Cyanidin-3-O-Glucoside Supplement Improves Sperm Quality and Spermatogenesis in a Mice Model of Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. C3G Preparation and Purification
2.2. Materials and Reagents
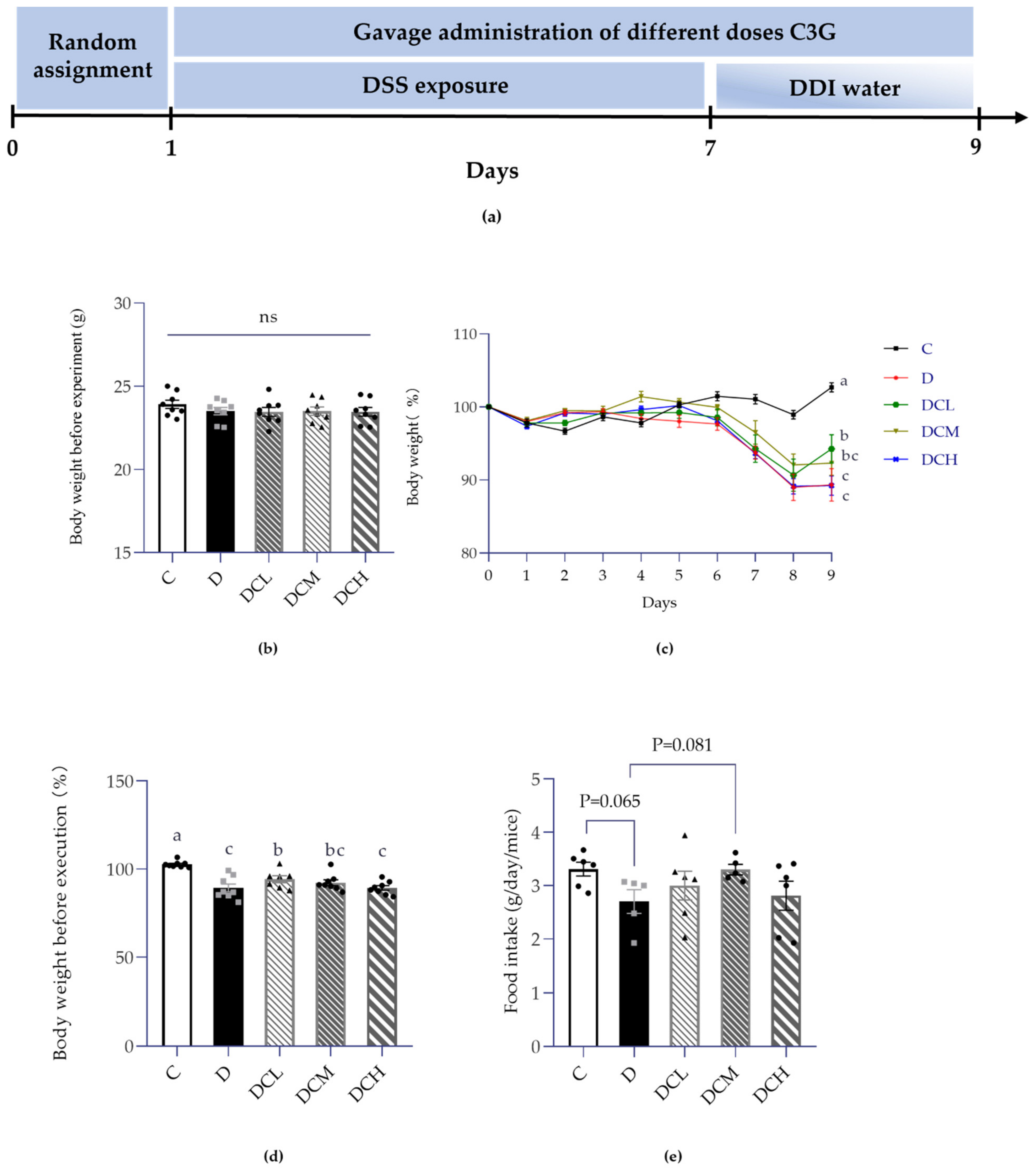
2.3. Experimental Protocol in Mice
2.3.1. Animals
2.3.2. Induction of UC Model and Treatment
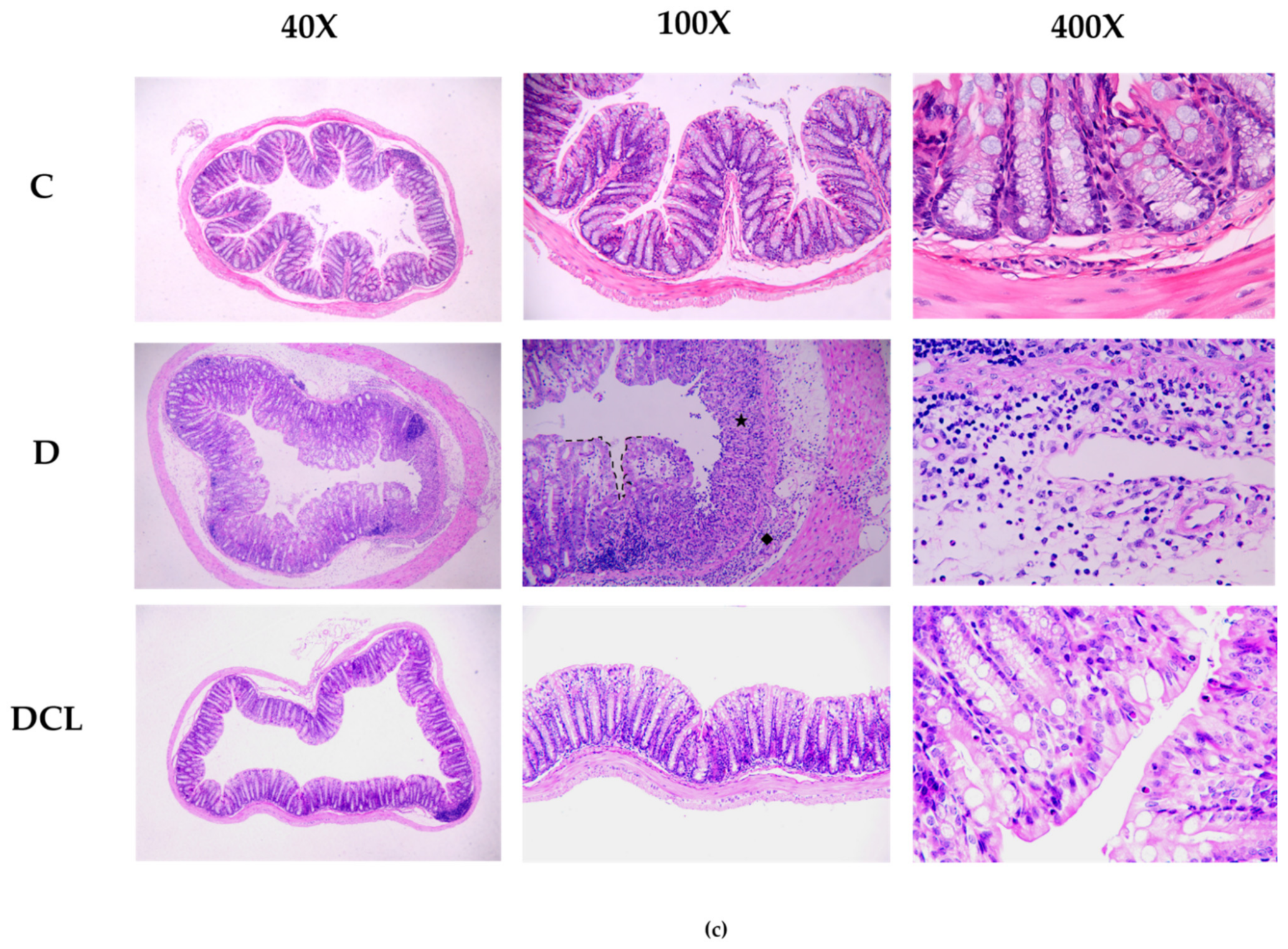
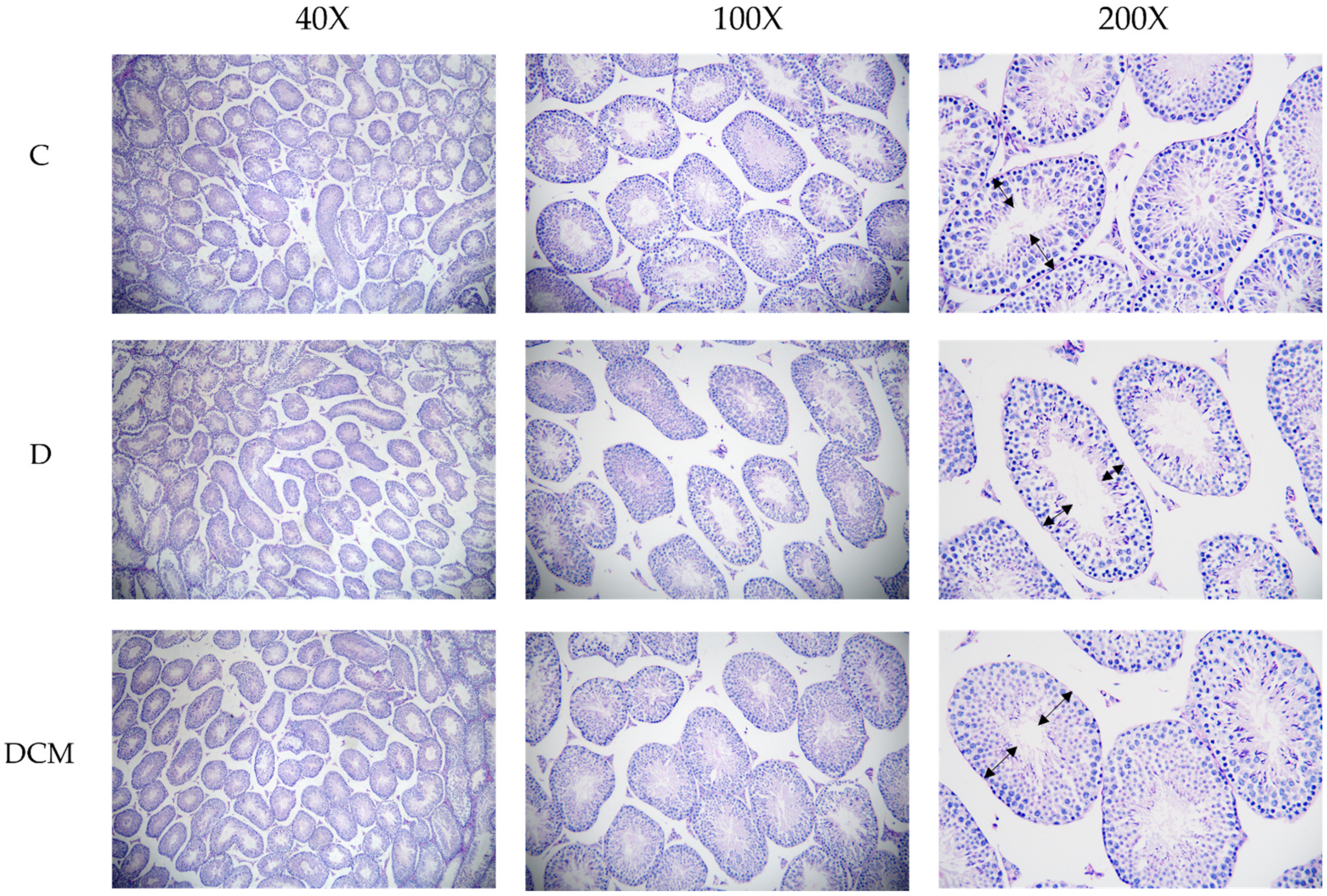
2.4. Evaluation of Histological Samples with H&E and PAS
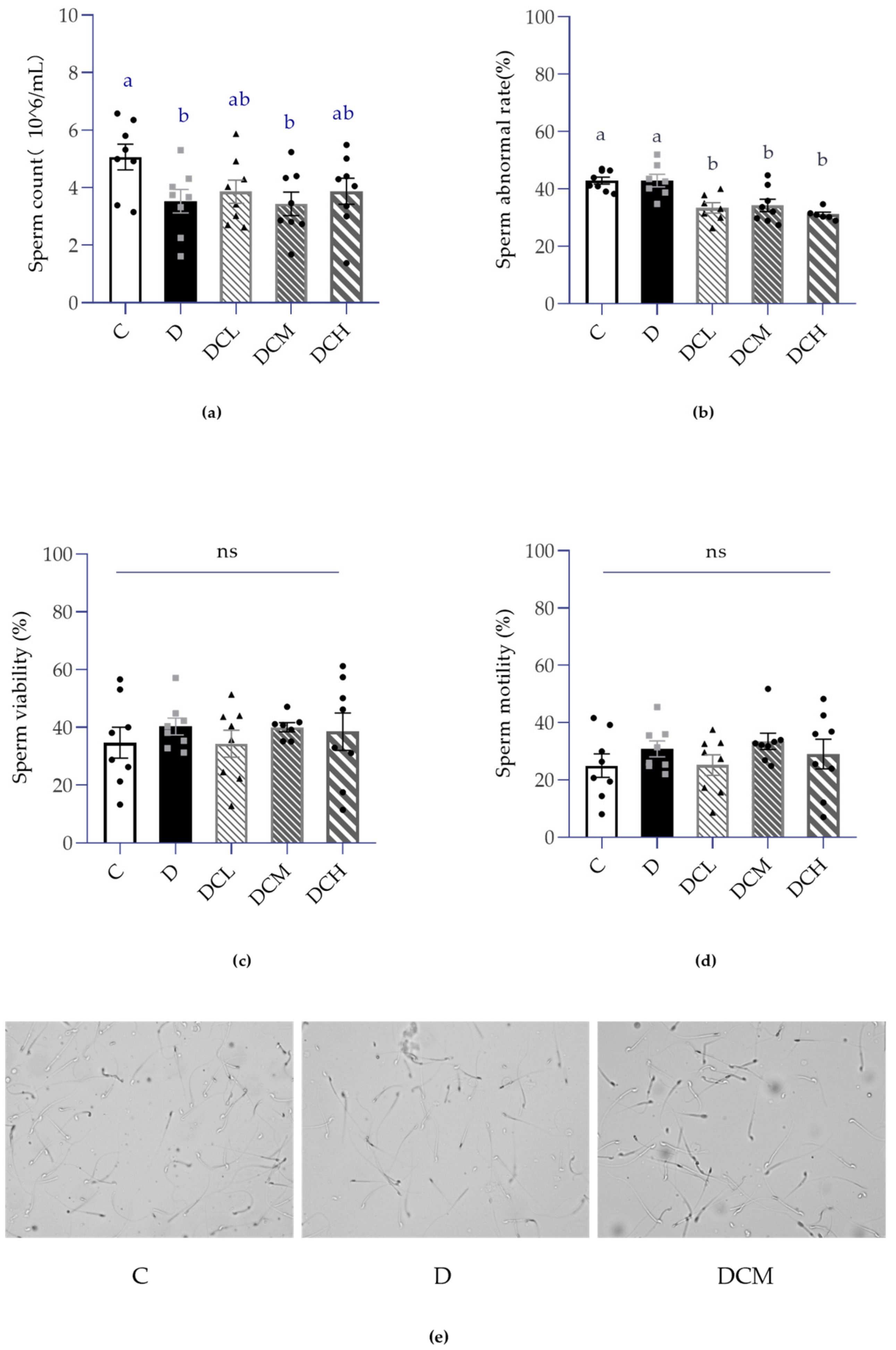
2.5. Measurement of Semen Quality
2.6. Analysis Serum of Parameters
2.7. Statistical Analysis
3. Results
3.1. Effect on Weight Gain and Food Intake
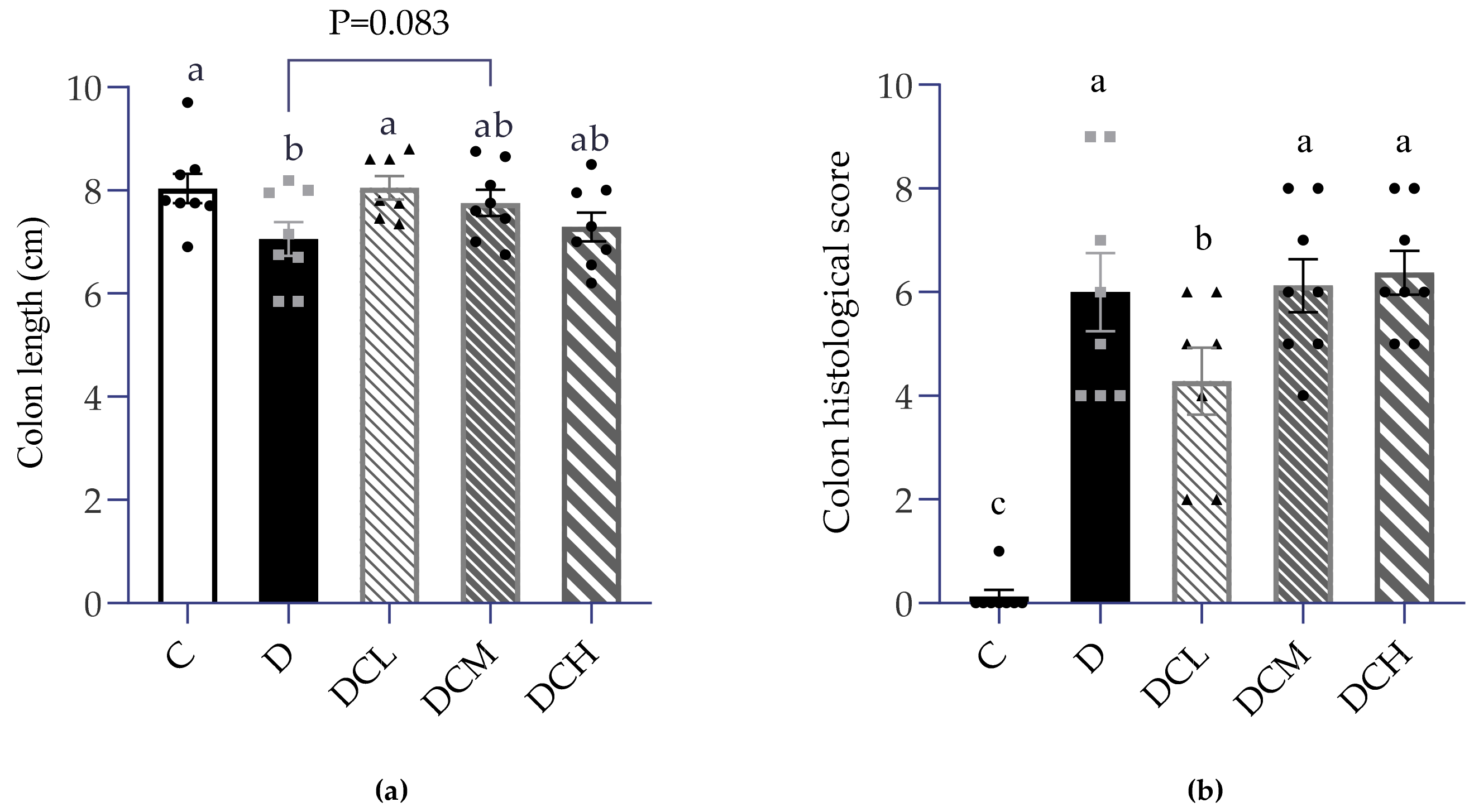
3.2. Low Dosage C3G Ameliorated Colonic Inflammation
3.3. Effects on Semen Parameters
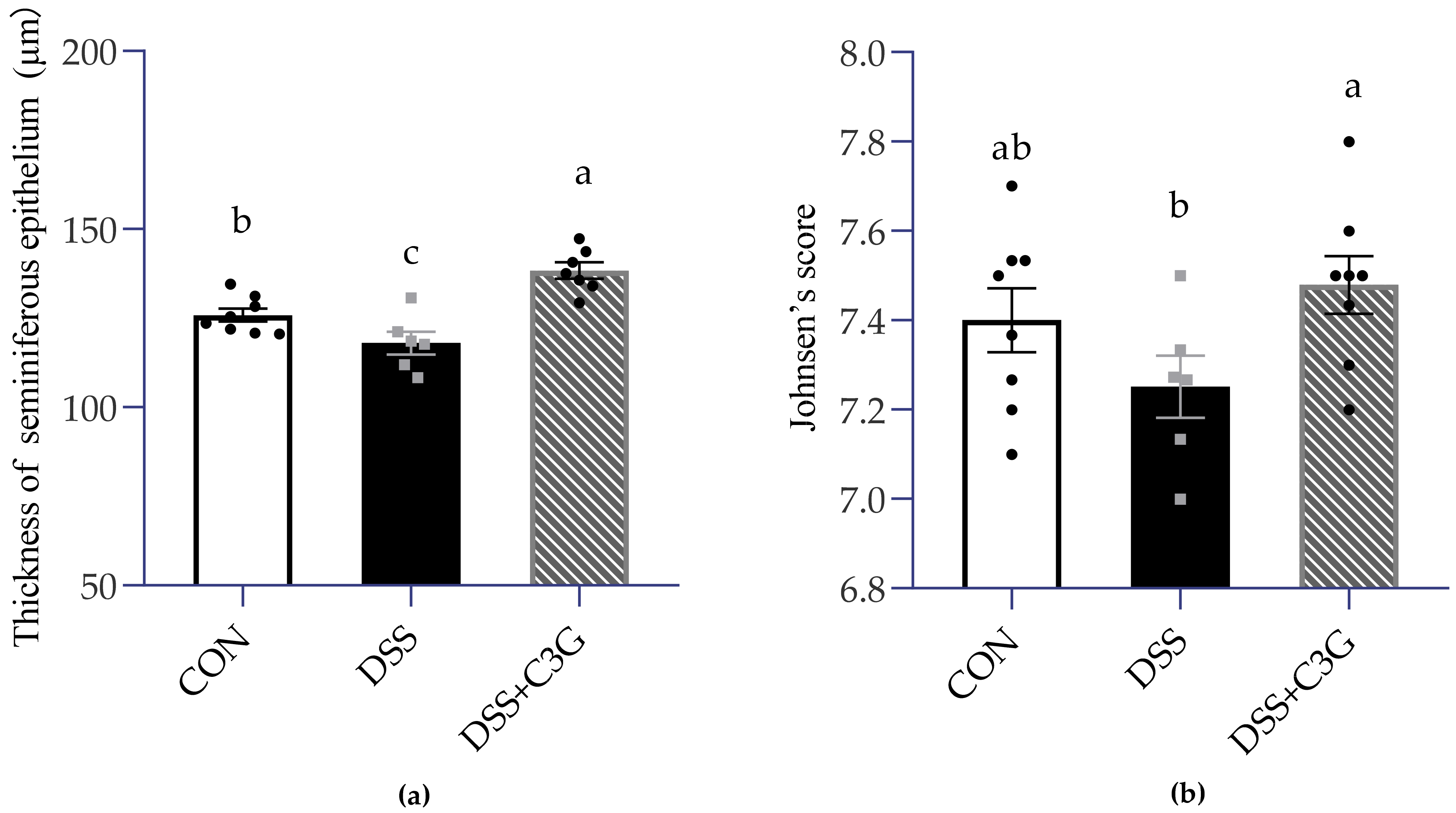
3.4. Impaired Spermatogenesis in the DSS-Induced Mice
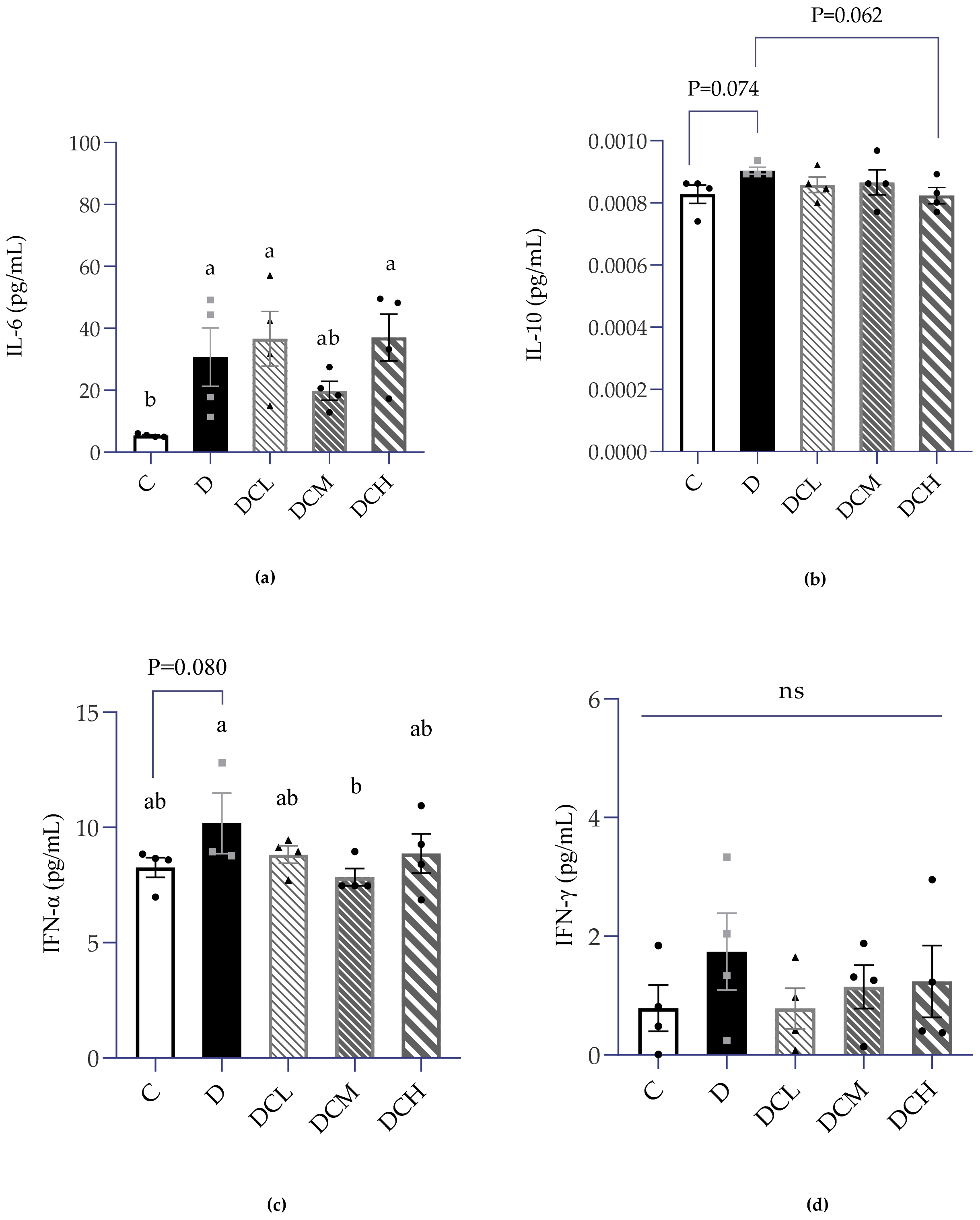
3.5. Expression of Inflammatory Factors in Serum
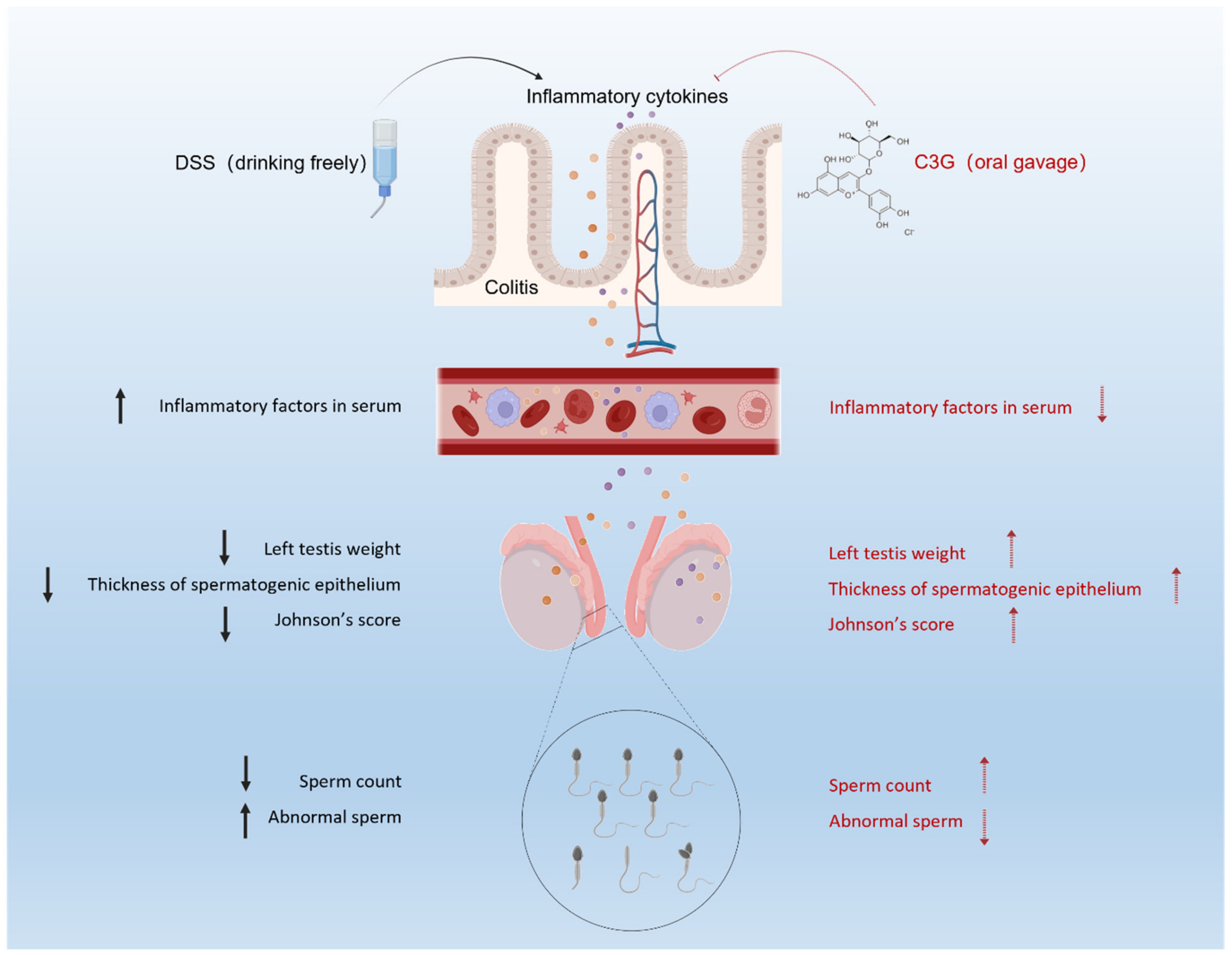
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Veysey, M.; Ersser, S.; Mason-Jones, A.; Galdas, P. The impact of inflammatory bowel disease on sexual health in men: A scoping review. J. Clin. Nurs. 2020, 29, 3638–3651. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Tamboli, C.P.; Jewell, D.; Colombel, J.F. Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology 2004, 126, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Karp, L.C. Defects in mucosal immunity leading to ulcerative colitis. Immunol. Rev. 2005, 206, 296–305. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A. A Systematic Review of Epidemiology and Risk Factors Associated with Chinese Inflammatory Bowel Disease. Front. Med. 2018, 5, 183. [Google Scholar] [CrossRef]
