Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating
Abstract
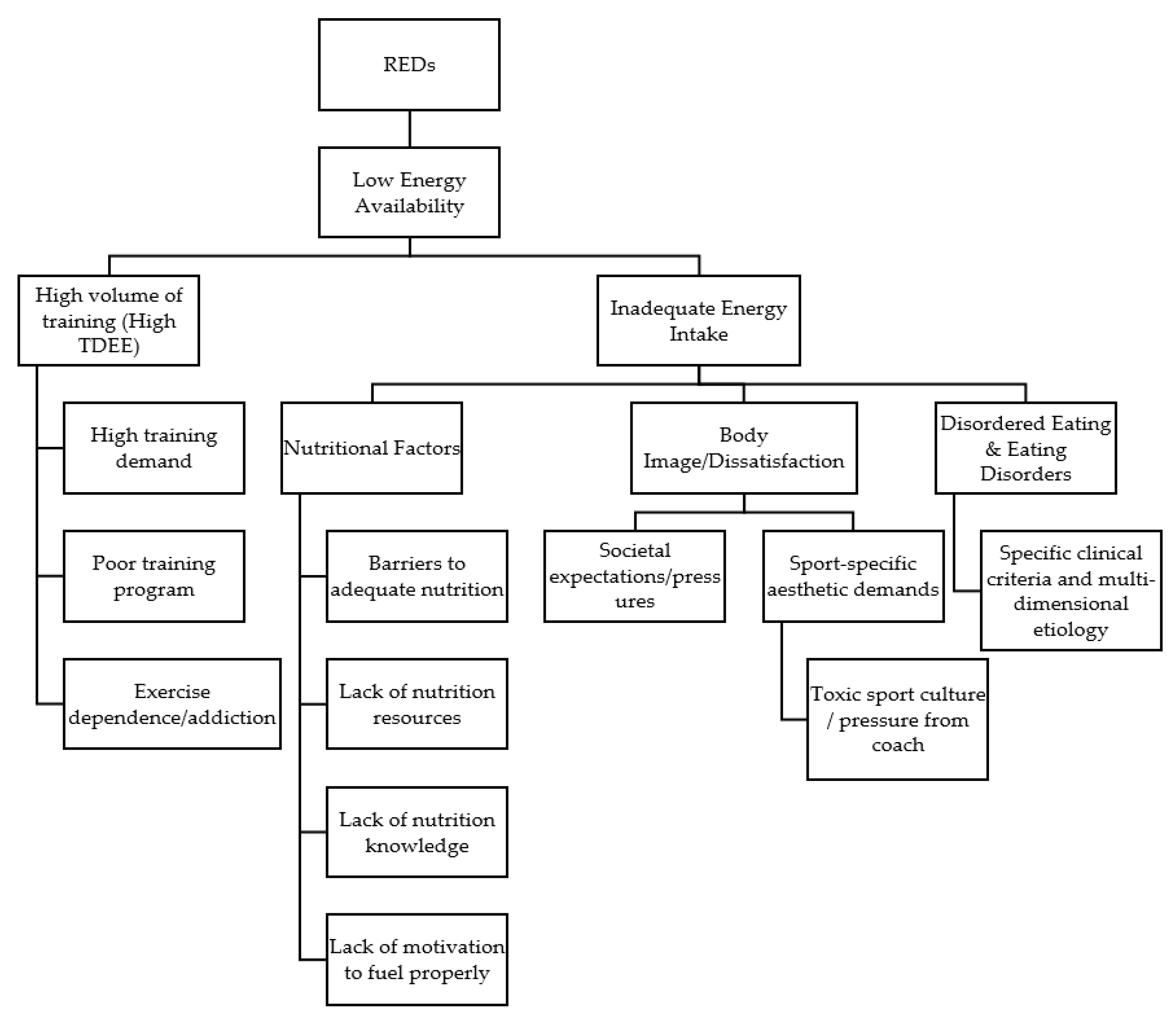
:1. Introduction
2. Prevalence of Low Energy Availability
3. Nutritional Factors Impacting Dietary Behaviors
4. Training Demands and Energy Expenditure
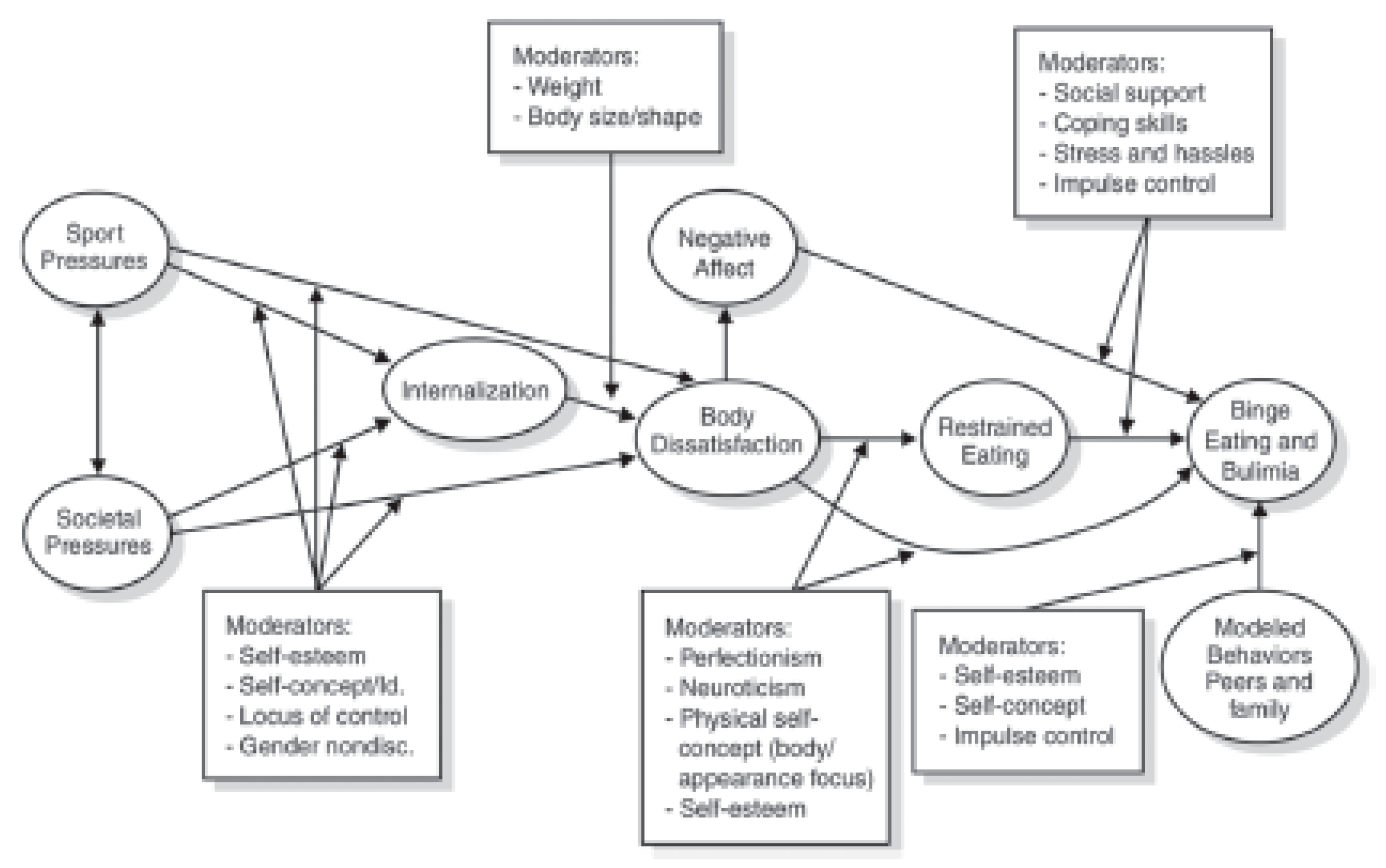
5. Body Image
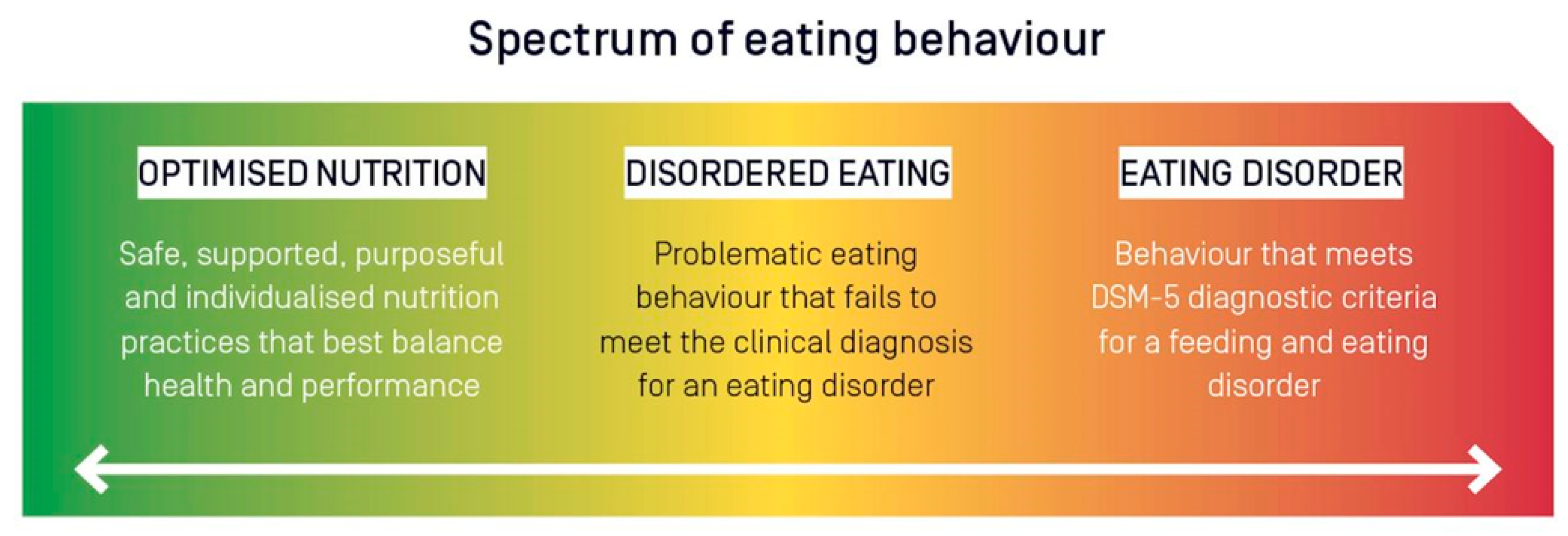
6. Disordered Eating
7. Practical Recommendations and Future Directions
- The development of more efficient screening tools to identify those at risk of RED-s and the female (and male) athlete triad, particularly those encompassing the multi-factorial nature of underlying risk factors.
