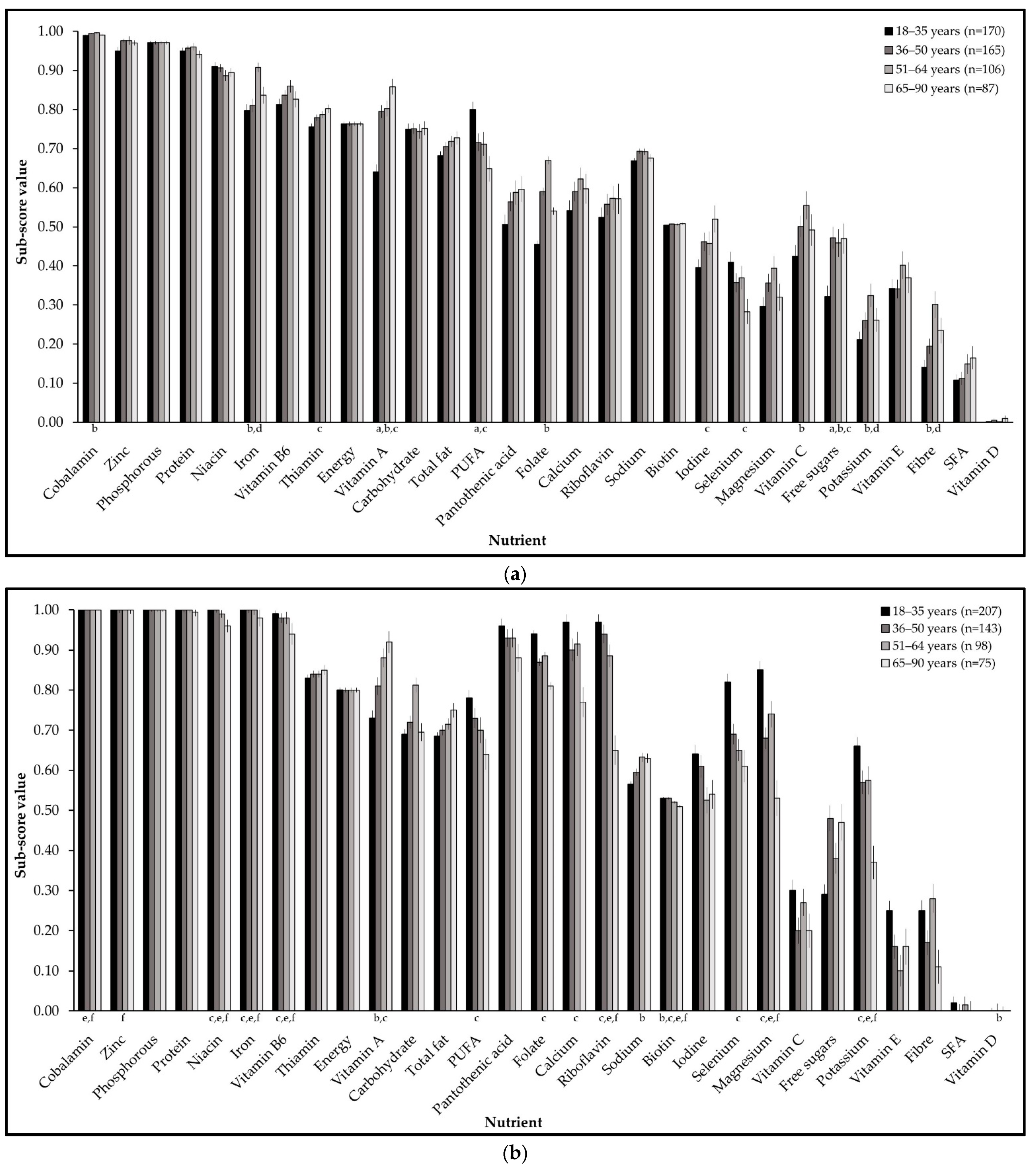
3.1. PANDiet Sub-Scores
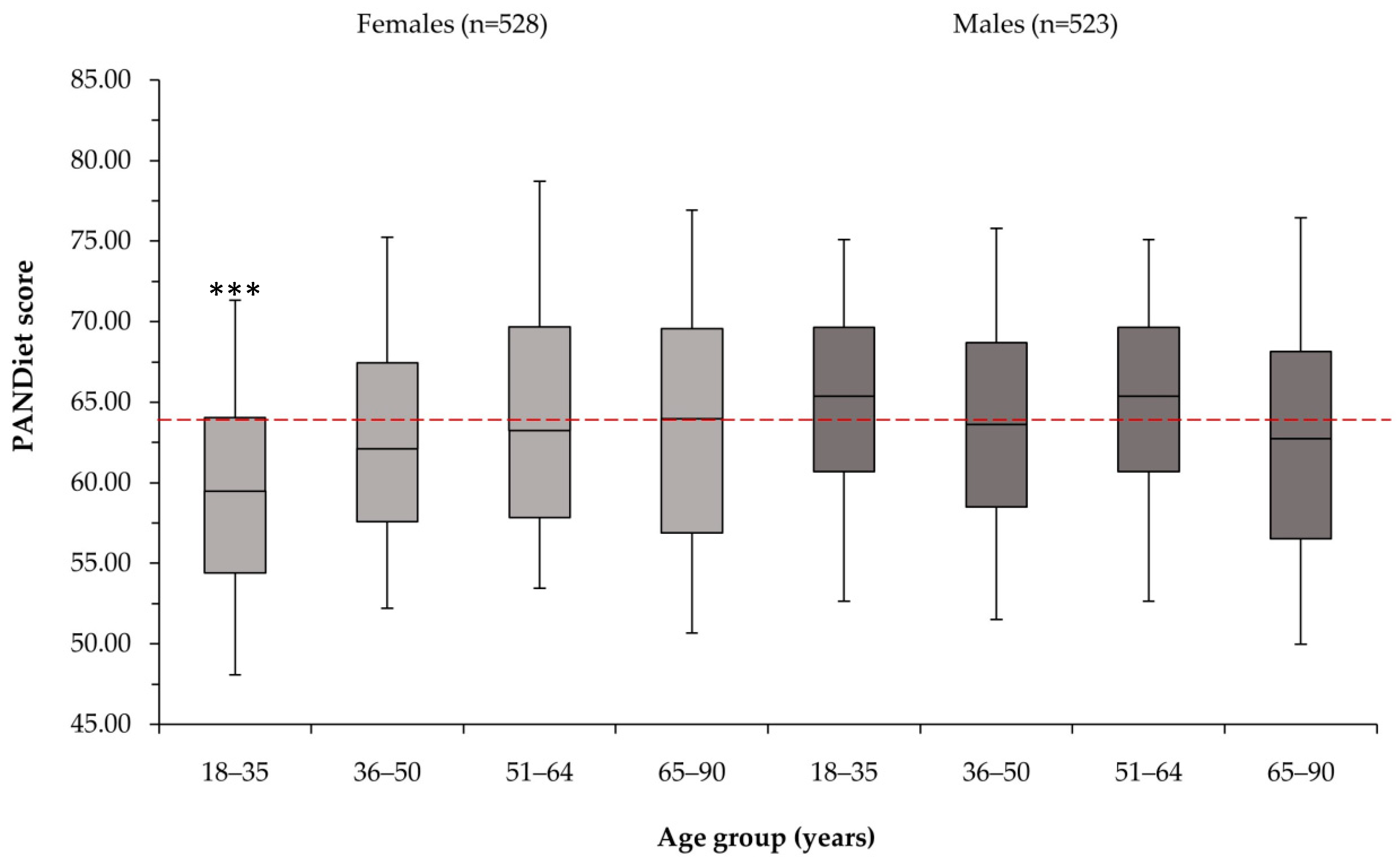
3.2. PANDiet Scores
3.3. Nutritional Adequacy, Demographic Factors, and Nutrition Biomarkers
3.4. Differences in Nutritional Adequacy by Gender and Age Group
3.5. Nutritional Adequacy and Food Group Intake at the Population Level
3.6. Food Group Intake Grouped by Age Group and Gender
3.7. Food Group Intake Grouped by Nutritional Adequacy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Females (n = 528) | Males (n = 523) | |
|---|---|---|
| Height (metres) | 1.62 ± 0.07 (1.43–1.81) | 1.76 ± 0.07 (1.55–1.99) |
| Weight (kg) | 67.8 ± 12.2 (42.8–129.0) | 82.6 ± 12.8 (53.1–144.0) |
| Basal energy expenditure (BEE) | 1365 ± 122 (1057–1894) | 1759 ± 165 (1356–2356) |
| Total energy expenditure (TEE) | 1879 ± 213 (1334–3115) | 2491 ± 266 (1791–3345) |
| Nutrient | CV a | Lower Limit a | Upper Limit b |
|---|---|---|---|
| Macronutrients | |||
| Total Energy (EI/TEE) | - | 0.95 | 1.05 |
| Protein (g/kg bw/day) | 12% | 0.66 | 2.20 |
| Carbohydrate (% EIEA) | 0% | 45 | 60 |
| Free Sugars (% EIEA) | 0% | - | 10 c |
| Dietary Fibre (g/day) | 10% | 25 | - |
| Total Fat (% EIEA) | 0% | 20 | 35 |
| Polyunsaturated Fatty Acids (% EIEA) | 12% | 5 | - |
| Saturated Fatty Acids (% EIEA) | 10% | - | 10 |
| Micronutrients | |||
| Vitamin A (µg RE/day) | 15% | 490–570 d | 3000 |
| Thiamin (mg/MJ) | 20% | 0.072 | - |
| Riboflavin (mg/day) | 10% | 1.6 | - |
| Niacin (mg NE/MJ) | 10% | 1.3 | 900 |
| Pantothenic acid (mg/day) | 10% | 5 e | - |
| Vitamin B6 (mg/day) | 10% | 1.3–1.5 d | 25 |
| Biotin (µg/day) | 10% | 40 e | - |
| Folate (µg DFE/day) | 15% | 250 | 1000 |
| Cobalamin (ug/day) | 10% | 4 e | - |
| Vitamin C (mg/day) | 10% | 80–90 d | 500 |
| Vitamin D (µg/day) | 10% | 15 e | 100 |
| Vitamin E (mg/day) | 10% | 11–13 d,e | 300 |
| Minerals | |||
| Calcium (mg/day) | 10% | 750–860 f | 2500 |
| Iodine (µg/day) | 20% | 150 e | 600 |
| Iron (mg/day) | 20% | 7–6 g | 28 |
| Magnesium (mg/day) | 10% | 300 e | 700 |
| Phosphorus (mg/day) | 10% | 550 e | 2500 |
| Potassium (mg/day) | 10% | 3500 e | - |
| Selenium (µg/day) | 10% | 70 e | 250 |
| Sodium (mg/day) | 10% | 2000 e | 2759 |
| Zinc (mg/day) | 10% | 12.7 | 25 |
| Energy (kcal)/TEE Difference | Sub-Score Assigned | n |
|---|---|---|
| <5% | 1 | 218 |
| 5–20% | 0.80 | 518 |
| 20–40% | 0.60 | 245 |
| 40–60% | 0.40 | 49 |
| 60–80% | 0.20 | 17 |
| >80% | 0 | 4 |
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; De Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- FAO. Sustainable Food Systems Concept and Framework; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Trijsburg, L.; Talsma, E.F.; De Vries, J.H.M.; Kennedy, G.; Kuijsten, A.; Brouwer, I.D. Diet quality indices for research in low- and middle-income countries: A systematic review. Nutr. Rev. 2019, 77, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Wirt, A.; Collins, C.E. Diet quality—What is it and does it matter? Public Health Nutr. 2009, 12, 2473–2492. [Google Scholar] [CrossRef]
- Lassale, C.; Gunter, M.J.; Romaguera, D.; Peelen, L.M.; Van der Schouw, Y.T.; Beulens, J.W.; Freisling, H.; Muller, D.C.; Ferrari, P.; Huybrechts, I.; et al. Diet Quality Scores and Prediction of All-Cause, Cardiovascular and Cancer Mortality in a Pan-European Cohort Study. PLoS ONE 2016, 11, e0159025. [Google Scholar] [CrossRef]
- Perignon, M.; Vieux, F.; Soler, L.-G.; Masset, G.; Darmon, N. Improving diet sustainability through evolution of food choices: Review of epidemiological studies on the environmental impact of diets. Nutr. Rev. 2017, 75, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Dalwood, P.; Marshall, S.; Burrows, T.L.; McIntosh, A.; Collins, C.E. Diet quality indices and their associations with health-related outcomes in children and adolescents: An updated systematic review. Nutr. J. 2020, 19, 118. [Google Scholar] [CrossRef]
- Mertens, E.; van’t Veer, P.; Hiddink, G.J.; Steijns, J.M.; Kuijsten, A. Operationalising the health aspects of sustainable diets: A review. Public Health Nutr. 2016, 20, 739–757. [Google Scholar] [CrossRef]