- Sturm, A.; White, L. Inflammatory Bowel Disease Nursing Manual; Springer: Cham, Switzerland, 2019; ISBN 3319750224. [Google Scholar]
- Grosen, A.; Bungum, M.; Christensen, L.A.; Cordelli, E.; Larsen, O.H.; Leter, G.; Julsgaard, M.; Vestergaard, T.; Villani, P.; Hvas, C.L.; et al. Semen Quality and Sperm DNA Integrity in Patients with Severe Active Inflammatory Bowel Disease and Effects of Tumour Necrosis Factor-alpha Inhibitors. J. Crohn’s Colitis 2019, 13, 564–571. [Google Scholar] [CrossRef]
- Parmar, A.R.; Trivedi, P.P.; Jena, G.B. Dextran sulfate sodium-induced ulcerative colitis leads to testicular toxicity in mice: Role of inflammation, oxidative stress and DNA damage. Reprod. Toxicol. 2014, 49, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Gujski, M.; Bojar, I.; Raczkiewicz, D.; Bartosinska, J.; Wdowiak-Filip, A.; Filip, R. Chronic Inflammation Impairs Male Fertility-A Case-Control Study in Ulcerative Colitis Patients. J. Clin. Med. 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Feagins, L.A.; Kane, S.V. Sexual and Reproductive Issues for Men with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2009, 104, 768–773. [Google Scholar] [CrossRef]
- Hammami, M.B.; Mahadevan, U. Men With Inflammatory Bowel Disease: Sexual Function, Fertility, Medication Safety, and Prostate Cancer. Am. J. Gastroenterol. 2020, 115, 526–534. [Google Scholar] [CrossRef]