- Develop a better understanding of the specific health and performance implications of energy deficiencies across a spectrum of energy availability ranges:
- Metabolic dysfunction;
- Hormonal disturbances;
- Menstrual dysfunction;
- Bone health;
- Immune function;
- Performance implications.
- Evaluate time-dependent effects of varying ranges of energy availability values to examine how long of a state of energy deficiency can be maintained prior to the onset of health and performance decrements.
- Assess the efficacy of refeeding strategies for athletes competing in weight-class or aesthetic based sports where a state of energy deficiency is often required to lose weight or reduce body fat.
- Evaluate the efficacy of various educational-based interventions to educate athletes on the importance of fueling strategies to support their performance and health.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell, S.M.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, Within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.I.; Koltun, K.J.; Strock, N.C.A.; De Souza, M.J. Female Athlete Triad and Relative Energy Deficiency in Sport: A Focus on Scientific Rigor. Exerc. Sport Sci. Rev. 2019, 47, 197–205. [Google Scholar] [CrossRef]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29, 15–24. [Google Scholar] [CrossRef]
- Kong, P.; Harris, L.M. The sporting body: Body image and eating disorder symptomatology among female athletes from leanness focused and nonleanness focused sports. J. Psychol. 2015, 149, 141–160. [Google Scholar] [CrossRef]
- Byrne, S.; McLean, N. Elite athletes: Effects of the pressure to be thin. J. Sci. Med. Sport 2002, 5, 80–94. [Google Scholar] [CrossRef]
- Kantanista, A.; Glapa, A.; Banio, A.; Firek, W.; Ingarden, A.; Malchrowicz-Mosko, E.; Markiewicz, P.; Ploszaj, K.; Ingarden, M.; Mackowiak, Z. Body Image of Highly Trained Female Athletes Engaged in Different Types of Sport. BioMed Res. Int. 2018, 2018, 6835751. [Google Scholar] [CrossRef]
- Bell, H.S.; Donovan, C.L.; Ramme, R. Is athletic really ideal? An examination of the mediating role of body dissatisfaction in predicting disordered eating and compulsive exercise. Eat. Behav. 2016, 21, 24–29. [Google Scholar] [CrossRef]
- Burke, L.M.; Lundy, B.; Fahrenholtz, I.L.; Melin, A.K. Pitfalls of Conducting and Interpreting Estimates of Energy Availability in Free-Living Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 350–363. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, A.B.; Skouby, S.; Faber, J.; Ritz, C.; Sjodin, A.; Sundgot-Borgen, J. The LEAF questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 2014, 48, 540–545. [Google Scholar] [CrossRef]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; Mc Donnell, S.J.; Corish, C.A. Low Energy Availability in Athletes: A Review of Prevalence, Dietary Patterns, Physiological Health, and Sports Performance. Sports Med. 2018, 48, 73–96. [Google Scholar] [CrossRef]
- Loucks, A.B. Low energy availability in the marathon and other endurance sports. Sports Med. 2007, 37, 348–352. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, A.B.; Skouby, S.; Moller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sidelmann, J.J.; Aziz, M.; Sjodin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports 2015, 25, 610–622. [Google Scholar] [CrossRef]
- Beermann, B.L.; Lee, D.G.; Almstedt, H.C.; McCormack, W.P. Nutritional Intake and Energy Availability of Collegiate Distance Runners. J. Am. Coll. Nutr. 2020, 39, 747–755. [Google Scholar] [CrossRef]
- Day, J.; Wengreen, H.; Heath, E.; Brown, K. Prevalence of low energy availabiilty in collegiate female runners and implementation of nutrition education intervention. Sports Nutr. Ther. 2015, 1, 101. [Google Scholar] [CrossRef] [Green Version]
- Magee, M.K.; Lockard, B.L.; Zabriskie, H.A.; Schaefer, A.Q.; Luedke, J.A.; Erickson, J.L.; Jones, M.T.; Jagim, A.R. Prevalence of Low Energy Availability in Collegiate Women Soccer Athletes. J. Funct. Morphol. Kinesiol. 2020, 5, 96. [Google Scholar] [CrossRef]
- Reed, J.L.; De Souza, M.J.; Williams, N.I. Changes in energy availability across the season in Division I female soccer players. J. Sports Sci. 2013, 31, 314–324. [Google Scholar] [CrossRef]
- Torres-McGehee, T.M.; Emerson, D.M.; Pritchett, K.; Moore, E.M.; Smith, A.B.; Uriegas, N.A. Energy Availability with or without Eating Disorder Risk in Collegiate Female Athletes and Performing Artists. J. Athl. Train. 2020, 56, 993–1002. [Google Scholar] [CrossRef]
- Schaal, K.; Tiollier, E.; Le Meur, Y.; Casazza, G.; Hausswirth, C. Elite synchronized swimmers display decreased energy availability during intensified training. Scand. J. Med. Sci. Sports 2017, 27, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.B.; Richmond, S.R.; Smith, C.R.; Currier, B.; Stecker, R.A.; Gieske, B.T.; Kemp, K.; Witherbee, K.E.; Kerksick, C.M. Physiologic, Metabolic, and Nutritional Attributes of Collegiate Synchronized Swimmers. Int. J. Sports Physiol. Perform. 2019, 14, 658–664. [Google Scholar] [CrossRef]
- Staal, S.; Sjodin, A.; Fahrenholtz, I.; Bonnesen, K.; Melin, A.K. Low RMRratio as a Surrogate Marker for Energy Deficiency, the Choice of Predictive Equation Vital for Correctly Identifying Male and Female Ballet Dancers at Risk. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 412–418. [Google Scholar] [CrossRef]
- Ihle, R.; Loucks, A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Min. Res. 2004, 19, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loucks, A.B.; Verdun, M.; Heath, E.M. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J. Appl. Physiol. 1998, 84, 37–46. [Google Scholar] [CrossRef]
- Loucks, A.B.; Heath, E.M. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am. J. Physiol. 1994, 266, R817–R823. [Google Scholar] [CrossRef]
- Reed, J.L.; De Souza, M.J.; Mallinson, R.J.; Scheid, J.L.; Williams, N.I. Energy availability discriminates clinical menstrual status in exercising women. J. Int. Soc. Sports Nutr. 2015, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Loucks, A.B. No Evidence of Absence of an Energy Availability Threshold for Menstrual Disturbances. Med. Sci. Sports Exerc. 2019, 51, 1790. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Peeling, P.; Pyne, D.B.; Tee, N.; Whitfield, J.; Sharma, A.P.; Heikura, I.A.; Burke, L.M. Six Days of Low Carbohydrate, Not Energy Availability, Alters the Iron and Immune Response to Exercise in Elite Athletes. Med. Sci. Sports Exerc. 2021, 54, 377–387. [Google Scholar] [CrossRef]
- Morehen, J.; Rosimus, C.; Cavanagh, B.P.; Hambly, C.; Speakman, J.R.; Elliot-Sale, K.J.; Hannon, M.P.; Morton, J.P. Energy Expenditure of Female International Standard Soccer Players: A Doubly Labelled Water Investigation. Med. Sci. Sports Exerc. 2021; Published ahead of Print. [Google Scholar] [CrossRef]
- Cherian, K.S.; Sainoji, A.; Nagalla, B.; Yagnambhatt, V.R. Energy Balance Coexists with Disproportionate Macronutrient Consumption across Pretraining, During Training, and Posttraining Among Indian Junior Soccer Players. Pediatr. Exerc. Sci. 2018, 30, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.L.; Randell, R.K.; Burgess, D.; Ridley, S.; Ócairealláin, C.; Allison, R.; Rollo, I. Assessment of energy availability and associated risk factors in professional female soccer players. Eur. J. Sport Sci. 2021, 21, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Heikura, I.A.; Uusitalo, A.L.T.; Stellingwerff, T.; Bergland, D.; Mero, A.A.; Burke, L.M. Low Energy Availability Is Difficult to Assess but Outcomes Have Large Impact on Bone Injury Rates in Elite Distance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Melin, A.; Tornberg, A.B.; Skouby, S.; Moller, S.S.; Faber, J.; Sundgot-Borgen, J.; Sjodin, A. Low-energy density and high fiber intake are dietary concerns in female endurance athletes. Scand. J. Med. Sci. Sports 2016, 26, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Schaal, K.; VanLoan, M.D.; Hausswirth, C.; Casazza, G.A. Decreased energy availability during training overload is associated with non-functional overreaching and suppressed ovarian function in female runners. Appl. Physiol. Nutr. Metab. 2021, 46, 1179–1188. [Google Scholar] [CrossRef]