- de Kroon, M.L.; Eussen, S.R.; Holmes, B.A.; Harthoorn, L.F.; Warners, M.J.; Bredenoord, A.J.; van Rhijn, B.D.; van Doorn, M.; Vlieg-Boerstra, B.J. The Habitual Diet of Dutch Adult Patients with Eosinophilic Esophagitis Has Pro-Inflammatory Properties and Low Diet Quality Scores. Nutrients 2021, 13, 214. [Google Scholar] [CrossRef]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods; World Health Organization Nutrition and Food Safety: Geneva, Switzerland, 2021. [Google Scholar]
- Verger, E.O.; Mariotti, F.; Holmes, B.A.; Paineau, D.; Huneau, J.-F. Evaluation of a Diet Quality Index Based on the Probability of Adequate Nutrient Intake (PANDiet) Using National French and US Dietary Surveys. PLoS ONE 2012, 7, e42155. [Google Scholar] [CrossRef]
- Wibisono, C.; Probst, Y.; Neale, E.; Tapsell, L. Changes in diet quality during a 12 month weight loss randomised controlled trial. BMC Nutr. 2017, 3, 38. [Google Scholar] [CrossRef][Green Version]
- Seconda, L.; Baudry, J.; Allès, B.; Touvier, M.; Hercberg, S.; Pointereau, P.; Lairon, D.; Kesse-Guyot, E. Prospective associations between sustainable dietary pattern assessed with the Sustainable Diet Index (SDI) and risk of cancer and cardiovascular diseases in the French NutriNet-Santé cohort. Eur. J. Epidemiol. 2020, 35, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Rabès, A.; Seconda, L.; Langevin, B.; Allès, B.; Touvier, M.; Hercberg, S.; Lairon, D.; Baudry, J.; Pointereau, P.; Kesse-Guyot, E. Greenhouse gas emissions, energy demand and land use associated with omnivorous, pesco-vegetarian, vegetarian, and vegan diets accounting for farming practices. Sustain. Prod. Consum. 2020, 22, 138–146. [Google Scholar] [CrossRef]
- Seconda, L.; Baudry, J.; Pointereau, P.; Lacour, C.; Langevin, B.; Hercberg, S.; Lairon, D.; Allès, B.; Kesse-Guyot, E. Development and validation of an individual sustainable diet index in the NutriNet-Sante study cohort. Br. J. Nutr. 2019, 121, 1166–1177. [Google Scholar] [CrossRef]
- Seconda, L.; Fouillet, H.; Huneau, J.F.; Pointereau, P.; Baudry, J.; Langevin, B.; Lairon, D.; Allès, B.; Touvier, M.; Hercberg, S.; et al. Conservative to disruptive diets for optimizing nutrition, environmental impacts and cost in French adults from the NutriNet-Santé cohort. Nat. Food 2021, 2, 174–182. [Google Scholar] [CrossRef]
- Seconda, L.; Baudry, J.; Allès, B.; Boizot-Szantai, C.; Soler, L.-G.; Galan, P.; Hercberg, S.; Langevin, B.; Lairon, D.; Pointereau, P.; et al. Comparing nutritional, economic, and environmental performances of diets according to their levels of greenhouse gas emissions. Clim. Chang. 2018, 148, 155–172. [Google Scholar] [CrossRef]
- Salomé, M.; De Gavelle, E.; Dufour, A.; Dubuisson, C.; Volatier, J.-L.; Fouillet, H.; Huneau, J.-F.; Mariotti, F. Plant-Protein Diversity Is Critical to Ensuring the Nutritional Adequacy of Diets When Replacing Animal with Plant Protein: Observed and Modeled Diets of French Adults (INCA3). J. Nutr. 2019, 150, 536–545. [Google Scholar] [CrossRef]
- Salomé, M.; Arrazat, L.; Wang, J.; Dufour, A.; Dubuisson, C.; Volatier, J.-L.; Huneau, J.-F.; Mariotti, F. Contrary to ultra-processed foods, the consumption of unprocessed or minimally processed foods is associated with favorable patterns of protein intake, diet quality and lower cardiometabolic risk in French adults (INCA3). Eur. J. Nutr. 2021, 60, 4055–4067. [Google Scholar] [CrossRef]
- Kadawathagedara, M.; Ahluwalia, N.; Dufourg, M.; Forhan, A.; Charles, M.A.; Lioret, S.; de Lauzon-Guillain, B. Diet during pregnancy: Influence of social characteristics and migration in the ELFE cohort. Matern. Child Nutr. 2021, 17, e13140. [Google Scholar] [CrossRef]
- Masset, G.; Vieux, F.; Verger, E.O.; Soler, L.-G.; Touazi, D.; Darmon, N. Reducing energy intake and energy density for a sustainable diet: A study based on self-selected diets in French adults. Am. J. Clin. Nutr. 2014, 99, 1460–1469. [Google Scholar] [CrossRef]