- Azooz, O.G.; Farthing, M.J.G.; Savage, M.O.; Ballinger, A.B. Delayed puberty and response to testosterone in a rat model of colitis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, R1483–R1491. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Sharma, R.K.; Ollero, M.; Saleh, R.A.; Lopez, M.C.; Thomas, A.J.; Evenson, D.P.; Agarwal, A. Increased DNA damage in sperm from leukocytospermic semen samples as determined by the sperm chromatin structure assay. Fertil. Steril. 2002, 78, 319–329. [Google Scholar] [CrossRef]
- Sanocka, D.; Jędrzejczak, P.; Szumała-Kaękol, A.; Frączek, M.; Kurpisz, M. Male Genital Tract Inflammation: The Role of Selected Interleukins in Regulation of Pro-Oxidant and Antioxidant Enzymatic Substances in Seminal Plasma. J. Androl. 2003, 24, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Adamopoulos, D.A.; Lawrence, D.M.; Vassilopoulos, P.; Contoyiannis, P.A.; Swyer, G.I. Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br. Med. J. 1978, 1, 1177–1180. [Google Scholar] [CrossRef] [Green Version]
- Dimitrova, D.; Kalaydjiev, S.; Mendizova, A.; Piryova, E.; Nakov, L. Circulating antibodies to human spermatozoa in patients with ulcerative colitis. Fertil. Steril. 2005, 84, 1533–1535. [Google Scholar] [CrossRef]
- Prideaux, L.; De Cruz, P.; Ng, S.C.; Kamm, M.A. Serological antibodies in inflammatory bowel disease: A systematic review. Inflamm. Bowel Dis. 2012, 18, 1340–1355. [Google Scholar] [CrossRef]
- Lin, A.; Micic, D. Nutrition Considerations in Inflammatory Bowel Disease. Nutr. Clin. Pract. 2021, 36, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Unal, N.G.; Oruc, N.; Tomey, O.; Omer Ozutemiz, A. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S. Leptin and the onset of puberty: Insights from rodent and human genetics. Semin. Reprod. Med. 2002, 20, 139–144. [Google Scholar] [CrossRef]