- Viner, R.T.; Harris, M.; Berning, J.R.; Meyer, N.L. Energy Availability and Dietary Patterns of Adult Male and Female Competitive Cyclists With Lower Than Expected Bone Mineral Density. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Zabriskie, H.A.; Currier, B.S.; Harty, P.S.; Stecker, R.A.; Jagim, A.R.; Kerksick, C.M. Energy Status and Body Composition Across a Collegiate Women’s Lacrosse Season. Nutrients 2019, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Zanders, B.R.; Currier, B.S.; Harty, P.S.; Zabriskie, H.A.; Smith, C.R.; Stecker, R.A.; Richmond, S.R.; Jagim, A.R.; Kerksick, C.M. Changes in Energy Expenditure, Dietary Intake, and Energy Availability Across an Entire Collegiate Women’s Basketball Season. J. Strength Cond. Res. 2021, 35, 804–810. [Google Scholar] [CrossRef]
- Braun, H.; von Andrian-Werburg, J.; Schanzer, W.; Thevis, M. Nutrition Status of Young Elite Female German Football Players. Pediatr. Exerc. Sci. 2018, 30, 157–167. [Google Scholar] [CrossRef]
- Woodruff, S.J.; Meloche, R.D. Energy availability of female varsity volleyball players. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 24–30. [Google Scholar] [CrossRef]
- Civil, R.; Lamb, A.; Loosmore, D.; Ross, L.; Livingstone, K.; Strachan, F.; Dick, J.R.; Stevenson, E.J.; Brown, M.A.; Witard, O.C. Assessment of Dietary Intake, Energy Status, and Factors Associated With RED-S in Vocational Female Ballet Students. Front. Nutr. 2018, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; O’Reilly, L.M.; Whybrow, S.; Fuller, Z.; Johnstone, A.M.; Livingstone, M.B.; Ritz, P.; Horgan, G.W. Measuring the difference between actual and reported food intakes in the context of energy balance under laboratory conditions. Br. J. Nutr. 2014, 111, 2032–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scagliusi, F.B.; Ferriolli, E.; Pfrimer, K.; Laureano, C.; Cunha, C.S.; Gualano, B.; Lourenco, B.H.; Lancha, A.H., Jr. Underreporting of energy intake in Brazilian women varies according to dietary assessment: A cross-sectional study using doubly labeled water. J. Am. Diet. Assoc. 2008, 108, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Guglielmetti, M.; Trentani, C.; Tagliabue, A. Assessment of Dietary Under-Reporting in Italian College Team Sport Athletes. Nutrients 2019, 11, 1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenner, S.L.; Buckley, G.L.; Belski, R.; Devlin, B.L.; Forsyth, A.K. Dietary Intakes of Professional and Semi-Professional Team Sport Athletes Do Not Meet Sport Nutrition Recommendations—A Systematic Literature Review. Nutrients 2019, 11, 1160. [Google Scholar] [CrossRef] [Green Version]
- Jagim, A.R.; Zabriskie, H.; Currier, B.; Harty, P.S.; Stecker, R.; Kerksick, C.M. Nutrient Status and perceptions of energy and macronutrient intake in a Group of Collegiate Female Lacrosse Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 43. [Google Scholar] [CrossRef] [Green Version]
- Jagim, A.R.; Fields, J.B.; Magee, M.; Kerksick, C.; Luedke, J.; Erickson, J.; Jones, M.T. The Influence of Sport Nutrition Knowledge on Body Composition and Perceptions of Dietary Requirements in Collegiate Athletes. Nutrients 2021, 13, 2239. [Google Scholar] [CrossRef]
- Heaney, S.; O’Connor, H.; Michael, S.; Gifford, J.; Naughton, G. Nutrition knowledge in athletes: A systematic review. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 248–261. [Google Scholar] [CrossRef] [Green Version]
- Hoogenboom, B.J.; Morris, J.; Morris, C.; Schaefer, K. Nutritional knowledge and eating behaviors of female, collegiate swimmers. N. Am. J. Sports Phys. Ther. NAJSPT 2009, 4, 139–148. [Google Scholar]
- Manore, M.M.; Patton-Lopez, M.M.; Meng, Y.; Wong, S.S. Sport Nutrition Knowledge, Behaviors and Beliefs of High School Soccer Players. Nutrients 2017, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Trakman, G.L.; Forsyth, A.; Devlin, B.L.; Belski, R. A Systematic Review of Athletes’ and Coaches’ Nutrition Knowledge and Reflections on the Quality of Current Nutrition Knowledge Measures. Nutrients 2016, 8, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, E.N.; Guadagni, A.J.; Pivarnik, J.M. Assessment of nutrition knowledge in division I college athletes. J. Am. Coll. Health 2020, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Condo, D.; Lohman, R.; Kelly, M.; Carr, A. Nutritional Intake, Sports Nutrition Knowledge and Energy Availability in Female Australian Rules Football Players. Nutrients 2019, 11, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessri, M.; Jessri, M.; RashidKhani, B.; Zinn, C. Evaluation of Iranian college athletes’ sport nutrition knowledge. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Rash, C.; Malinauskas, B.M.; Duffrin, M.W.; Barber-Heidal, K.; Overton, R.F. Nutrition-related knowledge, attitude, and dietary intake of colelge track athletes. Sport J. 2008, 11, 48–55. [Google Scholar]
- Rosenbloom, C.A.; Jonnalagadda, S.S.; Skinner, R. Nutrition knowledge of collegiate athletes in a Division I National Collegiate Athletic Association institution. J. Am. Diet. Assoc. 2002, 102, 418–420. [Google Scholar] [CrossRef]
- Spronk, I.; Heaney, S.E.; Prvan, T.; O’Connor, H.T. Relationship Between General Nutrition Knowledge and Dietary Quality in Elite Athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 243–251. [Google Scholar] [CrossRef]
- Torres-McGehee, T.M.; Pritchett, K.L.; Zippel, D.; Minton, D.M.; Cellamare, A.; Sibilia, M. Sports nutrition knowledge among collegiate athletes, coaches, athletic trainers, and strength and conditioning specialists. J. Athl. Train. 2012, 47, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Abood, D.A.; Black, D.R.; Birnbaum, R.D. Nutrition education intervention for college female athletes. J. Nutr. Educ. Behav. 2004, 36, 135–137. [Google Scholar] [CrossRef]
- Andrews, A.; Wojcik, J.R.; Boyd, J.M.; Bowers, C.J. Sports Nutrition Knowledge among Mid-Major Division I University Student-Athletes. J. Nutr. Metab. 2016, 2016, 3172460. [Google Scholar] [CrossRef] [Green Version]
- Cupisti, A.; D’Alessandro, C.; Castrogiovanni, S.; Barale, A.; Morelli, E. Nutrition knowledge and dietary composition in Italian adolescent female athletes and non-athletes. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 207–219. [Google Scholar] [CrossRef]
- Dunn, D.; Turner, L.W.; Denny, G. Nutrition Knowledge and Attitudes of College Athletes. Sport J. 2007, 10, 45–52. [Google Scholar]
- Hornstrom, G.R.; Friesen, C.A.; Ellery, J.E.; Pike, K. Nutrition Knowledge, Practices, Attitudes, and Information Sources of Mid-American Conference College Softball Players. Food Nutr. Sci. 2011, 2, 4528. [Google Scholar] [CrossRef] [Green Version]
- Trakman, G.L.; Forsyth, A.; Hoye, R.; Belski, R. Development and validation of a brief general and sports nutrition knowledge questionnaire and assessment of athletes’ nutrition knowledge. J. Int. Soc. Sports Nutr. 2018, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Zinn, C.; Schofield, G.; Wall, C. Development of a psychometrically valid and reliable sports nutrition knowledge questionnaire. J. Sci. Med. Sport 2005, 8, 346–351. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Theodoropoulou, E. Relationship between Nutrition Knowledge and Physical Fitness in Semiprofessional Soccer Players. Scientifica 2014, 2014, 180353. [Google Scholar] [CrossRef] [Green Version]
- Sedek, R.; Mohamad, M.; Kasim, Z.M. Knowledge, Attitudes and Practices on Hydration and Fluid Replacement among Endurance Sports Athletes in National University of Malaysia (UKM). Pak. J. Nutr. 2015, 14, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Shifflett, B.; Timm, C.; Kahanov, L. Understanding of athletes’ nutritional needs among athletes, coaches, and athletic trainers. Res. Q. Exerc. Sport 2002, 73, 357–362. [Google Scholar] [CrossRef]
- Heaney, S.; O’Connor, H.; Naughton, G.; Gifford, J. Towards an understanding of the barriers to good nutrition for elite athletes. Int. J. Sports Sci. Coach. 2008, 3, 391–401. [Google Scholar] [CrossRef]
- Thurecht, R.; Pelly, F. Key Factors Influencing the Food Choices of Athletes at two Distinct Major International Competitions. Nutrients 2020, 12, 924. [Google Scholar] [CrossRef] [Green Version]
- Hull, M.V.; Jagim, A.R.; Oliver, J.M.; Greenwood, M.; Busteed, D.R.; Jones, M.T. Gender differences and access to a sports dietitian influence dietary habits of collegiate athletes. J. Int. Soc. Sports Nutr. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, M.V.; Neddo, J.; Jagim, A.R.; Oliver, J.M.; Greenwood, M.; Jones, M.T. Availability of a sports dietitian may lead to improved performance and recovery of NCAA division I baseball athletes. J. Int. Soc. Sports Nutr. 2017, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front. Physiol. 2013, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Pikosky, M.A.; Smith, T.J.; Grediagin, A.; Castaneda-Sceppa, C.; Byerley, L.; Glickman, E.L.; Young, A.J. Increased protein maintains nitrogen balance during exercise-induced energy deficit. Med. Sci. Sports Exerc. 2008, 40, 505–512. [Google Scholar] [CrossRef]