- Schoen, S.; Jergens, S.; Barbaresko, J.; Nöthlings, U.; Kersting, M.; Remer, T.; Stelmach-Mardas, M.; Ziegler, A.-G.; Hummel, S. Diet Quality during Infancy and Early Childhood in Children with and without Risk of Type 1 Diabetes: A DEDIPAC Study. Nutrients 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Mistura, L.; D’Addezio, L.; Sette, S.; Piccinelli, R.; Turrini, A. Diet quality of Italian yogurt consumers: An application of the probability of adequate nutrient intake score (PANDiet). Int. J. Food Sci. Nutr. 2016, 67, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, M.; Zarrin, R.; Ayremlou, P. Adaptation and validity assessment of a diet quality index for patients with type 2 diabetes. J. Diabetes Metab. Disord. 2020, 19, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Verger, E.O.; Eussen, S.R.B.M.; Holmes, B.A. Evaluation of a nutrient-based diet quality index in UK young children and investigation into the diet quality of consumers of formula and infant foods. Public Health Nutr. 2016, 19, 1785–1794. [Google Scholar] [CrossRef]
- Bianchi, C.M.; Mariotti, F.; Lluch, A.; Journet, C.; Stehr, Y.; Beaussier, H.; Fournier, J.; Dervaux, S.; Cohen-Tanuggi, D.; Reulet, E.; et al. Computer-based tailored dietary counselling improves the nutrient adequacy of the diet of French pregnant women: A randomised controlled trial. Br. J. Nutr. 2020, 123, 220–231. [Google Scholar] [CrossRef]
- Verger, E.O.; Aron-Wisnewsky, J.; Dao, M.C.; Kayser, B.D.; Oppert, J.-M.; Bouillot, J.-L.; Torcivia, A.; Clément, K. Micronutrient and Protein Deficiencies After Gastric Bypass and Sleeve Gastrectomy: A 1-year Follow-Up. Obes. Surg. 2016, 26, 785–796. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Verger, E.; Bounaix, C.; Dao, M.C.; Oppert, J.-M.; Bouillot, J.-L.; Chevallier, J.-M.; Clément, K. Nutritional and Protein Deficiencies in the Short Term following Both Gastric Bypass and Gastric Banding. PLoS ONE 2016, 11, e0149588. [Google Scholar] [CrossRef]
- Leclercq, C.; Allemand, P.; Balcerzak, A.; Branca, F.; Sousa, R.F.; Lartey, A.; Lipp, M.; Quadros, V.P.; Verger, P. FAO/WHO GIFT (Global Individual Food consumption data Tool): A global repository for harmonised individual quantitative food consumption studies. Proc. Nutr. Soc. 2019, 78, 484–495. [Google Scholar] [CrossRef]
- Ioannidou, S.; Horváth, Z.; Arcella, D. Harmonised collection of national food consumption data in Europe. Food Policy 2020, 96, 101908. [Google Scholar] [CrossRef]
- EFSA. Creation of Open Access EU Food Composition Database (EU FCDB) and Related Datasets GP/EFSA/DATA/2021/02; D01.01-DATA-29; EFSA: Parma, Italy, 2021. [Google Scholar]
- Lenighan, Y.M.; Nugent, A.P.; Li, K.F.; Brennan, L.; Walton, J.; Flynn, A.; Roche, H.M.; McNulty, B.A. Processed red meat contribution to dietary patterns and the associated cardio-metabolic outcomes. Br. J. Nutr. 2017, 118, 222–228. [Google Scholar] [CrossRef]
- Feeney, E.; O’Sullivan, A.; Nugent, A.; McNulty, B.; Walton, J.; Flynn, A.; Gibney, E.R. Patterns of dairy food intake, body composition and markers of metabolic health in Ireland: Results from the National Adult Nutrition Survey. Nutr. Diabetes 2017, 7, e243. [Google Scholar] [CrossRef] [PubMed]
- Prendiville, O.; Walton, J.; Flynn, A.; Nugent, A.P.; McNulty, B.A.; Brennan, L. Classifying Individuals into a Dietary Pattern Based on Metabolomic Data. Mol. Nutr. Food Res. 2021, 65, 2001183. [Google Scholar] [CrossRef]
- Kiely, M.E.; McCarthy, E.K.; Hennessy, Á. Iron, iodine and vitamin D deficiencies during pregnancy: Epidemiology, risk factors and developmental impacts. Proc. Nutr. Soc. 2021, 80, 290–302. [Google Scholar] [CrossRef] [PubMed]
- McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Tlustos, C.; Gibney, M.J. Iodine intakes and status in Irish adults: Is there cause for concern? Br. J. Nutr. 2017, 117, 422–431. [Google Scholar] [CrossRef]
- CSO. Central Statistics Office Summary Report 2007; CSO: Dublin, Ireland, 2007. [Google Scholar]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. S1), 5–41. [Google Scholar] [PubMed]
- IUNA. National Adult Nutrition Survey (NANS) Summary Report on Food and Nutrient Intakes, Physical Measurements, Physical Activity Patterns and Food Choice Motives; Irish Universities Nutrition Alliance: Dublin, Ireland, 2011. [Google Scholar]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int. J. Epidemiol. 2002, 31, 168–174. [Google Scholar] [CrossRef]