- Kaminski, B.A.; Palmert, M.R. Genetic control of pubertal timing. Curr. Opin. Pediatr. 2008, 20, 458–464. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1913092. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The Anti-inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Ran, Y.; Li, X.; Jiang, X.; Chen, J.; Sun, J.; Tian, L.; Teerds, K.; Bai, W. Cyanidin-3-O-glucoside ameliorates cadmium induced uterine epithelium proliferation in mice. J. Hazard. Mater. 2022, 425, 127571. [Google Scholar] [CrossRef]
- Triebel, S.; Trieu, H.-L.; Richling, E. Modulation of Inflammatory Gene Expression by a Bilberry (Vaccinium myrtillus L.) Extract and Single Anthocyanins Considering Their Limited Stability under Cell Culture Conditions. J. Agric. Food Chem. 2012, 60, 8902–8910. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tian, L.-M.; Liu, Y.; Guo, K.-S.; Lv, M.; Li, Q.-T.; Hao, S.-Y.; Ma, C.-H.; Chen, Y.-X.; Tanaka, M.; et al. Low Dose of Cyanidin-3-O-Glucoside Alleviated Dextran Sulfate Sodium–Induced Colitis, Mediated by CD169+ Macrophage Pathway. Inflamm. Bowel Dis. 2019, 25, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Fu, Y.; Yang, L.; Chen, J.; Lei, H.; Liu, Q. Cyanidin-3-O-Glucoside and Cyanidin Protect Against Intestinal Barrier Damage and 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis. J. Med. Food 2020, 23, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixao, J.; Nunes, C.; Dinis, T.C.P.; Almeida, L.M. Cyanidin-3-Glucoside Suppresses Cytokine-Induced