- Joy, E.A.; Campbell, D. Stress fractures in the female athlete. Curr. Sports Med. Rep. 2005, 4, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Novas, A.; Rowbottom, D.; Jenkins, D. Total daily energy expenditure and incidence of upper respiratory tract infection symptoms in young females. Int. J. Sports Med. 2002, 23, 465–470. [Google Scholar] [CrossRef]
- Sinha, S.; Kurpad, A.V.; Kuriyan, R. Total energy expenditure (TEE) of young adults from urban South India: Revisiting their daily energy requirement. Eur. J. Clin. Nutr. 2021, 75, 845–851. [Google Scholar] [CrossRef]
- Moon, J.M.; Zabriskie, H.A.; Harty, P.S.; Currier, B.S.; Blumkaitis, J.C.; Stecker, R.A.; Jagim, A.; Kerksick, C.M. Comparison of Energy Expenditure Observed between Scheduled Activities in Collegiate Team-Sport Female Athletes. Sports 2021, 9, 50. [Google Scholar] [CrossRef]
- Mara, J.K.; Thompson, K.G.; Pumpa, K.L. Assessing the Energy Expenditure of Elite Female Soccer Players: A Preliminary Study. J. Strength Cond. Res. 2021, 29, 2780–2786. [Google Scholar] [CrossRef]
- Edwards, J.E.; Lindeman, A.K.; Mikesky, A.E.; Stager, J.M. Energy balance in highly trained female endurance runners. Med. Sci. Sports Exerc. 1993, 25, 1398–1404. [Google Scholar] [CrossRef]
- Loftin, M.; Sothern, M.; Koss, C.; Tuuri, G.; Vanvrancken, C.; Kontos, A.; Bonis, M. Energy expenditure and influence of physiologic factors during marathon running. J. Strength Cond. Res. 2007, 21, 1188–1191. [Google Scholar] [CrossRef]
- Schulz, L.O.; Alger, S.; Harper, I.; Wilmore, J.H.; Ravussin, E. Energy expenditure of elite female runners measured by respiratory chamber and doubly labeled water. J. Appl. Physiol. 1992, 72, 23–28. [Google Scholar] [CrossRef]
- Trappe, T.A.; Gastaldelli, A.; Jozsi, A.C.; Troup, J.P.; Wolfe, R.R. Energy expenditure of swimmers during high volume training. Med. Sci. Sports Exerc. 1997, 29, 950–954. [Google Scholar] [CrossRef]
- Costa, R.J.; Gill, S.K.; Hankey, J.; Wright, A.; Marczak, S. Perturbed energy balance and hydration status in ultra-endurance runners during a 24 h ultra-marathon. Br. J. Nutr. 2014, 112, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumahara, H.; Ohta, C.; Nabeshima, E.; Nakayama, A.; Mine, S.; Yamato, T. Dietary Intake and Energy Expenditure During Two Different Phases of Athletic Training in Female Collegiate Lacrosse Players. J. Strength Cond. Res. 2020, 34, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Matias, C.N.; Santos, D.A.; Thomas, D.; Bosy-Westphal, A.; Muller, M.J.; Heymsfield, S.B.; Sardinha, L.B. Energy Balance over One Athletic Season. Med. Sci. Sports Exerc. 2017, 49, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Santos, D.A.; Matias, C.N.; Minderico, C.S.; Schoeller, D.A.; Sardinha, L.B. Total energy expenditure assessment in elite junior basketball players: A validation study using doubly labeled water. J. Strength Cond. Res. 2013, 27, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, B.; Grzelak, A.; Klimek, A.T. Analysis of Daily Energy Expenditure of Elite Athletes in Relation to their Sport, the Measurement Method and Energy Requirement Norms. J. Hum. Kinet. 2019, 70, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.J.; Davies, P.S. Energy intake and energy expenditure in elite lightweight female rowers. Med. Sci. Sports Exerc. 2002, 34, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Hoeg, T.B.; Olson, E.M.; Skaggs, K.; Sainani, K.; Fredericson, M.; Roche, M.; Kraus, E. Prevalence of Female and Male Athlete Triad Risk Factors in Ultramarathon Runners. Clin. J. Sport Med. 2021. [Google Scholar] [CrossRef]
- Lichtenstein, M.B.; Hinze, C.J.; Emborg, B.; Thomsen, F.; Hemmingsen, S.D. Compulsive exercise: Links, risks and challenges faced. Psychol. Res. Behav. Manag. 2017, 10, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torstveit, M.K.; Fahrenholtz, I.L.; Lichtenstein, M.B.; Stenqvist, T.B.; Melin, A.K. Exercise dependence, eating disorder symptoms and biomarkers of Relative Energy Deficiency in Sports (RED-S) among male endurance athletes. BMJ Open Sport Exerc. Med. 2019, 5, e000439. [Google Scholar] [CrossRef] [Green Version]
- Bamber, D.J.; Cockerill, I.M.; Rodgers, S.; Carroll, D. Diagnostic criteria for exercise dependence in women. Br. J. Sports Med. 2003, 37, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuikman, M.A.; Mountjoy, M.; Burr, J.F. Examining the Relationship between Exercise Dependence, Disordered Eating, and Low Energy Availability. Nutrients 2021, 13, 2601. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, S.; Pribis, P.; Grajales, T.; Krivak, G. Gender differences in exercise dependence and eating disorders in young adults: A path analysis of a conceptual model. Nutrients 2014, 6, 4895–4905. [Google Scholar] [CrossRef]
- Oppliger, R.A.; Steen, S.A.; Scott, J.R. Weight loss practices of college wrestlers. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Stellingwerff, T.; Heikura, I.A.; Meeusen, R.; Bermon, S.; Seiler, S.; Mountjoy, M.L.; Burke, L.M. Overtraining Syndrome (OTS) and Relative Energy Deficiency in Sport (RED-S): Shared Pathways, Symptoms and Complexities. Sports Med. 2021, 51, 2251–2280. [Google Scholar] [CrossRef]
- Reale, R.; Slater, G.; Burke, L.M. Acute-Weight-Loss Strategies for Combat Sports and Applications to Olympic Success. Int. J. Sports Physiol. Perform. 2017, 12, 142–151. [Google Scholar] [CrossRef]
- de Bruin, A.P.; Oudejans, R.R.; Bakker, F.C.; Woertman, L. Contextual body image and athletes’ disordered eating: The contribution of athletic body image to disordered eating in high performance women athletes. Eur. Eat. Disord. Rev. 2011, 19, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Tiggemann, M. Body image across the adult life span: Stability and change. Body Image 2004, 1, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.P.; Murnen, S.K. “Everybody knows that mass media are/are not a cause of eating disorders”: A critical review of evidence for a causal link between media, negative body image, and disordered eating in females. J. Soc. Clin. Psychol. 2009, 28, 9–42. [Google Scholar] [CrossRef]
- Nerini, A. Media influence and body dissatisfaction in preadolescent ballet dancers and non-physically active girls. Psychol. Sport Exerc. 2015, 20, 76–83. [Google Scholar] [CrossRef]
- Kelley, C.C.; Neufeld, J.M.; Musher-Eizenman, D.R. Drive for thinness and drive for muscularity: Opposite ends of the continuum or separate constructs? Body Image 2010, 7, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Shroff, H.; Thompson, J.K. The tripartite influence model of body image and eating disturbance: A replication with adolescent girls. Body Image 2006, 3, 17–23. [Google Scholar] [CrossRef]
- Goldschmidt, A.B.; Wall, M.; Loth, K.A.; Le Grange, D.; Neumark-Sztainer, D. Which dieters are at risk for the onset of binge eating? A prospective study of adolescents and young adults. J. Adolesc. Health 2012, 51, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Wells, K.R.; Jeacocke, N.A.; Appaneal, R.; Smith, H.D.; Vlahovich, N.; Burke, L.M.; Hughes, D. The Australian Institute of Sport (AIS) and National Eating Disorders Collaboration (NEDC) position statement on disordered eating in high performance sport. Br. J. Sports Med. 2020, 54, 1247–1258. [Google Scholar] [CrossRef]
- Wasserfurth, P.; Palmowski, J.; Hahn, A.; Kruger, K. Reasons for and Consequences of Low Energy Availability in Female and Male Athletes: Social Environment, Adaptations, and Prevention. Sports Med.-Open 2020, 6, 44. [Google Scholar] [CrossRef]
- Gibson, C.; Hindle, C.; McLay-Cooke, R.; Slater, J.; Brown, R.; Smith, B.; Baker, D.; Healey, P.; Black, K. Body Image Among Elite Rugby Union Players. J. Strength Cond. Res. 2019, 33, 2217–2222. [Google Scholar] [CrossRef]