- Gerrior, S.; Juan, W.; Peter, B. An Easy Approach to Calculating Estimated Energy Requirements. Prev. Chronic Dis. 2006, 3, A129. [Google Scholar]
- Perry, I.B.; Loughrey, G.; Harrington, M.; Lutonski, J.; Fitzgerald, J.; Dietary, A. Salt Intake and Related Risk Factors in the Irish Population. A Report for Safefood Ireland; Safefood Ireland: Cork, Ireland, 2010. [Google Scholar]
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A.P.; McNulty, B.A.; Flynn, A.; Walton, J. Sodium and Potassium Intakes and Their Ratio in Adults (18–90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12, 938. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kinsella, M.; McNulty, B.A.; Walton, J.; Gibney, M.J.; Flynn, A.; Kiely, M. Dietary vitamin D2—A potentially underestimated contributor to vitamin D nutritional status of adults? Br. J. Nutr. 2014, 112, 193–202. [Google Scholar] [CrossRef]
- Kehoe, L.; Walton, J.; Hopkins, S.; McNulty, B.; Nugent, A.; McNulty, H.; Ward, M.; Flynn, A. Intake, status and dietary sources of riboflavin in a representative sample of Irish adults aged 18–90 years. Proc. Nutr. Soc. 2018, 77, E66. [Google Scholar] [CrossRef]
- IOM. Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients: Summary Report; EFSA Supporting Publication: Parma, Italy, 2017; Volume 14, p. e15121E. [Google Scholar]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Dietary Reference Values for Sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [PubMed]
- EFSA. Summary of Tolerable Upper Intake Levels—Version 4. Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies; Authority EFS: Parma, Italy, 2018. [Google Scholar]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar] [PubMed]
- FSAI. Updated Scientific Recommendations for Food-Based Dietary Guidelines for Older Adults Published; Food Safety Authority of Ireland: Dublin, Ireland, 2021. [Google Scholar]
- FSAI. Scientific Recommendations for Food-Based Dietary Guidelines for 1 to 5 Year-Olds in Ireland; Scientific Committee of the Food Safety Authority of Ireland: Dublin, Ireland, 2020. [Google Scholar]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- FSAI. Food Safety Authority of Ireland Total Diet Study; Food Safety Authority of Ireland: Dublin, Ireland, 2016. [Google Scholar]
- Sun, H.; Weaver, C.M. Decreased Iron Intake Parallels Rising Iron Deficiency Anemia and Related Mortality Rates in the US Population. J. Nutr. 2021, 151, 1947–1955. [Google Scholar] [CrossRef]
- van Vliet, S.; Bain, J.R.; Muehlbauer, M.J.; Provenza, F.D.; Kronberg, S.L.; Pieper, C.F.; Huffman, K.M. A metabolomics comparison of plant-based meat and grass-fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci. Rep. 2021, 11, 13828. [Google Scholar] [CrossRef]
- Lopes, C.T.; Oliveira, D.; Severo, A.; Guiomar, M.; Alarcão, S.; Vilela, V.; Ramos, S.; Rodrigues, E.; Oliveira, S.; Nicola, L.; et al. National Food, Nutrition and Physical Activity Survey of the Portuguese General Population; EFSA Supporting Publication: Parma, Italy, 2017; Volume 37, p. 1341E. [Google Scholar]
- McCourt, A.; McNulty, B.A.; Walton, J.; O’Sullivan, A. Efficacy and safety of food fortification to improve vitamin D intakes of older adults. Nutrition 2020, 75, 110767. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Nic Suibhne, T.; Cox, G.; Healy, M.; O’Morain, C. High prevalence of vitamin D insufficiency in healthy Irish adults. Ir. J. Med. Sci. 2008, 177, 131–134. [Google Scholar] [CrossRef]
- Zhao, Y.; Monahan, F.J.; McNulty, B.A.; Gibney, M.J.; Gibney, E.R. Effect of vitamin E intake from food and supplement sources on plasma α- and γ-tocopherol concentrations in a healthy Irish adult population. Br. J. Nutr. 2014, 112, 1575–1585. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef]
- IUNA. National Children Food Survey (NCFS) II Main Survey Report; Irish Universities Nutrition Alliance: Dublin, Ireland, 2019. [Google Scholar]
- IUNA. National Teens’ Food Survey II Summary Report; Irish Universities Nutrition Alliance: Dublin, Ireland, 2021; Available online: www.iuna.net (accessed on 26 January 2022).