Inflammatory Response in Human Intestinal Cells: Comparison with 5-Aminosalicylic Acid. PLoS ONE 2013, 8, e73001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Li, M.; Zou, F.; Bai, S.; Jiang, X.; Tian, L.; Ou, S.; Jiao, R.; Bai, W. Protection of cyanidin-3-O-glucoside against acrylamide- and glycidamide-induced reproductive toxicity in leydig cells. Food Chem. Toxicol. 2018, 119, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, C.; Li, X.; Sun, J.; Tian, L.; Bai, W. Cyanidin-3-O-glucoside at Low Doses Protected against 3-Chloro-1,2-propanediol Induced Testis Injury and Improved Spermatogenesis in Male Rats. J. Agric. Food Chem. 2018, 66, 12675–12684. [Google Scholar] [CrossRef]
- Li, X.; Yao, Z.; Yang, D.; Jiang, X.; Sun, J.; Tian, L.; Hu, J.; Wu, B.; Bai, W. Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium-exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J. Hazard. Mater. 2020, 387, 121706. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Zhu, C.; Sun, J.; Tian, L.; Chen, W.; Bai, W. The target cells of anthocyanins in metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2019, 59, 921–946. [Google Scholar] [CrossRef]
- Koga, N. Efficacy and Safety Measures for Low Density Lipoprotein Apheresis Treatment Using Dextran Sulfate Cellulose Columns. Ther. Apher. 1999, 3, 155–160. [Google Scholar] [CrossRef]
- Hamamoto, N.; Maemura, K.; Hirata, I.; Murano, M.; Sasaki, S.; Katsu, K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin. Exp. Immunol. 