- Wardle, J.; Haase, A.M.; Steptoe, A. Body image and weight control in young adults: International comparisons in university students from 22 countries. Int. J. Obes. 2006, 30, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Torres-McGehee, T.M.; Monsma, E.V.; Gay, J.L.; Minton, D.M.; Mady-Foster, A.N. Prevalence of eating disorder risk and body image distortion among National Collegiate Athletic Association Division I varsity equestrian athletes. J. Athl. Train. 2011, 46, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Bonci, C.M.; Bonci, L.J.; Granger, L.R.; Johnson, C.L.; Malina, R.M.; Milne, L.W.; Ryan, R.R.; Vanderbunt, E.M. National athletic trainers’ association position statement: Preventing, detecting, and managing disordered eating in athletes. J. Athl. Train. 2008, 43, 80–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardon, C.L.; Hainline, B.; Aron, C.M.; Baron, D.; Baum, A.L.; Bindra, A.; Budgett, R.; Campriani, N.; Castaldelli-Maia, J.M.; Currie, A.; et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br. J. Sports Med. 2019, 53, 667–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, J.C.; Williams, N.I.; De Souza, M.J. Prevalence of individual and combined components of the female athlete triad. Med. Sci. Sports Exerc. 2013, 45, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Galmiche, M.; Dechelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Greenleaf, C.; Petrie, T.A.; Carter, J.; Reel, J.J. Female collegiate athletes: Prevalence of eating disorders and disordered eating behaviors. J. Am. Coll. Health 2009, 57, 489–495. [Google Scholar] [CrossRef]
- Rosendahl, J.; Bormann, B.; Aschenbrenner, K.; Aschenbrenner, F.; Strauss, B. Dieting and disordered eating in German high school athletes and non-athletes. Scand. J. Med. Sci. Sports 2009, 19, 731–739. [Google Scholar] [CrossRef]
- Vardar, E.; Vardar, S.A.; Kurt, C. Anxiety of young female athletes with disordered eating behaviors. Eat. Behav. 2007, 8, 143–147. [Google Scholar] [CrossRef]
- Wells, E.K.; Chin, A.D.; Tacke, J.A.; Bunn, J.A. Risk of Disordered Eating Among Division I Female College Athletes. Int. J. Exerc. Sci. 2015, 8, 256–264. [Google Scholar]
- Rousselet, M.; Guerineau, B.; Paruit, M.C.; Guinot, M.; Lise, S.; Destrube, B.; Ruffio-Thery, S.; Dominguez, N.; Brisseau-Gimenez, S.; Dubois, V.; et al. Disordered eating in French high-level athletes: Association with type of sport, doping behavior, and psychological features. Eat. Weight Disord. 2017, 22, 61–68. [Google Scholar] [CrossRef]
- Torstveit, M.K.; Rosenvinge, J.H.; Sundgot-Borgen, J. Prevalence of eating disorders and the predictive power of risk models in female elite athletes: A controlled study. Scand. J. Med. Sci. Sports 2008, 18, 108–118. [Google Scholar] [CrossRef]
- Mancine, R.P.; Gusfa, D.W.; Moshrefi, A.; Kennedy, S.F. Prevalence of disordered eating in athletes categorized by emphasis on leanness and activity type—A systematic review. J. Eat. Disord. 2020, 8, 47. [Google Scholar] [CrossRef]
- Engel, S.G.; Johnson, C.; Powers, P.S.; Crosby, R.D.; Wonderlich, S.A.; Wittrock, D.A.; Mitchell, J.E. Predictors of disordered eating in a sample of elite Division I college athletes. Eat. Behav. 2003, 4, 333–343. [Google Scholar] [CrossRef]
- Arthur-Cameselle, J.; Quatromoni, P. Factors Related to the Onset of Eating Disorders Reported by Female Collegiate Athletes. Sport Psychol. 2010, 25, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ravi, S.; Ihalainen, J.K.; Taipale-Mikkonen, R.S.; Kujala, U.M.; Waller, B.; Mierlahti, L.; Lehto, J.; Valtonen, M. Self-Reported Restrictive Eating, Eating Disorders, Menstrual Dysfunction, and Injuries in Athletes Competing at Different Levels and Sports. Nutrients 2021, 13, 3275. [Google Scholar] [CrossRef] [PubMed]
- Surala, O.; Malczewska-Lenczowska, J.; Sadowska, D.; Grabowska, I.; Bialecka-Debek, A. Traits of Orthorexia Nervosa and the Determinants of These Behaviors in Elite Athletes. Nutrients 2020, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Segura-Garcia, C.; Papaianni, M.C.; Caglioti, F.; Procopio, L.; Nistico, C.G.; Bombardiere, L.; Ammendolia, A.; Rizza, P.; De Fazio, P.; Capranica, L. Orthorexia nervosa: A frequent eating disordered behavior in athletes. Eat. Weight Disord. 2012, 17, e226–e233. [Google Scholar] [CrossRef] [PubMed]
- Sundgot-Borgen, J. Risk and trigger factors for the development of eating disorders in female elite athletes. Med. Sci. Sports Exerc. 1994, 26, 414–419. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Stellingwerff, T.; Elliott-Sale, K.J.; Baltzell, A.; Cain, M.; Goucher, K.; Fleshman, L.; Mountjoy, M.L. #REDS (Relative Energy Deficiency in Sport): Time for a revolution in sports culture and systems to improve athlete health and performance. Br. J. Sports Med. 2020, 54, 369–370. [Google Scholar] [CrossRef]
- Slater, J.; McLay-Cooke, R.; Brown, R.; Black, K. Female Recreational Exercisers at Risk for Low Energy Availability. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 421–427. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Areta, J.L. Low energy availability in female athletes: From the lab to the field. Eur. J. Sport Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Black, D.R.; Larkin, L.J.; Coster, D.C.; Leverenz, L.J.; Abood, D.A. Physiologic Screening Test for Eating Disorders/Disordered Eating Among Female Collegiate Athletes. J. Athl. Train. 2003, 38, 286–297. [Google Scholar] [PubMed]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.C.; Peterson, C.B.; Frazier, P.; Crow, S.J. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. Int. J. Eat. Disord. 2012, 45, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, D.M. EDI-3—Eating Disorder Inventory: Professional Manual; Psychological Assessment Resources. Inc.: Lutz, FL, USA, 2004. [Google Scholar]
- Kennedy, S.F.; Kovan, J.; Werner, E.; Mancine, R.; Gusfa, D.; Kleiman, H. Initial validation of a screening tool for disordered eating in adolescent athletes. J. Eat. Disord. 2021, 9, 21. [Google Scholar] [CrossRef]
- Martinsen, M.; Holme, I.; Pensgaard, A.M.; Torstveit, M.K.; Sundgot-Borgen, J. The development of the brief eating disorder in athletes questionnaire. Med. Sci. Sports Exerc. 2014, 46, 1666–1675. [Google Scholar] [CrossRef]
- McNulty, K.Y.; Adams, C.H.; Anderson, J.M.; Affenito, S.G. Development and validation of a screening tool to identify eating disorders in female athletes. J. Am. Diet. Assoc. 2001, 101, 886–892. [Google Scholar] [CrossRef]
- Nagel, D.L.; Black, D.R.; Leverenz, L.J.; Coster, D.C. Evaluation of a screening test for female college athletes with eating disorders and disordered eating. J. Athl. Train. 2000, 35, 431–440. [Google Scholar]
- Stice, E.; Fisher, M.; Martinez, E. Eating disorder diagnostic scale: Additional evidence of reliability and validity. Psychol. Assess. 2004, 16, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Otis, C.L.; Drinkwater, B.; Johnson, M.; Loucks, A.; Wilmore, J. American College of Sports Medicine position stand. The Female Athlete Triad. Med. Sci. Sports Exerc. 1997, 29, i–ix. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Orbach, I.; Mikulincer, M. The Body Investment Scale: Construction and Validation of a Body Experience Scale. Psychol. Assess. 1998, 10, 415–425. [Google Scholar] [CrossRef]
- Sandoz, E.; Wilson, K.G.; Merwin, R.M.; Kellum, K.K. Assessment of body image flexibility: The Body Image-Acceptance and Action Questionnaire. J. Contextual Behav. Sci. 2013, 2, 39–48. [Google Scholar] [CrossRef]
- Lucena-Santos, P.; Carvalho, S.A.; Oliveira, M.D.S.; Pinto-Gouveia, J. Body-Image Acceptance and Action Questionnaire: Its deleterious influence on binge eating and psychometric validation. Int. J. Clin. Health Psychol. 2017, 17, 151–160. [Google Scholar] [CrossRef]
- Cash, T.; Szymanski, M.L. The development and validation of the body-image ideals questionnaire. J. Personal. Assess. 1995, 64, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.; Phillips, K.A.; Santos, M.T.; Hrabosky, J.I. Measuring “negative body image”: Validation of the Body Image Disturbance Questionnaire in a nonclinical population. Body Image 2004, 1, 363–372. [Google Scholar] [CrossRef]
- Cooper, P.; Taylor, M.J.; Cooper, Z.; Fairbum, C.G. The development and validation of the body shape questionnaire. Int. J. Eat. Disord. 1987, 6, 485–494. [Google Scholar] [CrossRef]
- Goltz, F.R.; Stenzel, L.M.; Schneider, C.D. Disordered eating behaviors and body image in male athletes. Braz. J. Psychiatry 2013, 35, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuikman, M.A.; Mountjoy, M.; Stellingwerff, T.; Burr, J.F. A Review of Nonpharmacological Strategies in the Treatment of Relative Energy Deficiency in Sport. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 268–275. [Google Scholar] [CrossRef]
- Fredericson, M.; Kussman, A.; Misra, M.; Barrack, M.T.; De Souza, M.J.; Kraus, E.; Koltun, K.J.; Williams, N.I.; Joy, E.; Nattiv, A. The Male Athlete Triad—A Consensus Statement From the Female and Male Athlete Triad Coalition Part II: Diagnosis, Treatment, and Return-To-Play. Clin. J. Sport Med. 2021, 31, 349–366. [Google Scholar] [CrossRef]