- Sundin, N.; Rosell, M.; Eriksson, M.; Jensen, C.; Bianchi, M. The climate impact of excess food intake—An avoidable environmental burden. Resour. Conserv. Recycl. 2021, 174, 105777. [Google Scholar] [CrossRef]
- EUPHA. Healthy and Sustainable Diets for European Countries; The European Public Health Association: Utrecht, The Netherlands, 2017. [Google Scholar]
- European Food Safety Authority (EFSA). Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar]
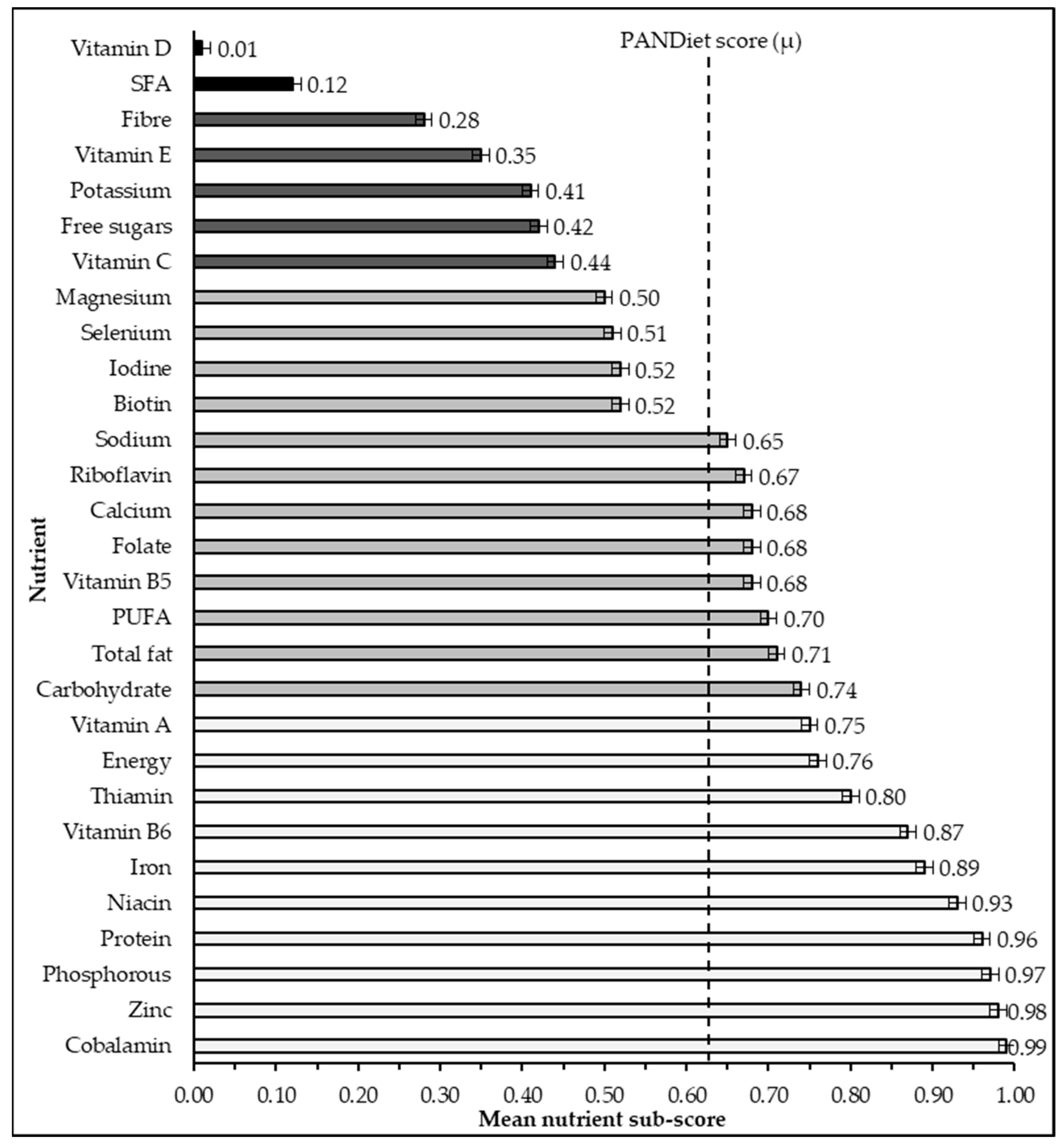

| Nutrient Sub-Score | Mean Score | ±SEM | Q1 | Q2 | Q3 | Q4 | Rs | p |
|---|---|---|---|---|---|---|---|---|
| Energy | 0.76 | 0.01 | 0.60 | 0.80 | 0.80 | 1.00 | 0.23 | <0.001 |
| Carbohydrate | 0.74 | 0.01 | 0.54 | 0.74 | 0.93 | 1.00 | 0.29 | <0.001 |
| Fibre | 0.28 | 0.01 | 0.02 | 0.12 | 0.48 | 1.00 | 0.62 | <0.001 |
| Free sugars | 0.42 | 0.01 | 0.04 | 0.37 | 0.79 | 1.00 | 0.23 | <0.001 |
| Fat | 0.71 | 0.00 | 0.99 | 1.00 | 1.00 | 1.00 | 0.50 | <0.001 |
| PUFA | 0.70 | 0.01 | 0.46 | 0.80 | 0.98 | 1.00 | −0.06 | <0.001 |
| SFA | 0.12 | 0.01 | 0.00 | 0.01 | 0.11 | 1.00 | 0.43 | 0.05 |
| Protein | 0.96 | 0.00 | 0.98 | 1.00 | 1.00 | 1.00 | 0.40 | <0.001 |
| Vitamin A | 0.75 | 0.01 | 0.56 | 0.82 | 0.97 | 1.00 | 0.31 | <0.001 |
| Thiamin | 0.80 | 0.00 | 0.72 | 0.80 | 0.88 | 1.00 | 0.57 | <0.001 |
| Riboflavin | 0.67 | 0.01 | 0.38 | 0.79 | 0.98 | 1.00 | 0.55 | <0.001 |
| Niacin | 0.93 | 0.00 | 0.91 | 0.98 | 1.00 | 1.00 | 0.40 | <0.001 |
| Pantothenic acid | 0.68 | 0.01 | 0.42 | 0.77 | 0.97 | 1.00 | 0.52 | <0.001 |
| Vitamin B6 | 0.87 | 0.01 | 0.81 | 0.95 | 1.00 | 1.00 | 0.56 | <0.001 |
| Biotin | 0.52 | 0.00 | 0.49 | 0.51 | 0.54 | 0.67 | 0.55 | <0.001 |
| Folate | 0.68 | 0.01 | 0.44 | 0.76 | 0.98 | 1.00 | 0.61 | <0.001 |
| Cobalamin | 0.99 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.49 | <0.001 |
| Vitamin C | 0.44 | 0.01 | 0.08 | 0.35 | 0.84 | 1.00 | 0.45 | <0.001 |
| Vitamin D | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.99 | 0.10 | <0.001 |
| Vitamin E | 0.35 | 0.01 | 0.04 | 0.22 | 0.64 | 1.00 | 0.37 | <0.001 |
| Calcium | 0.68 | 0.01 | 0.40 | 0.79 | 0.98 | 1.00 | 0.46 | <0.001 |
| Iodine | 0.52 | 0.01 | 0.22 | 0.50 | 0.82 | 1.00 | 0.44 | <0.001 |
| Iron | 0.89 | 0.01 | 0.85 | 0.98 | 1.00 | 1.00 | 0.49 | <0.001 |
| Magnesium | 0.50 | 0.01 | 0.15 | 0.47 | 0.87 | 1.00 | 0.62 | <0.001 |
| Phosphorous | 0.97 | 0.00 | 0.99 | 1.00 | 1.00 | 1.00 | 0.42 | <0.001 |
| Potassium | 0.41 | 0.01 | 0.07 | 0.32 | 0.72 | 1.00 | 0.60 | <0.001 |
| Selenium | 0.51 | 0.01 | 0.17 | 0.50 | 0.85 | 1.00 | 0.32 | <0.001 |
| Sodium | 0.65 | 0.00 | 0.55 | 0.66 | 0.75 | 0.78 | 0.14 | <0.001 |
| Zinc | 0.98 | 0.00 | 0.99 | 1.00 | 1.00 | 1.00 | 0.42 | <0.001 |
| Adequacy sub-score | 66.59 | 0.41 | 60.85 | 72.22 | 81.89 | 97.30 | 0.71 | <0.001 |
| Moderation sub-score | 60.78 | 0.32 | 50.00 | 57.67 | 65.00 | 94.83 | 0.46 | <0.001 |
| PANDiet score | 63.69 | 0.23 | 59.21 | 64.34 | 69.63 | 94.74 | - | - |
| β2 (5–95% CI) | p | |
|---|---|---|
| Demographic factors (n = 1051) | ||
| Age (years) | 0.03 ± 0.02 (0.00, 0.06) | 0.11 |
| Gender (female) | −4.07 ± 0.64 (−5.12, −3.02) | <0.001 |
| Education status | 0.73 ± 0.26 (0.30, 1.17) | <0.01 |
| Social class | −0.10 ± 0.21 (−0.44, 0.25) | 0.65 |
| Smoker | 1.07 ± 0.26 (0.63, 1.50) | <0.001 |
| Body fat (%) | 0.01 ± 0.04 (−0.05, 0.07) | 0.81 |
| Energy density (g/kcal) | −11.87 ± 0.75 (−13.11, −10.63) | <0.001 |
| Biomarkers (n = 771) | ||
| Serum calcium | −1.36 ± 1.41 (−3.67, 0.96) | 0.34 |
| Serum ferritin | 0.00 ± 0.00 (−0.01, 0.00) | 0.10 |
| Riboflavin status | −4.18 ± 1.29 (−6.30, −2.06) | <0.01 |
| Vitamin B6 status | 0.00 ± 0.00 (0, 0.01) | 0.25 |
| Serum folate | 0.04 ± 0.01 (0.02, 0.06) | <0.001 |
| Serum vitamin B12 | 0.00 ± 0.00 (0.00, 0.00) | 0.39 |