1999, 117, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jayachandran, M.; Zhang, W.; Chen, L.; Du, B.; Yu, Z.; Xu, B. Dietary Supplementation with Sea Bass (Lateolabrax maculatus) Ameliorates Ulcerative Colitis and Inflammation in Macrophages through Inhibiting Toll-Like Receptor 4-Linked Pathways. Int. J. Mol. Sci. 2019, 20, 2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsen, S.G. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, Y.; Zhou, J.; Tang, X.; Wang, P.; Shen, L.; Chen, S. Changes in serum inflammatory cytokine levels and intestinal flora in a self-healing dextran sodium sulfate-induced ulcerative colitis murine model. Life Sci. 2020, 263, 118587. [Google Scholar] [CrossRef]
- Wu, H.; Rao, Q.; Ma, G.-C.; Yu, X.-H.; Zhang, C.-E.; Ma, Z.-J. Effect of Triptolide on Dextran Sodium Sulfate-Induced Ulcerative Colitis and Gut Microbiota in Mice. Front. Pharmacol. 2020, 10, 1652. [Google Scholar] [CrossRef]
- Luo, S.; Wen, R.; Wang, Q.; Zhao, Z.; Nong, F.; Fu, Y.; Huan, S.; Chen, J.; Zhou, L.; Luo, X. Rhubarb Peony Decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J. Ethnopharmacol. 2019, 231, 39–49. [Google Scholar] [CrossRef]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.-F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [Green Version]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Pecoits, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol. Dial. Transplant. 2002, 17, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.-T.; Lin, C.-Y.; Ho, D.H.H.; Peng, J.; Chen, Y.; Tsang, S.-W.; Wong, M.; Zhang, X.-J.; Zhang, M.; Bian, Z.-X. Inhibitory Effect of the Gallotannin Corilagin on Dextran Sulfate Sodium-Induced Murine Ulcerative Colitis. J. Nat. Prod. 2013, 76, 2120–2125. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohle, G.R.; Elzanaty, S.; van Casteren, N.J. Testicular biopsy: Clinical practice and interpretation. Asian J. Androl. 2012, 14, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, T.A.; Pariz, J.R.; Dutra, R.T.; Saldiva, P.H.; Costa, E.; Hallak, J. Cut-off values of the Johnsen score and Copenhagen index as histopathological prognostic factors for postoperative semen quality in selected infertile patients undergoing microsurgical correction of bilateral subclinical varicocele. Transl. Androl. Urol. 2019, 8, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Lo, H.Y.; Cheng, S.P.; Chang, K.T.; Lin, X.F.; Liao, K.S.; Huang, J.H.; Hsieh, M.F.; Chan, C.K. The potential of a single injection of hUCMSCs in relevant testis injury and related severity of DSS induced acute and chronic colitis. Res. Sq. 2022, preprints. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Scholtz, I.; Keita, A.V. Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease-Focusing on Intestinal Barrier Function. Cells 2019, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Hosomi, S.; Watanabe, K.; Watanabe, K.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; Kamata, N.; et al. Serum interleukin-6 level is associated with response to infliximab in ulcerative colitis. Scand. J. Gastroenterol. 2018, 53, 579–585. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, L.; Li, H.; Yang, W.; Li, M. Protective effects of Lepidium draba L. leaves extract on testis histopathology, oxidative stress indicators, serum reproductive hormones and inflammatory signalling in oxymetholone-treated rat. Andrologia 2021, 53, 14239. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, M.; Zhang, J.; Li, J.; Gong, D.; Li, Y.; Zhang, J.; Lin, P.; Huang, L. IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway. Sci. Rep. 2016, 6, 28012. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Z.; Feng, P.; Ye, Z.; Li, R.; Liu, J.; Hu, J.; Kakade, A.; Liu, P.; Li, X. Lactobacillus plantarum TW1-1 Alleviates Diethylhexylphthalate-Induced Testicular Damage in Mice by Modulating Gut Microbiota and Decreasing Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Xu, B.; Bordiga, M.; Li, H.; Travaglia, F.; Bai, S.; Chen, J.; Bai, W. Cyanidin-3-O-Glucoside Supplement Improves Sperm Quality and Spermatogenesis in a Mice Model of Ulcerative Colitis. Nutrients 2022, 14, 984. https://doi.org/10.3390/nu14050984
Xiao Y, Xu B, Bordiga M, Li H, Travaglia F, Bai S, Chen J, Bai W. Cyanidin-3-O-Glucoside Supplement Improves Sperm Quality and Spermatogenesis in a Mice Model of Ulcerative Colitis. Nutrients. 2022; 14(5):984. https://doi.org/10.3390/nu14050984
Chicago/Turabian StyleXiao, Yuhang, Baojun Xu, Matteo Bordiga, Haiwei Li, Fabiano Travaglia, Shun Bai, Jiali Chen, and Weibin Bai. 2022. "Cyanidin-3-O-Glucoside Supplement Improves Sperm Quality and Spermatogenesis in a Mice Model of Ulcerative Colitis" Nutrients 14, no. 5: 984. https://doi.org/10.3390/nu14050984
APA StyleXiao, Y., Xu, B., Bordiga, M., Li, H., Travaglia, F., Bai, S., Chen, J., & Bai, W. (2022). Cyanidin-3-O-Glucoside Supplement Improves Sperm Quality and Spermatogenesis in a Mice Model of Ulcerative Colitis. Nutrients, 14(5), 984. https://doi.org/10.3390/nu14050984