- Joy, E.; De Souza, M.J.; Nattiv, A.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr. Sports Med. Rep. 2014, 13, 219–232. [Google Scholar] [CrossRef]

| Author Year | Athlete Population | Duration | LEA (kcal·kg FFM−1·Day−1) Mean ± SD | % Athletes with LEA | Implications of LEA |
|---|---|---|---|---|---|
| Soccer | |||||
| Magee 2020 [18] | NCAA DIII soccer (n = 18, height: 1.67 ± 0.05 m; body mass: 65.3 ± 7.9 kg; body fat %: 24.9 ± 5.6%) | 4 days | All: 27.5 ± 8.9 LEA: 23.0 ± 5.7 Non-LEA: 36.4 ± 7.3 | The screening tool classified 56.3% of athletes as at risk of LEA. Actual dietary intake identified 67% as LEA. | N/A |
| Morehen 2021 [31] | Professional soccer (n = 24; height: 168.1 ± 5.9 cm; weight: 62.1 ± 4.7 kg; body fat%: 20.6 ± 3.7% | 9-day international training camp (4 training days, 1 rest day, 2 travel days, 2 match days) | (n = 17) 18 ± 9 (range: 2–36) | <30 kcal·kg FFM−1·day−1, 88% of players | N/A |
| Cherian 2019 [32] | Junior soccer (n = 19; age: 12.2 ± 1.83 years; height: 1.54 ± 0.04 m; weight: 45.1 ± 6.58 kg; body fat%: 23.8 ± 3.46%) | 3-days | All: 27.1 ± 14.44 U12: 31.7 ± 10.10 U16: 24.1 ± 12.32 | <30 kcal·kg FFM−1·day−1, 58% of girls, of which 37% were Under-16 players. | N/A |
| Moss 2020 [33] | Professional soccer (n = 13) (age: 23.7 ± 3.4 yrs., height: 1.69 ± 0.08 m, body mass: 63.7 ± 7.0 kg) | 5 days during a competitive season | All Days: 35 ± 10 Rest Days: 42 ± 7 Light Days: 35 ± 11 Heavy Day: 29 ± 10 Match Day: 29 ± 16 | 30–45 kcal·kg FFM−1·day−1: 62% LEA (<30 kcal·kg FFM−1·day−1): 23% | LEA athletes met criteria for low resting metabolic rate. Other biochemical markers were inconclusive. |
| Reed 2013 [19] | NCAA DI soccer (n = 19, age: 19.23 ± 0.3 yrs.; height: 1.66 ± 0.0 m; weight: 60.6 ± 1.4 kg; body fat%: 22.5 ± 1.1% VO2 Max: 57.0 ± 1.0 mL kg(−1) min(−1)) | 3-day monitoring at Pre-, Mid-, and Post-season time points | Mid-season: 35.2 ± 3.7 Post-season: 44.5 ± 3.7 (p = 0.009) | Low energy availability (<30 kcal·kg FFM−1·day−1) was observed:
| N/A |
| Track & Field and endurance athletes | |||||
| Heikura 2018 [34] | National/world-class distance runners (n = 25; age: 23–27 years; height: 1.69–1.83 m; body mass: 52.9–70.5 kg) | 7-day monitoring | N/A | LEA: 11/35 (31%) | Amenorrheic and low testosterone athletes had significantly lower sex hormones, triiodothyronine, and bone mineral density, with a ~4.5-fold increased prevalence of bone injuries. |
| Beerman 2020 [16] | NCAA DI cross country (n = 20, age: 20.2 ± 1.7 years; height: 1.77 ± 0.06 m; body mass: 53.7 ± 6.5 kg; body fat %: 23.3 ± 3.6%) | 3-month average | 32.8 ± 16.1 | 30–44 kcal·kg FFM−1·day−1: 7 (41%) <30 kcal·kg FFM−1·day−1: 7 (41%) | N/A |
| Day 2015 [17] | NCAA DI track and field (n = 25, age: 19.5 ± 1.8 years; height: 1.69 ± 0.05 m; body mass: 61.1 ± 6.9 kg; body fat %: 22.3 ± 3.3%) | 3-day monitoring | 30.8 | <45 kcal·kg FFM−1·day−1: 23 (92%) <30 kcal·kg FFM−1·day−1: 13 (52%) | N/A |
| Melin 2014 [35] | Endurance athletes (n = 45; age: 26.6 ± 5.4; height: 1.6 9 ± 0.0; weight: 58.7 ± 6.8 kg; body fat %: 20.2 ± 3.4 | 7 consecutive days | All: 38.5 ± 13.9 At risk for LEA: 37.3 ± 13.1 Not at risk: 40.4 ± 15.3 | At Risk for LEA (LEAF-Q>8): 28/45 (62%) | N/A |
| Schaal 2021 [36] | Healthy distance runners: well-adapted [WA] (age: 29.4 ± 1.6 yrs.; height: 1.65 ± 0.2 m; weight: 57.6 ± 1.6 kg; body fat %: 22.5 ± 1.4) and non-functional overreaching (NFOR) (age: 27.7 ± 2.3 yrs.; height: 1.69 ± 0.2 m; weight: 59.1 ± 3.0 kg; body fat %: 23.5 ± 1.3) | Baseline: 24–35 Days Training Overload: 4 weeks Recovery Phase: 2 weeks | Baseline: WA:24.4 ± 3.7 NFOR: 30.4 ± 1.9 Training Overload: WA: 26.3 ± 3.8 NFOR: 24.8 ± 2.8 Recovery Phase: WA: 24.3 ± 4.0 NFOR: 26.8 ± 1.6 | N/A | Suppressed ovarian function. Decreased running performance. |
| Viner 2015 [37] | Competitive cyclists (n = 4; age: 38.4 ± 10.3 yrs.; height: 1.65 ± 0.06 m; weight: 62.8 ± 12.2 kg; body fat %: 24.9 ± 8.4%) | 3 days·month–1, through one cycling season. Records were completed on alternating days each month to represent all days of the week. | Pre-Season: 26.2 ± 14.1 Competition: 25.5 ± 3.1 Off-Season: 23.8 ± 8.9 | Low energy availability (<30 kcal·kg FFM−1·day−1): 100% at all time points. | N/A |
| Other Sports | |||||
| Zabriskie 2019 [38] | NCAA DII lacrosse (n = 20, age: 20.4 ± 1.8 years; height: 1.68 ± 0.06 m; body mass: 68.8 ± 8.9 kg; body fat %: 27.9 ± 3.0%) | 5 periods, of 4-day monitoring | Off-season: 30.4 ± 11.0 Off-season: 26.2 ± 10.5 Pre-season: 22.9 ± 8.5 In-season: 28.7 ± 9.5 In-season: 28.9 ± 9.2 | Off-season: 10/20 (50%) Off-season: 12/20 (60%) Pre-season: 15/20 (75%) In-season: 12/20 (60%) In-season: 12/20 (60%) | Associated with reduced sleep quality and perceived rest. |
| Zanders 2021 [39] | NCAA DII basketball (n = 13; age: 19.8 ± 1.3 yrs.; height: 1.74 ± 0.1 m; weight: 74.6 ± 9.1 kg; body fat %: 27.1 ± 3.2%) | 5 periods, of 4-day monitoring | In-Season (non-conf): 21.8 ± 7.8 In-Season (conf): 22.3 ± 13.7 In-Season (playoffs): 22.5 ± 11.2 Off-Season I: 31.8 ± 8.1 Off-Season II: 30.6 ± 9.5 | In-Season (non-conf): 10/11 (91%) In-Season (conf): 10/11 (91%) In-Season (playoffs): 5/9 (56%) Off-Season I: 3/10 (30%) Off-season II: 6/11 (55%) | N/A |
| Braun 2018 [40] | Elite soccer (n = 56; age: 14.8 ± 0.7 yrs.; height: 166 ± 5 m; weight: 56.8 ± 6.1 kg; body fat%: 17.2 ± 3.9%) | 7-day food & activity records | 30.0 ± 7.3 Range: 20.3 to 51.0 | LEA (i.e., <30 kcal·kg FFM−1·day−1): 53% | N/A |
| Woodruff 2013 [41] | University volleyball (n = 10; age: 20.9 ± 1.4 yrs.; height: 1.77 ± 0.05 m; weight: 75.0 ± 9.7 kg; body fat %: 25.2 ± 6.9%) | 7-day food & activity records | 42.5 | LEA (i.e., <30 kcal·kg FFM−1·day−1): 2/10 (2%) | N/A |
| Schaal 2017 [21] | Synchronized swimming (n = 11; age: 20.4 ± 0.4 yrs.; weight: 58.9 ± 1.8 kg; body fat%: 17.3 ± 0.6%) | 4-day food & activity monitoring period | Baseline: 25.0 ± 3.2 Week 2: 22.3 ± 1.9 Week4: 18.0 ± 2.8 | LEA (<30 kcal·kg FFM−1·day−1): 11/11 (100%) | Associated with perceived fatigue and endocrine signs of conservation (i.e., increase ghrelin and decrease in leptin). |
| Costa 2018 [22] | Collegiate female synchronized swimmers (n = 21, 20.4 ± 1.6 yrs.; height: 168 ± 4.9 cm; weight: 64.4 ± 8.7 kg; body fat%: 28.4 ± 4.5% fat) | 4-day food & activity monitoring. AEE was estimated using MET values | Low AEE estimate: 30.27 ± 12.6 kcal/kg FFM High AEE estimated: 26.1 ± 12.4 kcal/kg FFM | 52% (11/21) were below 30 kcal·kg FFM−1·day−1 while an additional 38% (8/21) were between 30–45 kcal·kg FFM−1·day−1 | N/A |
| Civil 2018 [42] | Vocational ballet students (n = 20; age: 18.1 ± 1.1 years; body mass index: 19.0 ± 1.6 kg·m2; body fat: 22.8 ± 3.4%) | 7 days, including 5 weekdays (with dance training) and 2 weekend days (without scheduled dance training) | Weekdays 38 ± 13 Weekend days 44 ± 13 (p = 0.110). | Reduced energy availability (30–45 kcal·kg FFM·day−1: 44% LEA (i.e., <30 kcal·kg FFM−1·day−1): 22% | Association with menstrual dysfunction. |
| Torres-McGehee 2021 [20] | Collegiate athletes and performing artists (n = 121; age: 19.8 6 ± 2.0 yrs.; height: 168.9 ± 7.7 cm, body mass: 63.6 ± 9.3 kg); equestrian (n = 28), soccer (n = 20), beach volleyball (n = 18), softball (n = 17), volleyball (n = 12), and ballet (n = 26) | 7 consecutive days | All: 19.5 ± 16.1 Equestrian: 21.9 ± 9.9 Volleyball: 18.6 ± 10.9 Softball: 7.8 ± 6.4 Beach Volleyball: 12.44 ± 9.6 | All: 81% (96/121) Equestrian: 82.1% (23/28) Volleyball: 83.3% (10/12) Softball: 100% (17/17) Beach Volleyball: 94.4% (17/18) Ballet: 96.2% (25/26) Soccer: 30% (6/20) | N/A |
| Author Year | Athlete Population | Primary Variables | Results (% of Questions Answered Correctly) |
|---|---|---|---|
| Abood 2004 [60] | Collegiate soccer and basketball (n = 30; height: 167.4 ± 6.1 cm; body mass: 61.9 ± 5.9 kg) | 42-item true/false questionnaire related to total calories, carbohydrate, fat, protein, calcium iron, and zinc | 67–70% |
| Andrews 2016 [61] | NCAA DI (n = 47) | 20-item questionnaire related to macronutrients, micronutrients, supplements, weight management, eating disorders, and hydration | 56.5% |
| Cupisti 2002 [62] | Elite national adolescent (n = 60; height: 167.0 ± 6.0 cm; body mass: 55.8 ± 9.0 kg) | 20-item questionnaire on fats, carbohydrates, proteins, vitamins, minerals, and fiber | 77.6% |
| Dunn 2007 [63] | NCAA DI (n = 98) | Nutrition and Knowledge Questionnaire [9] | 51.49 ± 13.57% |