| Vitamin D (s25OHD) | 0.02 ± 0.01 (0.00, 0.03) | <0.05 |
| Urinary creatinine | 0.00 ± 0.00 (0.00, 0.00) | 0.51 |
| Urinary potassium | 0.01 ± 0.01 (−0.01, 0.02) | 0.53 |
| Urinary sodium | 0.00 ± 0.01 (−0.01, 0.01) | 0.64 |
| Age Group (Years) | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| 18–35 n = 170 | 36–50 n = 165 | 51–64 n = 106 | 65–90 n = 87 | 18–35 n = 207 | 36–50 n = 143 | 51–64 n = 98 | 65–90 n = 75 | |
| Food Group (grams per day) | ||||||||
| Alcoholic beverages †,‡ | 782 ± 952 a | 484 ± 662 b | 390 ± 612 b,c | 243 ± 469 c | 266 ± 380 a | 169 ± 261 b | 106 ± 162 b | 33.2 ± 69.6 b |
| Biscuits, cakes, and pastries †,‡ | 28.2 ± 39.8 a | 39.8 ± 40.9 b | 52.7 ± 53.7 b | 39.1 ± 42.6 | 24.9 ± 26.9 a | 35.1 ± 32.1 | 39.0 ± 34.9 b | 30.3 ± 28.4 |
| Butters, fat spreads, and hard cooking fats †,‡ | 12.5 ± 13.6 a | 15.2 ± 17.0 | 17.7 ± 19.8 | 18.9 ± 28.4 b | 7.42 ± 9.7 | 10.6 ± 10.3 | 10.7 ± 16.2 | 14.0 ± 19.0 |
| Cheeses †,‡ | 20.1 ± 22.8 a | 18.1 ± 24.1 b | 14.0 ± 16.5 | 16.6 ± 23.1 b | 15.8 ± 16.9 | 11.4 ± 12.9 | 11.5 ± 14.9 | 7.73 ± 10.7 |
| Chips and processed potatoes †,‡ | 70.4 ± 71.8 a | 62.1 ± 58.7 b | 46.1 ± 52.5 b,c | 29.0 ± 43.2 c | 58.6 ± 56.3 a | 44.8 ± 49.4 a | 36.4 ± 39.1 | 20.3 ± 34.4 b |
| Confectionary †,‡ | 22.6 ± 31.2 a | 14.1 ± 19.8 b | 11.4 ± 18.0 b,c | 3.77 ± 9.9 c | 21.4 ± 22.5 a | 13.6 ± 16.9 b | 11.7 ± 15.3 b,c | 6.02 ± 11.0 c |
| Creams, ice-creams, rice puddings, and custard | 15.8 ± 34.9 | 18.1 ± 29.2 | 30.6 ± 46.4 | 41.6 ± 55.8 | 22.9 ± 38.3 a | 23.1 ± 32.6 a | 24.9 ± 37.0 | 32.7 ± 41.6 b |
| Eggs and egg dishes ‡ | 19.7 ± 31.1 | 18.1 ± 24.2 | 21.2 ± 22.4 | 22.4 ± 28.9 | 13.9 ± 20.2 | 16.2 ± 22.1 | 16.7 ± 23.2 | 14.9 ± 18.5 |
| Fish, fish dishes, and fish products †,‡ | 21.5 ± 36.4 | 30.1 ± 46.6 | 39.3 ± 51.9 | 45.6 ± 54.4 | 21.6 ± 32.1 a | 30.3 ± 36.9 | 32.8 ± 38.4 b | 33.4 ± 39.9 b |
| Fruit † | 76.1 ± 100 a | 80.6 ± 94.6 b | 115 ± 120 b | 122 ± 148 b | 61.6 ± 82.7 a | 102 ± 104 | 175 ± 147 b | 141 ± 122 b |
| Fruit juices and smoothies ‡ | 82.1 ± 126 a | 55.3 ± 106 | 44.5 ± 81.1 c | 43.5 ± 67.7 | 62.7 ± 89.8 a | 45.5 ± 77.8 | 32.0 ± 63.4 b | 48.2 ± 75.9 b |
| High-energy beverages | 191 ± 210 a | 113 ± 198 b | 58.4 ± 126 b | 15.1 ± 43.9 b | 151 ± 231 a | 57.0 ± 131 b | 31.2 ± 73.9 b,c | 7.0 ± 24.2 c |
| Low-energy beverages †,‡ | 1178 ± 759 a | 1118 ± 627 b | 1090 ± 465 b | 1009 ± 745 | 1049 ± 626 | 1290 ± 698 | 1283 ± 583 | 1138 ± 538 |
| Low-fat and skimmed milks †,‡ | 88.8 ± 155 a | 112 ± 192 | 90.6 ± 159 | 103 ± 142 b | 77.6 ± 110 | 105 ± 148 | 112 ± 122 | 131 ± 170 |
| Low-fat spreads and oils ‡ | 2.0 ± 4.6 a | 4.3 ± 10.2 a | 8.8 ± 17.5 b | 11.8 ± 20.5 | 2.5 ± 5.9 a | 3.51 ± 7.4 a | 7.8 ± 13.9 b | 5.2 ± 10.3 b |
| Other breakfast cereals † | 18.2 ± 50.6 a | 38.3 ± 91.6 a | 60.0 ± 114 b | 82.8 ± 108 b | 15.0 ± 46.5 a | 31.8 ± 69.4 a | 64.8 ± 100 | 78.0 ± 103 b |
| Other milks and milk-based beverages ‡ | 15.8 ± 67.9 | 9.9 ± 53.1 | 9.8 ± 44.5 | 6.91 ± 38.7 | 25.0 ± 58.4 | 22.6 ± 61.7 | 11.1 ± 39.1 | 18.1 ± 55.7 |
| Potatoes †,‡ | 72.7 ± 87.5 a | 89.9 ± 89.0 b | 124 ± 93.0 b,c | 43.7 ± 49.6 c | 42.0 ± 53.1 a | 64.5 ± 60.2 a | 78.4 ± 67.0 b | 20.9 ± 22.5 b |
| Processed red meat †,‡ | 67.5 ± 58.9 a | 58.9 ± 54 | 46.2 ± 42.9 b | 11.6 ± 27.6 b | 37.6 ± 39.4 a | 28.7 ± 35.3 | 25.5 ± 27 b | 6.8 ± 19.9 b |
| Processed white meat ‡ | 30.0 ± 40.7 a | 13.4 ± 27.1 | 11.3 ± 23.8 b | 15.9 ± 47.5 b | 17.9 ± 29.2 a | 11.7 ± 24.7 b | 7.78 ± 21.2 b | 15.2 ± 30.5 b |
| Ready-to-eat breakfast cereals (RTEBC) † | 59.3 ± 75.4 | 37.4 ± 47.9 | 31.0 ± 54.2 | 15.8 ± 25.2 | 34.3 ± 46.3 a | 32.0 ± 41.3 a | 23.2 ± 32.5 | 16.6 ± 22.7 b |