| Grete 2011 [64] | NCAA softball (n = 185) | 80-item questionnaire that ranged in topic from general nutrition to specific effects of nutrients | 45.7 ± 4.7% |
| Jagim 2021 [48] | NCAA Division III (n = 42, height: 169.9 ± 6.9 cm; body mass: 67.1 ± 8.6 kg; fat-free mass: 51.3 ± 6.6 kg; body fat per cent: 24.2 ± 5.3%) | Abridged Sports Nutrition Knowledge Questionnaire [65] | 47.03 ± 11.04% |
| Condo 2019 [54] | Australian rules football (n = 30) (age: 24.15 ± 4.1 yrs.; weight: 64.5 kg ± 8.0; height: 168.2 cm ± 7.6) | Sports Nutrition Knowledge Questionnaire (SNKQ) [66] | Median (IQR), % correct General Nutrition Concepts (46): 28 (7), 60.8% Fluid (9): 6 (7), 66.7% Recovery (7): 4 (3), 57.1% Weight Control (15): 7 (3), 46.7% Supplements (11): 2 (3), 18.2% Total Nutrition Knowledge (88): 48 (12), 54.5% |
| Jessri 2010 [55] | International collegiate (n = 98) | 88-item nutrition knowledge questionnaire on nutrient type (n = 46), recovery (n = 7), fluids (n = 9), weight control (n = 15) and supplements (n = 11) | Nutrient type: 42.6% ± 18.6% Recovery: 38.4 ± 15.2% Fluids: 38.2 ± 17.5% Weight control: 39.1 ± 16.7% Supplements: 35.3 ± 15.4% |
| Manore 2017 [51] | High school (n = 297) | 40-item questionnaire on dietary and hydration practices, attitudes towards nutrition and hydration, nutrition knowledge, and sources of nutritional information | All: 45.1% White: 48.4% Latino: 38.8% |
| Nikolaidis 2014 [67] | Semiprofessional soccer (n = 185; height: 177.5 ± 6.4 cm; body mass: 72.3 ± 8.4kg) | 11-item nutrition knowledge questionnaire | 5.4 ± 1.7 |
| Rash 2008 [56] | NCAA DI track (n = 52) | Questionnaire related to carbohydrates, protein, vitamins and minerals, vitamin C, and vitamin E | All: 57.8 ± 1.8% Carbohydrates: 74.5 ± 17.3% Protein: 54.2 ± 16.0% Vitamins and Minerals: 62.5% Vitamin C: 33.7 ± 36.7% Vitamin E: 47.1 ± 33.8% |
| Rosenbloom 2002 [57] | NCAA DI (n = 91) | 11-item questionnaire related to macronutrients, hydration, and micronutrients | Average knowledge score was 5.7 ± 1.9 (out of 11) 54% knew that carbohydrate and fat are the main energy source for activity 75% knew that eating carbohydrates would not make them fat 49% knew that high-fat meals should not be eaten 2–3 h before an event 71% believed that sugar before an event would adversely affect performance 92% knew that dehydration negatively impacts performance 95% knew that fluids should be replaced pre-, during, and post-training 80% knew that thirst is not an indicator of fluid need 53% believed vitamin and mineral supplements increase energy |
| Sedek 2014 [68] | Pakistani University (n = 50; height: 160 ± 10cm; body mass: 53.1 ± 8.6 kg) | 29-item questionnaire Nutrition knowledge was classified as very good (85–100%), good (70–84%), moderate (55–69%), and weak (<55%) | 57% classified as having a “good” understanding of nutrition knowledge 43% classified as having a “very good” understanding of nutrition knowledge |
| Shifflett 2002 [69] | NCAA D I, II and III (n = 52) | 20-item questionnaire related to information of perceived understanding of nutritional needs, importance of healthy diet, quality of eating habits, and sources of nutrition information | 52.5% |
| Spronk 2015 [58] | Elite (n = 64) | General Nutrition Knowledge Questionnaire | Total: 59.5% Sources of Nutrients: 66.2% Choosing Foods: 61.0% Diet-disease Relationships: 43% |
| Torres-McGehee 2012 [59] | NCAA DI, II, and III (n = 111) | 20-item questionnaire related to micronutrients, macronutrients, supplements, weight management, eating disorders, and hydration | All: 54.9 ± 13.5% Micronutrient and Macronutrient: 51.8 ± 20.5% Dietary Supplements: 66.3 ± 19.9% Weight Management and Eating Disorders: 47.0 ± 21.9% Hydration: 54.7 ± 24.2% |
| Author Year | Athlete Population | Duration | Activity Energy Expenditure | Total Daily Energy Expenditure | Physical Activity Level (PAL) |
|---|---|---|---|---|---|
| Endurance Sports | |||||
| Day 2015 [17] | NCAA DI track and field (n = 27; height: 168.8 ± 4.7 cm; body mass: 61.1 ± 6.9 kg; body fat %: 22.3 ± 3.3%) | 3 consecutive days at 2 different time points (6 days total) | 711 ± 524 kcal | N/A | N/A |
| Edwards 1993 [81] | Elite distance runners (n = 9; body mass: 55.29 ± 6.18; height: 169.1 ± 5.5 cm; BMI: 19.32 ± 1.67 kg·m−2; body fat %: 13.0 ± 3.2%) | 7-day monitoring period | N/A | 2990 ± 415 kcal·day−1 | N/A |
| Loftin 2007 [82] | Recreational marathon runners (n = 10; age: 43 ± 12 yr.; body mass: 60.8 ± 5.7 kg; height: 162 ± 13 cm; body fat: 24.9 ± 5.5%) | Indirect open-circuit calorimetry to estimate EE of recent marathon performance | 2436 ± 297 kcal | N/A | N/A |
| Schulz 1992 [83] | Elite distance runners (n = 9; age: 26 ± 3 yr.; 53 ± 4 kg, 12 ± 3% body fat, and VO2 max: 66 ± 4 mL·kg−1·min−l) | 6-day monitoring period training mileage (10 ± 3 miles/day). | 1087 ± 244 kcal | 2826 ± 312 kcal·day−1 | 1.99 ± 0.3 |
| Trappe 1997 [84] | Swimmers (n = 5; age, 19 ± 1 yr.; height: 178.3 ± 2.2 cm; body mass: 65.4 ± 1.6 kg) | 5-day high volume training period (17.5 ± 1.0 km.d−1) | N/A | 5593 ± 495 kcal·day−1 | 3.0 ± 0.2 |
| Ultra-distance | |||||
| Costa 2014 [85] | Ultra-endurance runners (n = 25; age: 39 ± 7 yr.; body mass: 78 ± 11 kg; height: 177 ± 8 cm) | 24 h ultra-marathon (distance range: 122–208 km) | 10,755 ± 1912 kcal (equivalent to 454 kcal/h) | ||
| Soccer | |||||
| Moss 2020 [33] | Professional soccer (n = 13; height: 1.69 ± 0.08 m, body mass: 63.7 ± 7.0 kg) | 5-day monitoring period | Rest days: 15 ± 54 kcal Light training days: 299 ± 78 kcal Heavy training days: 786 ± 159 kcal Match days: 881 ± 473 kcal | N/A | N/A |
| Morehen 2021 [31] | Professional soccer (n = 24; height: 168.1 ± 5.9 cm; weight: 62.1 ± 4.7 kg; body fat%: 20.6 ± 3.7% | 9-day international training camp in (4 training days, 1 rest day, 2 travel days, 2 match days) | 1058 ± 352 kcal·day−1 (range: 155–1549 kcal·day−1) | 2693 ± 432 kcal·day−1 (range: 2105–3507 kcal·day−1) 43 ± 6 kcal·kg·day−1 (range: 33–55 kcal·kg·day−1) 54 ± 6 kcal·kg·day−1 FFM (range: 45–68 kcal·kg·day−1 FFM). | 1.79 ± 0.24 (range: 1.4–2.2) |
| Mara 2015 [80] | Elite soccer (n = 8; height: 172.9 ± 5.5 cm; body mass: 65.1 ± 5.9 kg; body fat %: 23.2 ± 6.2%) | 7-day monitoring period | Friendly game: 644 ± 72 kcal Training session: 607 ± 76 kcal | Game days: 2925 ± 144 kcal·day−1 Training days: 2794 ± 65 kcal·day−1 Rest days: 2274 ± 88 kcal·day−1 | N/A |
| Yli-Piipari 2019 | Division I collegiate (age: 19.86 ± 1.35 yr.) (n = 18: 5 tennis, 13 soccer) | 4-day monitoring period (1 game/ match, 2 training sessions, and 1 rest day) | Game/Match days: 2848 ± 304 kcal·day−1 Training days: 2622 ± 248 kcal·day−1 Rest days: 1833 ± 959 kcal·day−1 | ||
| Reed 2013 [19] | NCAA DI soccer (n = 19; height: 165.6 ± 1.2 cm; body mass: 60.6 ± 1.4 kg; body fat %: 22.5 ± 1.1%) | 3 consecutive days at 3 different time points (9 days total) | Pre-season: 819 ± 57 kcal Mid-season: 642 ± 26 kcal Post-season: 159 ± 28 kcal | N/A | N/A |
| Lacrosse and basketball | |||||
| Zabriskie 2019 [38] | NCAA DII lacrosse (n = 20; height: 168.5 ± 6.6 cm; body mass: 68.8 ± 8.9 kg; body fat %: 27.9 ± 3.0%) | 4 consecutive days at 3 different time points (20 days total) | Off-season: 842 ± 267 kcal Off-season: 804 ± 244 kcal Pre-season: 1001 ± 267 kcal In-season: 749 ± 161 kcal In-season: 817 ± 235 kcal | Off-season: 2608 ± 378 kcal·day−1 Off-season: 2579 ± 376 kcal·day−1 Pre-season: 2798 ± 391 kcal·day−1 In-season: 2513 ± 248 kcal·day−1 In-season: 2582 ± 303 kcal·day−1 | Off-season: 1.75 ± 0.19 Off-season: 1.72 ± 0.14 Pre-season: 1.87 ± 0.15 In-season: 1.69 ± 0.15 In-season: 1.73 ± 0.18 |
| Kumahara 2020 [86] | Japanese collegiate lacrosse (n = 17; age: 20 ± 1 yr.; height: 159.0 ± 5.7 cm; body mass: 53.0 ± 5.3 kg; body mass index: 20.9 ± 1.7 kg·m−2 | 1-week period during: Preparatory [P-phase] (approximately 9-week period) Transition [T-phase] (approximately 2-week period) | P-phase 410 ± 144 kcal T-phase: 3 ± 12 kcal | P-phase 2168 ± 248 kcal·day−1 T-phase: 1744 ± 138 kcal·day−1 | |
| Moon 2021 [79] | NCAA DII basketball (n = 13; height: 173 ± 13.6 cm; body mass: 74.6 ± 9.1 kg; body fat %: 27.1 ± 3.2%) and lacrosse (n = 20; height: 168.4 ± 6.6 cm; body mass: 68.8 ± 8.9 kg; body fat %: 27.9 ± 3.0%) | 4 consecutive days at 5 different time points (20 days total) | Game Lacrosse: ~1050 kcal Basketball: ~1600 kcal Practice Lacrosse: ~980 kcal Basketball: ~1150 kcal Conditioning Lacrosse: ~800 kcal Basketball: ~1150 kcal Lacrosse: ~625 kcal | Game Lacrosse: ~2850 kcal·day−1 Basketball: ~3500 kcal·day−1 Practice Lacrosse: ~2700 kcal·day−1 Basketball: ~3000 kcal·day−1 Conditioning Lacrosse: ~2550 kcal·day−1 Basketball: ~3050 kcal·day−1 Off day Lacrosse: ~2400 kcal·day−1 Basketball: ~2480 kcal·day−1 | Game Lacrosse: 1.85 Basketball: 2.2 Practice Lacrosse: 1.8 Basketball: 1.9 Conditioning Lacrosse: 1.7 Basketball: 1.9 Off day Lacrosse: 1.58 Basketball: 1.55 |