| Rice, pasta, flours, and starches †,‡ | 35.7 ± 40.8 a | 31.2 ± 33.3 a | 24.0 ± 26.8 | 11.8 ± 30.0 b | 21.7 ± 24.4 a | 21.1 ± 24.9 b | 16.6 ± 24.2 b | 6.9 ± 16.9 b |
| Savouries | 62.3 ± 80.5 a | 31.7 ± 44.1 b | 27.6 ± 73.6 b | 0.9 ± 4.2 b | 41.8 ± 54.5 b | 18.4 ± 28 b | 15.1 ± 29.1 b | 3.7 ± 12.4 b |
| Savoury snacks †,‡ | 16.3 ± 22.1 a | 9.1 ± 16.0 b | 5.9 ± 15.2 b,c | 80.8 ± 92.8 c | 15.1 ± 17.6 a,b | 9.5 ± 12.6 b | 6.7 ± 12.2 a,b | 57.9 ± 69.4 c |
| Soups, sauces, and condiments ‡ | 68.8 ± 72.6 | 55.8 ± 61.0 | 53.7 ± 73.8 | 25.9 ± 29.7 | 59.6 ± 63.0 | 48.9 ± 69.8 | 68.3 ± 80.3 | 11.8 ± 12.4 |
| Sugars, syrups, preserves, and sweeteners †,‡ | 8.77 ± 12.5 a | 16.2 ± 19 | 19.5 ± 21.1 c | 103 ± 72.6 | 7.41 ± 11.4 a | 8.93 ± 12.0 b | 12.2 ± 20.7 b,c | 78.3 ± 72.1 c |
| Unprocessed red meat †,‡ | 89.5 ± 76.3 a | 98.8 ± 75.6 | 98.0 ± 77.5 | 21.3 ± 36.3 b | 55.6 ± 59.3 | 67.1 ± 61.3 | 74.5 ± 61.2 | 37.0 ± 45.9 |
| Unprocessed white meat †,‡ | 68.5 ± 70.6 a | 60.1 ± 66.8 b | 40.3 ± 46.7 | 111.0 ± 79.5 | 51.1 ± 50.6 a | 37.2 ± 45.1 a | 38.6 ± 48.3 | 128.0 ± 68.8 b |
| Vegetables and vegetable dishes †,‡ | 96.1 ± 82.9 a | 118 ± 82.9 b | 132 ± 65.3 b | 81.5 ± 73.2 | 102 ± 89.2 a | 137 ± 98.5 | 142 ± 88.6 b | 44.2 ± 45.8 |
| White bread, rolls, scones, and croissants † | 77.6 ± 60.4 | 83.6 ± 64.3 | 83.7 ± 69.1 | 83.3 ± 113 | 56.3 ± 46.6 | 57.0 ± 45.8 | 47.5 ± 51.8 | 95.9 ± 149 |
| Whole milk † | 187 ± 260 a | 158 ± 192 | 141 ± 173 | 75.3 ± 74 b | 71.8 ± 109 a | 94.7 ± 165 | 51.4 ± 96.6 | 56.9 ± 47.2 b |
| Wholemeal/brown bread and rolls † | 55.9 ± 57.0 | 65.2 ± 72.1 b | 78.1 ± 74.6 b | 24.7 ± 41.4 b | 38.6 ± 38.0 a | 56.9 ± 50.3 | 64.6 ± 48.0 b | 49.3 ± 66.6 |
| Yogurts †,‡ | 28.6 ± 49.4 a | 25.2 ± 46.5 c | 32.1 ± 57.7 b | 130 ± 99 b,c | 27.9 ± 41.5 | 31.1 ± 46.9 | 57.3 ± 66.4 | 87.8 ± 58.7 |
| Mean PANDiet score | 59.9 | 63.3 | 64.9 | 64.1 | 64.7 | 64.7 | 65.7 | 63.5 |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | Low | Moderate | High | |
| n = 176 | n = 176 | n = 176 | n = 174 | n = 174 | n = 175 | |
| Food Group (g/mL per day) | ||||||
| Alcoholic beverages †,‡ | 189 ± 344 | 175 ± 279 | 131 ± 209 | 576 ± 853 | 657 ± 863 a | 417 ± 601 b |
| Biscuits, cakes, and pastries †,‡ | 28.4 ± 31.1 | 34.3 ± 33.1 | 32.7 ± 28.3 | 32.7 ± 42.8 | 43.9 ± 50.9 | 36.0 ± 37.4 |
| Butters, fat spreads, and hard cooking fats †,‡ | 14.5 ± 18.3 a | 9.0 ± 9.9 b | 6.9 ± 8.8 b | 20.1 ± 23.9 a | 15.5 ± 16.1 a | 9.95 ± 12.5 b |
| Cheeses †,‡ | 12.0 ± 14.4 | 13.7 ± 15.3 | 10.9 ± 14.2 | 19.3 ± 25.1 | 19.2 ± 20.2 | 15.3 ± 21.1 |
| Chips and processed potatoes †,‡ | 46.9 ± 54.4 | 45.9 ± 49.5 | 37.7 ± 43.7 | 62.7 ± 63.3 a | 64.2 ± 67.8 a | 46.1 ± 55.9 b |
| Confectionary †,‡ | 16.5 ± 21.3 a | 15.6 ± 17.4 | 11.4 ± 16.6 b | 15.5 ± 24.4 | 18.1 ± 27.4 | 12.8 ± 21.8 |
| Creams, ice-creams, rice puddings, and custard | 20.6 ± 29.7 | 27.4 ± 40.1 | 26.9 ± 40.0 | 19.1 ± 37.0 | 20.7 ± 36.9 | 28.8 ± 46.5 |
| Eggs and egg dishes ‡ | 14.5 ± 19.8 | 16.5 ± 23.9 | 15.0 ± 19.5 | 18.8 ± 28.1 | 20.8 ± 28.9 | 20.2 ± 25.5 |
| Fish, fish dishes, and fish products †,‡ | 18.1 ± 26.8 a | 30.1 ± 39.6 b | 37.3 ± 39.1 b | 24.0 ± 38.9 a | 24.2 ± 42.9 a | 43.6 ± 52.5 b |
| Fruit † | 59.0 ± 76.7 a | 90.4 ± 97.1 b | 181 ± 138 c | 40.0 ± 59.1 a | 81.0 ± 96.1 b | 152 ± 136 c |
| Fruit juices and smoothies ‡ | 46.8 ± 84.1 | 56.6 ± 88.0 | 43.0 ± 64.5 | 37.9 ± 80.7 a | 53.7 ± 91.5 a | 94.8 ± 133 b |
| High-energy beverages | 114 ± 212 a | 69.1 ± 159 b | 38.0 ± 87.4 b | 162 ± 222 a | 124 ± 178 a | 72.3 ± 149 b |
| Low-energy beverages †,‡ | 998 ± 606.0 a | 1153 ± 565 a | 1407 ± 666 b | 985 ± 593 a | 1115 ± 632 | 1261 ± 764 b |
| Low-fat and skimmed milks †,‡ | 42.9 ± 74.1 a | 94.6 ± 134.0 b | 168.0 ± 158 b | 34.2 ± 86.8 a | 85.3 ± 142 b | 173 ± 211 c |
| Low-fat spreads and oils ‡ | 2.1 ± 6.1 a | 4.4 ± 10.4 | 6.4 ± 10.6 b | 2.47 ± 6.37 a | 4.99 ± 14.7 a | 8.51 ± 14.9 b |
| Other breakfast cereals † | 20.5 ± 49.1 a | 37.9 ± 75.5 a | 63.6 ± 102 b | 33.5 ± 89.0 | 42.3 ± 86.5 | 46.6 ± 90.9 |
| Other milks and milk-based beverages ‡ | 16.7 ± 43.8 | 19.4 ± 48.8 | 24.8 ± 71.1 | 10.7 ± 55.5 | 12.4 ± 62.5 | 12.3 ± 50.9 |
| Potatoes †,‡ | 52.1 ± 53.6 a | 64.7 ± 59.6 | 74.8 ± 68.6 b | 71.6 ± 73.7 a | 86.3 ± 87.6 a | 127 ± 107 b |
| Processed red meat †,‡ | 38.8 ± 42.9 | 30.4 ± 32.7 | 19.7 ± 19.1 | 73.5 ± 62.1 a | 58.4 ± 53.2 b | 41.4 ± 40.9 c |
| Processed white meat ‡ | 14.2 ± 26.7 a | 11.7 ± 22.7 b | 10.5 ± 26.1 b | 24.0 ± 37.0 | 18.6 ± 32.1 | 15.4 ± 31.6 |
| Ready-to-eat breakfast cereals (RTEBC) † | 11.4 ± 16.5 a | 16.5 ± 19.9 a | 31.1 ± 29.9 b | 14.9 ± 21.3 a | 28.1 ± 33.4 b | 45.2 ± 40.9 c |
| Rice, pasta, flours, and starches †,‡ | 19.5 ± 37.0 a | 29.3 ± 37.4 a | 35.8 ± 44.8 b | 23.7 ± 41.1 a | 51.8 ± 71.7 b | 49.8 ± 68.3 b |
| Savouries | 28.7 ± 45.1 a | 24.1 ± 40.7 | 17.2 ± 31.9 b | 39.7 ± 62.6 | 51.4 ± 83.4 a | 29.6 ± 51.7 b |
| Savoury snacks †,‡ | 9.6 ± 15.3 | 10.6 ± 14.2 | 9.16 ± 15.0 | 8.18 ± 14.8 a | 14.4 ± 21.8 b | 8.01 ± 17.3 a |
| Soups, sauces, and condiments ‡ | 50.1 ± 54.0 | 57.2 ± 75.0 | 65.9 ± 78.1 | 60.2 ± 71.2 | 71.4 ± 69.8 | 60.9 ± 79.2 |
| Sugars, syrups, preserves, and sweeteners †,‡ | 11.7 ± 19.1 a | 9.2 ± 11.4 | 7.8 ± 10.3 b | 19.1 ± 22.9 a | 13.5 ± 18.2 b | 13.2 ± 18.5 b |
| Unprocessed red meat †,‡ | 61.8 ± 63.2 | 70.2 ± 67.2 | 68.2 ± 58.2 | 85.8 ± 70.6 | 102 ± 80.9 | 98.6 ± 74.8 |
| Unprocessed white meat †,‡ | 34.6 ± 38.8 a | 42.9 ± 48.5 | 48.3 ± 54.7 b | 44.9 ± 57.0 a | 53.5 ± 55.6 | 63.9 ± 75.5 b |
| Vegetables and vegetable dishes †,‡ | 94.4 ± 69.0 a | 116 ± 77.8 a | 165 ± 106.0 b | 88.2 ± 60.3 a | 107 ± 84.1 a | 138 ± 86.4 b |
| White bread, rolls, scones, and croissants † | 61.2 ± 51.9 a | 51.8 ± 46.3 | 45.2 ± 42.7 b | 92.9 ± 67.9 a | 81.9 ± 63.7 | 68.1 ± 61.1 b |
| Whole milk † | 78.7 ± 126 | 84.3 ± 148.0 | 73.5 ± 129.0 | 129 ± 145.0 | 179 ± 225.0 | 158 ± 252.0 |
| Wholemeal/brown bread and rolls † | 36.7 ± 43.3 a | 54.1 ± 42.1 b | 66.8 ± 49.4 c | 43.3 ± 54.3 a | 64.5 ± 58.9 b | 88.3 ± 79.8 c |
| Yogurts †,‡ | 19.4 ± 35.7 a | 38.4 ± 54.4 b | 57.3 ± 63.4 c | 16.9 ± 37.7 a | 27.8 ± 52.3 | 38.5 ± 53.8 b |
| PANDiet score μ | 54.3 | 62.3 | 71.3 | 57.3 | 64.8 | 72.1 |
| PANDiet score range | 38.3–58.8 | 58.8–66.1 | 66.1–89.6 | 42.0–62.1 | 62.2–63.3 | 67.5–89.7 |
| Age (years) μ | 43.3 | 43.2 | 50.7 | 43.3 | 40.6 | 44.5 |
| Age (years) range | 18–87 | 18–90 | 18–82 | 18–86 | 18–88 | 18–82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirwan, L.B.; Walton, J.; Flynn, A.; Nugent, A.P.; McNulty, B.A. An Evaluation of Probability of Adequate Nutrient Intake (PANDiet) Scores as a Diet Quality Metric in Irish National Food Consumption Data. Nutrients 2022, 14, 994. https://doi.org/10.3390/nu14050994
Kirwan LB, Walton J, Flynn A, Nugent AP, McNulty BA. An Evaluation of Probability of Adequate Nutrient Intake (PANDiet) Scores as a Diet Quality Metric in Irish National Food Consumption Data. Nutrients. 2022; 14(5):994. https://doi.org/10.3390/nu14050994
Chicago/Turabian StyleKirwan, Laura B., Janette Walton, Albert Flynn, Anne P. Nugent, and Breige A. McNulty. 2022. "An Evaluation of Probability of Adequate Nutrient Intake (PANDiet) Scores as a Diet Quality Metric in Irish National Food Consumption Data" Nutrients 14, no. 5: 994. https://doi.org/10.3390/nu14050994
APA StyleKirwan, L. B., Walton, J., Flynn, A., Nugent, A. P., & McNulty, B. A. (2022). An Evaluation of Probability of Adequate Nutrient Intake (PANDiet) Scores as a Diet Quality Metric in Irish National Food Consumption Data. Nutrients, 14(5), 994. https://doi.org/10.3390/nu14050994