| Zanders 2021 [39] | NCAA DII basketball (n = 13; height: 173 ± 13.6 cm; body mass: 74.6 ± 9.1 kg; body fat %: 27.1 ± 3.2%) | 4 consecutive days at 5 different time points (20 days total) | Phase 1: 1196 ± 296 kcal Phase 2: 1252 ± 157 kcal Phase 3: 1028 ± 157 kcal Phase 4: 819 ± 160 kcal Phase 5: 969 ± 362 kcal | Phase 1: 3065 ± 361 kcal·day−1 Phase 2: 2866 ± 363 kcal·day−1 Phase 3: 2850 ± 159 kcal·day−1 Phase 4: 2674 ± 216 kcal·day−1 Phase 5: 2806 ± 419 kcal·day−1 | Phase 1: 1.75 ± 0.27 Phase 2: 1.63 ± 0.22 Phase 3: 1.62 ± 0.15 Phase 4: 1.52 ± 0.17 Phase 5: 1.59 ± 0.23 |
| Silva 2017 [87] | Elite basketball, handball, volleyball, triathlon, and swimming (n = 18; height: 172.4 ± 5.6 cm; body mass: 62.3 ± 7.4 kg; fat mass: 14.3 ± 2.7 kg) | 1 day at 2 different time points (2 days total) | Beginning of the season: 1297 ± 498 kcal Main stage of the competition: 1670 ± 301 kcal | Beginning of the season: 3126 ± 520 kcal·day−1 Main stage of the competition: 3549 ± 317 kcal·day−1 | N/A |
| Silva 2013 [88] | Elite junior basketball (n = 7; height 173.1 ± 3.3 cm; body mass: 64.0 ± 5.4 kg; body fat%: 20.0 ± 4.6%) | 7-day monitoring period In season | 2103 ± 272 kcal | 3493 ± 242 kcal·day−1 | 2.6 ± 0.3 |
| Other | |||||
| Torres-McGehee 2020 [20] | NCAA DI equestrian, soccer, beach volleyball, softball, volleyball, ballet (n = 121; height: 168.9 ± 7.7 cm; body mass: 63.6 ± 9.3 kg; fat-free mass: 48.4 ± 4.9 kg; body fat %: 26.1 ± 5.4) | 7-day monitoring period | Total: 825.8 ± 350.3 kcal Equestrian: 403 ± 62 kcal Beach Volleyball: 1109 ± 158 kcal Softball: 811 ± 131 kcal Volleyball: 838 ± 78 kcal/day Ballet: 811 ± 408 kcal Soccer: 1187 ± 40 kcal | Total: 2428 ± 145 kcal·day−1 Equestrian: 2389 ± 117 kcal·day−1 Beach Volleyball: 2447 ± 86 kcal·day−1 Softball: 2550 ± 172 kcal/day Volleyball: 2357 ± 114 kcal·day−1 Ballet: 2468 ± 127 kcal·day−1 Soccer: 2468 ± 61 kcal·day−1 | N/A |
| Fraczek 2019 [89] | Elite speed skating, cross-country skiing, mountain biking, volleyball, downhill skiing, middle-distance running, kayaking (n = 15, height: 172.5 ± 6.2 cm; body mass: 63.7 ± 5.2; fat-free mass: 50.5 ± 4.4 kg; body fat%: 21.2 ± 5.2%) | 7-day monitoring period | N/A | Accelerometer: 2289 ± 286 kcal·day−1 Relative: 35.9 kcal·kg·day−1 Self-completed questionnaire: 3156 ± 620 kcal·day−1 Relative: 49.5 kcal·kg·day−1 | 1.75–2.0 |
| Hill 2002 [90] | Elite lightweight rowing (n = 7; height: 168.8 ± 4.7 cm; body mass: 60.9 ± 2.3 kg; body fat %: 22.8 ± 5.1%) | 14-day monitoring period | N/A | 3957 ± 1219 kcal·day−1 | N/A |
| Woodruff 2013 [41] | Inter-university volleyball (n = 10; height: 177 ± 5 cm; body mass: 75 ± 9.7 kg; body fat percent: 25.2 ± 6.9% | 7-day monitoring period | Starters: 392–892 kcal Reserve: 416–734 kcal | 3479 ± 604 kcal·day−1 | N/A |
| Author Year | Name of Tool/Metric | Primary Focus | Direct or Indirect | Target Population |
|---|---|---|---|---|
| Low Energy Availability | ||||
| Loucks 1994, 2011 [6,27] | Energy Availability Assessment | Energy availability | Direct | All athletes |
| Melin 2014 [12] | Low Energy Availability in Females Questionnaire (LEAF-Q) | Identify those at risk of LEA | Indirect | Adult female athletes |
| Slater 2016 [130] | Low Energy Availability Amongst New Zealand Athletes (LEANZA) questionnaire | Identify those at risk of LEA | Indirect | Adult female athletes |
| Heikura 2021 [131] | Lab markers (i.e., T3, T4, LH, hepcidin, Testosterone, etc.) | Identify biomarkers that may indicate risk of LEA | Indirect | Female and male athletes |
| Staal 2018 [23] | Resting Metabolic Rate Ratio | Identify those at risk of LEA via suppressed metabolic rate | Indirect | Female ballet dancers |
| Eating Disorders | ||||
| Black 2003 [132] | The Bulimia Test-Revised | Screening test designed to assess bulimia-type characteristics | Indirect | Female athletes |
| Garner 1982 [133] | Eating Attitudes Test (EAT) | Screening tool for anorexia nervosa | Indirect | Adult females |
| Berg 2012 [134] | Eating Disorder Examination Questionnaire | Self-reported questionnaire for the assessment and diagnoses of the DSM-IV eating disorders | Indirect | Adult females |
| Garner 2004 [135] | Eating Disorder Inventory (EDI-3) | Self-reported measure for identifying eating disorder patterns and associated psychological constructs | Indirect | Valid in individuals aged 13 to 53 years for identifying disordered eating patterns and has high reliability (Cronbach a mean = 0.94, range = 0.90–0.97) |
| Kennedy 2021 [136] | Disordered Eating Screening Tool for Athletes (DESA-6) | Screening tool for disordered eating | Indirect | Adolescent athletes |
| Martinsen 2014 [137] | Brief Eating Disorder in Athletes Questionnaire (BEDA-Q) | Brief questionnaire able to discriminate between female elite athletes with and without an eating disorder | Indirect | High school female athletes |
| McNulty 2001 [138] | Female Athlete Screening Tool (FAST) | Screening tool to identify eating disorders | Indirect | Collegiate female athletes |
| Nagel 2000 [139] | Athletic Milieu Direct Questionnaire (AMDQ) version 2 | Screening tool to identify eating disorders | Indirect | Female athletes |
| Stice 2004 [140] | Eating Disorder Diagnostic Scale | A brief self-report measure for diagnosing anorexia nervosa, bulimia nervosa, and binge eating disorder | Indirect | Adolescent, collegiate, and adult females |
| Female Athlete Triad | ||||
| Otis 1997 [141] | Menstrual Cycle | Used to assess delayed menarche, menstrual irregularities or amenorrhea | Direct | Female athletes |
| Otis 1997 [141] | Bone Mineral Density | Low BMC or BMD * is defined as a BMC or areal BMD Z-score that is ≤−2.0, adjusted for age, gender and body size, as appropriate. [142] * American College of Sports Medicine (ACSM) defines low BMC or BMD as a Z-score that is less than −1.0 in female athletes in weight-bearing sports | Direct | Multiple populations |
| Otis 1997 [141] | Eating Disorder (see above examples: FAST. AMDQ, DESA-6, EAT, EDI-3, BEDA-Q) | See above examples | Indirect | |
| Body Image/Dissatisfaction | ||||
| Orbach 1998 [143] | Body Image Investment Scale | Identify those at risk for body image issues | Indirect | Boys and girls (age: 13–19 years) 3–19 years |
| Garner 2004 [135] | Eating Disorder Inventory-3 subscale c: body dissatisfaction | Identify risk factors for eating disorder associated with body dissatisfaction | Indirect | Valid in individuals aged 13 to 53 years for identifying disordered eating patterns and has high reliability (Cronbach a mean = 0.94, range = 0.90–0.97) |
| Sandoz 2013 [144] Lucena-Santos 2017 [145] | The Body Image-Acceptance and Action Questionnaire | Evaluates body image flexibility and dissatisfaction | Indirect | Females |
| Cash 1995 [146] | Body-Image Ideals Questionnaire | Attitudinal body-image assessment that considers physical attributes | Indirect | Female college students |
| Cash 2004 [147] | Body Image Disturbance Questionnaire | Assessed body image disturbance | Indirect | Male and female college students |
| Kong 2013 [7] | Figure Rating Scale | Identify body satisfaction and views on body shape | Indirect | Female athletes |
| Cooper 1987 [148] Goltz 2013 [149] | Body Shape Questionnaire | Identify concerns associated with body image | Indirect | Young men and women, athletes and non-athletes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagim, A.R.; Fields, J.; Magee, M.K.; Kerksick, C.M.; Jones, M.T. Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating. Nutrients 2022, 14, 986. https://doi.org/10.3390/nu14050986
Jagim AR, Fields J, Magee MK, Kerksick CM, Jones MT. Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating. Nutrients. 2022; 14(5):986. https://doi.org/10.3390/nu14050986
Chicago/Turabian StyleJagim, Andrew R., Jennifer Fields, Meghan K. Magee, Chad M. Kerksick, and Margaret T. Jones. 2022. "Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating" Nutrients 14, no. 5: 986. https://doi.org/10.3390/nu14050986
APA StyleJagim, A. R., Fields, J., Magee, M. K., Kerksick, C. M., & Jones, M. T. (2022). Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating. Nutrients, 14(5), 986. https://doi.org/10.3390/nu14050986







